Abstract
Background
Mesotherapy, commonly known as “biorejuvenation” or “biorevitalization”, is a technique used to rejuvenate the skin by means of a transdermal injection of a multivitamin solution and natural plant extracts that are thought to improve the signs of skin aging.
Objectives
This prospective study aimed to evaluate the clinical effect of mesotherapy applied to periorbital wrinkles and to quantitatively evaluate histological changes in the skin occurring in response to the same treatment.
Methods
Six volunteers with Fitzpatrick skin types III or IV and Glogau class I–III wrinkles were subjected to a three-month course of mesotherapy injections in the periocular area (six sessions administered at two-week intervals). Standard photographs and skin biopsies were obtained from the treatment area at baseline, at the end of treatment, and at three months post-treatment. Quantitative evaluation of collagen types I, III, and VII, newly synthesized collagen, total elastin, and tropoelastin was performed using a computerized morphometric analysis.
Results
The clinical evaluation of volunteers at baseline, end of treatment, and three months post-treatment revealed no significant differences. Histological and immunostaining analysis of collagen types I, III, and VII, newly synthesized collagen, total elastin, and tropoelastin showed no statistically significant changes (P > 0.05) after mesotherapy injection.
Conclusions
The present study indicates that mesotherapy for skin rejuvenation does not result in statistically significant histological changes or clinical improvement.
Introduction
Aging is a complex, multifactorial process that affects every organ in the body, including the skin.1,2 Clinically, aged skin is characterized as thin, dry, and pale, with noticeable wrinkles and decreased elasticity.3,4 The histological changes associated with aging in skin include the accumulation of elastotic material in the papillary and mid-dermis, a process known as solar elastosis, and quantitative changes in collagen, which are reflected in a decline in biosynthesis and content.4
Mesotherapy, initially described in France by Michel Pistor,5 is one of the modalities recently used to rejuvenate and tone the skin.6,7 It involves the non-invasive transdermal injection of vitamins, enzymes, hormones, hyaluronic acid, and natural plant extracts into the skin to stimulate the biosynthetic ability of fibroblasts and facilitate interaction between cells and is intended to increase collagen and elastin production.8,9 Different injection techniques can be used in mesotherapy: (i) the intra-epidermal technique; (ii) the papular technique, in which reagents are injected into the dermo–epidermal junction; (ii) the nappage method, in which injections penetrate to a depth of 2–4 mm and are delivered at an angle of 30–60°; and (iv) point-by-point injection into the deep dermis.10,11
Over the past few years, the use of mesotherapy for skin rejuvenation has increased rapidly at a relatively high financial cost to patients. As no in-depth, evidence-based studies have explored the safety or efficacy of mesotherapy, questions remain about the scientific validity of this popular approach. The purpose of this study was to evaluate the clinical effects of this treatment and to objectively quantify the corresponding histological changes associated with mesotherapy injection as a non-invasive method of skin rejuvenation.
Materials and methods
Volunteers and treatment protocol
Six female volunteers with Fitzpatrick skin types III or IV and Glogau class I–III wrinkles12 were subjected to three months of treatment with mesotherapy injection (delivered in six sessions held at 2-week intervals). The volunteers were recruited from the dermatology outpatient clinic at Al-Minya University Hospital, Al-Minya, Egypt, which they had attended for treatment of periorbital wrinkles. Their mean age was 43.1 ± 4.7 years (range: 37–49 years). The treatment and study details were fully explained to the subjects, all of whom signed informed consent forms pertaining to treatment and participation in the study, which included consent to photography and skin biopsy before treatment, at three months (end of treatment), and at six months after the start of treatment (three months post-treatment).
