Abstract
Robotic thyroidectomy is an emerging technique with postoperative outcomes that are at least comparable to those of conventional endoscopic thyroidectomy, with some end-points appearing superior. Our multicenter series represents the largest comparison of robotic and endoscopic thyroidectomy to date, with results suggesting a comparable robot technology we used that could overcome some of the technical limitations associated with conventional endoscopic procedures, with reduced operation times and increased lymph node retrieval. Moreover, we found that the learning curve for robotic thyroidectomy was shorter than that for endoscopic thyroidectomy.
1. Introduction
Endoscopic surgical techniques for thyroid cancer surgery can benefit patients by eliminating the anterior neck incision utilized in the traditional open approach. In addition to superior cosmetic results, endoscopic thyroidectomy can reduce postoperative pain and discomfort, shorten hospital stay, and enhance postoperative recovery [1–4]. Despite these advantages, however, endoscopic thyroidectomy has technical limitations, including the use of straight, rigid endoscopic instruments without articulation and a 2Dimensional (2D) view.
The recent introduction of the da Vinci robot surgical system may be a major improvement in extracervical approaches for thyroid surgery and may be more ergonomic for surgeons than the endoscopic approach [5–7]. Among the advantages of the da Vinci robot are improved visualization via a 3D view, magnification, a tremor-filtering system, and instrument flexibility. To date, however, few studies have compared the postoperative outcomes in patients undergoing robotic and endoscopic thyroid surgery [8–11].
At present, endoscopic techniques are regarded as too time consuming and technically demanding to be adopted on a large-scale. The learning curve for endoscopic thyroidectomy performed by skilled endocrine surgeons has been estimated to be approximately 60 patients [12]. Use of a robot in thyroid surgery may shorten the learning curve, by providing a broader view of the surgical field and easier access to deep and narrow spaces through the use of multiarticulated instruments. We have previously reported the results of a multicenter study of learning curves for robotic thyroidectomy, based on a scientific analysis of a range of perioperative parameters [13]. However, there have been few comparisons of learning curves for robotic and endoscopic thyroidectomies.
At present, the benefits of robotic thyroidectomy relative to endoscopic thyroidectomy, as determined by oncologic and functional outcomes, have not been fully clarified. Moreover, there have been no multicenter studies comparing learning curves for these two methods. We therefore compared the operative outcomes and surgical learning curves of robotic and endoscopic thyroidectomy in patients with differentiated thyroid carcinoma (DTC).
2. Materials and Methods
2.1. Study Patients
This comparative, multicenter study evaluated patients who underwent robotic or endoscopic thyroidectomy at three large-volume centers with considerable experience in thyroid cancer surgery. Clinical and pathological data were collected retrospectively at each institution and entered into a dedicated database for analysis. Between November 2001 and June 2010, 2,612 patients with DTC underwent thyroidectomies at three centers, with all operations performed by four surgeons. Of these patients, 1796 underwent robotic thyroidectomy and 843 underwent conventional endoscopic thyroidectomy. At the time of surgery there was no intention to compare the two procedures. This study was approved by the institutional review boards (IRBs).
At all institutions, perioperative workup included physical examination, high-resolution ultrasonography (US), and neck computed tomography (CT) and/or neck magnetic resonance imaging (MRI); preoperative staging US and neck CT (or MRI) was utilized to evaluate the degree of tumor invasion, including tumor size, extrathyroidal invasion, tumor infiltration of adjacent structures, and nodal involvement. Sonographic findings, such as loss of an echogenic thyroid capsule at the contact site of the primary tumor or contact with an adjacent thyroid capsule along more than 25% of the boundary of a tumor, were considered indicative of extrathyroidal extension [14].
In accordance with American Thyroid association (ATA) guidelines, a less than total thyroidectomy was performed in patients <45 years old, with a single lesion <1 cm in size, no definitive evidence of extrathyroidal invasion or lymph node metastasis, no personal history of radiation therapy to the head or neck, and no first-degree family history of DTC [15]. A total thyroidectomy was performed in patients with multiple or bilateral lesions, or if definite extrathyroidal invasion was discovered during surgery. All patients underwent prophylactic ipsilateral pretracheal, prelaryngeal, and paraesophageal central compartment neck dissection (CCND).
