Abstract
Background
Functional hemispherectomy is effective in carefully selected patients, resulting in a reduction of seizure burden up to complete resolution, improvement of intellectual development, and developmental benefit despite possible additional neurological deficit. Despite apparent hemispheric pathology on brain magnetic resonance imaging (MRI) or other imaging tests, scalp electroencephalography (EEG) could be suggestive of bilateral ictal onset or even ictal onset contralateral to the dominant imaging abnormality. We aimed to investigate the role of scalp EEG lateralization pre-operatively in predicting outcome.
Methods
We retrospectively reviewed 54 patients who underwent hemispherectomy between 1991 and 2009 at Medical College of Georgia (1991–2006) and Cincinnati Children’s Hospital Medical Center (2006–2009) and had at least one year post-operative follow-up. All preoperative EEGs were reviewed, and classified as either lateralizing or nonlateralizing, for both ictal and interictal EEG recordings.
Results
Of 54 patients, 42 (78%) became seizure free. Twenty-four (44%) of 54 had a nonlateralizing ictal or interictal EEG. Further analysis was based on etiology of epilepsy, including malformation of cortical development (MCD), Rasmussen syndrome (RS), and stroke (CVA). EEG nonlateralization did not predict poor outcome in any of the etiology groups evaluated.
Conclusion
Scalp EEG abnormalities in contralateral or bilateral hemispheres do not, in isolation, predict a poor outcome from hemispherectomy. Results of other non-invasive and invasive evaluations should be used to determine candidacy.
Keywords: Hemispherectomy, Hemispherotomy, EEG, Bilateral, Outcome
1. Introduction
Hemispherectomy, originally described as the anatomical removal of one hemisphere of the brain, has been the treatment of choice for patients with catastrophic epilepsy due to hemispheric pathology, whether congenital or acquired. Even though the surgical approach has now evolved to functional hemispherectomy or hemispherotomy, the goal and result remain the same: disconnection of the hemisphere causing epileptic encephalopathy.1 There is no difference in seizure outcome based on the different surgical approaches.2 Therefore, the conventional term “hemispherectomy” will be used in this report. Anticipated adverse effects of hemispherectomy include worsening hemiparesis, hemianopia, language impairment, and loss of fine motor control in the affected hand depending on the timing of original insult and surgery, and the language dominance of the hemisphere, in addition to typical surgical risks which include bleeding, infection, and potential for development of hydrocephalus. The potential benefits on cognition and development from hemispherectomy have increased the urgency to properly evaluate and treat candidates earlier in their course. In children with epileptic encephalopathy resulting in progressive cognitive deterioration, hemispherectomy results in a stabilization of developmental velocity.3 Seizure free outcome, including all etiologies and clinical risk factors, has been reported ranging from 65% to 95%.4–6
Appropriate selection of patients who will become seizure free after the procedure is imperative. Scalp electroencephalogram (EEG) is a standard component in the evaluation for hemispherectomy candidates, yet there are conflicting reports regarding the utility of EEG in predicting seizure outcome.7–9 Specifically, it is not clear whether scalp EEG abnormalities arising from the “normal” hemisphere confer a poor prognosis. Our primary aim was to determine if bilateral or contralateral EEG abnormalities are a poor prognostic factor for seizure freedom. Our secondary aim was to determine seizure outcomes in the major subgroups of Rasmussen syndrome (RS), stroke (CVA) and malformations of cortical development (MCD), and relate these outcomes to lateralization or nonlateralization of preoperative scalp EEG.
