Abstract
Background
We sought to describe the incidence, pathogen distribution, and mortality associated with blood culture-proven sepsis in young infants with congenital heart disease (CHD) admitted to a neonatal intensive care unit (NICU).
Methods
Cohort study of all blood cultures obtained from infants with CHD between 4 and 120 days of age cared for in250 NICUs managed by the Pediatrix Medical Group in the United States between 1996 and 2007.
Results
Of 11,638 infants with CHD, 656 (6%) had 821 episodes of sepsis: a cumulative incidence of 71/1000 admissions. Gram-positive organisms were the most common cause (64%), and coagulase-negative Staphylococcus and Staphylococcus aureus were the most frequently isolated species. On multivariable regression, infants with sepsis were more likely to die compared to infants with sterile blood cultures (odds ratio [OR] = 1.53 [95% confidence interval: 1.09, 2.13]). Infants with gram-negative bacteremia and candidemia were more likely to die than infants with sterile blood cultures (OR = 2.01 [1.20, 3.37], and OR = 3.18 [1.60, 6.34], respectively).
Conclusion
Infants with CHD have a high incidence of culture-proven sepsis, especially with staphylococcal organisms. Gram-negative bacteremia and candidemia are strongly associated with increased mortality in this group of young infants.
Keywords: infant, sepsis, infection, congenital heart disease, epidemiology, outcomes
1. Introduction
Congenital heart disease (CHD) is 1 of the most common groups of congenital malformations, with a cumulative incidence of 0.8% of all live births [1, 2]. CHD is associated with substantial morbidity, including functional limitations and neurodevelopmental delay [3, 4], and accounts for 4% of all neonatal deaths [5]. In addition, half of all deaths among patients with CHD occur during the first year of life [6]. Previous reports have indicated a high incidence of nosocomial sepsis among infants with CHD [7–12]. Infants with CHD are frequently exposed to major surgical procedures, cardiopulmonary bypass, extracorporeal membrane oxygenation, multiple transfusions, multiple intravascular devices, prolonged central venous access, and prolonged hospital stays that may increase their risk of sepsis [13–20].
There is little published research on the epidemiology of sepsis in infants with CHD, and the data are limited to single-center studies [13–19]. Studies of sepsis in the neonatal intensive care unit (NICU) general population show a high burden of gram-positive sepsis caused by staphylococcal species [21–26]. While gram-positive sepsis is associated with significant morbidity in infants, gram-negative sepsis is associated with higher mortality and fulminant illness [21–23]. Given these data and growing concern about the development of vancomycin resistance [27], ampicillin and gentamicin are commonly used for empirical antimicrobial therapy in the NICU, especially for suspected early-onset sepsis. These antimicrobials provide gram-negative coverage but do not cover staphylococcal organisms.
The purpose of this study was to define the incidence of sepsis; to describe the pathogen distribution; and to evaluate the impact of sepsis on mortality in a large multicenter cohort of infants with CHD admitted to the NICU.
2. Methods
2.1. Study design and setting
We identified all infants with CHD admitted to 250 NICUs managed by the Pediatrix Medical Group from 1996 through 2007. Infants eligible for inclusion in the study were diagnosed with CHD and were 4–120 days of age. We excluded the following observations from the analysis: infants with isolated patent ductus arteriosus, infants with isolated patent foramen ovale, infants with an unspecified type of CHD, and infants with DiGeorge syndrome.
2.2. Data source
The data were obtained from an administrative database that prospectively captures information from daily progress notes generated by clinicians using a computer-assisted tool on all infants cared for by the Pediatrix Medical Group. Data are collected each day from admission until death or discharge on multiple aspects of care and entered into the system to generate admission notes, daily progress notes, and discharge summaries. Information is collected regarding maternal history and demographics, physical exam findings, vital signs, medications, laboratory results, culture results, diagnoses, and other aspects of clinical care. Collected data are de-identified and assembled in a common database.