2.2. Postoperative Outcomes
Clinical parameters analyzed included patient characteristics, operative variables, extent of surgery, pathologic findings, and short-term operative outcomes. Pathologic examinations included assessments of disease tumor-node-metastasis (TNM) stage, number of lymph nodes harvested, and number of metastatic lymph nodes. We also assessed perioperative complications, including hematoma, seroma, vocal cord palsy, hypocalcemia, trachea injury, esophageal injury, chyle leakage, and brachial plexus neuropraxia. All patients were followed up in the same manner at the three centers, including clinical examinations within 1 week of discharge and a 3-to-6-month follow up that included a physical examination, neck US, assay of tumor markers (serum thyroglobulin concentration), and/or a 131radioactive iodine (131RAI) scan.
2.3. Learning Curves
To evaluate the learning curve for robotic versus endoscopic thyroidectomy, we used a protocol similar to those previously described for the evaluation of learning curves for thyroid surgery [5, 9]. That is, we assessed groups of about 100 (96–130) patients who underwent robotic and endoscopic less than total thyroidectomy performed by four surgeons at the time they started performing these operations independently. Each patient was assigned a case number without regard to tumor size or lymph node metastasis. Operation time was defined as the time from first incision to the completion of skin closure and included docking and undocking of the robot. Surgical learning curves were analyzed using a moving average method. The surgical techniques we use for endoscopic and robotic thyroidectomy have been described in detail elsewhere [8, 17–19].
2.4. Statistics
Data were analyzed using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). All data are expressed as means ± standard deviations (SDs), proportions, or absolute numbers. Continuous data were compared using Student's t-tests, and categorical data were analyzed using chi-squared or Fisher's exact tests. A P value < 0.05 was considered statistically significant.
A moving average method was used to analyze operation time and learning curves. Creating an average that “moves” with the addition of new data results in “smoothing” of the process being analyzed, thus reducing the effects of fluctuations. We used a moving average of 20 to reduce variations and accentuate trends [20].
3. Results
3.1. Demographic and Clinical Data
Table 1 shows the demographic data and extent of surgery for the 2,612 patients included in this study. Mean age and gender distribution were equivalent for the robotic and endoscopic groups, but types of operation differed significantly. Less than total thyroidectomy was performed in 693 patients (82.2%) in the endoscopic group and in 1,063 (60.1%) in the robotic group, whereas total thyroidectomy was performed in 150 patients (17.8%) in the endoscopic group and in 706 (39.9%) in the robotic group (P = 0.004). Modified radical neck dissection (MRND) was also performed more frequently in the robotic than in the endoscopic group (3.4% versus 1.7%, P < 0.001).
Table 1.
Demographics and extent of surgery in patients with thyroid carcinoma treated by robotic thyroidectomy versus endoscopic thyroidectomy.
| Robot group (n = 1769) | Endoscopy group (n = 843) | P value | |
|---|---|---|---|
| Age, mean (years), range | 39.4 ± 9.1 (13–70) | 37.5 ± 9.4 (6–50) | NS* |
| Gender | |||
| Female | 1637 (92.5%) | 824 (97.7%) | NS |
| Male | 132 (7.5%) | 19 (2.3%) | |
| Extent of surgery | |||
| Less than total | 1063 (60.1%) | 693 (82.2%) | 0.004 |
| Unilateral total | 595 (33.6%) | 307 (36.4%) | |
| Unilateral total + partial | 177 (10.0%) | 208 (24.7%) | |
| Unilateral total + subtotal | 291 (16.4%) | 178 (21.1%) | |
| Total | 706 (39.9%) | 150 (17.8%) | |
| Extent of neck node dissection | |||
| No dissection | 23 (1.3%) | 59 (7.0%) | <0.001 |
| CCND** | 1675 (94.7%) | 770 (91.3%) | NS |
| Selective node dissection | 11 (0.6%) | 0 (0%) | NS |
| MRND§ | 60 (3.4%) | 14 (1.7%) | <0.001 |
NS*: nonspecific finding, CCND**: central compartment neck dissection, MRND§: modified radical neck dissection.