Mesotherapy injection is a minimally painful procedure and requires no anesthesia. Before treatment, the volunteer was positioned for treatment and the periorbital area on both sides of the face (treatment area) was marked. The sterile solutions used for injection were composed of a cocktail of a multivitamin solution and non-cross-linked, high-viscosity hyaluronic acid, provided in two separate vials in a packaged kit (Revitacare®, Bio-Revitalisation; Laboratoire Revitacare, Saint Ouen l'Aumône, France). The first vial contained 4 ml of non-cross-linked hyaluronic acid at 1% biotechnological origin and water for injectable preparation. The second vial contained 10 ml of a multivitamin solution of retinol 10,000 IU (5.5 mg), thiamine 4 mg, riboflavin 0.6 mg, nicotinamide 20 mg, dexpanthenol 8 mg, pyridoxine 4 mg, ascorbic acid 100 mg, ergocalciferol 2000 IU (2 mg), tocopherol 4 mg, Solutol® HS 15.52 mg, and water for injectable preparation. Materials were stored in a refrigerator, mixed immediately before injection, and transferred to 1-ml syringes to accommodate the high viscosity of the hyaluronic acid. For each volunteer, the injection material was prepared and mixed as a diluted suspension of multivitamin solution for injection and non-cross-linked, high-viscosity hyaluronic acid at a ratio of 9 : 1.
The injection process was performed using a meso-system gun [Anti-Aging Medical Systems (AAMS), Montrodat, France] controlled by a microprocessor with eight different injection programs. The gun maintained automatic control of the syringe and allowed the depth and dose of the injection to be adjusted electronically. The gun was positioned at an angle of 60° to the surface of the skin. In each injection, approximately 0.01 ml of solution was injected into the skin at a depth of 2 mm using the nappage technique in a horizontal direction; the total amount of solution injected per session on both sides of the face was 2 ml.
The clinical status of the skin in terms of wrinkles, tautness, and texture was evaluated and rated by the volunteer, two dermatologists, and two independent observers before treatment and at three and six months after the start of treatment. Rating was based on a 5-point scale on which improvement was rated as none (0%), mild (1–25%), moderate (26–50%), good (51–75%), or very good (76–100%).
Punch biopsies (3 mm) were obtained from the facial skin (treatment site) at baseline, at the end of treatment, and at three months post-treatment. Biopsies obtained after treatment were taken from a site near to that of the pretreatment biopsies. Tissues were fixed in 10% buffered formalin, embedded in paraffin, and sliced into sections 5 μm thick. All histological and immunostaining evaluations were carried out in the Department of Dermatology and Cutaneous Biology, Thomas Jefferson University, Philadelphia, PA, USA.
Histochemical staining
Specimens were stained for standard hematoxylin and eosin (H&E), Verhoeff–van Gieson (elastic fibers) (HT25A; Sigma-Aldrich Corp., St Louis, MO, USA) and picrosirius red staining (Direct Red 80; Sigma-Aldrich Corp.) for newly synthesized collagen.13
Histological measurements
Histological measurements were performed on standard H&E stained sections using computer-based software (Image-Pro Plus 6.1; Media Cybernetics, Inc., Silver Spring, MD, USA). Epidermal thickness was measured from the top of the granular cell layer to the dermo–epidermal junction. Five measurements were calculated for each section.
Immunohistochemical staining
We evaluated collagen types I and III, as well as total elastin, using the immunoperoxidase technique. Following antigen retrieval by the microwave method in 0.1 M sodium citrate (pH 6.0) for five minutes, nonspecific sites were blocked using 5% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.02% Triton X-100 (TX-100) in phosphate buffered saline (PBS). Tissues were incubated at 4 °C overnight with antibodies to type I collagen (1 : 400) (sc-59772; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), type III collagen (1 : 600) (ab6310; Abcam, Inc., Cambridge, MA, USA), and total elastin (1 : 300) (E4013; Sigma-Aldrich Corp.). Tissues were developed using biotinylated secondary antibody (1 : 200) (PK-6102; Vector Laboratories, Inc., Burlingame, CA, USA), ABC reagent (Vectastain Elite ABC Peroxidase Kits Mouse, PK-6102; Vector Laboratories, Inc.) and DAB Chromogen Substrate Kit (K3468; Dako North America, Inc., Carpinteria, CA, USA), and were counterstained with hematoxylin (7211; Fisher Thermo Scientific, Inc., Waltham, MA, USA).
Indirect immunofluorescence (IF) staining was used to detect collagen type VII and tropoelastin. After antigen retrieval and blocking, specimens were incubated with antibodies to type VII collagen (1 : 600) (sc-33710; Santa Cruz Biotechnology, Inc.), secondary antibody Alexa Fluor® goat anti-mouse IgG 594 (1 : 400) (Molecular Probes, Inc., Eugene, OR, USA) and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (1 : 1000) (D8417; Sigma-Aldrich Corp.) for nuclear staining.