We collected the results of all blood cultures drawn between 4 and 120 days of life, and neonatal demographic data including: birth weight, estimated gestational age at birth, sex, and 5-minute Apgar score. We also collected maternal demographic data including: race, ethnicity, route of delivery, and use of antenatal steroids. We documented the type of CHD as recorded by the attending neonatologist but did not have information on surgical timing or type of intervention, or presence or type of intravascular catheters. We also did not collect information on the use of empiric or prophylactic antibiotics prior to surgery or the use of prostaglandin infusion. Infants with multiple cardiac anomalies (e.g., atrial septal defect and transposition of the great arteries) were classified by the most significant cardiac lesion present.
2.3. Definitions
We defined sepsis as a positive blood culture. Multiple positive blood cultures for the same organism within a 21-day period were considered a single sepsis episode. If a blood culture was positive for multiple pathogenic organisms, we counted each 1 as a separate episode of sepsis. We looked at sepsis episodes beginning between 4 and 120 days of life. If a blood culture was positive during the first 3 days of life, subsequent positive blood cultures of the same organism within a 21-day period were not considered part of a sepsis episode.
We defined definite coagulase-negative staphylococci (CoNS) sepsis as 2 positive blood cultures for CoNS drawn on the same day [28]; probable CoNS sepsis as 2 positive blood cultures for CoNS within a 4-day period, 3 positive blood cultures for CoNS within a 7-day period, or 4 positive blood cultures for CoNS within a 10-day period; and possible CoNS sepsis as a culture positive for CoNS that did not meet criteria for definite or probable CoNS sepsis. We included definite and probable CoNS sepsis in the analysis.
We defined sterile blood cultures as either negative blood cultures (n=1837), possible CoNS (n=520), or blood cultures positive for organisms considered contaminants, including: non-speciated streptococci (n=16), alpha Streptococcus (n=10), Bacillus sp. (n=6), gram-positive rods (n=6, not including Listeria sp.), Micrococcus sp. (n=3), Corynebacterium sp. (n=1), Bacteroides sp. (n=1), Stomatococcus sp. (n=2), and Bifidobacterium sp. (n=1).
2.4. Statistical methods
The unit of observation for this analysis was the infant. We determined the number of sepsis episodes by type of CHD and by organism, calculated as number of sepsis episodes per 1000 NICU admissions. We assessed overall mortality by organism, and we used logistic regression controlling for cyanotic CHD, gestational age, and ventilator support on the first day of life to evaluate the effect of sepsis on overall mortality and the effect of gram-positive bacteremia, gram-negative bacteremia, and candidemia on overall mortality. We included sepsis, cyanotic CHD, and ventilator support on the first day of life as dichotomous variables, and gestational age as a continuous variable.
STATA 10 (College Station, TX, USA) was used to perform the statistical analysis. Significance for all tests was established at a p<0.05. Permission to conduct this analysis without written informed consent was provided by the Duke University Institutional Review Board because the analysis was performed on data without patient identifiers.
3. Results
3.1. Demographics
From 1996 through 2007, the Pediatrix Medical Group cared for 11,638 infants with CHD between 4 and 120 days of life. The mean birth weight and gestational age at birth of the cohort were 2356 g (5%, 95% percentile: 761, 3935) and 34.6 weeks (26, 40), respectively (Table 1). Among infants with blood culture-positive sepsis, the mean birth weight and gestational age at birth were 1420 g (549, 3401) and 29.8 weeks (24, 39). The most common types of CHD in the study population were ventricular septal defect (n=5250), atrial septal defect (n=2208), tetralogy of Fallot (n=688), atrioventricular canal (n=600), aortic coarctation (n=578), pulmonary stenosis (n=575), transposition of the great arteries (n=319), and hypoplastic left heart syndrome (n=265) (Table 2).
Table 1.