3.2. Pathologic Findings and Perioperative Outcomes
Pathologic results are shown in Table 2. There were no significant between differences groups in type of tumor, tumor size, multifocality, and bilaterality. However, the mean number of harvested lymph nodes was significantly higher in the robotic than in the endoscopic group (4.5 ± 2.6 versus 2.9 ± 1.7, P < 0.001). More advanced T stage (P = 0.011), N stage (P = 0.001), and TNM stage (P = 0.002) tumors occurred more frequently in the robotic group. All 8 patients with T4a lesions showed local invasion of recurrent laryngeal nerve detected during operation, which could be not be noticed in preoperative imaging study, and underwent Maryland or endoscopic dissector shaving procedure.
Table 2.
Comparison of pathologic findings between patients treated with robotic thyroidectomy and endoscopic thyroidectomy.
| Robot group (n = 1769) | Endoscopy group (n = 843) | P value | |
|---|---|---|---|
| Pathology | NS | ||
| Papillary carcinoma | 1758 (99.3%) | 837 (99.3%) | |
| Follicular carcinoma | 5 (0.3%) | 6 (0.7%) | |
| Medullary carcinoma | 5 (0.3%) | ||
| Hurthle cell carcinoma | 1 (0.1%) | ||
| Tumor size, mean (cm) | 0.5 ± 0.5 | 0.4 ± 0.5 | NS |
| Multifocality | NS | ||
| Yes | 469 (26.5) | 110 (13.0%) | |
| No | 1300 (73.5) | 733 (87.0%) | |
| Bilaterality | NS | ||
| Yes | 208 (11.8%) | 72 (8.5%) | |
| No | 1561 (88.2%) | 771 (91.5%) | |
| Mean retrieved central LN (n) | 4.5 ± 2.6 | 2.9 ± 1.7 | <0.001 |
| Mean metastatic central LN (n) | 1.2 ± 0.9 | 1.0 ± 0.7 | NS |
| TNM stage | |||
| T1 | 905 (51.2%) | 513 (60.9%) | 0.011 |
| T2 | 16 (0.9%) | 15 (1.8%) | |
| T3 | 841 (47.5%) | 314 (37.2%) | |
| T4a | 7 (0.4%) | 1 (0.1%) | |
| N0 | 1131 (64.0%) | 605 (71.7%) | 0.001 |
| N1a | 570 (32.2%) | 224 (26.6%) | |
| N1b | 68 (3.8%) | 14 (1.7%) | |
| Stage | 0.002 | ||
| Stage I | 1480 (83.7%) | 750 (89.0%) | |
| Stage II | 275 (15.5%) | 92 (10.9%) | |
| Stage IVa | 14 (0.8%) | 1 (0.1%) |
LN: lymph node.
TNM: tumor-node-metastasis.
Total operation time for subtotal thyroidectomy was similar in the two groups, but was significantly shorter in the robotic than in the endoscopic group for total thyroidectomy (P < 0.001). Postoperative hospital stay did not differ significantly (Table 3). Among the perioperative complications observed, transient hypocalcemia and transient hoarseness were the most frequent causes of postoperative morbidity, but there were no between differences groups in incidence. Transient hoarseness resolved in all patients within 6 months, as confirmed by postoperative laryngoscopy, and transient hypocalcemia resolved within 3 months. Transient traction injury from brachial plexus neuropraxia was observed in 3 patients in the robotic and 1 in the endoscopic group. This impairment resulted in pain and movement disorder of the shoulder and upper arm, but resolved spontaneously within 3 months.
Table 3.
Comparison of perioperative outcomes between patients treated with robotic thyroidectomy and endoscopic thyroidectomy.