The microprobe system (FD-188-10A; Fisher Thermo Scientific, Inc.) was used to detect tropoelastin using reagents obtained from Open Biosystems, Inc. (Huntsville, AL, USA) as previously described.14 Antibodies used were tropoelastin GA317 (1 : 400) (Elastin Products Co. Inc., Owensville, MO, USA) and Alexa Fluor® goat anti-rabbit IgG 594 (1 : 400) (Molecular Probes, Inc.). Tissues were incubated with DAPI for 1–2 minutes.
Quantitative evaluation and statistical analysis
Quantitative evaluation of picrosirius red stained and immunostained tissues was carried out using Image-Pro Plus 6.1; all values were normalized to baseline values. A Nikon microscope equipped with filters to provide circularly polarized illumination was used to evaluate picrosirius red stained tissues. Data were tabulated and analyzed using spss Version 16 (SPSS, Inc., Chicago, IL, USA). Statistical analysis was performed using Wilcoxon matched-pairs signed ranks and chi-squared tests. Data were expressed as the mean ± standard deviation (SD). Statistical significance was defined as P ≤ 0.05.
Results
Clinical evaluation
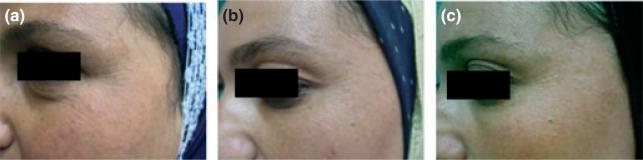
All six volunteers completed the mesotherapy treatment and follow-up period. No side effects were observed. Two of the six subjects showed mild changes (after the third session) in terms of increased glowing of the skin, but none reported improvement in skin tightening or wrinkles. Clinical evaluations did not show any statistically significant effect on skin texture, tightening, or wrinkles in response to mesotherapy injection (Fig. 1). Results obtained from a structured questionnaire were tabulated and compared with data obtained at baseline to evaluate the statistical significance of any differences using Pearson's chi-squared test. Subjects showed a 10–15% improvement in skin tightening (P = 0.06) and skin texture (P = 0.09) at the end of treatment versus a 5–10% improvement in skin tightening (P = 0.7) and skin texture (P = 0.8) at three months post-treatment, compared with baseline. Clinical improvement in wrinkles ranged from none to mild (0–5%) at the end of treatment and at three months post-treatment compared with baseline (P = 0.621).
Figure 1.
Clinical evaluation of a volunteer's response to mesotherapy injection. Photographs of the periorbital area show no significant changes in skin from (a) baseline to (b) the end of treatment or (c) 3 months post-treatment
Histometric changes
Microscopic evaluation of H&E stained sections revealed no difference in epidermal thickness (64.1 ± 2.2 μm before treatment, 67.0 ± 2.8 μm at the end of treatment, 63.3 ± 3.3 μm at three months post-treatment; P = 0.07 and P = 0.29, respectively).
Histological changes
It has been proposed that skin rejuvenation might be promoted by intradermal injection of multivitamins, which stimulate fibroblasts to produce more collagen and elastin. In addition, hyaluronic acid injection is supposed to facilitate fibroblast activation and extracellular matrix remodeling.9,15 We examined the effects of mesotherapy treatment on collagen types I and III by measuring the percentage of the dermis occupied by immunohistochemically detectable collagen and compared these values with measurements obtained at baseline to assess the statistical significance of any changes.
Immunoperoxidase staining for collagen type I did not show significant differences between values obtained before treatment (68.7 ± 5.6%) and those obtained at the end of treatment (69.6 ± 4.9%) and at three months post-treatment (69.1 ± 4.6%) (P = 0.765 and P = 0.889, respectively) (Table 1, Fig. 2a). Evaluation of type III collagen showed a slight increase to 65.0 ± 4.7% at the end of treatment (P = 0.615) and 64.0 ± 4.6% at three months post-treatment (P = 0.829) compared with the baseline value of 63.3 ± 6.1%, although the changes were not statistically significant (Table 1, Fig. 2b).
Table 1.
