Abstract
Background & Aims
Epstein-Barr virus (EBV) has been causally associated with cancer; some gastric carcinomas have a monoclonal EBV genome in every cancer cell, indicating that they arose from a single infected progenitor cell. However, the proportion of EBV-positive gastric carcinomas is uncertain and the etiological significance is unknown.
Methods
We conducted a meta-analysis of 70 studies including 15,952 cases of gastric cancer assessed by in situ hybridization for EBV-encoded small RNA.
Results
The pooled prevalence estimate of EBV-positivity was 8.7% (95% CI: 7.5, 10.0) overall, with a two-fold difference by sex: 11.1% (95% CI: 8.7, 14.1) of gastric cancer cases in males vs. 5.2% (95% CI: 3.6, 7.4) of cases in females. Tumors arising in the gastric cardia (13.6%) or corpus (13.1%) were more than twice as likely to be EBV-positive as those in the antrum (5.2%; p<0.01 for both comparisons). EBV-prevalence was four times higher (35.1%) for tumors in post-surgical gastric stump/remnants. Over 90% of lymphoepithelioma-like carcinomas were EBV-positive but only 15 studies reported any cases of this type; prevalence did not significantly differ between the more common diffuse (7.6%) and intestinal (9.5%) histologies. EBV-prevalence was similar in cases from Asia (8.3%), Europe (9.2%), and the Americas (9.9%).
Conclusions
EBV-positive gastric cancers greatly differ from other gastric carcinomas based on sex, anatomic subsite, and surgically disrupted anatomy, indicating that it is a distinct etiologic entity. Epidemiologic studies comparing EBV-positive and -negative gastric cancers are warranted to investigate EBV’s role in gastric carcinogenesis.
Keywords: Epstein-Barr virus, gastric cancer, meta-analysis, prevalence
Introduction
Despite its decline in incidence during the 20th century, gastric cancer (GC) remains the fourth most commonly diagnosed cancer and the second leading cause of cancer-related mortality worldwide1. Risk of gastric cancer is now believed to be modulated by a complex interaction between Helicobacter pylori (H. pylori) and a myriad of human genetic polymorphisms, as well as a number of other environmental and lifestyle factors2–4.
Epstein-Barr Virus (EBV) is a ubiquitous gamma-1 herpes virus usually acquired during childhood via salivary transmission which establishes a life-long persistent infection of B-cells in over 90% of adults5. EBV is an established cause of Burkitt lymphoma, sinonasal angiocentric T-cell lymphoma, immunosuppression-related lymphoma, Hodgkin’s lymphoma and nasopharyngeal carcinoma 6. The oncogenic effects of the virus are likely exerted via the expression of EBV nuclear antigens (EBNAs) and latent membrane proteins (LMPs) which interact with a number of tumor suppressor genes and signaling pathways7–10.
EBV is known to be present in a small percentage of gastric carcinomas; estimates vary widely but EBV-positive GC has been reported to constitute between 2 and 16% of cases11. In EBV-positive cases, virtually 100% of the carcinoma cells contain EBV nucleic acid sequences12, and the EBV terminal repeat sequences are always uniform13–15. These observations imply that the tumor arose from a single EBV infected cell and that the EBV genome was retained during malignant transformation and proliferation. Moreover, EBV is routinely detected in an uncommon histologic entity, undifferentiated lymphoepithelioma-like gastric carcinoma (also known as medullary carcinoma), the microscopic appearance of which resembles nasopharyngeal lymphoepithelioma16, 17.
Recent reviews18, 19 have qualitatively described some of the epidemiological and clinicopathological features of Epstein-Barr virus associated GC. However, to date, there has not been a formal overview of published prevalence estimates. We therefore undertook a rigorous meta-analysis of papers demonstrating EBV tumor positivity using the demonstrated gold standard (in situ hybridization). This type of formal meta-analysis technique using a random effects model allowed our prevalence estimate to include consideration of within and between study variation in estimating the overall prevalence of EBV-positive GC and assessing variation by regional, clinical and tumor characteristics.
Material and Methods
We used PubMed® software tosearch Medline (U.S. National Library of Medicine, Bethesda, MD) using the following search terms: “Epstein Barr Virus AND gastric cancer”, “EBV and gastric cancer”, “Epstein Barr Virus AND stomach cancer”, “EBV AND stomach cancer” for studies listed on or before September 30th 2008. Eligibility criteria for inclusion were: (i) studies must have ascertained EBV status of gastric tumor tissue using EBER in situ hybridization (the accepted gold standard in determining EBV-positivity in tumor tissue) and (ii) studies had to report prevalence of EBV-positivity in unselected GC cases, or provide enough information to calculate this estimate.
A total of 407 papers were identified and their titles and abstracts reviewed for relevance. 157 papers were discounted as irrelevant and 64 as duplications from a single population already represented. 28 papers were excluded due to patient selection (thereby making calculation of true prevalence impossible), 17 had not used EBER in situ hybridization, 19 were in languages other than English and 56 were found to be review articles. Thus, 70 papers met the inclusion criteria and were abstracted for prevalence data. Of the 63 studies which were included in the analyses of adenocarcinoma (defined from here onwards as primary GC tumors which are not stump/remnant cancers), 12 studies also included lymphoepithelioma-like gastric carcinoma tumors and 4 included stump/remnant cancers. In addition, 3 studies were included that exclusively described lymphoepithelioma-like gastric carcinoma as were 5 describing stump/remnant cancers only. Separate analyses were conducted for gastric adenocarcinoma (63 studies), lymphoepithelioma-like gastric carcinoma tumors (15 studies) and stump/remnant cancers (9 studies). One publication clearly differentiated between ethnic Japanese and non-Japanese GC cases in Brazil20 and is, therefore, included in the meta-analysis of gastric adenocarcinoma as two separate studies.