| Robot group (n = 1769) | Endoscopy group (n = 843) | P value | |
|---|---|---|---|
| Total operation time (min), range | |||
| Total thyroidectomy | 149.2 ± 32.3 | 172.7 ± 66.7 | <0.001 |
| Subtotal thyroidectomy | 122.3 ± 32.4 | 127.2 ± 41.3 | NS |
| Postoperative hospital stay (days) | 3.3 ± 1.3 | 3.4 ± 1.1 | NS |
| Postoperative complications (n) | |||
| Transient hypocalcemia | 276/706 (39.1%) | 55/150 (36.7%) | NS |
| Permanent hypocalcemia | 0 (0%) | 2 (0.2%) | |
| Transient hoarseness | 68 (3.8%) | 41 (4.9%) | |
| Permanent hoarseness | 8 (0.5%) | 1 (0.1%) | |
| Flap hematoma | 10 (0.6%) | 8 (0.9%) | |
| Observation | 8 (0.5%) | 5 (0.6%) | |
| Reoperation | 2 (0.1%) | 3 (0.4%) | |
| Seroma | 40 (2.3%) | 19 (0.3%) | |
| Tracheal injury | 3 (0.2%) | 4 (0.5%) | |
| Esophageal injury | 0 (0%) | 0 (0%) | |
| Transient chyle leakage | 6 (0.3%) | 3 (0.4%) | |
| Transient traction injury | 3 (0.2%) | 1 (0.1%) | |
| d/t brachial plexus neuropraxia |
Of the 856 patients who underwent bilateral total thyroidectomy, 481 underwent 131RAI ablation (range, 30–150 mCi) and a 131RAI scan 5–7 days after 131RAI ablation. The remaining 375 patients did not undergo 131RAI ablation as they were deemed low risk (356 patients) or chose not to undergo such treatment (19 patients). No patient showed abnormal uptake on 131I whole body scans. At the time of 131RAI ablation (TSH stimulated), mean serum thyroglobulin levels were checked, serum thyroglobulin in 362 (75.2%) was <1 ng/mL and in the remaining 119 (24.8%) was >1 ng/mL (3.1 ± 1.8, range 1.2~9.6 ng/mL). Serum thyroglobulin levels were measured in 1,851 patients at 6–12 months postoperatively (TSH suppressed), with most being maintained at the lowest level (<1 ng/mL). Mean thyroglobulin concentrations did not differ significantly in the robotic and endoscopic groups (0.72 ± 1.84 ng/mL versus 0.61 ± 1.99 ng/mL). Follow-up neck US and neck CT showed recurrence in six patients, 3 in each group, with 3 recurrences in a lateral neck node and 3 in the contralateral thyroid gland.
3.3. Learning Curves for Robotic and Endoscopic Thyroidectomies
When we compared the data on about 100 individual patients who underwent robotic and endoscopic less than total thyroidectomy by all involved surgeons, we observed no significant differences in patient-selection criteria. For both procedures, the operation times gradually decreased with accumulating experience. For all 4 surgeons, operation times reached a plateau after 35–45 robotic and 55–70 endoscopic less than total thyroidectomies (Figure 1).
Figure 1.
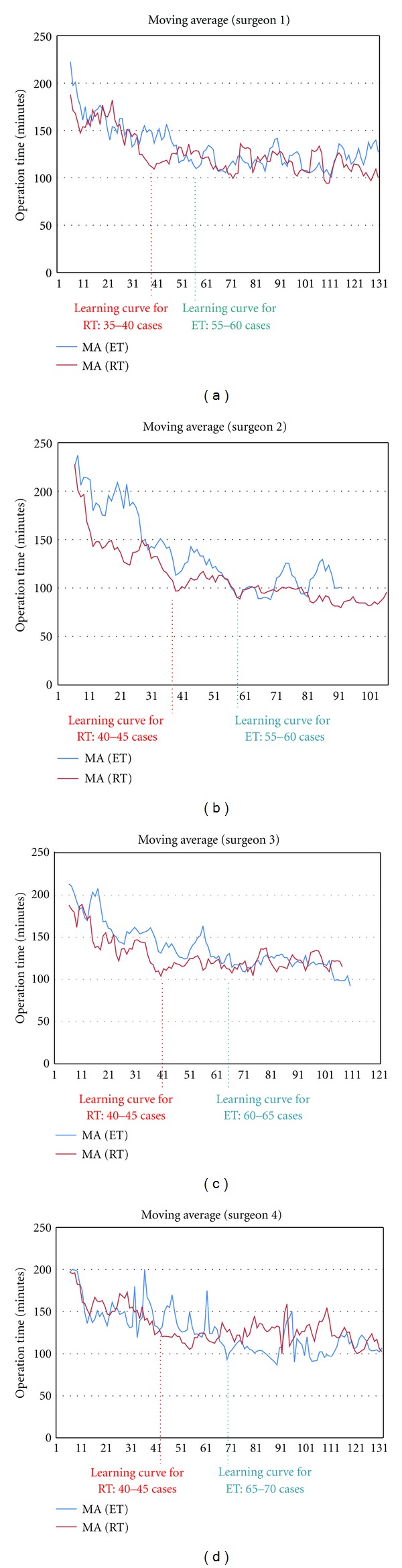
(a–d) Individual learning curves for robotic thyroidectomy (RT) and endoscopic thyroidectomy (ET); The graph plots the time taken to perform each procedure as a function of the number of patients. The moving average method was used to determine changes in operation times for each surgeon. The time required for each surgeon to perform RT decreased after 35–50 patients, whereas the time required by each to perform ET decreased after 55–70 patients.