Outcomes in six volunteers undergoing treatment with mesotherapy administered over 3 months
| Dermis positive, %, mean ± SD | P-value | |||||
|---|---|---|---|---|---|---|
| Parameter | Baseline | End of treatment | 3 months post-treatment | Baseline vs. end of treatment | End of treatment vs. 3 months post-treatment | Baseline vs. 3 months post-treatment |
| Collagen type I | 68.7 ± 5.6 | 69.6 ± 4.9 | 69.1 ± 4.6 | 0.765 | 0.855 | 0.889 |
| Collagen type III | 63.3 ± 6.1 | 65.0 ± 4.7 | 64.0 ± 4.6 | 0.615 | 0.738 | 0.829 |
| Collagen type VII | 9.8 ± 1.1 | 10.2 ± 1.2 | 9.9 ± 1.3 | 0.644 | 0.680 | 0.949 |
| Newly synthesized collagen | 14.7 ± 2.1 | 16.6 ± 1.7 | 15.5 ± 2.4 | 0.104 | 0.360 | 0.575 |
| Total elastin | 60.7 ± 4.0 | 55.1 ± 3.6 | 58.0 ± 5.3 | 0.071 | 0.290 | 0.357 |
SD, standard deviation.
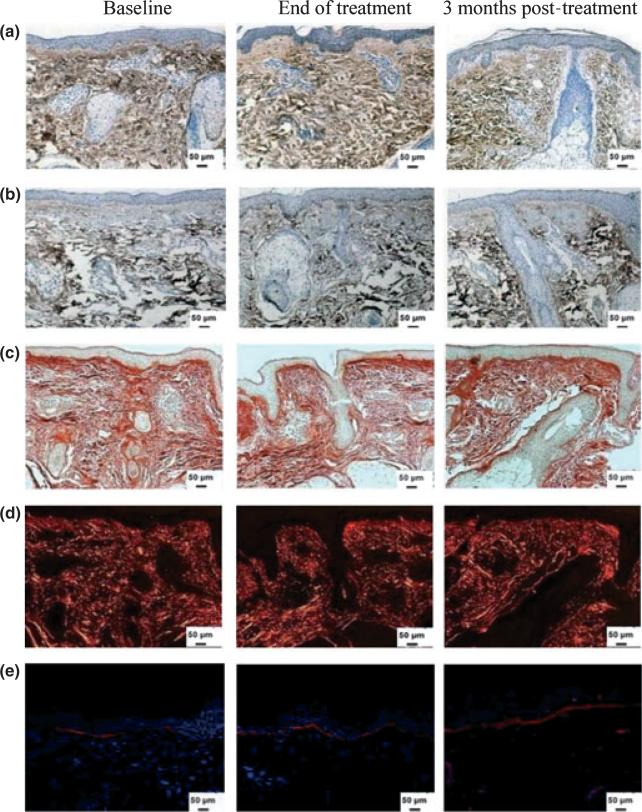
Figure 2.
Dermal collagen content after mesotherapy treatment. Immunoperoxidase staining of skin specimens for collagen (a) type I and (b) type III shows no change in collagen content. Representative samples of skin stained with picrosirius red and viewed under a (c) bright or (d) polarized field show no noticeable change in yellow or red colors. (e) Collagen type VII expression by immunofluorescence shows no change in response to mesotherapy injection. Nuclei stained in blue with DAPI (original magnification × 200)
Newly synthesized collagen can be detected by picrosirius red staining under polarized microscopy: large fibers stain red and thinner fibers, which represent newly synthesized fibers, show a yellow–orange stain.13,16 Mesotherapy injection did not appear to have any noticeable effect on collagen formation as picrosirius red staining revealed no significant difference in newly synthesized collagen before treatment (14.7 ± 2.1%) compared with after treatment (16.6 ± 1.7%; P = 0.104) and at three months post-treatment (15.5 ± 2.4; P = 0.575) (Table 1, Fig. 2c,d).