2. Methods
Electronic records and paper medical charts were reviewed. All hemispherectomies at Medical College of Georgia (MCG) from 1991 to 2006, and at Cincinnati Children’s Hospital Medical Center (CCHMC) from 2006 to 2009, were reviewed. The surgical techniques used included functional hemispherectomy and functional hemispherotomy. A minimum 1 year postoperative outcome was obtained by review of clinic and phone records. All preoperative EEG studies were reviewed. In all cases at least one seizure was captured on video EEG. Each patient’s EEGs were categorized as one of the following: (1) interictal lateralizing or (2) interictal nonlateralizing and either (1) ictal lateralizing or (2) ictal nonlateralizing. Abnormalities were restricted to epileptiform abnormalities (spikes, sharp-waves, or seizures) and excluded focal or generalized slowing. If any of a patient’s EEGs (interictal, ictal or both) demonstrated bilateral or contralateral abnormalities to the eventual surgical site, the patient was categorized as nonlateralizing.
Etiology was determined by review of postoperative pathology reports. Categorical data were analyzed for statistically significant differences in distribution of proportions using Fisher’s exact test with SAS® statistical software version 9.2. Outcome was measured 1 year post-operatively and subsequently on an annual basis. If a second surgery was necessary as a result of incomplete disconnection, the outcome after the second surgery was reported as the end point. This study was approved by both institutional review boards.
3. Results
3.1. Illustrative case
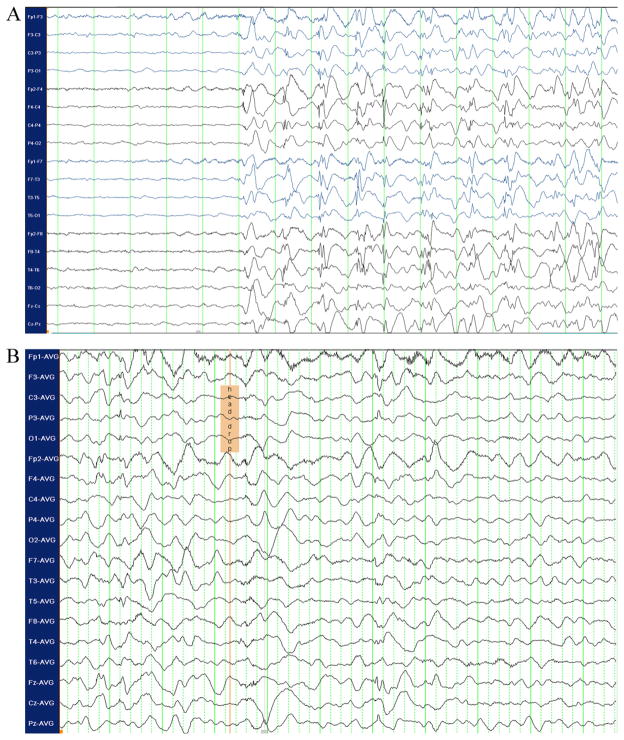
An 18 month old male presented with daily occurrence of multiple seizure types since 5 months of age. The MRI showed extensive left hemispheric cortical dysplasia (Fig. 1). The child’s typical generalized seizure began with rhythmic jerking of the right arm, but the scalp EEG was non-lateralizing (Fig. 2A). More common head drop seizures with facial myoclonus showed somewhat different, nonetheless, non-lateralizing EEG (Fig. 2B). Despite the discouraging EEG findings, he underwent left functional hemispherectomy and became seizure-free. Pathology confirmed Palmini type 2A cortical dysplasia.
Fig. 1.