The following data were abstracted as available: first author, year of publication, sample size, EBV prevalence (or EBV-positive cases), sex, country of origin, regional group (Asia, Europe, Americas), histologic type (Laurén classification21) and tumor anatomic subsite (cardia, middle/corpus or antrum).
Statistical analysis
Meta-analyses were performed with Stata version 10 (StataCorp, College Station, TX), using the “metan” command22. Summary estimates (% prevalence), standard errors and 95% confidence intervals (CIs) were calculated, using the Wilson method23, for each study. As the meta-analysis technique assumes normally distributed data, we logarithmically transformed all prevalence estimates24, which necessitated adding a correction factor of 0.5 to both numerator and denominator25 for reported prevalence of 0.
We first computed pooled summary estimates using the Mantel-Haenszel method assuming a fixed effects model26. However, as we found significant heterogeneity in prevalence estimates across studies, we also employed the random effect model of DerSimonian and Laird27 and focus on those results in our presentation. Heterogeneity was described using the I2 statistic, that represents the approximate proportion of total variability in point estimates that can be attributed to heterogeneity28:
where σ2 denotes the within-study variance and τ2 denotes the between-studies variance component.
Meta-regression models were estimated using the “metareg” command in Stata v10.1, to analyze associations of EBV prevalence in GC with national incidence rates, study size and study quality. Incidence rates of GC among males were obtained from GLOBOCAN estimates for individual countries29; national incidence was treated both as a continuous variable and as a categorical variable, comparing countries in the top quintile (>21.7 cases per 100,000 population) to all other countries1. Study size was categorized according to whether the prevalence estimate was based on more than or less than 100 GC cases. Although we have no direct measure of ‘quality’ across reports we calculated a surrogate measure based on the number of variables (0–3) included among the following: (i) sex, (ii) anatomic subsite and (iii) histologic type.
Meta-analytic assumptions were assessed with Egger’s test (“metabias”) of funnel plot asymmetry (publication bias). This test identified no evidence of publication bias (P = 0.49). The influence of individual studies on the summary effect estimate was analyzed using the using the “metainf” command30, which graphically compares meta-analytic estimates computed by omitting each study in turn. None of the included studies appeared to dominate the overall meta-analysis.
Results
A total of 70 studies were chosen for inclusion in the meta-analysis; these represented hospital cancer case series, together reporting a grand total of 15,952 GC cases. The earliest study was published in 199215 and the most recent studies in 200831, 32, the largest study included 2966 GC cases33 and the smallest, 19 cases34. The majority of the 70 studies included originated in Asia (45/70), with a similar number of studies from Europe (12/70) and America (13/70). Of the 70 studies included, 47 provided information on patients’ sex.
Of the 63 studies of primary non-remnant GC, 43 included information on patients’ sex. 31 studies included information on histological type and 20 had information on anatomic sub-site. Of the 12 studies that included both lymphoepithelioma-like gastric carcinoma and adenocarcinoma, lymphoepithelioma-like gastric carcinoma cases comprised between 0.9%34 and 15%35 of the respective series. Only 3 of the lymphoepithelioma-like gastric carcinoma studies included information on sex 36–38. Of the 5 studies describing both gastric stump/remnant cancer and adenocarcinoma, gastric stump/remnant cancer comprised between 2%39 and 25%40 of the series. Five of the gastric stump/remnant studies included information on sex40–44. Together, the 70 studies reported a grand total of 15,952 GC cases.
Gastric adenocarcinoma meta-analysis
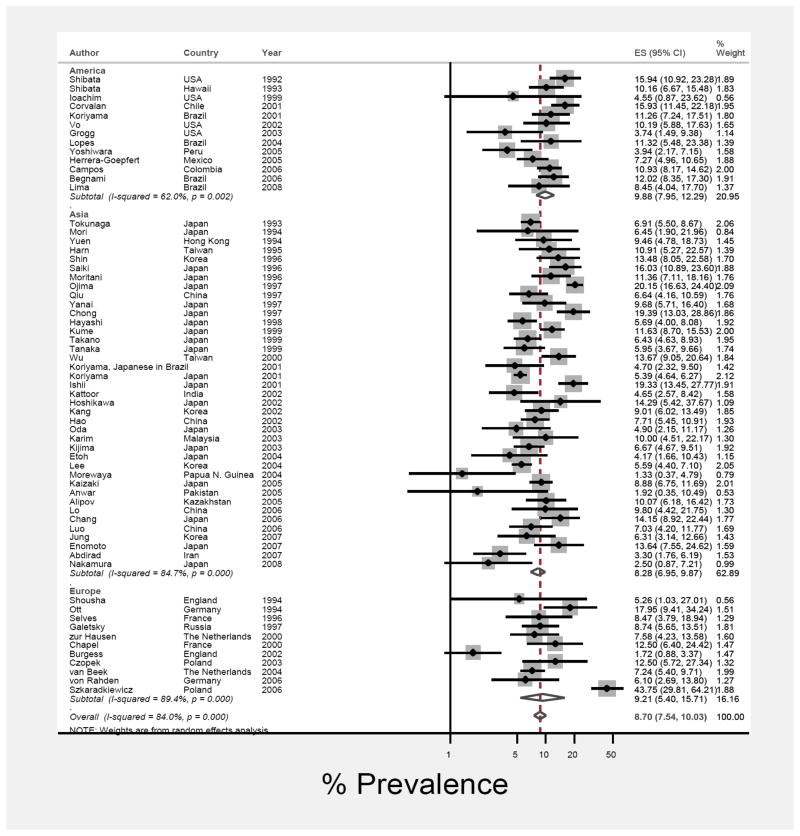
Figure 1 shows EBV prevalence and 95% CI estimates from individual studies based on the random effects model. The pooled prevalence of EBV-positive GC, as a proportion of gastric adenocarcinoma was 8.7% (95% CI: 7.5%, 10.0%). EBV prevalence was similar in cases from each of the three geographic regions: 9.9% (95% CI: 7.9%, 12.3%) for cases from America, 8.3% (95% CI: 6.9%, 9.9%) for Asian cases, and 9.2% (95% CI: 5.4%, 15.7%) for European cases (Figure 1).