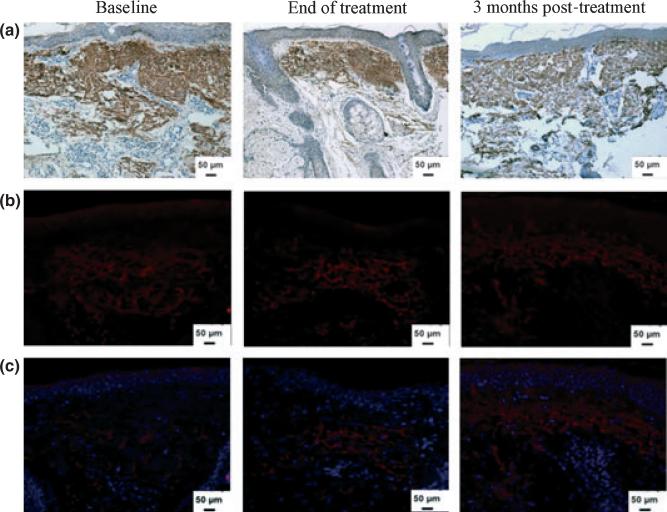
Type VII collagen is the main component of anchoring fibrils and is synthesized by both fibroblasts and keratinocytes; it mediates the dermo–epidermal adherence in human skin.17 The effect of the aging process on collagen VII biosynthesis and degradation has been previously noted.18,19 Quantitative evaluation of type VII collagen did not reveal statistically significant differences between levels at baseline (9.8 ± 1.1%) and at the end of treatment (10.2 ± 1.2%; P = 0.644) or at three months post-treatment (9.9 ± 1.3%; P = 0.949) (Table 1, Fig. 2e).
Elastic fibers, which are responsible for the elasticity and resilience of normal human skin, constitute <2–4% of the extracellular matrix.14,20,21 The accumulation of thickened, tangled, and amorphous elastic structures in the dermis is known as solar elastosis and represents the histopathological mark of skin aging.21–24 The effect of mesotherapy injection on total dermal elastin was examined by measuring the percentage area of dermis occupied by immunohistochemically detectable elastin; values were then normalized to baseline. Our data revealed a statistically insignificant decrease in the level of total elastin expression from 60.7 ± 4.0% at baseline to 55.1 ± 3.6% at the end of treatment (P = 0.07). This was followed by a slight, but statistically insignificant, increase in elastin level to 58.0 ± 5.3% at three months post-treatment (P = 0.290) (Table 1, Fig. 3a). All tissues were also stained with Verhoeff–van Gieson special stain to differentiate elastic fibers (blue–black to black) from collagen fibers (red); this stain showed no changes in response to treatment.
Figure 3.
Dermal elastin in response to mesotherapy treatment. (a) Immunoperoxidase staining of skin tissues for total elastin shows no significant change in levels at the end of treatment or at 3 months post-treatment. (b) Immunofluorescence staining of tropoelastin shows no effect of treatment on newly synthesized tropoelastin in the dermis. (c) The same tissues were counterstained for nuclei (blue) with DAPI (original magnification × 200)
Elastic fibers are composed mainly of elastin, which is initially synthesized as tropoelastin.4,14 The biosynthetic rate of elastin was evaluated by quantifying newly synthesized tropoelastin. Mesotherapy injection did not appear to bring about any significant differences between tropoelastin levels prior to treatment (13.6 ± 1.3%) and levels at the end of treatment (13.9 ± 3.3%; P = 0.636) or at three months post-treatment (13.0 ± 1.9%; P = 0.586) (Table 1, Fig. 3b,c).
Discussion
Mesotherapy, which derives its name from the Greek words “mesos” (middle) and “therapeia” (to treat medically), was originally developed in Europe, is one of the newest techniques in cosmetic medicine proposed to rejuvenate aging skin, and can be performed by medical and non-medical professionals.6,11 Although the US Food and Drug Administration (FDA) has approved most of the ingredients used in mesotherapy injection, these components are being applied for indications for which they currently unapproved.6,25,26 The efficacy, treatment protocols, pharmacokinetics, and safety of mesotherapy are still of concern and under debate.8,27 Improvements in wrinkles, increased elasticity, and enhanced skin texture have been attributed to mesotherapy injection but have not been rigorously proven.8,10,28,29 At present, the basic concerns pertaining to the mesotherapy approach to skin rejuvenation are that peer-reviewed publications dealing with the efficacy of mesotherapy are few, and published evidence-based scientific studies do not support the treatment rationale or address the issue of whether the effects, if any, are temporary or permanent.10 To the best of our knowledge, no previous work concerning objective quantitative changes in newly formed collagen (using picrosirius red stain), collagen types I, III and VII, elastin, and tropoelastin (using immunohistochemistry) after mesotherapy skin rejuvenation has been presented.