Demographics
| Positive blood culture | Negative blood culture | No blood culture | |
|---|---|---|---|
| N=656 (%) | N=2403 (%) | N=8579 (%) | |
| Birth weight (g) | |||
| <750 | 25 | 11 | 1 |
| 750–1499 | 43 | 36 | 12 |
| 1500–2499 | 16 | 25 | 33 |
| 2500–3499 | 12 | 21 | 37 |
| ≥3500 | 4 | 7 | 16 |
| Gestational age (weeks) | |||
| <26 | 23 | 9 | 1 |
| 26–28 | 30 | 19 | 4 |
| 29–33 | 21 | 30 | 20 |
| 34–36 | 11 | 15 | 27 |
| ≥37 | 15 | 27 | 48 |
| Female | 49 | 49 | 48 |
| Race/ethnicity | |||
| White | 49 | 50 | 59 |
| Black | 19 | 15 | 12 |
| Hispanic | 24 | 30 | 23 |
| Asian | 7 | 5 | 6 |
| 5-minute Apgar score | |||
| 0–3 | 6 | 3 | 1 |
| 4–6 | 15 | 14 | 7 |
| 7–10 | 79 | 83 | 92 |
| Received antenatal steroids | 55 | 43 | 21 |
| Vaginal delivery | 31 | 38 | 45 |
| Respiratory supporta | |||
| Room air | 10 | 14 | 35 |
| Nasal cannula or hood O2 | 9 | 16 | 28 |
| CPAP | 7 | 12 | 12 |
| Ventilator | 54 | 46 | 22 |
| HFV | 21 | 12 | 3 |
| Died | 11 | 6 | 3 |
CPAP, continuous positive airway; HFV, high-frequency ventilation.
Respiratory support on the first day of life.
Table 2.
Cumulative Incidence of Sepsis by Type of Congenital Heart Disease
| Type of CHD | # Infants | Mean birth weight (g) | Infants with sepsis |
|---|---|---|---|
| N=11,638 (%) | (5%, 95% percentile) | n, (%) | |
| Cyanotic CHD | 2104 (18%) | 2694 (1239, 3891) | 104 (4%) |
| Tetralogy of Fallot | 688 (6%) | 2502 (1123, 3767) | 31 (5%) |
| Transposition of the great arteries | 319 (3%) | 3026 (1390, 4056) | 6 (2%) |
| Hypoplastic left heart syndrome | 265 (2%) | 2896 (1490, 4090) | 16 (6%) |
| Double outlet right ventricle | 193 (2%) | 2469 (1160, 3580) | 15 (8%) |
| Ebstein’s anomaly | 132 (1%) | 3015 (1622, 4027) | 4 (3%) |
| Truncus arteriosus | 114 (1%) | 2649 (1093, 3723) | 6 (5%) |
| Total anomalous pulmonary venous return | 99 (1%) | 2806 (1260, 3895) | 5 (5%) |
| Pulmonary atresia | 86 (1%) | 2702 (900, 3884) | 4 (5%) |
| Tricuspid atresia | 86 (1%) | 2731 (1425, 3885) | 0 (0%) |
| Other cyanotic CHDa | 122 (1%) | 2826 (1505, 3950) | 4 (3%) |
| Acyanotic CHD | 9534 (82%) | 2260 (724, 3947) | 552 (6%) |
| Ventricular septal defect | 5250 (45%) | 2307 (795, 3947) | 265 (5%) |
| Atrial septal defect | 2208 (19%) | 1998 (645, 3839) | 184 (8%) |
| Atrioventricular canal | 600 (5%) | 2681 (1260, 3861) | 17 (3%) |
| Aortic coarctation | 578 (5%) | 2651 (970, 4070) | 24 (4%) |
| Pulmonary stenosis | 575 (5%) | 2045 (650, 3940) | 62 (11%) |
| Aortic stenosis | 166 (1%) | 2564 (802, 3982) | 10 (6%) |
| Other acyanotic CHDb | 157 (1%) | 2805 (1155, 4170) | 3 (2%) |
CHD, congenital heart disease.
Other cyanotic CHD includes single ventricle and interrupted aortic arch.
Other acyanotic CHD includes subaortic stenosis, endocardial fibroelastosis, and dextrocardia.