4. Discussion
Robotic thyroidectomy is an emerging technique with early outcomes that are at least comparable to those of conventional endoscopic thyroidectomy, with some end points appearing superior. Our series represents the largest comparison of robotic and endoscopic thyroidectomy to date, with results suggesting comparable operation times, perioperative outcomes, and complications. In addition, early-term oncologic outcomes demonstrated that robotic thyroidectomy resulted in acceptably complete resection and radical dissection, with an extremely low rate of recurrent disease at follow up. Moreover, to our knowledge, this study is the first multicenter trial to compare the learning curves of robotic and endoscopic thyroidectomy.
4.1. Comparison of Perioperative Outcomes with Robotic and Endoscopic Thyroidectomy
The goal of thyroidectomy plus neck dissection in patients with thyroid cancer is the complete surgical removal of the entire thyroid gland, along with radical cervical lymphadenectomy when necessary. Other goals include rapid convalescence and complete preservation of the recurrent and superior laryngeal nerves and the parathyroid gland. Only a few previous large series have compared perioperative outcomes in patients undergoing robotic and endoscopic thyroidectomy [8–11] (Table 4). Our previous experience suggests that robotic thyroidectomy may be associated with decreased operation times and increased harvest of cervical LNs, outcomes related to the operative dexterity of the flexible robotic instruments, and a 3D surgical view [5–9]. In contrast, endoscopic thyroid dissection and lymph node retrieval may be more difficult and time consuming because of the straight angles of the endoscopic instruments and a 2D operative field [1–4]. Another superiority of robotic thyroidectomy over conventional endoscopic thyroidectomy is that surgeons use three arms during operation. In conventional endoscopy, the surgeon can steer only two arms during dissection, and this makes it difficult to create appropriate conditions for dissection. However, in robot technique, the surgeon can handle the Prograsp forceps during using the Maryland dissector, optimal dissection planes can be obtained by applying traction and countertraction to thyroid gland, and could position the thyroid gland to allow fine, dexterous dissection after swapping arms by controlling robotic arms himself [5–8].
Table 4.
Endoscopic versus robotic thyroidectomy studies. Case series comparing perioperative outcomes.
| Author, year | Study design | No. (patients) | Approach | Comparative parameters | Perioperative results | Complication rate | Oncologic safety (surgical completeness such as harvested lymph node) | Other notable findings |
|---|---|---|---|---|---|---|---|---|
| Lee et al. (2011) [9] | Prospective, controlled, single surgeon | RT* (163) versus ET§ (96) | GT** | Perioperative outcomes, surgical learning curve |
Operation time (ET > RT) Learning curve (ET > RT) Advanced cancer (RT > ET) |
No difference | Retrieved LN (RT > ET) | First comparative study between RT and ET. Showed superiority of RT in terms of operation time, lymph node retrieval, and learning curve |
|
| ||||||||
| Lang and Chow (2011) [11] | Retrospective, controlled, single surgeon | RT (7) ET (39) | GT | Perioperative outcomes | Operation time (RT > ET) |
No difference | No data | Described initial experience of RT in Hong Kong |
|
| ||||||||
| Lee et al. (2011) [8] | Retrospective, controlled, single center | RT (580) ET (570) |
GT | Perioperative outcomes | Operation time (ET > RT) Advanced cancer (RT > ET) |
Transient- hypoparathyroidism (RT > ET) |
Retrieved LN (RT > ET) | RT was found to be superior to ET in terms of operation time, and LN retrieval. |
|
| ||||||||
| Kim et al. (2011) [10] | Retrospective, single center | RT (69) ET (95) OT (138) |
BABA§§ | Perioperative outcomes | Operation time (RT > ET > OT#) |
No difference | Surgical completeness (RT = OT > ET) Retrieved LN (RT = OT = ET) |
First comparative study of RT versus OT by analyzing postoperative outcomes |
RT*: robotic thyroidectomy, GT**: gasless transaxillary approach, ET§: endoscopic thyroidectomy, BABA§§: bilateral axillo-breast approach, OT#: open thyroidectomy.
