Abstract
Regeneration of normal shape, architecture, and function of craniofacial tissues following congenital abnormality, trauma, or surgical treatment presents special problems to tissue engineering. Because of the great variations in properties of these tissues, currently available treatment options fall short of adequate care. We propose that the engineering of personalized bone graft customized to the patient and the specific clinical condition would revolutionize the way we currently treat craniofacial defects and discuss some of the current and emerging treatment modalities.
Keywords: Stem cell, Tissue regeneration, Bone, Bone marrow stromal cells, Cellular therapy
Introduction
Reconstruction of Head and Face: Recent Advances and Current Needs
Defects in head and face due to trauma, tumor removal, or congenital abnormalities not only leave patients with reduced tissue structure and function but also render them psychologically scarred. The burden of craniofacial injuries extends far beyond medical expenses, as these injuries often impair the patient's social integration and ability to engage in economic activity. Because of the complexity of craniofacial reconstructions [1, 2], the currently available treatment options fall short of adequate care [3, 4]. There is a pressing need for functional and esthetic restoration of a multitude of bones, including temporomandibular joint (TMJ), zygomatic arch, cranial, nose, temple, mandible, and orbital bone. The current market for craniofacial bone is estimated to be $390 million for trauma alone, based on the overall market for trauma of $3 billion in 2010, with facial trauma representing 13% of all traumatic bone injuries [5].
Numerous bone-grafting options exist for head and face reconstruction to fulfill various needs, depending on the specifics of the defect and the patient's clinical condition. Autologous bone implantation is widely adopted because of the superior osteogenic, osteoinductive, and osteoconductive properties of native bone grafts [6]. Sources of autologous grafts determine their quality and functionality in the craniofacial complex. The membranous bone grafts, such as those harvested from cranium [7], are superior to endochondral bone grafts in terms of the volume maintenance [8], whereas highly vascularized grafts, such as vascularized bone flap from fibula or iliac bone [9], have an advantage of rapid incorporation into the host bone and vascular flow [6]. Finally, osteochondral grafts can repair composite defects of bone and fibrocartilage [9, 10]. Autologous grafts are considered a gold standard for head and face reconstruction because of their bioactivity, mechanical competence, and immediate cellular function. However, the restricted volume of the bone available for harvest, donor site morbidity, the lack of precision in carving delicate shapes of craniofacial bones, and differences in the structure and biomechanics of bones from different parts of the body call for alternative methods.
Bone allografts and alloplastic substitutes provide unlimited off-the-shelf supply of implants in a range of sizes and shapes. Bone allografts are osteoconductive and may also have some osteoinductive capability [11, 12]. For comparison, a demineralized bone matrix (DBM) exhibits significant osteoinductive behavior and imparts osteoconductivity to the collagen matrix [13]. However, the use of bone allografts is often associated with infection, disease transmission, and immunological rejection. Processing bone allografts into a demineralized bone matrix can significantly reduce these complications [12]. In contrast, alloplastic grafts are fabricated from synthetic materials, such as hydroxyapatite, calcium phosphates, polymers, plastics, and metals, and carry less burden of infection or immune rejection. Also, nonbiological grafts can be designed to provide desired mechanical strength, size, and shape to meet specific implantation requirements. However, the osteoinductivity and osteoconductivity of alloplastic grafts are generally inferior to those exhibited by autologous or allogeneic bone grafts [6]. Also, the nonbiological nature of alloplastic grafts does not provide metabolic function, adaptation, and remodeling of bone (e.g., in response to mechanical loading and aging), all of which are critical for long-term function of implanted grafts. Existing bone grafting techniques offer a variety of tools to reconstruct head and face defects, with tolerable success. Nevertheless, disadvantages and complications of existing bone grafts inspire clinicians, scientists, and engineers to develop more effective bone treatment modalities. A major trend in this direction is the development of personalized bone grafts that are autologous in nature and have properties tailored to the patient and the specific clinical situation.