3.2. Incidence of sepsis
Twenty-six percent of infants with CHD (3059/11,638) had at least 1 blood culture obtained (Fig. 1). The median day of life of a positive blood culture was 11 days (interquartile range: 7, 18). Of those who were evaluated with blood cultures, 21% (656/3059) had a total of 821 sepsis episodes: 533 (81%) of these infants had 1 sepsis episode, 91 (14%) infants had 2 sepsis episodes and 32 (5%) infants had ≥3 sepsis episodes. Sepsis was most common in infants with pulmonary stenosis (11%), atrial septal defect (8%), hypoplastic left heart syndrome (6%), and ventricular septal defect (5%) (Table 2). The cumulative incidence of sepsis over the study period was 71/1000 NICU admissions.
Fig. 1.
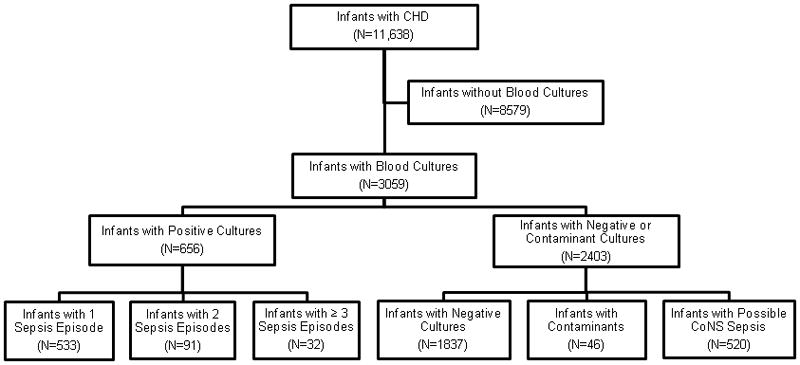
Study population. CHD, congenital heart disease; CoNS, coagulase-negative Staphylococcus.
3.3. Microbiologic etiology and associated mortality
Gram-positive organisms were the most common cause of sepsis (64% [527/821]). Gram-negative organisms and Candida sp. accounted for 26% (216/821) and 9% (73/821) of sepsis episodes, respectively. The most common gram-positive organisms were CoNS (23%, 188/821), non-speciated gram-positive cocci (17%, 137/821), Staphylococcus aureus (13%, 109/821), and Enterococcus sp. (9%, 71/821). Of the 188 episodes of CoNS sepsis, 22% were classified as definite (41/188) and 78% as probable (147/188). The majority of gram-negative bacteremia were caused by Klebsiella sp. (7%, 60/821), Escherichia coli (E. coli) (6%, 49/821), and Enterobacter sp. (5%, 44/821) (Table 3).
Table 3.
Causative Organisms and Mortality
| Organism | Episodes (%) | Episodes/1000 admissions | Mortality (%) |
|---|---|---|---|
| Gram-positive organisms | 64 | 45.3 | 9 |
| Coagulase-negative staphylococci | 23 | 16.2 | 10 |
| Staphylococcus aureus | 13 | 9.4 | 7 |
| Enterococcus sp. | 9 | 6.1 | 8 |
| Group B Streptococcus | 3 | 1.9 | 0 |
| Other gram-positive | 17 | 11.8 | 13 |
| Gram-negative organisms | 26 | 18.6 | 13 |
| Escherichia coli | 6 | 4.2 | 6 |
| Klebsiella sp. | 7 | 5.2 | 16 |
| Enterobacter sp. | 5 | 3.8 | 9 |
| Pseudomonas | 2 | 1.6 | 32 |
| Serratia sp. | 2 | 1.5 | 12 |
| Other gram-negative | 2 | 1.4 | 16 |
| Candida sp. | 9 | 6.3 | 21 |
Mortality among all infants during the study was 4% (473/11,594). Among the infants with sepsis, 11% died (71/650). Mortality was highest in infants with sepsis caused by Pseudomonas sp. (32%, 6/19) and Candida sp. (21%, 15/73) (Table 3).