Figure 1.
Forest plot (random-effects model) of prevalence of EBV-positivity in gastric cancer cases by region of residence, in order of year of publication. Rhomboids indicate pooled prevalence for each region and in all studies.
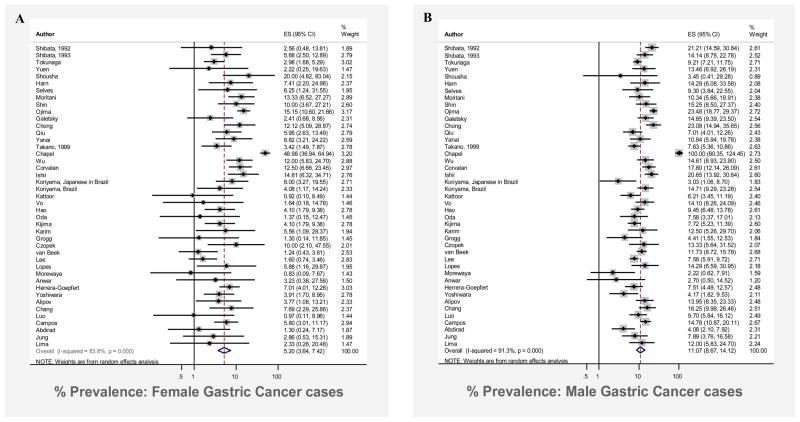
There was a two-fold difference in the proportion of EBV-positive GC among cases in males compared to cases in females, with a prevalence of 11.1% (95% CI: 8.7%, 14.1%) in male GC cases compared to 5.2% (95% CI: 3.6%, 7.4%) in female cases (Figure 2). Notably, nine studies31, 45–52 reported no cases of EBV-positive GC in females, whereas only a single study reported no cases among males34.
Figure 2.
Forest plot (random effects model) of prevalence of EBV-positivity in gastric cancer cases in females (Panel A) and males (Panel B), in order of year of publication. Rhomboids indicate pooled prevalence for each sex.
Of gastric tumors from the antrum, 5.2% (95% CI: 3.8, 7.0) were EBV-positive, which was significantly lower than the proportion for either cardia (13.6%; 95% CI: 9.9, 18.7) or middle/corpus (13.1%; 95% CI: 10.4, 16.5) stomach tumors. In contrast, there was no statistically significant difference in the proportion EBV-positive for tumors of intestinal (9.5%; 95% CI: 7.2, 12.5) relative to diffuse (7.6%; 95% CI: 5.7, 10.3) histology.
Prevalence of EBV-positivity was similar for cases from high GC incidence countries (8.6; 95% CI: 7.2, 10.4) compared to all others (8.7; 95% CI: 6.9, 11.0). Analyzed as a continuous variable, there was no significant correlation between EBV-prevalence and national incidence (r = 0.08; p=0.51).
Heterogeneity was notably high in that the overall I2 for the meta-analysis was 84% (τ2 = 0.24; p < 0.001). A number of variables (distribution of cases by sex, anatomic subsite and histologic type, national incidence rates, regional group and year of publication) were alternately added to the meta-regression model to examine the extent to which one or more of these covariates might explain the heterogeneity between studies. Size of study was significantly associated with EBV-positive GC prevalence so that for each ten-fold increase in the total number of GC cases, the prevalence of EBV decreased by 0.6% (95% CI: 0.5, 0.8). When sex, histological type, and study size were included together, I2 was reduced to 63%. The number of “quality” variables reported was also associated with heterogeneity in that studies providing prevalence estimates alone (and none of the 3 quality variables) had an I2 of 89% (τ2 = 0.43) whereas studies which also included sex, anatomic subsite and histologic type had an I2 of 56% (τ2= 0.08).
Lymphoepithelioma-like gastric carcinoma
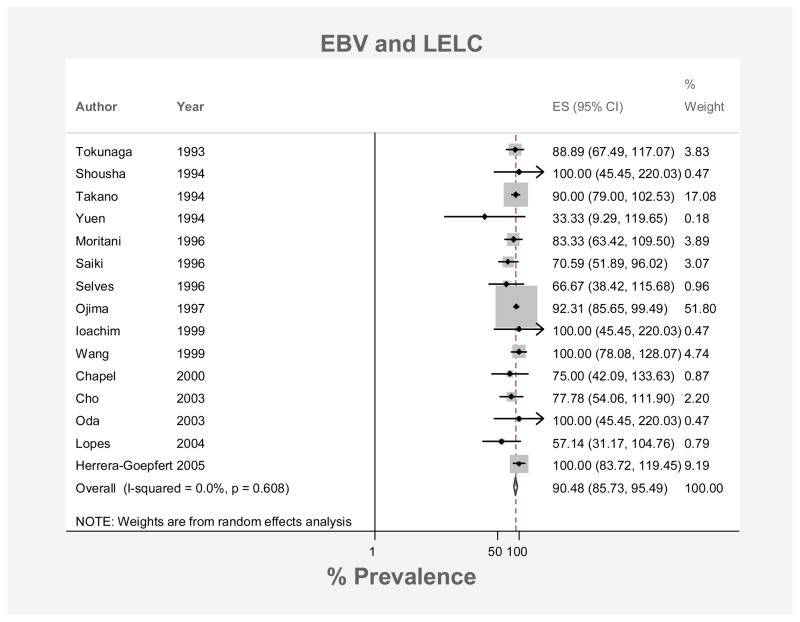
The pooled prevalence of EBV-positivity among lymphoepithelioma-like gastric carcinoma cases was 90.5% (95% CI: 85.7%, 95.5%; Figure 3). Prevalence estimates did not differ significantly by regional group: 88.9% (95% CI: 64.2%, 100%) for American cases, 90.3% (95% CI: 85.2%, 95.6%) for Asian cases and 75.7% (95% CI: 53.0%, 100%) for cases from Europe. There was very little heterogeneity across the 15 lymphoepithelioma-like gastric carcinoma studies (I2 = 0.0%, p=0.61).