In the present study, we objectively quantified both the clinical and histological effects of mesotherapy on photoaging and wrinkle reduction. The evaluation of our volunteers revealed no clinical improvement, and the histological effects were statistically insignificant. Although some improvement was seen in the skin texture of two subjects after the third session, most volunteers were unimpressed with the results throughout the treatment and follow-up period.
The results of the present work are consistent with those of a previous study8 which reported that mesotherapy injection did not result in any photographically discernible differences between treated subjects and controls, and no significant clinical or histological changes in collagen fibers were detected using H&E staining and electron microscopy. Moreover, in the present study, we tried to strengthen the subjective evaluation by quantitatively evaluating changes in collagen and elastin using immunohistochemistry.
It is also important to point out that the American Society of Plastic Surgeons has not approved the use of injectable mesotherapy treatments for any indication but has recommended that further research into the safety and efficacy of such treatments is required.28
By contrast, Lacarrubba et al.9 treated 20 women with signs of photoaging on the dorsum of the hands with hyaluronic acid salts (of biotechnological origin) and multivitamin injections (weekly for four weeks). The authors, using ultrasonography, reported that 15 of 19 patients who completed the study showed an increase in the subepidermal low-echogenic band (SLEB) and concluded that mesotherapy may be an effective treatment for skin photoaging.9 However, Sandby-Moller and Wulf reported that the SLEB layer was not detected in children, whereas an increase in the SLEB layer was observed in over 50% of adults aged >40 years. The age-related increase in the SLEB layer was attributed to chronic ultraviolet B (UVB) exposure.30 Thus, Lacarrubba et al.9 may have erroneously concluded that mesotherapy may be an effective treatment for skin photoaging. Furthermore, these authors did not confirm their clinical data by histological evaluation of the treated area.
Conclusions
As with any new procedure, it is important to assess the benefits, safety, efficacy, and standardization of mesotherapy before it can be advocated for skin rejuvenation treatments. In the present study, although mild clinical improvement in skin texture was observed in some study participants (n = 2) after the third session, subjective, as well as objective, quantitative evaluation suggested that mesotherapy treatment utilizing the protocol established in this study has no significant beneficial effect in reversing the signs of skin aging, either clinically or histologically. However, further in-depth, long-term studies on a large cohort of patients are required to identify the maximum duration of treatment necessary to achieve clinical or histological benefits if, indeed, these are to be obtained.
Acknowledgments
The authors would like to thank the Cultural and Educational Bureau of Egypt for its financial support of this work. We also thank Alicia Dowling for critically reviewing this manuscript.
Funding: This work was supported by a grant from the Cultural and Educational Bureau of Egypt to WM, and a National Institutes of Health grant to JU (R01 AR28450).
Footnotes
Conflicts of interest: None.
References
- 1.Yaar M, Gilchrest BA. Photoaging: mechanism, prevention and therapy. Br J Dermatol. 2007;157:874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 2.Farage MA, Miller KW, Berardesca E, et al. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10:73–86. doi: 10.2165/00128071-200910020-00001. [DOI] [PubMed] [Google Scholar]
- 3.Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119:40–50. doi: 10.1196/annals.1404.027. [DOI] [PubMed] [Google Scholar]
- 4.El-Domyati M, El-Ammawi TS, Medhat W, et al. Electro-optical synergy technique: a new and effective non-ablative approach to skin aging. J Clin Aesthet Dermatol. 2010;3:22–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Pistor M. What is mesotherapy? Chir Dent Fr. 1976;46:59–60. [PubMed] [Google Scholar]
- 6.Atiyeh BS, Ibrahim AE, Dibo SA. Cosmetic mesotherapy: between scientific evidence, science fiction, and lucrative business. Aesth Plast Surg. 2008;32:842–849. doi: 10.1007/s00266-008-9195-x. [DOI] [PubMed] [Google Scholar]