During development, the craniofacial and skeletal bones form by two distinct processes: intramembranous and endochondral ossification, respectively. In intramembranous ossification, cells of the condensed mesenchymal tissue differentiate into osteoblasts and directly form bone. In contrast, endochondral ossification involves the formation of cartilaginous anlage, which then undergoes calcification and invasion by blood vessels, resulting in the formation of new bone by mesenchymal stem cells. The developmental differences are necessarily reflected in tissue engineering protocols for the two types of bone.
The important criteria for developing functional bone grafts for head and face reconstruction include (a) superior bioactivity for successful graft incorporation, (b) native-like graft shape and architecture for esthetic purposes, and (c) maintenance of tissue volume during and beyond the bone remodeling period. Patient-specific bone grafts can be designed to synergize the advantages of autologous and alloplastic grafts. A personalized graft is readily accepted by the patient's immune system and designed to match, with great precision, the structural and biomechanical features of the donor site to ensure full reconstruction of both esthetics and function. The availability of personalized human bone graft customized to the patient and the specific clinical condition would revolutionize the way we currently treat craniofacial defects.
Engineering Personalized Human Bone Grafts
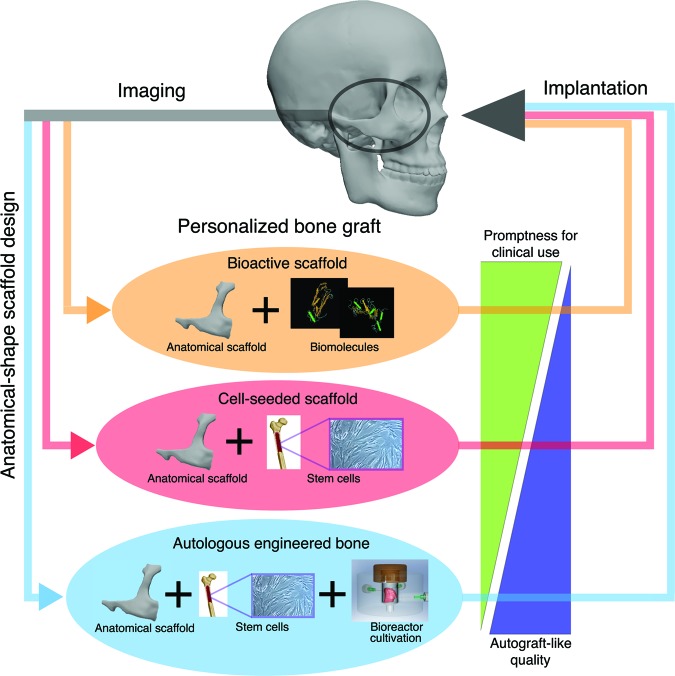
A fundamental requirement for personalized bone reconstruction is to fulfill the functional and esthetic properties by fabricating anatomical shape grafts with the ability for integration with the host bone, adjacent soft tissues, and vascular supply and the capacity for load-bearing and remodeling. We highlight three strategies of great interest for engineering personalized bone grafts that rely on the use of (a) bioactive scaffolds, (b) cell-seeded scaffolds, and (c) autologous bone grafts grown in vitro (Fig. 1). These three strategies result in different maturity of the implanted tissue (from scaffold alone, over immature cellular graft, to preformed bone) and the promptness for translation (with cell-free scaffolds being closer to clinical application than cellular or bone grafts).
Figure 1.
Key strategies for engineering personalized bone grafts. (a) Bioactive scaffolds with incorporation of bioactive molecules, designed to recruit the host cells, (b) cell-seeded scaffolds, with or without additional bioactive factors, designed to foster rapid bone growth inside a scaffold providing structural and mechanical competence, and (c) autologous bone grafts grown in vitro to various levels of maturity, designed to provide immediate function along with the capacity for integration with the adjacent tissues and blood supply. The similarity to native bone tissue increases from bioactive scaffolds to cell-seeded scaffolds and to preformed bone, whereas the readiness for clinical application decreases in this same order.