Infants with sepsis were more likely to die compared to infants with sterile blood cultures (OR = 1.53 [95% confidence interval {CI}: 1.09, 2.13]). In addition, infants with candidemia and gram-negative bacteremia were more likely to die compared to infants with sterile blood cultures (OR = 3.18 [95% CI: 1.60, 6.34] and OR = 2.01 [95% CI: 1.20, 3.37], respectively). Infants with gram-positive bacteremia were more likely to die compared to infants with sterile blood cultures, but the estimate was not statistically significant (OR = 1.31 [95% CI: 0.88, 1.96]). Infants with gram-positive bacteremia were less likely to die compared to infants with candidemia (OR = 0.38 [95% CI: 0.20, 0.75]), as well as in comparison to infants with gram-negative bacteremia, although the latter estimate was not statistically significant (OR = 0.68 [95% CI: 0.40, 1.15]).
4. Discussion
In the present study, 26% of all infants with CHD admitted to the NICU had at least 1 blood culture obtained between day of life 4 and 120. Overall, 6% (656/11,638) of infants in our cohort had 1 or more episodes of blood culture-positive sepsis. This is in the lower range of estimates previously reported in this population (6–15%) [13, 15, 16, 19, 29]. The lower incidence of sepsis in our study may be explained by different study settings (NICU vs. pediatric cardiac intensive care unit), risk periods (ICU stay vs. post-cardiac surgery), and population differences in cardiac complexity, gestational age, birth weight, and the proportion of infants undergoing cardiac surgery.
Because sepsis is strongly associated with low birth weight and gestational age, previous multicenter reports describing the epidemiology of sepsis in the NICU often limited their evaluation to premature infants [21, 23, 30]. We included infants with CHD regardless of prematurity, which was important because nearly half of the infants in our cohort with blood cultures had a birth weight >1500 g. Of the infants with blood culture-positive sepsis, we found that over 30% had a birth weight >1500 g, and 15% were term. The large proportion of more mature infants with blood culture-positive sepsis may be explained by infants with CHD requiring prolonged lengths of stay in the NICU [31, 32].
Prior multicenter studies in premature infants found that up to 70% of late-onset sepsis episodes are caused by gram-positive organisms, with CoNS accounting for 50–60%, and nearly 15% are caused by gram-negative organisms [21, 23, 25]. We observed a similar proportion of gram-positive bacteremia in our study, but less CoNS and more gram-negative bacteremia. Differences in our study’s pathogen distribution may be explained by differences in exposure to nosocomial pathogens between CHD and non-CHD infants admitted to the NICU and by differences in birth weight. For example, previous studies of postoperative sepsis in pediatric cardiac surgery patients show a high proportion of gram-negative organisms [13, 15]. Also, differences in the definitions of CoNS sepsis used in prior studies may have overestimated CoNS counts due to contaminants [23]. The low incidence of group B Streptococcus and the high incidence of E. coli in our study are consistent with contemporary studies performed in the era of intra-partum group B Streptococcus antibiotic prophylaxis [33, 34].
Prior studies of mortality associated with sepsis in infants with CHD are limited by small sample sizes, with mortality ranging from 11% (3/27) to 83% (5/6) [13, 14, 35, 36]. We observed an all-cause mortality of 11% associated with blood culture-positive sepsis (71/650). In multivariable analysis, infants with blood culture-positive sepsis were more likely to die than infants with a sterile blood culture. In addition, we observed higher mortality associated with gram-negative bacteremia and candidemia compared to infants with sterile blood cultures. Mortality associated with gram-positive bacteremia in our study (9%) was similar to prior multicenter NICU studies, but we observed that mortality associated with gram-negative bacteremia and candidemia (13% and 21%, respectively) were lower than previously reported [21, 23, 30]. A study in premature infants reported a crude mortality of 11% associated with gram-positive sepsis, 36% with gram-negative sepsis, and 32% with Candida sepsis [23]. In addition, the study found infants with gram-positive sepsis were significantly less likely to die compared to infants with other sepsis infections (OR = 0.26 [95% CI: 0.19, 0.35]), whereas infants with gram-negative sepsis and Candida sepsis were more likely to die compared to other sepsis infections (OR = 3.5 [95% CI: 2.5, 4.9] and OR = 2.0 [95% CI: 1.3, 3.0], respectively) [23]. A second study in premature infants reported crude mortality associated with gram-negative sepsis and Candida sepsis almost 3 times as high as gram-positive sepsis (25%, 29%, and 9% died, respectively) [30]. In our study, the similar mortality between organism groups may be attributed to improved care captured in our contemporary dataset. In addition, more infants in our cohort were more mature and may have been more likely to survive severe gram-negative bacteremia and candidemia.
