Abstract
Medically refractory status epilepticus (RSE) causes high morbidity and mortality in children. There are no evidence-based guidelines for treatment. Epilepsy surgery is a treatment option for RSE. We describe a 9-year-old boy treated successfully for RSE with complete corpus callosotomy (CC). Epilepsy surgery should be considered for prolonged RSE. In the absence of evidence of focal epileptogenesis, complete corpus callosotomy may be effective in select cases.
Keywords: Pediatric epilepsy, Status epilepticus, Refractory status epilepticus, Corpus callosotomy, Epilepsy surgery
1. Introduction
Status epilepticus (SE) is refractory to initial intravenous therapy with lorazepam in about 30% of cases.1 Second and third line therapy aborts SE in some, but those patients refractory to this initial round of treatment, going on to pharmacologic coma therapy, represent a significant fraction and the major cause of morbidity and mortality in SE. In adults persistent SE despite trials of anesthesia for cerebral suppression is referred to as refractory or “malignant status epilepticus”, and represents 20% of cases.2 Medically refractory status epilepticus (RSE) has a high morbidity and mortality in children, estimated at 16–32%.3,4 Neurosurgical intervention is a treatment option for RSE. We describe a case of a 9-year-old boy treated with complete corpus callosotomy (CC) to abort an episode of recurrent RSE.
2. Case report
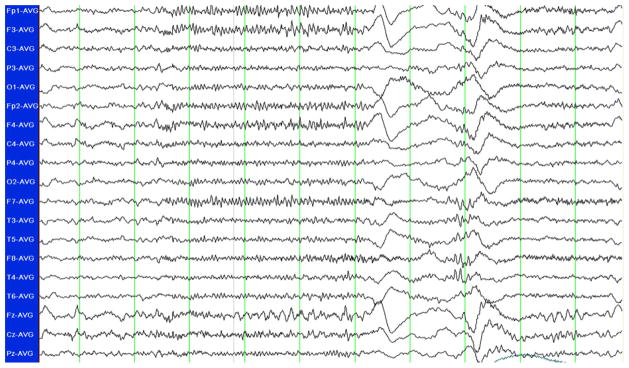
A 9-year-old Caucasian male with a history of medically refractory epilepsy presented with altered mentation for 2 days. Parents reported a viral syndrome with low-grade fever 5 days before, but he had been afebrile for 3 days prior to admission. Typically seizures occurred at baseline one to two times a night, consisting of brief eye opening and tonic stiffening of both arms. On presentation he was having more frequent clinical events, and on examination showed signs of acute encephalopathy. Electroencephalogram (EEG) showed very frequent bursts of generalized beta rhythms followed by slow waves, correlating with eye opening and eyelid fluttering on video (Fig. 1). This pattern evolved into continuous rhythmic slow waves, consistent with SE.
Fig. 1.
Standard 10–20 system EEG displayed in average referential montage. Frequent bursts of ictal beta rhythms, generalized but maximal in the bifrontal regions, were observed. On close inspection of the video, this activity correlated with eye opening and eyelid fluttering.
The patient had a history of multiple seizure types beginning at age three (myoclonic, tonic and drop seizures) and three prior episodes of SE. One episode occurring 6 months prior to presentation was refractory requiring pharmacologic coma. With this prior episode, he presented with low-grade fever and upper respiratory symptoms. There was a clustering of his typical tonic seizures evolving to SE, refractory to intravenous benzodiazepines, valproate and fosphenytoin. He was electively intubated and treated for 4 days with pentobarbital for cerebral suppression. The patient returned to his typical seizure frequency and was discharged 2 weeks from admission. Upon review, there was no history of febrile or hyperthermia-induced seizures, and no family history of febrile seizures or epilepsy. Detailed neuropsychological testing with the Wechsler Intelligence Scale for Children (WISC-IV) revealed global difficulties in many indices preventing formal score calculation. The perceptual reasoning (PRI) score was extremely low at 55. Fine and gross motor skills were reportedly normal.
3. Hospital course
Trials of intravenous benzodiazepines and valproate were ineffective. On day two he was transferred to the intensive care unit (ICU) for pharmacologic coma with intravenous midazolam. Burst suppression was eventually achieved with high dose continuous pentobarbital and ketamine. Over the next 30 days, he cycled in and out of burst suppression four times, and developed recurring SE with each attempted wean from pharmacologic coma. Several anticonvulsant trials and the ketogenic diet were ineffective. Medical complications of his ICU stay included ventilator-associated pneumonia and hemodynamic instability requiring vasopressors.