Figure 3.
Forest plot (random effects model) of prevalence of EBV positivity in gastric lymphoepithelioma-like carcinoma in order of year of publication. Rhomboid indicates pooled prevalence.
Gastric stump/remnant cancer
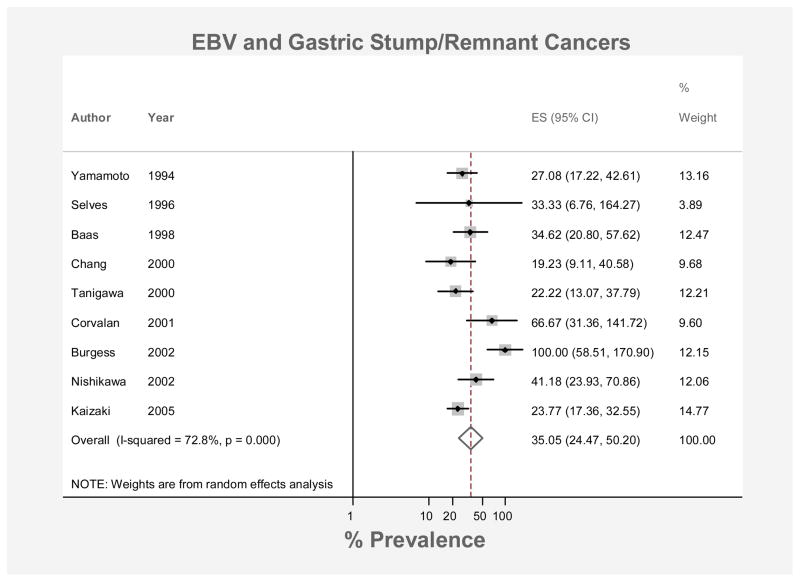
The pooled prevalence of EBV-positivity among stump/remnant cancers was 35.1% (95% CI: 24.5, 50.2; Figure 4). From the 5 studies40–43, 53 which also listed information on sex, the prevalence among stump/remnant cancers in males was estimated at 40% (± 7.44%). 3 studies included cases in females40, 41, 43 where the EBV-positive cancer prevalence was 17% (± 9.6%). These studies were markedly heterogeneous (I2 = 99.5%, p < 0.001).
Figure 4.
Forest plot (random effects model) of prevalence of EBV positivity in gastric stump/remnant cancers in order of year of publication. Rhomboid indicates pooled prevalence.
Discussion
We conducted a formal meta-analysis of the published literature to estimate the prevalence of EBV-positivity in GC. The overall pooled prevalence was estimated at 8.7% (95% CI: 7.5%, 10.0%) and was four times higher in gastric stump/remnant cancers than in primary non-remnant GC. Male GC patients were twice as likely to have EBV-positive tumors as female patients and antral tumors were half as likely to be EBV-positive as tumors from other sub-sites. In contrast, we found no quantitative evidence of regional variation, which had been previously assumed to be important18,19, and no significant difference between intestinal and diffuse histologies. As has been reported previously, over 90% of lymphoepithelioma-like gastric carcinoma cancers were EBV-positive.
Heterogeneity across studies for the overall meta-analysis was substantial. We investigated possible sources for this heterogeneity using meta-regression methods; however, most of the residual heterogeneity was not explained by measured covariates. Laboratory methodology should not have been a large source of variation since we included only studies using EBER in situ hybridization on paraffin embedded tissue, however, minor differences in technical quality cannot be ruled out.
The 70 studies provided sparse patient and clinical data and it is possible that the observed heterogeneity might relate to such factors. For example, associations with age have been reported previously observed although results have been discordant: some studies find an age-dependent decrease in EBV-positivity rates54, 55 and others an age-dependent increase in rates36. If there is an age association overall as well as variation between studies in patient age, this factor might explain some of the residual heterogeneity, however, patient age was generally not reported.
Our calculated prevalence estimate of 8% in gastric carcinomas contrasts sharply with the consistent absence of gastric EBV infection in non-cancer control conditions. In two studies with 16%15 and 13%56 EBV prevalence in gastric carcinomas, gastric biopsies from patients with Barrett’s esophagitis15, gastric ulcer disease15 and healthy control subjects15, 56 were uniformly EBV-negative (odds ratios: incalculable). These early and definitive studies associated gastric EBV infection only with the presence of malignancy. Normal gastric tissue surrounding neoplastia is also EBV-negative39, 42, 50, 51 further demonstrating the specificity of EBV infection.
Contrary to previous suggestions in the literature, we found no evidence that background incidence of GC is associated with rates of EBV-positivity in gastric adenocarcinoma. However, there are more reports and more cases in the published literature from Asia than from other regions of the world. Since regions with particularly high risk of GC, outside of Asia, are underrepresented in the literature, our analysis may not provide a complete picture.
To date, there is little evidence of interaction or antagonism of EBV with H. pylori, the agent most strongly implicated in gastric carcinogenesis48, 57. However, some in vitro experimental data suggests that H. pylori-associated monochloramine may induce EBV lytic conversion in gastric epithelium latently infected with EBV58. Results of previous clinical studies have demonstrated no correlation between H. pylori and EBV infections, with H. pylori equally likely regardless of EBV status 48, 59, 60. EBV may be acting as a co-factor in H. pylori related gastric carcinogenesis, contributing to the likelihood of malignant transformation. Importantly, the relative predilection of EBV-positive tumors for the non-antral stomach may be analogous to the stronger association of H. pylori with non-cardia cancer, implying distinctive etiologies for gastric carcinogenesis at different subsites61.