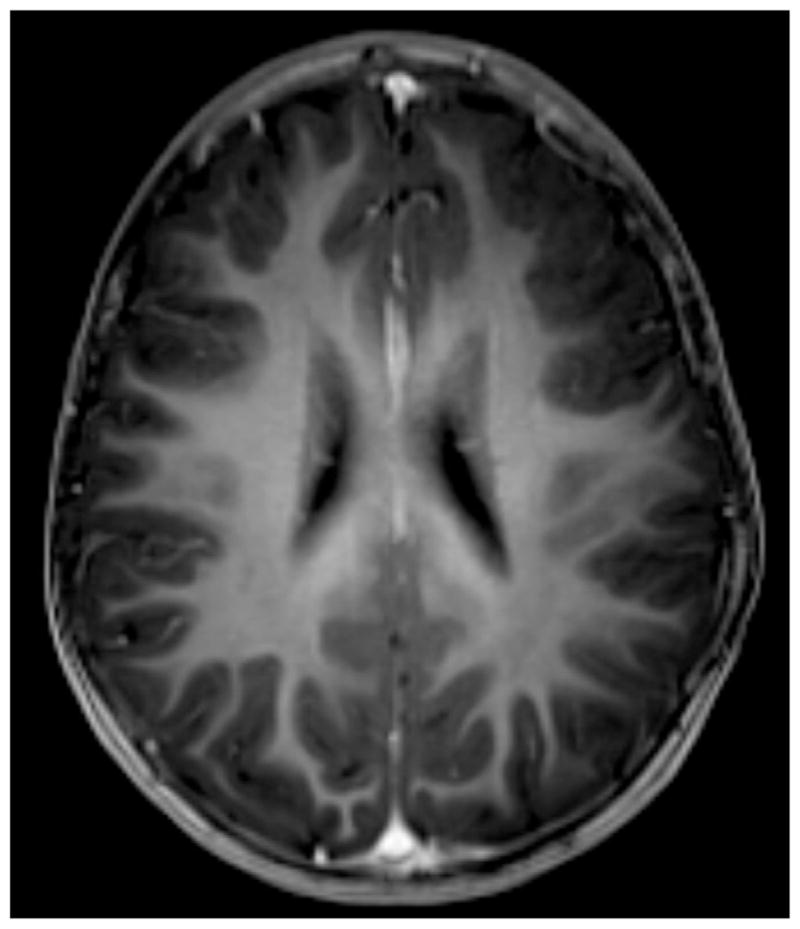
Axial T1-based MRI sequence demonstrating cortical thickening and simplified arborization pattern in the left frontal lobe.
Fig. 2.
(A) Ictal EEG with bipolar montage (standard 10–20 electrode system) demonstrating diffuse onset of rhythmic spike–polyspike and wave discharges during rhythmic clonic jerking of the right arm; (B) ictal EEG with average referential montage of the same patient during a head drop seizure. Diffuse slow wave and attenuation is noted at clinical onset.
3.2. Demographics
Fifty-four patients were identified, 37 from MCG and 17 from CCHMC. Median age of seizure onset was 0.9 (SD 4.2) years. The mean duration of epilepsy was 7.6 years prior to hemispherectomy. At the time of operation their ages were 6 months to 40 years, with a median of 8.5 (SD 9.4) years of age. The patients were followed for a minimum of 1 year, with a mean of 5.3 (SD 4.4) years. Of the 54 patients, 21 had an etiology of MCD, 20 CVA, and 10 RS. Two patients had Sturge-Weber syndrome (SWS), and one patient suffered from head trauma (Table 1).
Table 1.
Demographics. All data reported in years, including median age at seizure onset, median age at hemispherectomy, and mean years of postoperative follow-up.
| Etiology | Age at onset (years) | Duration of epilepsy | Age at surgery | Last follow-up |
|---|---|---|---|---|
| All, n = 54 | 0.9 (SD 4.2) | 7.6 | 8.5 (SD 9.4) | 5.3 (SD 4.4) |
| CVA, n = 20 | 1.5 (SD 6) | 11.2 | 12.7 (SD 11.2) | 8.5 (SD 4.5) |
| MCD, n = 21 | 0.3 (SD 0.8) | 7.2 | 7.5 (SD 5.8) | 2 (SD 4.6) |
| RS, n = 10 | 4.4 (SD 2.5) | 5.0 | 7.5 (SD 8.2) | 2.8 (SD 3) |
| SWS, n = 2 | 0.4 (SD 0.2) | 1.4 | 1.8 (SD 1.1) | 1.5 (SD 0.7) |
| Trauma, n = 1 | 4 | 12.8 | 16.8 | 8 |
SD = standard deviation; CVA = stroke; MCD = malformation of cortical development; RS = Rasmussen syndrome; SWS = Sturge Weber syndrome.