The results of a previous presurgical workup were reviewed, including prior video-EEG monitoring and structural and functional imaging. Detailed 3-Tesla (3 T) magnetic resonance imaging (MRI) had been negative for lesional cause. Fluorodeoxyglucose positron emission tomography (FDG-PET) had shown subtle left temporal and anterior parietal hypometabolism. Ictal single photon emission computed tomography (SPECT) with subtraction ictal SPECT co-registered to MRI (SISCOM) and previous scalp EEG were not clearly lateralizing. After this workup, the family had been offered the option of CC or bilateral strip electrode placement to determine lateralization and subsequent possible resection, but had postponed this decision. Based on available information at the time of the most recent status epilepticus episode, our surgical team concluded he was not a resective surgery candidate. Therefore the option of complete CC to abort RSE and palliate seizures was offered.
Two days prior to CC, the patient had breakthrough SE requiring higher doses of ketamine and pentobarbital infusions. Immediately preoperatively the EEG pattern was burst suppression. Intraoperatively, the pentobarbital infusion was continued. Neither electro-corticography nor scalp EEG was performed intraoperatively, since it is expected this would have demonstrated a burst suppression pattern and added a slightly increased risk for morbidity. After the procedure, he was monitored with limited scalp EEG electrodes. The ketamine and pentobarbital were weaned over a period of 17 days without recurrence of SE. He was maintained on phenobarbital and valproate, which had been previously tried preoperatively. After extubation and stabilization, he had a severe global encephalopathy. A component of akinesis related to callosal disconnection was suspected, but a specific disconnection syndrome was not identified. He received 5 weeks of inpatient rehabilitation. On discharge he had evidence of moderate neurological insult compared to prior baseline, including impaired frontal lobe function and incoordination. He required gait assistance. Six months after surgery, neurological function had recovered to baseline. At 12 month follow-up, seizure frequency was one to two per day, similar to prior to admission; there had been no recurrences of SE.
4. Discussion
The benefits of CC to prevent recurrence of SE have been previously described.5 To our knowledge, this is the first reported case of successful surgical treatment of ongoing pediatric RSE with CC alone: previous reports describe CC as part of a resective procedure, such as hemispherectomy or focal resection with CC. In our report, anticonvulsant medications which were ineffective preoperatively were sufficient to prevent relapse into RSE postoperatively while the same anesthetic agents were weaned. There are only six case reports of successful disconnective or disruptive epilepsy surgery (excluding hemispherectomy) for pediatric RSE.6,7
There is little evidence-based guidance for treating RSE. Treatment strategies may follow from consideration of the differential diagnosis—in our patient with a history of prior RSE, an infectious or inflammation-mediated encephalopathy was considered unlikely. Pharmacologic coma is often initiated, but in some cases cerebral suppression proves ineffective when the patient re-emerges from coma with SE. Ma et al. recommend consideration of surgical treatment after failure of three trials of pharmacologic coma therapy over 2 weeks.8 In the setting of focal epilepsy, particularly with lesional findings on magnetic resonance imaging (MRI), RSE is clearly amenable to focal resection or hemispherectomy. In a retrospective study of 10 patients with focal epileptogenesis, all were treated surgically, successfully aborting RSE and without mortality.9 In our case, a prior presurgical workup identified the patient as a poor resective surgical candidate. Since resective surgery could not be accomplished, additional trials of anesthetic agents were attempted before a palliative procedure was considered.
In the absence of a clear anatomical focus for seizures or in the presence of signs suggestive of generalized epilepsy, resective surgery is not an option. Disconnective or disruptive procedures, including CC, multiple subpial transections, vagal nerve stimulation (VNS) and electroconvulsive therapy may effectively abort RSE. Complete CC was chosen in our case, since it is probably superior in the treatment of tonic seizures, the patient’s habitual seizure type.10 For this indication, the added benefit of complete disconnection in complete CC compared to anterior two-thirds sectioning may outweigh the difference in surgical risk, as some reports demonstrate lower relapse rate in tonic and drop seizures with complete CC.11,12 Complete callosal section is associated with improved seizure outcome in prepubescent children and children with global cerebral dysfunction.13,14 Our patient’s young age and cognitive impairment were factors favoring complete CC in clinical decision-making. While the extent of callosal section correlates with the risk for development of a disconnection syndrome, studies in prepubescent children show that functional gain and family satisfaction post-callosotomy are most strongly correlated with seizure control, not extent of callosal section.13–15 The mechanism by which CC may abort seizures, rather than simply prevent generalization, is unclear. However, it is well-documented that CC can reduce partial seizures, indicating that it may have some antiepileptic effect.16,17 Further, presurgical evaluation after CC may identify a resectable focus in some patients; favorable seizure outcome can be obtained from subsequent surgery following CC.18,19
The limitations of the report include a lack of direct evidence that CC was responsible for terminating the patient’s RSE. It is possible that the natural history of the RSE episode was such that it would have relented without surgical intervention. Since patients with RSE are typically in a pharmacologic coma necessitated by the diagnosis, it is not always practical to demonstrate direct evidence of termination of RSE intraoperatively. The most common anesthetic agent used is a barbiturate; even after discontinuation barbiturates continue to cause cerebral suppression for several hours. That the same medications tried ineffectually preoperatively were sufficient postoperatively, and that pharmacologic coma was able to be discontinued, is indicative of the benefit of CC to terminate RSE in this case.