EBV infection in GC seems to be associated with increased inflammation, as seen in lymphoepithelioma-like gastric carcinoma with its the high-degree of lymphocytic infiltrate. The monoclonal nature of infection of tumor cells14 necessarily implies that the clonal progenitor cell was EBV positive. Thus EBV must have been present at tumor inception regardless of whether the onset of inflammation was earlier or later. Furthermore, although it has been demonstrated that EBV is not present in normal epithelium adjacent to EBV-positive GC37, it is unknown whether EBV is associated with inflammation in pre-cancerous states, such as gastritis62, 63. Even so, EBV positivity of inflammatory infiltrate would not demonstrate causality, since recruited lymphocytes may be coincidentally EBV-positive.
The mechanism of entry of EBV into gastric epithelial cells is not understood. At initial infection of B cells, EBV binds via CD21 receptors; however, whether CD21 is expressed on epithelial cells and/or carcinoma tissue has not been definitively determined64. CD21 independent mechanisms of EBV transfer have also been proposed, including direct cell-to-cell contact with virus-infected B cells and IgA mediated transport64. Interestingly, frequent salty food intake and occupational exposure to wood dust and/or iron filings have been associated with EBV-positive GC18, 65, which has been interpreted as indicating that mechanical injury to gastric epithelia increases susceptibility to EBV infection.
The relatively high proportion of GC stump/remnant cancers which are EBV-positive is thought to result from bile reflux. As well as directly damaging the mucosal barrier66 prolonged exposure to bile acids and pancreatic juices may mediate EBV entry into normally non-susceptible epithelial cells, for instance by inducing the fusion with infected B cells41. Interestingly, very high prevalence of EBER expression has been reported in both ulcerative colitis and Crohn’s disease67, which suggests that epithelial damage more generally is associated with increased vulnerability to EBV colonization.
Likewise, the etiologic role of the virus in gastric carcinogenesis remains largely unknown. EBV-positive GC exhibits the EBV latency I pattern also seen in Burkitt lymphoma, in contrast to nasopharyngeal carcinoma and Hodgkin’s lymphoma which both express latency II pattern16. The absence of the known transforming proteins LMP1 and EBNA2 brings into question the oncogenic potential of the latency I pattern EBV. However, the viral genes that are expressed in EBV-positive GC also display carcinogenic activities: EBER-1 and -2 have been shown to upregulate insulin-like growth factor expression in vitro, BARF1 can transform rodent fibroblasts in vitro and is known to increase the bcl-1 to bax ratio which promotes cell survival, and LMP2A has been found to confer resistance to apoptosis68.
Data on lymphoepithelioma-like gastric carcinoma were only reported in a limited number of studies. The uneven reporting across studies and the absence of standardized criteria for its histologic appearance may indicate that GC cases classified as lymphoepithelioma-like gastric carcinoma by some pathologists are classified as ‘carcinoma with heavy lymphocytic infiltrate’ by others. This speculation and the finding that lymphoepithelioma-like gastric carcinoma is >90% EBV-positive has led us to question the separate classification of lymphoepithelioma-like gastric carcinoma, for which the extra-ordinary lymphocytic infiltrate might merely indicate one extreme of host response to an EBV-positive tumor.
Worldwide, men are twice as likely as women to develop GC69, which the established risk factors for GC (H. pylori and smoking) do not fully explain this sex difference in incidence rates70. Given this sex difference in incidence rates overall, the two-fold sex difference in EBV-positivity implies that the incidence of EBV-positive GC is four times higher in males than females. The reasons for this large sex difference are, as yet, unknown but may relate to differing exposure to lifestyle or occupational factors risk factors for EBV-positive GC18. Alternatively intrinsic biological and/or hormonal factors, might also be responsible71. Interestingly, other EBV associated cancers including Hodgkin’s lymphoma, Burkitt lymphoma and nasopharyngeal carcinoma are two to three times as common in men72–75. The “immunocompetence” hypothesis has been put forward as one possible explanation for sex difference in these and other cancers relating to infection, based on data suggesting that testosterone causes immunosuppression76. The hypothesis holds that men are thus more susceptible to infections and their sequelae which increase morbidity and mortality throughout all ages of life77, 78.
EBV-positive GC is a distinct entity accounting for 8.7% of GC worldwide. Considering the worldwide burden of GC, the paucity of data regarding the significance of EBV-positivity is remarkable. Further epidemiologic studies are warranted to determine the role of EBV with respect to etiology, treatment and prognosis.
Supplementary Material
Acknowledgments
Funding: Dr Murphy is supported by the Ireland-Northern Ireland-National Cancer Institute Cancer Consortium and the Health Research Board of Ireland. This project is supported by the Intramural Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute.