Since the facial skeleton has complex shapes with anisotropic structural and mechanical properties that vary from one individual to another, imaging-guided tissue engineering technologies are of great interest. Computer-assisted imaging and preoperative planning are increasingly used to predetermine the surgical location and to facilitate manufacturing of patient-specific implants. Rudman et al. summarized the innovations in computer-assisted reconstructive surgery, including anatomic considerations, implant materials available, technologies for preoperative planning, and the process of obtaining a patient-specific graft [14]. Clinical experience demonstrates that virtual planning, rapid prototype modeling, and stereotactic navigation for complex craniofacial defects can provide the reconstructive surgeon with innovative options for treating challenging and patient-specific reconstructions [15]. The use of computer-assisted imaging allows an exact fabrication of a personalized graft matching the esthetic requirements of head and face reconstructions.
Option 1: Bioactive Acellular Scaffolds.
The first option for personalized bone reconstruction is the use of scaffolds made of biodegradable synthetic materials with incorporated osteoinductive factors that can recruit the host cells and guide bone ingrowth. Ideally, bone healing responses to implantation of these scaffolds involve host inflammatory reaction, cell proliferation, migration, differentiation, revascularization, and new bone formation [6]. The biodegradation can be provided either through hydrolysis or inflammatory and osteoclastic resorption at rates allowing isomorphic replacement of scaffold material with the new bone tissue. The bioactive function should support all processes involved in bone tissue formation—vascularization, osteogenic differentiation, and prevention of scar formation. In addition, mechanical properties of the scaffold are important, especially for load bearing sites, such as the temporomandibular joint (TMJ). Two techniques are being actively pursued toward designing bioactive scaffolds: (a) fabrication of hierarchical structures resembling those of the native tissue to provide mechanical support and direct cell migration and differentiation and (b) incorporation of biomolecules to recruit cells and guide bone formation.
Considerations for scaffold architecture include porous channels for cell migration, surface features for cell attachment, and mass-transport conduits for cell nutrition. Because of the complexity of facial bones, an “ideal” scaffold would have a hierarchical porous structure to attain desired mechanical function and mass transport and allow for manufacturing of complex three-dimensional anatomical shapes [16]. Common methods for fabrication of anatomically shaped scaffolds include molding and machining [17, 18]. Examples of highly anisotropic structure include the native skull bone and the TMJ that contains vertical lamina of cancellous bone, with the matrix density decreasing from superior to inferior across the condyle [19, 20]. Another example is the zygomatic arch (cheek bone) that has a porous central region and a compact rim, with trabeculae arranged vertically and anteroposteriorly [21–24].
Recently, manufacturing techniques known as solid free-form fabrication (SFF) and rapid prototyping have been successfully used to fabricate complex scaffolds. SFF builds parts by selectively adding materials, layer by layer, as specified by a computer program [25]. Using this technique, scaffolds can be fabricated to match the anisotropic structure of human mandibular condyle from polycaprolactone (Fig. 2A) [16]. Optimizing architecture and scaffolding material for biocompatibility and osteoconductive properties would greatly improve the quality of tissue-engineered human bone graft.
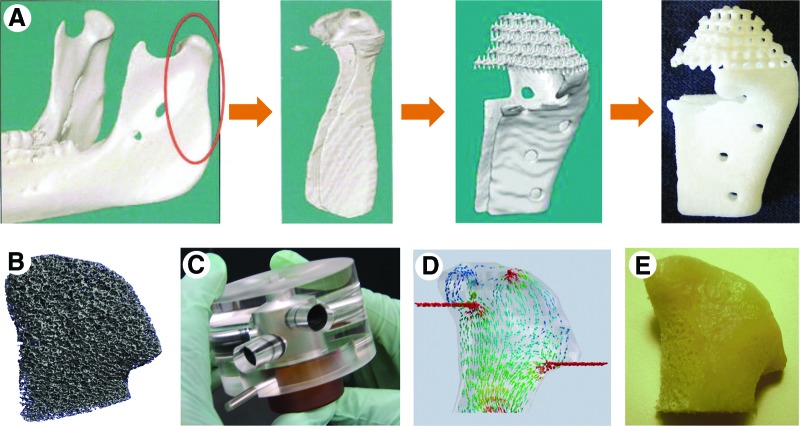
Figure 2.