A recent retrospective comparison of 580 patients who underwent robotic thyroidectomy and 570 who underwent conventional endoscopic thyroidectomy at a single center found that the operation time was shorter and the mean number of central LNs retrieved greater in the robotic than in the endoscopic group [8]. These findings were also observed in a recent comparison of robotic and endoscopic thyroidectomy performed by a single surgeon [9], providing further evidence that the robotic technique provides better results that conventional endoscopy in thyroid cancer patients [8–11]. Table 4 summarizes the published findings in studies comparing robotic and endoscopic thyroidectomy. Our findings were similar, in that mean operation times were 13.6% shorter (P < 0.001) and the numbers of LNs retrieved significantly greater (P < 0.001) for robotic than for endoscopic total thyroidectomy.
Objective comparisons are limited, however, by the lack of long-term surgical outcomes and by nonuniformity in defining, assessing, and reporting postoperative outcomes. Larger prospective studies, with uniform definitions of parameters, methodologies of data collection, and times of assessment are needed to compare operative outcomes of the two procedures.
4.2. Learning Curves for Robotic and Endoscopic Thyroidectomy
The operation time required by a single surgeon to perform robotic thyroidectomy using a gasless transaxillary approach was found to reach a plateau after 40–45 operations [5]. In addition, the learning curve for inexperienced surgeons to perform robotic thyroidectomy was 35–40 operations, whereas that for endoscopic thyroidectomy using a gasless transaxillary approach was 55–60 operations [9]. An extensive review of the outcomes of learning curves for robotic thyroidectomy in the Republic of Korea categorized patients into two groups, those treated by surgeons with and without experience in robotic thyroid surgery [13]. The learning curves for robotic thyroidectomy were 50 for total and 40 for less than total thyroidectomies. Moreover, once through the learning curve period, operation times and perioperative parameters for previously inexperienced surgeons were similar to those of the experienced surgeons, indicating that the former had acquired the necessary technical skills to perform robotic thyroidectomy successfully [13]. Despite different methodologies and variables in different studies, the results of these investigations of surgical learning curves for robotic thyroidectomy showed similar operation time-related results [5, 9, 13, 16] (Table 5). Similar to these studies, we found that the learning curve was significantly shorter for the robotic than for the endoscopic technique. For all 4 enrolled surgeons at 3 centers, the learning curves were found to be 35–45 patients for robotic and 55–70 for endoscopic less than total thyroidectomy. In this study, we could not perform the comparative evaluation for learning curves between robotic and endoscopic total thyroidectomy, because the number of endoscopic total thyroidectomy cases was too low to analyze the learning curve for this procedure.
Table 5.
Published data for surgical learning curves for robotic thyroidectomy.