Abbreviations
- EBV
Epstein-Barr virus
- GC
gastric adenocarcinoma
- EBNAs
EBV nuclear antigens
- LMPs
latent membrane proteins
Footnotes
Disclosures: None to declare
Conflict of interest: GM: No conflicts of interest exist
RP: No conflicts of interest exist
MCC: No conflicts of interest exist
CSR: No conflicts of interest exist
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.El-Omar EM, Carrington M, Chow W-H, McColl KEL, Bream JH, Young HA, Herrera J, Lissowska J, Yuan C-C, Rothman N, Lanyon G, Martin M, Fraumeni JF, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 3.Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, Schatzkin A. A Prospective Study of Tobacco, Alcohol, and the Risk of Esophageal and Gastric Cancer Subtypes. 2007;165:1424–1433. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 4.Blaser MJ. Science, medicine, and the future - Helicobacter pylori and gastric diseases. British Medical Journal. 1998;316:1507–1510. doi: 10.1136/bmj.316.7143.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–68. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 6.IARC Monogr Eval Carcinog Risks Hum. Vol. 70. Lyon: WHO; 1997. Epstein-Barr virus; pp. 47–373. [PMC free article] [PubMed] [Google Scholar]
- 7.Szekely L, Selivanova G, Magnusson KP, Klein G, Wiman KG. EBNA-5, an Epstein-Barr virus-encoded nuclear antigen, binds to the retinoblastoma and p53 proteins. Proc Natl Acad Sci U S A. 1993;90:5455–9. doi: 10.1073/pnas.90.12.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh JJ, Henkel T, Salmon P, Robey E, Peterson MG, Hayward SD. Truncated mammalian Notch1 activates CBF1/RBPJk-repressed genes by a mechanism resembling that of Epstein-Barr virus EBNA2. Mol Cell Biol. 1996;16:952–9. doi: 10.1128/mcb.16.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huen DS, Henderson SA, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–60. [PubMed] [Google Scholar]
- 10.Stewart S, Dawson CW, Takada K, Curnow J, Moody CA, Sixbey JW, Young LS. Epstein-Barr virus-encoded LMP2A regulates viral and cellular gene expression by modulation of the NF-kappaB transcription factor pathway. Proc Natl Acad Sci U S A. 2004;101:15730–5. doi: 10.1073/pnas.0402135101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess DE, Woodman CB, Flavell KJ, Rowlands DC, Crocker J, Scott K, Biddulph JP, Young LS, Murray PG. Low prevalence of Epstein-Barr virus in incident gastric adenocarcinomas from the United Kingdom. Br J Cancer. 2002;86:702–4. doi: 10.1038/sj.bjc.6600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada K. Epstein-Barr virus and gastric carcinoma. Mol Pathol. 2000;53:255–61. doi: 10.1136/mp.53.5.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukayama M, Hayashi Y, Iwasaki Y, Chong J, Ooba T, Takizawa T, Koike M, Mizutani S, Miyaki M, Hirai K. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest. 1994;71:73–81. [PubMed] [Google Scholar]
- 14.Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci U S A. 1994;91:9131–5. doi: 10.1073/pnas.91.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769–74. [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann K, Niedobitek G. Epstein-Barr virus-associated carcinomas: facts and fiction. J Pathol. 2003;199:140–5. doi: 10.1002/path.1296. [DOI] [PubMed] [Google Scholar]
- 17.Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377–80. [PubMed] [Google Scholar]
- 18.Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. 2008;99:195–201. doi: 10.1111/j.1349-7006.2007.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sousa H, Pinto-Correia AL, Medeiros R, Dinis-Ribeiro M. Epstein-Barr virus is associated with gastric carcinoma: the question is what is the significance? World J Gastroenterol. 2008;14:4347–51. doi: 10.3748/wjg.14.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koriyama C, Akiba S, Iriya K, Yamaguti T, Hamada GS, Itoh T, Eizuru Y, Aikou T, Watanabe S, Tsugane S, Tokunaga M. Epstein-Barr virus-associated gastric carcinoma in Japanese Brazilians and non-Japanese Brazilians in Sao Paulo. Jpn J Cancer Res. 2001;92:911–7. doi: 10.1111/j.1349-7006.2001.tb01180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. an Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Harris R. metan: fixed- and random-effects meta-analysis. Stata Journal. 2008;8:3–28. [Google Scholar]
- 23.Wilson EB. Probable inference, the law of succession, and statistical inference. Journal of the American Statistical Association. 1927;22:209–212. [Google Scholar]
- 24.Lipsey MWD. Volume Applied social research methods series. Vol. 49. California Sage Publications; 2001. Practical meta-analysis. [Google Scholar]
- 25.Deeks JJH, JPT, Altman DG. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006] 2006. Analysing and presenting results. [Google Scholar]
- 26.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37:1158–60. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 29.Ferlay J, Bray F, Pisani P, Parkin DM. IARC Cancer Base No. 5. version 2.0. Lyon: IARC Press; 2004. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. [Google Scholar]
- 30.Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;47:15–17. [Google Scholar]
- 31.Lima VP, de Lima MA, Andre AR, Ferreira MV, Barros MA, Rabenhorst SH. H pylori (CagA) and Epstein-Barr virus infection in gastric carcinomas: correlation with p53 mutation and c-Myc, Bcl-2 and Bax expression. World J Gastroenterol. 2008;14:884–91. doi: 10.3748/wjg.14.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura Y, Yanai H, Kitoh T, Matsubara Y, Hirano A, Okamoto T, Yoshida T, Nishikawa J, Okita K. The clinical meaning of mucin phenotype and Epstein-Barr virus infection in gastric cancer. Hepatogastroenterology. 2008;55:41–5. [PubMed] [Google Scholar]