Tissue engineering of anatomically shaped, personalized bone grafts. Two representative, distinctly different approaches are shown for human temporomandibular joint condyle (TMJ). (A): Imaging-guided fabrication of a nonbiological scaffold by rapid prototyping (adapted from Hollister [16]; reprinted by permission of Macmillan Publishers Ltd. (B-E): Tissue engineering of living bone (adapted from Grayson et al. [50]; reprinted by permission of Proc. Natl. Acad. Sci. USA). (B): Image guided fabrication of a scaffold by micromachining of decellularized bone. (C): Perfused bioreactor with an anatomically shaped chamber. (D): Analysis and optimization of the interstitial flow through the cultured bone. (E): Living TMJ graft engineered using human mesenchymal stem cells from bone marrow.
Incorporation of biomolecules into alloplastic scaffolds could be used to recruit specific cell types into the scaffold in a way leading to bone regeneration. With advances in scaffold fabrication and controlled release of bioactive factors [26, 27], “smart” scaffolds are becoming available to provide both a structural template and the temporal control of osteoinductive factors. The use of growth factors, including transforming growth factor β, bone morphogenetic proteins (BMPs), fibroblast growth factors, insulin-like growth factors, and platelet-derived growth factor, in the repair of bone has previously been reviewed [28]. Scaffolds releasing multiple factors in a timely fashion—for example, initial release of cytokines to recruit cell migration followed by the release of vasculogenic and osteogenic factors to direct bone formation—would be most effective for guiding bone regeneration. Techniques in anatomical shape alloplastic graft fabrication and incorporation of cytokines, such as BMP-2, are currently being used commercially, whereas the more complex systems, such as multiple cytokines incorporation, are only being investigated in vitro.
Option 2: Cell-seeded Scaffolds.
Cellularized grafts, in general, provide better integration with the host tissues and remodeling than acellular scaffolds. Cell-seeded scaffolds combine the benefits of adding exogenous cells with the incorporation of bioactive molecules and the use of customized scaffolds. In order to prevent immunological response, the graft can be made using autologous materials. Harvesting osteoblasts from native tissue would cause donor site morbidity and is, therefore, not a method of choice. Instead, less invasive harvesting methods are preferred to obtain autologous cells from whole bone marrow (BM) or adipose tissue and the platelet-rich plasma (PRP).
Bone marrow is rich in biomolecules and cells responsible for normal maintenance of bone, including three types of stem cells: hematopoietic, mesenchymal, and vascular precursor cells. Autologous PRP is commonly used in clinical settings to treat injury and defects. PRP consists of concentrated platelets in a small volume of plasma extracted from whole blood. It comprises seven fundamental protein growth factors proved to be actively secreted by platelets to initiate wound healing and three blood proteins known to act as cell adhesion molecules for osteoconduction and synthesis of connective tissues and epithelial migration [29]. Both the bone marrow and PRP can be harvested and seeded into anatomical shape scaffolds in a point-of-care setting right prior to implantation.
We focus, in this review, on the types of adult stem cells that have demonstrated high clinical potential in the application of personalized human bone graft for reconstructing head and face, including mesenchymal stem cells from bone marrow (BMSCs), adipose-derived stem cells (ASCs), and dental pulp stem cells (DPSCs). Other sources of cells—such as embryonic and fetal stem cells—are not discussed, as they still have not shown advantages over adult stem cells or induced pluripotent stem (iPS) cells in term of osteogenic differentiation and bone formation.