The distribution of sepsis pathogens and pathogen-specific morbidity and mortality are important considerations for choosing appropriate empirical antimicrobial therapy. Despite the high frequency of gram-positive sepsis in the NICU, empiric antibiotics for suspected sepsis typically cover gram-negative organisms owing to their association with higher mortality and the high proportion of early-onset infections [21–23, 37]. We specifically looked at blood culture-positive sepsis in infants with CHD. Nearly two-thirds of infections were caused by gram-positive organisms, and we estimated similar mortality associated with gram-positive bacteremia and gram-negative bacteremia. While ampicillin and gentamicin are effective empirical therapy for suspected sepsis in premature infants, especially early-onset sepsis, our data suggest empirical antistaphylococcal therapy should be considered for suspected nosocomial sepsis in infants with CHD.
There are several limitations to our study. These data do not include information on cardiac surgical procedures and intravascular catheterizations, which are likely the main determinants of sepsis in this population. The absence of information regarding surgical timing and complexity and central catheter presence and duration makes comparisons to previous studies stratifying by these exposures difficult. We also lacked clinical data regarding underlying illnesses; risk factors such as type, timing, and duration of maternal antibiotic therapy, mechanical ventilation beyond the first day of life, extracorporeal membrane oxygenation use, number of blood transfusions, and total parenteral nutrition; source of blood culture (central or peripheral); and cause of death. We also did not have information on the empirical use of antibiotics in the NICU or prophylactic antibiotic use prior to sternotomy.
To our knowledge, this study is the largest evaluation of sepsis in infants with CHD and the first multicenter study that specifically describes sepsis in this unique population. Strengths of this study include a large sample size; population diversity from academic and community institutions; quantification of every blood culture obtained for each patient during the hospitalization; inclusion of maternal and infant demographic risk factors for sepsis; information on type of CHD; and a contemporary study period after the introduction of intra-partum group B Streptococcus antibiotic prophylaxis. This study provides a point of reference for monitoring the epidemiology of sepsis in infants with CHD. Knowledge of the pathogens and mortality associated with sepsis in infants with CHD is important for targeting efforts to prevent infections and selecting appropriate antibiotic therapy.
Footnotes
Conflict of interest statement
D.K.B. Jr. receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and Government Contract HHSN267200700051C) and the nonprofit organization Thrasher Research Foundation for his work in neonatal candidiasis (www.thrasherresearch.org). He also is a consultant for Pfizer, Cerexa, Biosynexus, and Johnson & Johnson and a principal investigator for Astellas Pharma, AstraZeneca, and UCB Pharma. P.B.S. received support from NICHD 1K23HD060040-01 and DHHS-1R18AE000028-01; he is also a consultant for Pfizer, Cubist Pharmaceuticals, Pangen Biosystems, Johnson & Johnson, and Astellas Pharma and a principal investigator for Cubist Pharmaceuticals. M.C.W. received support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin), the nonprofit organization Thrasher Research Foundation, and from NICHD 1K23HD064814-01. He is also a consultant and a principal investigator for Pfizer. All other authors report no potential conflicts of interest relevant to this article.
This study used CTSA biostatistical services through the Division of Pediatric Quantitative Sciences (NIH-5UL-1RR024128-01).
The funding organizations played no role in the study design, collection, analysis, and interpretation of the data, the writing of the manuscript, and the decision to submit the manuscript for publication.