- 33.Koriyama C, Shinkura R, Hamasaki Y, Fujiyoshi T, Eizuru Y, Tokunaga M. Human leukocyte antigens related to Epstein-Barr virus-associated gastric carcinoma in Japanese patients. Eur J Cancer Prev. 2001;10:69–75. doi: 10.1097/00008469-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Shousha S, Luqmani YA. Epstein-Barr virus in gastric carcinoma and adjacent normal gastric and duodenal mucosa. J Clin Pathol. 1994;47:695–8. doi: 10.1136/jcp.47.8.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ojima H, Fukuda T, Nakajima T, Nagamachi Y. Infrequent overexpression of p53 protein in Epstein-Barr virus-associated gastric carcinomas. Jpn J Cancer Res. 1997;88:262–6. doi: 10.1111/j.1349-7006.1997.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrera-Goepfert R, Akiba S, Koriyama C, Ding S, Reyes E, Itoh T, Minakami Y, Eizuru Y. Epstein-Barr virus-associated gastric carcinoma: Evidence of age-dependence among a Mexican population. World J Gastroenterol. 2005;11:6096–103. doi: 10.3748/wjg.v11.i39.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokunaga M, Land CE, Uemura Y, Tokudome T, Tanaka S, Sato E. Epstein-Barr virus in gastric carcinoma. Am J Pathol. 1993;143:1250–4. [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HH, Wu MS, Shun CT, Wang HP, Lin CC, Lin JT. Lymphoepithelioma-like carcinoma of the stomach: a subset of gastric carcinoma with distinct clinicopathological features and high prevalence of Epstein-Barr virus infection. Hepatogastroenterology. 1999;46:1214–9. [PubMed] [Google Scholar]
- 39.Selves J, Bibeau F, Brousset P, Meggetto F, Mazerolles C, Voigt JJ, Pradere B, Chiotasso P, Delsol G. Epstein-Barr virus latent and replicative gene expression in gastric carcinoma. Histopathology. 1996;28:121–7. doi: 10.1046/j.1365-2559.1996.287333.x. [DOI] [PubMed] [Google Scholar]
- 40.Chang MS, Lee JH, Kim JP, Kim HS, Lee HS, Kim CW, Kim YI, Kim WH. Microsatellite instability and Epstein-Barr virus infection in gastric remnant cancers. Pathol Int. 2000;50:486–92. doi: 10.1046/j.1440-1827.2000.01072.x. [DOI] [PubMed] [Google Scholar]
- 41.Baas IO, van Rees BP, Musler A, Craanen ME, Tytgat GN, van den Berg FM, Offerhaus GJ. Helicobacter pylori and Epstein-Barr virus infection and the p53 tumour suppressor pathway in gastric stump cancer compared with carcinoma in the non-operated stomach. J Clin Pathol. 1998;51:662–6. doi: 10.1136/jcp.51.9.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corvalan A, Koriyama C, Akiba S, Eizuru Y, Backhouse C, Palma M, Argandona J, Tokunaga M. Epstein-Barr virus in gastric carcinoma is associated with location in the cardia and with a diffuse histology: a study in one area of Chile. Int J Cancer. 2001;94:527–30. doi: 10.1002/ijc.1510. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa J, Yanai H, Hirano A, Okamoto T, Nakamura H, Matsusaki K, Kawano T, Miura O, Okita K. High prevalence of Epstein-Barr virus in gastric remnant carcinoma after Billroth-II reconstruction. Scand J Gastroenterol. 2002;37:825–9. [PubMed] [Google Scholar]
- 44.Tanigawa H, Uesugi H, Mitomi H, Saigenji K, Okayasu I. Possible association of active gastritis, featuring accelerated cell turnover and p53 overexpression, with cancer development at anastomoses after gastrojejunostomy. Comparison with gastroduodenostomy. Am J Clin Pathol. 2000;114:354–63. doi: 10.1093/ajcp/114.3.354. [DOI] [PubMed] [Google Scholar]
- 45.Anwar M, Koriyama C, Naveed IA, Hamid S, Ahmad M, Itoh T, Minakami Y, Eizuru Y, Akiba S. Epstein-barr virus detection in tumors of upper gastrointestinal tract. An in situ hybridization study in Pakistan. J Exp Clin Cancer Res. 2005;24:379–85. [PubMed] [Google Scholar]
- 46.Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003;16:641–51. doi: 10.1097/01.MP.0000076980.73826.C0. [DOI] [PubMed] [Google Scholar]
- 47.Kattoor J, Koriyama C, Akiba S, Itoh T, Ding S, Eizuru Y, Abraham EK, Chandralekha B, Amma NS, Nair MK. Epstein-Barr virus-associated gastric carcinoma in southern India: A comparison with a large-scale Japanese series. J Med Virol. 2002;68:384–9. doi: 10.1002/jmv.10215. [DOI] [PubMed] [Google Scholar]
- 48.Luo B, Wang Y, Wang XF, Gao Y, Huang BH, Zhao P. Correlation of Epstein-Barr virus and its encoded proteins with Helicobacter pylori and expression of c-met and c-myc in gastric carcinoma. World J Gastroenterol. 2006;12:1842–8. doi: 10.3748/wjg.v12.i12.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morewaya J, Koriyama C, Akiba S, Shan D, Itoh T, Eizuru Y. Epstein-Barr virus-associated gastric carcinoma in Papua New Guinea. Oncol Rep. 2004;12:1093–8. [PubMed] [Google Scholar]
- 50.Oda K, Koda K, Takiguchi N, Nunomura M, Seike K, Miyazaki M. Detection of Epstein-Barr virus in gastric carcinoma cells and surrounding lymphocytes. Gastric Cancer. 2003;6:173–8. doi: 10.1007/s10120-003-0247-2. [DOI] [PubMed] [Google Scholar]
- 51.Vo QN, Geradts J, Gulley ML, Boudreau DA, Bravo JC, Schneider BG. Epstein-Barr virus in gastric adenocarcinomas: association with ethnicity and CDKN2A promoter methylation. J Clin Pathol. 2002;55:669–75. doi: 10.1136/jcp.55.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuen ST, Chung LP, Leung SY, Luk IS, Chan SY, Ho J. In situ detection of Epstein-Barr virus in gastric and colorectal adenocarcinomas. Am J Surg Pathol. 1994;18:1158–63. doi: 10.1097/00000478-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Kaizaki Y, Hosokawa O, Sakurai S, Fukayama M. Epstein-Barr virus-associated gastric carcinoma in the remnant stomach: de novo and metachronous gastric remnant carcinoma. J Gastroenterol. 2005;40:570–7. doi: 10.1007/s00535-005-1590-3. [DOI] [PubMed] [Google Scholar]