BMSCs are the most extensively investigated and used therapeutic cells for bone regeneration. These cells have had quite successful use in orthopedic cell-based reconstructive therapies [30]. ASCs have similar immunophenotype, morphology, and multilineage potential to BMSC. Because ASCs are more abundant and more accessible than BMSCs, causing very little donor site morbidity, these cells are actively investigated as an alternative to BMSCs [31, 32]. Both BMSCs and ASCs were shown to stabilize vascular infiltration and to form functional bone. Much less investigated, yet interesting, cell sources are DPSCs, the cells entrapped within dental pulp, which are a source of stromal stem cells that can be obtained at the time of tooth extraction and stored for future use [33]. Similar to BMSCs and ASCs, DPSCs are a source of osteoblasts and are able to form mineralized bone tissue in vitro [34]. The use of any type of adult stem cells in cellularized bone grafts requires isolation, purification, and in vitro expansion in order to obtain an adequate number of high-quality stem cells.
Immature cellularized grafts, obtained by seeding of scaffolds immediately prior to implantation, enhance osteogenesis in the site of the bone defect by their constituent cells and biological factors. The enhancement in craniofacial reconstruction was investigated using various combinations of cellular and scaffolding materials in several autologous implantation models: BM in a scaffold [35], PRP in a scaffold [29], BMSC in a scaffold [36], and BMSC with PRP [37]. In general, grafts containing cells were superior over their respective no-cell controls, conclusively suggesting that implanted autologous cells contributed to the reconstruction process. The uses of cell-seeded scaffold were conducted in an animal model as an in vivo proof-of-concept and to determine its efficacy. Challenges for fabricating personalized immature cellular graft include obtaining sufficient amounts of cellular material, spatially uniform and rapidly seeding a large anatomical shape scaffold, maintaining graft survival postimplantation, and controlling cells to undergo tissue-specific formation in vivo.
Option 3: Customized Autologous Bone Grafts.
Knowledge in stem cells biology has advanced to the point that allows biologists and bioengineers to manipulate cells into specific tissue types and form in vitro bone tissues of various levels of maturity. BMSCs, ASCs, and DPSCs have the capability to proliferate in vitro and differentiate into multiple cell phenotypes, including adipogenic, chondrogenic, and, most importantly in this context, osteogenic [31, 38, 39]. Adult stem cells quality (cell numbers, purity, and proliferative and differentiating abilities) alter from one individual to another and by age [40]. Consequently, selecting one cell phenotype over another for engineering personalized autologous bone grafts is highly patient specific and requires adequate assessment criteria. Human induced pluripotent stem cells (iPSCs) that have recently become available offer significant potential for engineering of a multitude of tissues, as these cells are both autologous and pluripotent [41]. Osteogenic potential of iPSC has been demonstrated through direct differentiation and derivation of MSC-like cells [42]. However, the clinical applicability of iPSC is still far from fruition. iPSCs evolve very slowly in culture, and the yields can be rather low [41]. In addition, the iPSC used for therapeutic purposes must be free of genomic insertions of transgene sequences [42]. Therefore, novel techniques must be developed to overcome these obstacles prior to clinical use.
Engineering clinically sized autologous bone grafts requires a large number of cells and advanced cultivation systems providing cell seeding of anatomically shaped scaffolds, sufficient nutrition to the cells within scaffolds, and regulatory signals for cell differentiation and functional assembly [43]. Various technologies have been developed for engineering human bone grafts by using various scaffolds and bioreactor configurations [43–45]. In a suitable environment and with adequate stimulation, stem cells differentiate into osteogenic cells, producing bone proteins and minerals [46, 47]. Furthermore, when an appropriate amount of osteoconductive scaffolding material is initially incorporated into a protein matrix, BMSCs differentiate into osteogenic lineages and form trabecular bone-like structure, with significant enhancement of the graft mechanical properties [48]. Notably, tissue engineered bone grafts are superior to either scaffold alone or cell-seeded scaffold in terms of graft incorporation into the critical size mouse calvarial bone defects [49].