References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008;93:F33–5. doi: 10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 3.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C, et al. Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics. 2001;108:1325–31. doi: 10.1542/peds.108.6.1325. [DOI] [PubMed] [Google Scholar]
- 4.Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148:72–7. doi: 10.1016/j.jpeds.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Racial differences by gestational age in neonatal deaths attributable to congenital heart defects—United States, 2003–2006. MMWR Morb Mortal Wkly Rep. 2010;59:1208–11. [PubMed] [Google Scholar]
- 6.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103:2376–81. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 7.Olsen AL, Reinholdt J, Jensen AM, Andersen LP, Jensen ET. Nosocomial infection in a Danish neonatal intensive care unit: a prospective study. Acta Paediatr. 2009;98:1294–9. doi: 10.1111/j.1651-2227.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- 8.Yogaraj JS, Elward AM, Fraser VJ. Rate, risk factors, and outcomes of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2002;110:481–5. doi: 10.1542/peds.110.3.481. [DOI] [PubMed] [Google Scholar]
- 9.Elward AM, Fraser VJ. Risk factors for nosocomial primary bloodstream infection in pediatric intensive care unit patients: a 2-year prospective cohort study. Infect Control Hosp Epidemiol. 2006;27:553–60. doi: 10.1086/505096. [DOI] [PubMed] [Google Scholar]
- 10.Bendig EA, Singh J, Butler TJ, Arrieta AC. The impact of the central venous catheter on the diagnosis of infectious endocarditis using Duke criteria in children with Staphylococcus aureus bacteremia. Pediatr Infect Dis J. 2008;27:636–9. doi: 10.1097/INF.0b013e31816b78c8. [DOI] [PubMed] [Google Scholar]
- 11.Valente AM, Jain R, Scheurer M, Fowler VG, Jr, Corey GR, Bengur AR, et al. Frequency of infective endocarditis among infants and children with Staphylococcus aureus bacteremia. Pediatrics. 2005;115:e15–9. doi: 10.1542/peds.2004-1152. [DOI] [PubMed] [Google Scholar]
- 12.Singh-Naz N, Sprague BM, Patel KM, Pollack MM. Risk factors for nosocomial infection in critically ill children: a prospective cohort study. Crit Care Med. 1996;24:875–8. doi: 10.1097/00003246-199605000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Abou Elella R, Najm HK, Balkhy H, Bullard L, Kabbani MS. Impact of bloodstream infection on the outcome of children undergoing cardiac surgery. Pediatr Cardiol. 2010;31:483–9. doi: 10.1007/s00246-009-9624-x. [DOI] [PubMed] [Google Scholar]
- 14.Prasad PA, Dominguez TE, Zaoutis TE, Shah SS, Teszner E, Gaynor JW, et al. Risk factors for catheter-associated bloodstream infections in a pediatric cardiac intensive care unit. Pediatr Infect Dis J. 2010;29:812–5. doi: 10.1097/01.inf.0000388000.39298.b7. [DOI] [PubMed] [Google Scholar]
- 15.Levy I, Ovadia B, Erez E, Rinat S, Ashkenazi S, Birk E, et al. Nosocomial infections after cardiac surgery in infants and children: incidence and risk factors. J Hosp Infect. 2003;53:111–6. doi: 10.1053/jhin.2002.1359. [DOI] [PubMed] [Google Scholar]
- 16.Valera M, Scolfaro C, Cappello N, Gramaglia E, Grassitelli S, Abbate MT, et al. Nosocomial infections in pediatric cardiac surgery, Italy. Infect Control Hosp Epidemiol. 2001;22:771–5. doi: 10.1086/501861. [DOI] [PubMed] [Google Scholar]
- 17.Costello JM, Graham DA, Morrow DF, Potter-Bynoe G, Sandora TJ, Laussen PC. Risk factors for central line-associated bloodstream infection in a pediatric cardiac intensive care unit. Pediatr Crit Care Med. 2009;10:453–9. doi: 10.1097/PCC.0b013e318198b19a. [DOI] [PubMed] [Google Scholar]
- 18.Grisaru-Soen G, Paret G, Yahav D, Boyko V, Lerner-Geva L. Nosocomial infections in pediatric cardiovascular surgery patients: a 4-year survey. Pediatr Crit Care Med. 2009;10:202–6. doi: 10.1097/PCC.0b013e31819a37c5. [DOI] [PubMed] [Google Scholar]