Acknowledgments
This work is not supported by a funding source.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
References
- 1.Treiman DM, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339(12):792–8. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 2.Holtkamp M, et al. A “malignant” variant of status epilepticus. Arch Neurol. 2005;62(9):1428–31. doi: 10.1001/archneur.62.9.1428. [DOI] [PubMed] [Google Scholar]
- 3.Sahin M, et al. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42(11):1461–7. doi: 10.1046/j.1528-1157.2001.21301.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert DL, Gartside PS, Glauser TA. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children: a meta-analysis. J Child Neurol. 1999;14(9):602–9. doi: 10.1177/088307389901400909. [DOI] [PubMed] [Google Scholar]
- 5.Vossler DG, Lee JK, Ko TS. Treatment of seizures in subcortical laminar heterotopia with corpus callosotomy and lamotrigine. J Child Neurol. 1999;14(5):282–8. doi: 10.1177/088307389901400503. [DOI] [PubMed] [Google Scholar]
- 6.Vendrame M, Loddenkemper T. Surgical treatment of refractory status epilepticus in children: candidate selection and outcome. Semin Pediatr Neurol. 2010;17(3):182–9. doi: 10.1016/j.spen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Shin HW, et al. Successful ECT treatment for medically refractory nonconvulsive status epilepticus in pediatric patient. Seizure. 2011;20(5):433–6. doi: 10.1016/j.seizure.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, et al. Neurosurgical treatment of medically intractable status epilepticus. Epilepsy Res. 2001;46(1):33–8. doi: 10.1016/s0920-1211(01)00252-2. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos A, et al. Resective surgery to treat refractory status epilepticus in children with focal epileptogenesis. Neurology. 2005;64(3):567–70. doi: 10.1212/01.WNL.0000150580.40019.63. [DOI] [PubMed] [Google Scholar]
- 10.Nei M, et al. Refractory generalized seizures: response to corpus callosotomy and vagal nerve stimulation. Epilepsia. 2006;47(1):115–22. doi: 10.1111/j.1528-1167.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 11.Spencer SS, et al. Ictal EEG changes with corpus callosum section. Epilepsia. 1993;34(3):568–73. doi: 10.1111/j.1528-1157.1993.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 12.Sunaga S, Shimizu H, Sugano H. Long-term follow-up of seizure outcomes after corpus callosotomy. Seizure. 2009;18(2):124–8. doi: 10.1016/j.seizure.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Maehara T, Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001;42(1):67–71. doi: 10.1046/j.1528-1157.2001.081422.x. [DOI] [PubMed] [Google Scholar]
- 14.Pinard JM, et al. Callosotomy for epilepsy after West syndrome. Epilepsia. 1999;40(12):1727–34. doi: 10.1111/j.1528-1157.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 15.Lassonde M, Sauerwein C. Neuropsychological outcome of corpus callosotomy in children and adolescents. J Neurosurg Sci. 1997;41(1):67–73. [PubMed] [Google Scholar]
- 16.Asadi-Pooya AA, et al. Corpus callosotomy. Epilepsy Behav. 2008;13(2):271–8. doi: 10.1016/j.yebeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Tanriverdi T, et al. Long-term seizure outcome after corpus callosotomy: a retrospective analysis of 95 patients. J Neurosurg. 2009;110(2):332–42. doi: 10.3171/2008.3.17570. [DOI] [PubMed] [Google Scholar]
- 18.Clarke DF, et al. Corpus callosotomy: a palliative therapeutic technique may help identify resectable epileptogenic foci. Seizure. 2007;16(6):545–53. doi: 10.1016/j.seizure.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Ono T, et al. Callosotomy and subsequent surgery for children with refractory epilepsy. Epilepsy Res. 2011;93(2–3):185–91. doi: 10.1016/j.eplepsyres.2010.12.011. [DOI] [PubMed] [Google Scholar]