Maintaining cellularity in large, anatomically shaped bone grafts in vitro can be a challenging task. Recently, Grayson et al. [50] reported that clinically sized, anatomically shaped, viable human bone grafts can be engineered in vitro using human mesenchymal stem cells and a “biomimetic” scaffold-bioreactor system (Fig. 2B–2E). Human TMJ was selected as a model because of the tremendous clinical importance of TMJ and the challenges associated with reconstructing its complex shape and load-bearing function. Anatomically shaped scaffolds were generated from fully decellularized trabecular bone using digitized clinical images (Fig. 2B) and seeded with human mesenchymal stem cells. A novel bioreactor with a chamber in the exact shape of a human TMJ was designed for controllable perfusion throughout the engineered construct (Fig. 2C). By using computer software to analyze fluid flow patterns, the medium perfusion was optimized to ensure nutrient transport within the forming tissue (Fig. 2D).
By five weeks of cultivation, tissue growth was evidenced by the formation of confluent layers of lamellar bone (by scanning electron microscopy), markedly increased volume of mineralized matrix (by quantitative microcomputer tomography), and the formation of osteoids (histologically). For the first time for the bone grafts of this size and complexity, cells were fully viable at a physiologic density, likely an important factor of graft function (Fig. 2E). The density and architecture of the bone matrix correlated with the intensity and pattern of the interstitial flow, as determined in experimental and modeling studies. This approach has potential to overcome a critical hurdle—in vitro cultivation of viable bone grafts of complex geometries—and provide patient-specific bone grafts for craniofacial and orthopedic reconstructions.
The potential of engineering autologous bone grafts with precise anatomical shapes, internal architectures, and biomechanical properties matching the properties of native tissues is tremendous. This approach is currently being investigated on benchtop and in large animal models, and it could impact research in developmental biology (where high-fidelity tissue models can be used to study bone formation), as much as clinical translation (by providing surgeons with large and viable anatomically shaped bone grafts for treating craniofacial or orthopedic defects). Current developments are nearing a point when such an approach could become clinically feasible for reconstruction of small to moderate-sized bone defects.
One major challenge in reconstructing large bone defects remains in the ability to vascularize the graft and establish blood perfusion immediately following implantation to maintain graft viability. Vascularization has been identified as a major factor influencing bone development, healing, remodeling, and graft repair. In fact, bone is not vascularized during development, but instead, the vascular network develops first and serves as a template for the assembly of the bone matrix. The techniques under research include prevascularization in vivo, prevascularization in vitro, and incorporation of angiogenic factors. The success of large autologous bone graft entails further investigation in large animal models for rigorous evaluation of the safety and efficacy of bone tissue engineering at scales and under loading conditions comparable to human. The considerations for translational work also include the graft maturity (which affects both the survival and function), the effectiveness of prevascularization, and provisions for the long-term graft function and remodeling.
Summary
Bone defects in head and face due to trauma, tumor removal, or congenital abnormalities have far-reaching consequences, as these injuries most directly impair all aspects of the patient's life. Regeneration of normal shape, architecture, and function of craniofacial tissues presents special problems to tissue engineering. Because of the great variation in properties of these tissues among different grafts and from one person to another, currently available treatment options fall short of adequate care. In general, the clinical utility of craniofacial tissue engineering depends upon our ability to direct cells to form tissues with site-specific properties across different hierarchical scales. The availability of customized living tissues engineered in vitro would revolutionize the way we currently treat craniofacial defects. Engineering of living bone grafts would also have tremendous value for controlled studies of the development and regeneration of craniofacial tissues. Recent years have brought significant advances in the synergistic use of stem cells and bioengineering for craniofacial reconstruction and identified many of the remaining challenges in translating these advances into clinical reality.
Acknowledgments
We gratefully acknowledge funding support of this work by the NIH (DE161525 and EB02520) and the New York Stem Cell Foundation (CU09-3055).