| Author, year | Study design | No. (patients) | Approach | Pathology | Operation | Operation time | Complications (Major)* | Methods for analysis | Surgical learning curve for robotic thyroidectomy |
|---|---|---|---|---|---|---|---|---|---|
| Kang et al. (2009) [5] | Retrospective (single surgeon) |
338 | GT** | PTC§ (332) Benign (6) |
TT§§ & CCND# (104) LTT∆& CCND (234) |
Total: 144.0 ± 43.5 Console: 59.1±25.7 |
5/338 (1.5%) | Moving average | RT## (Console time): 40–45 cases |
|
| |||||||||
| Lee et al. (2011) [9] | Retrospective (single surgeon) |
163 | GT | PTC (151) FTCγ (1) Benign (11) |
TT (48), LTT (115) CCND (149) |
Total: 110.1 ± 50.7 Console: 50.9 ± 11.4 |
2/163 (1.2%) | Moving average | RT: 35-40 cases, ET∆∆:50–60 cases |
|
| |||||||||
| Lee et al. (2011) [13] |
Prospective (multicenter study) |
644 | GT | PTC (616) FTC (13) Benign (15) |
TT & CCND (353) LTT & CCND (291) |
Total: 181.5 ± 78.2 (≤50γγ)→141.5 ± 33.0 (>50) Console: 50.9 ± 11.4 |
2/644 (0.3%) | Comparative analysis between beginners and experience surgeons | RT (LTT): 40 cases RT (TT): 50 cases |
|
| |||||||||
| Kandil et al. (2012) [16] | Prospective (single surgeon) |
100 | GT | No data | TT (22) LTT (69) CT√ (9) |
Total: 121.9 ± 63.8 (≤45√√)→104.2 ± 71.5 (>45) Console: 51.3 ± 31.1 (≤45) →45 ± 36.4 (>45) |
1/100 (1.0%) | Comparative analysis between early experience and late experience | RT: 45 cases |
Complications (Major)*: major complications mean permanent damages such as recurrent laryngeal nerve injury, permanent hypocalcemia, hematoma of muscle flap need to reoperation, hemorrhage of a major vessel need to reoperation, trachea injury, Honor's syndrome, major chyle leakage, and brachial plexus neuropraxia (not including minor complications such as transient hypocalcemia, transient hoarseness, wound seroma, wound infection, and hematoma of muscle flap only need to conservative management), GT**: gasless transaxillary approach, PTC§: papillary thyroid carcinoma, TT§§: total thyroidectomy, CCND#: central compartment node dissection, RT##: robotic thyroidectomy, LTT∆: less than total thyroidectomy, ET∆∆: endoscopic thyroidectomy, FTCγ: follicular thyroid carcinoma, ≤50γγ: experience for robotic thyroidectomy were less than 50 cases, CT√: completion thyroidectomy, ≤45√√: experience for robotic thyroidectomy was less than 45 cases.
This study had potential shortcomings. First, although all 4 surgeons in our study used the same technique, all procedures could not be completed with the same quality of manipulations. Second, patients were not randomized, as it was neither ethically nor geographically possible to move patients from one center to another. This was likely a result of selection bias of our patients and our overall experience with endoscopic and robotic surgery. Third, the short history of robotic thyroidectomy makes assertions on the oncologic safety and the surgical learning curve premature. In addition, the surgeon had experience in performing several endoscopic thyroidectomies prior to any robotic thyroidectomies, and this is likely to have influenced comparisons of operation times and learning curves. Therefore, further study is required to confirm these findings and to assess the surgical learning curve for surgeons who have no experience of endoscopic surgery. Moreover, a prospective and randomized study with longer follow-up period should be conducted to evaluate the role of robotic surgery in thyroid disease.
In conclusion, we have shown that the robot technology we used could overcome some of the technical limitations associated with conventional endoscopic procedures, with reduced operation times and increased lymph node retrieval. Moreover, we found that the learning curve for robotic thyroidectomy was shorter than that for endoscopic thyroidectomy. Prospective randomized studies are required to evaluate the actual learning curves of inexperienced endoscopic surgeons for robotic thyroidectomy and lymph node dissection.
Conflict of Interests
All authors including Drs. J. Lee, J. H. Yun, U. J. Choi, S.-W. Kang, J. J. Jeong, and W. Y. Chung have no conflict of interests or financial ties to disclose.