3.3. Seizure outcome
Seizure freedom after a minimum of 1 year follow-up was seen in 42/54 (78% Engel Class 1). Of the remaining 12 patients, five had greater than 90% reduction in seizures. Only seven (13%) had frequent or unchanged seizures postoperatively (Table 2). In four cases, a second surgery was performed after seizure recurrence was determined to be the result of an incomplete surgical disconnection. Three of four patients were seizure free after second surgery. The RS population had the best seizure outcome (seizure free 90%, 9/10). When typically unihemispheric pathologies, including RS, CVA, and SWS, were combined, their seizure-free outcome (25/32, 78%) was similar to the outcome in MCD (17/21, 81%).
Table 2.
Outcome by etiology.
| Etiology | Engel Cl.1 | Engel Cl.2 | Engel Cl.3 | Engel Cl.4 |
|---|---|---|---|---|
| All, n = 54 (%) | 42 (78) | 5 (9) | 5 (9) | 2 (4) |
| CVA, n = 20 | 15 (75) | 4 (20) | 1 (5) | |
| MCD, n = 21 | 17 (81) | 3 (14) | 1 (5) | |
| RS, n = 10 | 9 (90) | 1 | ||
| SWS, n = 2 | 1 (50) | 1 (50) | ||
| Trauma, n = 1 | 1 (100) |
CVA = stroke; MCD = malformation of cortical development; RS = Rasmussen syndrome; SWS = Sturge Weber syndrome. Outcome in standard Engel classification.
3.4. Neuropsychological outcome
Neuropsychological or neurodevelopmental assessment was available only for the patients from CCHMC (n = 17). Formal assessment could not be completed in very young patients with encephalopathy so severe that they could not comply with the behavioral demands of assessment. Formal preoperative evaluations were available in six patients. Several cognitive assessment tools were used, depending on age and functional status. Five of the six patients with numeric scores preoperatively had follow-up testing. Three of the patients who were not tested due to encephalopathy preoperatively, improved sufficiently postoperatively to allow for formal evaluations. In all nine patients with numeric data available, cognitive assessment demonstrated they were normatively stable intellectually. Additionally, many families reported subjective and qualitative improvement with regard to level of engagement with environment (Table 3).
Table 3.
Cognitive assessments pre-and-postoperatively. Qualitative assessments reflect parents and neuropsychologist.
| Patient | Age (years) | Preop. | Postop. | Qualitative |
|---|---|---|---|---|
| 1 | 7.5 | 71a | 77 | NC-I |
| 2 | 14.4 | 20b | 29 | NC |
| 3 | 12.2 | NT | (I) 65 and (II) 73a | I |
| 4 | 12.7 | 58a | – | – |
| 5 | 2.5 | NT | 81c | |
| 6 | 1.6 | 60c | 62–72 | NC |
| 7 | 1.7 | 55c | 55 | NC |
| 8 | 15.5 | 40a | 42 | NC |
| 9 | 2.9 | NT | 86c | I |
NC = no change; I = improved; NC-I = no change to improved; NT = not testable.
Wechsler intelligence scale for children (WISCS) IV.
Peabody picture vocabulary test – three and four (PPVT-III or PPVT-IV).
Bayley cognitive score.
Wechsler preschool and primary scale of intelligence, third edition (WPPSI-III).
3.5. Prevalence of nonlateralization
Findings of epileptic discharges on the side contralateral to the surgery site were very common interictally, ictally or both. Twenty-four of 54 (44%) had evidence of nonlateralization. We found 16 patients with contralateral interictal discharges, and 22 patients with either unlocalizing ictal onset, contralateral ictal onset, or both. Fourteen (26%) patients demonstrated nonlateralization both interictally and ictally. Three of the 54 patients had seizures originating independently from the contralateral hemisphere. In 13/54 (24%), interictal and ictal EEGs were not concordant, such that interictal EEG lateralization was not a reliable predictor of ictal EEG lateralization.