- 19.Shah SS, Kagen J, Lautenbach E, Bilker WB, Matro J, Dominguez TE, et al. Bloodstream infections after median sternotomy at a children’s hospital. J Thorac Cardiovasc Surg. 2007;133:435–40. doi: 10.1016/j.jtcvs.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Gaynor JW. The effect of modified ultrafiltration on the postoperative course in patients with congenital heart disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2003;6:128–39. doi: 10.1053/pcsu.2003.50006. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin DK, DeLong E, Cotten CM, Garges HP, Steinbach WJ, Clark RH. Mortality following blood culture in premature infants: increased with gram-negative bacteremia and candidemia, but not gram-positive bacteremia. J Perinatol. 2004;24:175–80. doi: 10.1038/sj.jp.7211068. [DOI] [PubMed] [Google Scholar]
- 22.Karlowicz MG, Buescher ES, Surka AE. Fulminant late-onset sepsis in a neonatal intensive care unit, 1988–1997, and the impact of avoiding empiric vancomycin therapy. Pediatrics. 2000;106:1387–90. doi: 10.1542/peds.106.6.1387. [DOI] [PubMed] [Google Scholar]
- 23.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very-low-birth-weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 24.Cohen-Wolkowiez M, Moran C, Benjamin DK, Cotten CM, Clark RH, Benjamin DK, Jr, et al. Early- and late-onset sepsis in late preterm infants. Pediatr Infect Dis J. 2009;28:1052–6. doi: 10.1097/inf.0b013e3181acf6bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodie SB, Sands KE, Gray JE, Parker RA, Goldmann DA, Davis RB, et al. Occurrence of nosocomial bloodstream infections in six neonatal intensive care units. Pediatr Infect Dis J. 2000;19:56–65. doi: 10.1097/00006454-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. Very-low-birth-weight preterm infants with early-onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24:635–9. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 27.Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16:105–13. doi: 10.1086/647066. [DOI] [PubMed] [Google Scholar]
- 28.Zaidi AK, Harrell LJ, Rost JR, Reller LB. Assessment of similarity among coagulase-negative staphylococci from sequential blood cultures of neonates and children by pulsed-field gel electrophoresis. J Infect Dis. 1996;174:1010–4. doi: 10.1093/infdis/174.5.1010. [DOI] [PubMed] [Google Scholar]
- 29.Pollock EM, Ford-Jones EL, Rebeyka I, Mindorff CM, Bohn DJ, Edmonds JF, et al. Early nosocomial infections in pediatric cardiovascular surgery patients. Crit Care Med. 1990;18:378–84. doi: 10.1097/00003246-199004000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very-low-birth-weight infants in Israel: a national survey. Pediatrics. 2002;109:34–9. doi: 10.1542/peds.109.1.34. [DOI] [PubMed] [Google Scholar]
- 31.Gaynes RP, Edwards JR, Jarvis WR, Culver DH, Tolson JS, Martone WJ. Nosocomial infections among neonates in high-risk nurseries in the United States. National Nosocomial Infections Surveillance System. Pediatrics. 1996;98:357–61. [PubMed] [Google Scholar]
- 32.Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very-low-birth-weight infants. Pediatrics. 2004;114:348–55. doi: 10.1542/peds.114.2.348. [DOI] [PubMed] [Google Scholar]
- 33.Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 34.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121:689–96. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- 35.Chakrabarti C, Sood SK, Parnell V, Rubin LG. Prolonged candidemia in infants following surgery for congenital heart disease. Infect Control Hosp Epidemiol. 2003;24:753–7. doi: 10.1086/502126. [DOI] [PubMed] [Google Scholar]
- 36.San Miguel LG, Cobo J, Otheo E, Martos I, Muriel A, Fortun J, et al. Candidemia in pediatric patients with congenital heart disease. Diagn Microbiol Infect Dis. 2006;55:203–7. doi: 10.1016/j.diagmicrobio.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early-onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]