- 7.Toledo LS. Emerging techniques in aesthetic plastic surgery. Clin Plast Surg. 2009;36:177–180. doi: 10.1016/j.cps.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Amin SP, Phelps RG, Goldberg DJ. Mesotherapy for facial skin rejuvenation: a clinical, histologic, and electron microscopic evaluation. Dermatol Surg. 2006;32:1467–1472. doi: 10.1111/j.1524-4725.2006.32353.x. [DOI] [PubMed] [Google Scholar]
- 9.Lacarrubba F, Tedeschi A, Nardone B, et al. Mesotherapy for skin rejuvenation: assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-sectional B-mode scanning. Dermatol Ther. 2008;21(Suppl.):1–5. doi: 10.1111/j.1529-8019.2008.00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Vedamurthy M. Mesotherapy. Indian J Dermatol Venereol Leprol. 2007;73:60–62. doi: 10.4103/0378-6323.30661. [DOI] [PubMed] [Google Scholar]
- 11.Iorizzo M, De Padova MP, Tosti A. Biorejuvenation: theory and practice. Clin Dermatol. 2008;26:177–181. doi: 10.1016/j.clindermatol.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 1996;15:134–138. doi: 10.1016/s1085-5629(96)80003-4. [DOI] [PubMed] [Google Scholar]
- 13.Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22:97–104. [Google Scholar]
- 14.Mahoney MG, Brennan D, Starcher B, et al. Extracellular matrix in cutaneous aging: the effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis. Exp Dermatol. 2009;18:205–211. doi: 10.1111/j.1600-0625.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 15.Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg. 2006;32:465–480. doi: 10.1111/j.1524-4725.2006.32100.x. [DOI] [PubMed] [Google Scholar]
- 16.Whittaker P, Kloner RA, Boughner DR, et al. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 17.Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28:93–105. doi: 10.1016/j.det.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kon A, Takeda H, Ito N, et al. Tissue-specific downregulation of type VII collagen gene (COL7A1) transcription in cultured epidermal keratinocytes by ultraviolet A radiation (UVA) and UVA-inducible cytokines, with special reference to cutaneous photoaging. J Dermatol Sci. 2005;1(Suppl.):29–35. [Google Scholar]
- 19.Amano S. Possible involvement of basement membrane damage in skin photoaging. J Investig Dermatol Symp Proc. 2009;14:2–7. doi: 10.1038/jidsymp.2009.5. [DOI] [PubMed] [Google Scholar]
- 20.El-Domyati M, Attia S, Saleh F, et al. Trichloroacetic acid peeling versus dermabrasion: a histometric, immunohistochemical, and ultrastructural comparison. Dermatol Surg. 2004;30:179–188. doi: 10.1111/j.1524-4725.2004.30061.x. [DOI] [PubMed] [Google Scholar]
- 21.Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7(Suppl.):12–16. [PubMed] [Google Scholar]
- 22.Uitto J. Understanding premature skin aging. N Engl J Med. 1997;337:1463–1465. doi: 10.1056/NEJM199711133372011. [DOI] [PubMed] [Google Scholar]
- 23.El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 24.El-Domyati M, Attia S, Saleh F, et al. Effect of topical tretinoin on photoaged facial skin: a histometric, immunohistochemical and ultrastructural study. J Cosmet Dermatol. 2004;3:191–201. doi: 10.1111/j.1473-2130.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- 25.Rotunda AM, Avram MM, Avram AS. Cellulite: is there a role for injectables? J Cosmet Laser Ther. 2005;7:147–154. doi: 10.1080/14764170500430234. [DOI] [PubMed] [Google Scholar]
- 26.Rohrich RJ, Janis JE, Reisman NR. Use of off-label and non-approved drugs and devices in plastic surgery. Plast Reconstr Surg. 2003;112:241–243. doi: 10.1097/01.PRS.0000067912.54634.56. [DOI] [PubMed] [Google Scholar]
- 27.Brown SA. The science of mesotherapy: chemical anarchy. Aesthet Surg J. 2006;26:95–98. doi: 10.1016/j.asj.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Rohrich RJ. Mesotherapy: what is it? Does it work? Plast Reconstr Surg. 2005;115:>1425. doi: 10.1097/01.prs.0000162243.34988.90. [DOI] [PubMed] [Google Scholar]
- 29.Stachowski J, Botts K, Rine L, et al. Mesotherapy: cosmetic applications. Int J Pharmaceut Compound. 2006;10:331–334. [PubMed] [Google Scholar]
- 30.Sandby-Moller J, Wulf HC. Ultrasonographic subepidermal low-echogenic band, dependence of age and body site. Skin Res Technol. 2004;10:57–63. doi: 10.1111/j.1600-0846.2004.00056.x. [DOI] [PubMed] [Google Scholar]