3.6. Effect of nonlateralization on outcome
Overall the seizure free outcome of patients with non-lateralizing EEGs (17/24; 71%) was the same as that of patients with lateralized EEGs (25/30; 81%). Of the patients who were seizure free, 17/42 (43%) had a nonlateralizing EEG. On the other hand, of the patients who continued to have seizures postoperatively, 7/12 (58%) had a nonlateralizing EEG, either ictal or interictal, or both (p = 0.27). While this may have indicated a trend toward a relationship between nonlateralization and poor seizure outcome, this trend did not hold in patients with both ictal and interictal nonlateralization. Of the 14 patients with both ictal and interictal nonlateralization, 12 (86%) became seizure free. Of those with interictal nonlateralization, 13/16 (81%) became seizure free. Of those with nonlateralizing ictal EEGs, 16/22 (73%) became seizure free. Comparatively, 26/32 (81%) of patients with lateralizing ictal EEGs were seizure free (p = 0.20, Table 4). There was no statistically significant relationship between scalp EEG lateralization and seizure outcome. Three patients had evidence of paradoxical, contralateral independent seizures, not just diffuse or nonlateralizing seizures. Two of these three patients were seizure free at last follow-up.
Table 4.
Outcome by EEG lateralization/nonlateralization.
| EEG abnormality | Seizure free | Not seizure free | Total |
|---|---|---|---|
| Interictal L | 29 | 9 | 38 |
| Interictal N-L | 13 | 3 | 16 (p = 0.27) |
| Ictal L | 26 | 6 | 32 |
| Ictal N-L | 16 | 6 | 22 (p = 0.20) |
L = lateralizing EEG; N-L = nonlateralizing EEG, including contralateral or bilateral spikes, sharps, or seizures, or diffuse onset of electrographic seizures.
3.7. Alternate EEG classification for lateralization
In order to incorporate both interictal and ictal EEG patterns and to distinguish between nonlateralizing EEG abnormality and contralateral EEG abnormality, an alternate classification was applied to make three categories: (1) ipsilateral EEG abnormalities to surgical side (ictal onset lateralizing to the surgical hemisphere or nonlateralizing ictal EEG but interictal discharges lateralizing to the surgical side); (2) nonlateralizing EEG abnormalities (interictal and ictal discharges showing no lateralization to either side); and (3) contralateral EEG abnormalities (ictal onset contralateral to the surgical hemisphere or nonlateralizing ictal EEG but interictal discharges contralateral to the surgical side) (Table 5). Applying this classification increased the number of patients categorized as lateralizing, since it added weight to interictal discharges in the setting of a nonlateralizing ictal EEG. In category 1 with ipsilateral EEG abnormalities, 31/41 (76%) became seizure free. Examining this group, it was found that 5/9 (56%) defined as lateralizing based on interictal discharges with a nonlateralizing ictal EEG were seizure free compared to 26/32 (81%) seizure free when lateralization based on ictal EEG. While this was not statistically significant, there was a trend toward poorer outcome in the ipsilateral EEG group defined by interictal discharges alone (p = 0.18). Combining categories 2 and 3, nonlateralizing or truly contralateral EEGs, 11/13 (85%) became seizure free. Using this alternate classification, no significant difference in outcome was obtained.
Table 5.
Alternate EEG classification and surgical outcome. EEGs were interpreted based on both ictal and interictal EEG abnormalities as either (1) showing dominant abnormalities ipsilateral to surgical side; (2) nonlateralizing; (3) showing dominant abnormalities contralateral to surgical side.