References
- 1.Ikeda Y, Takami H, Sasaki Y, Takayama J, Niimi M, Kan S. Clinical benefits in endoscopic thyroidectomy by the axillary approach. Journal of the American College of Surgeons. 2003;196(2):189–195. doi: 10.1016/S1072-7515(02)01665-4. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda Y, Takami H, Sasaki Y, Takayama JI, Kurihara H. Are there significant benefits of minimally invasive endoscopic thyroidectomy? World Journal of Surgery. 2004;28(11):1075–1078. doi: 10.1007/s00268-004-7655-2. [DOI] [PubMed] [Google Scholar]
- 3.Chung YS, Choe JH, Kang KH, et al. Endoscopic thyroidectomy for thyroid malignancies: comparison with conventional open thyroidectomy. World Journal of Surgery. 2007;31(12):2302–2306. doi: 10.1007/s00268-007-9117-0. [DOI] [PubMed] [Google Scholar]
- 4.Koh YW, Park JH, Kim JW, Lee SW, Choi EC. Endoscopic hemithyroidectomy with prophylactic ipsilateral central neck dissection via an unilateral axillo-breast approach without gas insufflation for unilateral micropapillary thyroid carcinoma: preliminary report. Surgical Endoscopy and Other Interventional Techniques. 2010;24(1):188–197. doi: 10.1007/s00464-009-0646-5. [DOI] [PubMed] [Google Scholar]
- 5.Kang SW, Lee SC, Lee SH, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery. 2009;146(6):1048–1055. doi: 10.1016/j.surg.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Yun JH, Nam KH, Choi UJ, Chung WY, Soh EY. Perioperative clinical outcomes after robotic thyroidectomy for thyroid carcinoma: a multicenter study. Surgical Endoscopy and Other Interventional Techniques. 2011;25(3):906–912. doi: 10.1007/s00464-010-1296-3. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Kang SW, Jung JJ, et al. Multicenter study of robotic thyroidectomy: short-term postoperative outcomes and surgeon ergonomic considerations. Annals of Surgical Oncology. 2011;18(9):2538–2547. doi: 10.1245/s10434-011-1628-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Ryu HR, Park JH, et al. Excellence in robotic thyroid surgery: a comparative study of robot-assisted versus conventional endoscopic thyroidectomy in papillary thyroid microcarcinoma patients. Annals of Surgery. 2011;253(6):1060–1066. doi: 10.1097/SLA.0b013e3182138b54. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Lee JH, Nah KY, Soh EY, Chung WY. Comparison of endoscopic and robotic thyroidectomy. Annals of Surgical Oncology. 2011;18(5):1439–1446. doi: 10.1245/s10434-010-1486-1. [DOI] [PubMed] [Google Scholar]
- 10.Kim WW, Kim JS, Hur SM, et al. Is robotic surgery superior to endoscopic and open surgeries in thyroid cancer? World Journal of Surgery. 2011;35(4):779–784. doi: 10.1007/s00268-011-0960-7. [DOI] [PubMed] [Google Scholar]
- 11.Lang BHH, Chow MP. A comparison of surgical outcomes between endoscopic and robotically assisted thyroidectomy: the authors’ initial experience. Surgical Endoscopy and Other Interventional Techniques. 2011;25(5):1617–1623. doi: 10.1007/s00464-010-1450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Qiu M, Jiang DZ, et al. The learning curve for endoscopic thyroidectomy: a single surgeon’s experience. Surgical Endoscopy and Other Interventional Techniques. 2009;23(8):1802–1806. doi: 10.1007/s00464-009-0332-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Yun JH, Nam KH, Soh EY, Chung WY. The learning curve for robotic thyroidectomy: a multicenter study. Annals of Surgical Oncology. 2011;18(1):226–232. doi: 10.1245/s10434-010-1220-z. [DOI] [PubMed] [Google Scholar]
- 14.Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. American Journal of Roentgenology. 2009;193(3):871–878. doi: 10.2214/AJR.09.2386. [DOI] [PubMed] [Google Scholar]
- 15.Cooper DS, Doherty GM, Haugen BR, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 16.Kandil EH, Noureldine SI, Yao L, Slakey DP. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. Journal of the American College of Surgeons. 2012;214(4):558–564. doi: 10.1016/j.jamcollsurg.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Yoon JH, Park CH, Chung WY. Gasless endoscopic thyroidectomy via an axillary approach: experience of 30 cases. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques. 2006;16(4):226–231. doi: 10.1097/00129689-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Jong JJ, Kang SW, Yun JS, et al. Comparative study of endoscopic thyroidectomy versus conventional open thyroidectomy in papillary thyroid microcarcinoma (PTMC) patients. Journal of Surgical Oncology. 2009;100(6):477–480. doi: 10.1002/jso.21367. [DOI] [PubMed] [Google Scholar]
- 19.Kang SW, Jeong JJ, Yun JS, et al. Gasless endoscopic thyroidectomy using trans-axillary approach; surgical outcome of 581 patients. Endocrine Journal. 2009;56(3):361–369. doi: 10.1507/endocrj.k08e-306. [DOI] [PubMed] [Google Scholar]
- 20.Diggle P. Time Series: A Biostatistical Introduction. London, UK: Oxford University Press; [Google Scholar]