- 54.Chang MS, Lee HS, Kim HS, Kim SH, Choi SI, Lee BL, Kim CW, Kim YI, Yang M, Kim WH. Epstein-Barr virus and microsatellite instability in gastric carcinogenesis. J Pathol. 2003;199:447–52. doi: 10.1002/path.1302. [DOI] [PubMed] [Google Scholar]
- 55.van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, Meijer CJ, Bloemena E. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22:664–70. doi: 10.1200/JCO.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 56.Shin WS, Kang MW, Kang JH, Choi MK, Ahn BM, Kim JK, Sun HS, Min KW. Epstein-Barr virus-associated gastric adenocarcinomas among Koreans. Am J Clin Pathol. 1996;105:174–81. doi: 10.1093/ajcp/105.2.174. [DOI] [PubMed] [Google Scholar]
- 57.Arikawa J, Tokunaga M, Satoh E, Tanaka S, Land CE. Morphological characteristics of Epstein-Barr virus-related early gastric carcinoma: a case-control study. Pathol Int. 1997;47:360–7. doi: 10.1111/j.1440-1827.1997.tb04509.x. [DOI] [PubMed] [Google Scholar]
- 58.Minoura-Etoh J, Gotoh K, Sato R, Ogata M, Kaku N, Fujioka T, Nishizono A. Helicobacter pylori-associated oxidant monochloramine induces reactivation of Epstein-Barr virus (EBV) in gastric epithelial cells latently infected with EBV. J Med Microbiol. 2006;55:905–11. doi: 10.1099/jmm.0.46580-0. [DOI] [PubMed] [Google Scholar]
- 59.Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, Wang HP, Lin JT. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology. 2000;118:1031–8. doi: 10.1016/s0016-5085(00)70355-6. [DOI] [PubMed] [Google Scholar]
- 60.Yanai H, Murakami T, Yoshiyama H, Takeuchi H, Nishikawa J, Nakamura H, Okita K, Miura O, Shimizu N, Takada K. Epstein-Barr virus-associated gastric carcinoma and atrophic gastritis. J Clin Gastroenterol. 1999;29:39–43. doi: 10.1097/00004836-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Hansen S, Vollset SE, Derakhshan MH, Fyfe V, Melby KK, Aase S, Jellum E, McColl KEL. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. 2007;56:918–925. doi: 10.1136/gut.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Molot R. Severe gastritis secondary to Epstein-Barr viral infection. Unusual presentation of infectious mononucleosis and associated diffuse lymphoid hyperplasia in gastric mucosa. Arch Pathol Lab Med. 2003;127:478–80. doi: 10.5858/2003-127-0478-SGSTEV. [DOI] [PubMed] [Google Scholar]
- 63.Saxena A, Nath Prasad K, Chand Ghoshal U, Krishnani N, Roshan Bhagat M, Husain N. Association of Helicobacter pylori and Epstein-Barr virus with gastric cancer and peptic ulcer disease. Scand J Gastroenterol. 2008;43:669–74. doi: 10.1080/00365520801909660. [DOI] [PubMed] [Google Scholar]
- 64.Kim YS, Paik SR, Kim HK, Yeom BW, Kim I, Lee D. Epstein-Barr virus and CD21 expression in gastrointestinal tumors. Pathol Res Pract. 1998;194:705–11. doi: 10.1016/S0344-0338(98)80130-1. [DOI] [PubMed] [Google Scholar]
- 65.Koriyama C, Akiba S, Minakami Y, Eizuru Y. Environmental factors related to Epstein-Barr virus-associated gastric cancer in Japan. J Exp Clin Cancer Res. 2005;24:547–53. [PubMed] [Google Scholar]
- 66.Dougherty SH, Foster CA, Eisenberg MM. Stomach cancer following gastric surgery for benign disease. Arch Surg. 1982;117:294–7. doi: 10.1001/archsurg.1982.01380270022005. [DOI] [PubMed] [Google Scholar]
- 67.Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K. Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol. 1999;94:1582–6. doi: 10.1111/j.1572-0241.1999.01148.x. [DOI] [PubMed] [Google Scholar]
- 68.Uozaki H, Fukayama M. Epstein-Barr Virus and Gastric Carcinoma - Viral Carcinogenesis through Epigenetic Mechanisms. Int J Clin Exp Pathol. 2008;1:198–216. [PMC free article] [PubMed] [Google Scholar]
- 69.Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213–9. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]
- 70.Freedman ND, Chow WH, Gao YT, Shu XO, Ji BT, Yang G, Lubin JH, Li HL, Rothman N, Zheng W, Abnet CC. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007;56:1671–7. doi: 10.1136/gut.2007.129411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 72.Hirayama T. IARC Sci Publ. 1978. Descriptive and analytical epidemiology of nasopharyngeal cancer; pp. 167–89. [PubMed] [Google Scholar]
- 73.Chang ET, Zheng T, Lennette ET, Weir EG, Borowitz M, Mann RB, Spiegelman D, Mueller NE. Heterogeneity of risk factors and antibody profiles in epstein-barr virus genome-positive and -negative hodgkin lymphoma. J Infect Dis. 2004;189:2271–81. doi: 10.1086/420886. [DOI] [PubMed] [Google Scholar]
- 74.Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O’Grady J, Hummel M, Preciado MV, Knecht H, Chan JK, Claviez A. Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70:375–82. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 75.Shapira J, Peylan-Ramu N. Burkitt’s Lymphoma. Oral Oncology. 1998;34:15–23. doi: 10.1016/s1368-8375(97)00041-9. [DOI] [PubMed] [Google Scholar]
- 76.Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20:91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crimmins EM, Finch CE. Commentary: do older men and women gain equally from improving childhood conditions? Int J Epidemiol. 2006;35:1270–1. doi: 10.1093/ije/dyl194. [DOI] [PubMed] [Google Scholar]
- 78.Owens IP. Ecology and evolution. Sex differences in mortality rate. Science. 2002;297:2008–9. doi: 10.1126/science.1076813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.