Author Contributions
S.B.: conception and design, data analysis and interpretation, and manuscript writing; G.V.-N.: conception and design, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Disclosures Of Potential Conflict Of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423:326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- 2.Herring SW, Ochareon P. Bone—special problems of the craniofacial region. Orthod Craniofac Res. 2005;8:174–182. doi: 10.1111/j.1601-6343.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor JA. Bilateral orbitozygomatic reconstruction with tissue-engineered bone. J Craniofac Surg. 2010;21:1612–1614. doi: 10.1097/SCS.0b013e3181edc829. [DOI] [PubMed] [Google Scholar]
- 4.Zizelmann C, Schoen R, Metzger MC, et al. Bone formation after sinus augmentation with engineered bone. Clin Oral Implants Res. 2007;18:69–73. doi: 10.1111/j.1600-0501.2006.01295.x. [DOI] [PubMed] [Google Scholar]
- 5.New York, NY: Frost & Sullivan; 2008. U.S. Trauma Fixation Markets. [Google Scholar]
- 6.Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000:10–27. [PubMed] [Google Scholar]
- 7.Zins JE, Langevin CJ, Nasir S. Controversies in skull reconstruction. J Craniofac Surg. 2010;21:1755–1760. doi: 10.1097/SCS.0b013e3181c34675. [DOI] [PubMed] [Google Scholar]
- 8.Zins JE, Whitaker LA. Membranous versus endochondral bone: Implications for craniofacial reconstruction. Plast Reconstr Surg. 1983;72:778–785. doi: 10.1097/00006534-198312000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Engroff SL. Fibula flap reconstruction of the condyle in disarticulation resections of the mandible: A case report and review of the technique. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:661–665. doi: 10.1016/j.tripleo.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahimi A, Ashford BG. Advances in temporomandibular joint reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2010;18:255–2560. doi: 10.1097/MOO.0b013e32833af88c. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson S. Biology of bone grafts. Orthop Clin North Am. 1999;30:543–552. doi: 10.1016/s0030-5898(05)70107-3. [DOI] [PubMed] [Google Scholar]
- 12.Eppley BL, Pietrzak WS, Blanton MW. Allograft and alloplastic bone substitutes: A review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16:981–989. doi: 10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- 13.Davy DT. Biomechanical issues in bone transplantation. Orthop Clin North Am. 1999;30:553–563. doi: 10.1016/s0030-5898(05)70108-5. [DOI] [PubMed] [Google Scholar]
- 14.Rudman K, Hoekzema C, Rhee J. Computer-assisted innovations in craniofacial surgery. Facial Plast Surg. 2011;27:358–365. doi: 10.1055/s-0031-1283054. [DOI] [PubMed] [Google Scholar]
- 15.Hanasono MM, Kridel RW, Pastorek NJ, et al. Correction of the soft tissue pollybeak using triamcinolone injection. Arch Facial Plast Surg. 2002;4:26–30. doi: 10.1001/archfaci.4.1.26. [DOI] [PubMed] [Google Scholar]
- 16.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 17.Singare S, Dichen L, Bingheng L, et al. Design and fabrication of custom mandible titanium tray based on rapid prototyping. Med Eng Phys. 2004;26:671–676. doi: 10.1016/j.medengphy.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Vats A, Tolley NS, Polak JM, et al. Scaffolds and biomaterials for tissue engineering: A review of clinical applications. Clin Otolaryngol Allied Sci. 2003;28:165–172. doi: 10.1046/j.1365-2273.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- 19.Giesen EB, Ding M, Dalstra M, et al. Mechanical properties of cancellous bone in the human mandibular condyle are anisotropic. J Biomech. 2001;34:799–803. doi: 10.1016/s0021-9290(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 20.Giesen EB, van Eijden TM. The three-dimensional cancellous bone architecture of the human mandibular condyle. J Dent Res. 2000;79:957–963. doi: 10.1177/00220345000790041101. [DOI] [PubMed] [Google Scholar]
- 21.Kato Y, Kizu Y, Tonogi M, et al. Internal structure of zygomatic bone related to zygomatic fixture. J Oral Maxillofac Surg. 2005;63:1325–1329. doi: 10.1016/j.joms.2005.05.313. [DOI] [PubMed] [Google Scholar]
- 22.Peterson J, Dechow PC. Material properties of the human cranial vault and zygoma. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:785–797. doi: 10.1002/ar.a.10096. [DOI] [PubMed] [Google Scholar]
- 23.Rafferty KL, Herring SW, Artese F. Three-dimensional loading and growth of the zygomatic arch. J Exp Biol. 2000;203:2093–2104. doi: 10.1242/jeb.203.14.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng S, Choi IW, Herring SW, et al. Stereological analysis of bone architecture in the pig zygomatic arch. Anat Rec. 1997;248:205–213. doi: 10.1002/(sici)1097-0185(199706)248:2<205::aid-ar7>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: Rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22:354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Sinha VR, Trehan A. Biodegradable microspheres for parenteral delivery. Crit Rev Ther Drug Carrier Syst. 2005;22:535–602. doi: 10.1615/critrevtherdrugcarriersyst.v22.i6.20. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard EM, Kaplan DL. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin Drug Deliv. 2011;8:797–811. doi: 10.1517/17425247.2011.568936. [DOI] [PubMed] [Google Scholar]
- 28.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Caplan AI. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 31.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monaco E, Bionaz M, Hollister SJ, et al. Strategies for regeneration of the bone using porcine adult adipose-derived mesenchymal stem cells. Theriogenology. 2011;75:1381–1399. doi: 10.1016/j.theriogenology.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laino G, Graziano A, d'Aquino R, et al. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006;206:693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- 35.Mendonça JJ, Juiz-Lopez P. Regenerative facial reconstruction of terminal stage osteoradionecrosis and other advanced craniofacial diseases with adult cultured stem and progenitor cells. Plast Reconstr Surg. 2010;126:1699–1709. doi: 10.1097/PRS.0b013e3181f24164. [DOI] [PubMed] [Google Scholar]
- 36.Boo JS, Yamada Y, Okazaki Y, et al. Tissue-engineered bone using mesenchymal stem cells and a biodegradable scaffold. J Craniofac Surg. 2002;13:231–239. doi: 10.1097/00001665-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Yamada Y, Ueda M, Naiki T, et al. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: Tissue-engineered bone regeneration. Tissue Eng. 2004;10:955–964. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 38.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Walboomers XF, Shi S, et al. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 40.Barrilleaux B, Phinney DG, Prockop DJ, et al. Review: Ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:3007–3019. doi: 10.1089/ten.2006.12.3007. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Illich DJ, Demir N, Stojkovic M, et al. Concise review: Induced pluripotent stem cells and lineage reprogramming: Prospects for bone regeneration. Stem Cells. 2011;29:555–563. doi: 10.1002/stem.611. [DOI] [PubMed] [Google Scholar]
- 43.Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell. 2011;8:252–261. doi: 10.1016/j.stem.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: State of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 45.Rauh J, Milan F, Günther KP, et al. Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev. 2011;17:263–280. doi: 10.1089/ten.TEB.2010.0612. [DOI] [PubMed] [Google Scholar]
- 46.Fröhlich M, Grayson WL, Marolt D, et al. Bone grafts engineered from human adipose-derived stem cells in perfusion bioreactor culture. Tissue Eng Part A. 2010;16:179–189. doi: 10.1089/ten.tea.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grayson WL, Marolt D, Bhumiratana S, et al. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;108:1159–1170. doi: 10.1002/bit.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhumiratana S, Grayson WL, Castaneda A, et al. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials. 2011;32:2812–2820. doi: 10.1016/j.biomaterials.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meinel L, Fajardo R, Hofmann S, et al. Silk implants for the healing of critical size bone defects. Bone. 2005;37:688–698. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Grayson WG, Fröhlich M, Yeager K, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107:3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]