| Clinical category | EEG abnormality | Seizure free | Not seizure free | Total |
|---|---|---|---|---|
| Ipsilateral | Ictal L | 26 | 6 | 32 |
| Ipsilateral | Interictal L/ictal NL | 5 | 4 | 9 |
| Total ipsilateral | 31 | 10 | 41 | |
| Nonlateralizing | Interictal/ictal NL | 10 | 1 | 11 |
| Contralateral | Ictal ContraL | 1 | 1 | 2 |
| Total NL/ContraL | 11 | 2 | 13 | |
| Total | 42 | 12 | 54 |
Ictal L = seizure onset ipsilateral to surgical side; interictal L/ictal NL = interictal abnormalities clearly lateralizing to surgical side, seizure onset nonlateralizing; interictal NL = interictal abnormalities in both hemispheres; interictal/ictal NL = both ictal and interictal abnormalities which are either bilateral independent or nonlateralizing; ictal ContraL = seizure onset exclusively contralateral to surgical side.
3.8. Effect of etiology and EEG nonlateralization on outcome
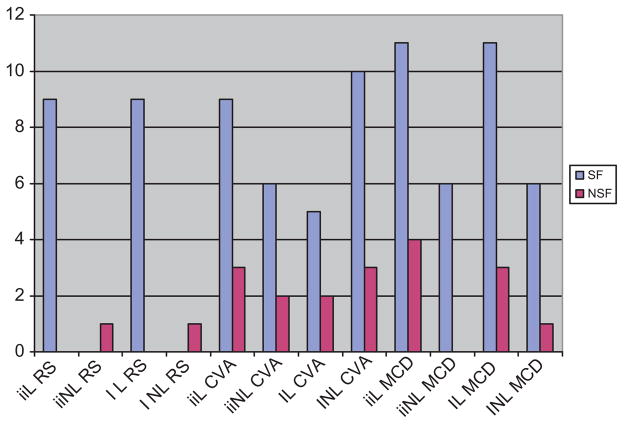
There were no significant differences in surgical outcome seen among different etiologies. Nonlateralization was seen in all etiology groups, including classically “unihemispheric” pathologies. In fact, the most common etiology in patients who had nonlateralization of ictal EEG was CVA, seen in 13/20 (65%) of CVA patients. This compares to only 7/21 (33%) in MCD patients with nonlateralizing ictal EEG (Table 3). Only one RS (1/10) patient had a nonlateralizing EEG, and the seizure outcome was poor in this case. The only patient with a preoperative diagnosis of trauma had a lateralizing EEG but poor seizure outcome. No statistically significant differences relating etiology, EEG nonlateralization (whether interictal or ictal) and outcome were found (Fig. 3).
Fig. 3.
Outcome based on etiology and lateralization/nonlateralization of EEG. SF = seizure free. NSF = not seizure free. ii = interictal; I = ictal. L = lateralizing EEG; NL = nonlateralizing EEG. RS = Rasmussen syndrome; CVA = stroke; MCD = malformation of cortical development.
3.9. Relationship between age, duration of epilepsy, nonlateralization and outcome
Neither older age nor longer duration of epilepsy was associated with a difference in seizure outcome (p = 0.40). Nonlateralization did correlate with a longer duration of epilepsy (p = 0.04). However, age at seizure onset was not associated with nonlateralization. Selecting only those patients with nonlateralizing EEG for analysis, logistic regression found no significant impact of duration of epilepsy on outcome (p = 0.41). There was no difference of outcome based on etiology.
3.10. Postoperative EEG
Six patients had postoperative EEGs, most often for characterization of questionable spells of epileptic origin. Preoperatively, four of the six patients had lateralizing EEG. Postoperatively, three had lateralization on EEG. The patient with new nonlateralization postoperatively presented as an infant with MCD treated with functional hemispherectomy at 2 months of age. Two years later, electroclinical seizures were recorded from the hemisphere contralateral to the surgery.
4. Discussion
Counterintuitively, scalp EEG is often nonlateralizing or even paradoxically contralateralizing to the known structural lesion in hemispherectomy candidates. A published series of 68 children undergoing hemispherectomy found that six (9%) had paradoxical ictal onset of seizures.10 Five of these six patients were seizure free after greater than one year of follow-up. In our report two out of three patients with paradoxical ictal onset were seizure free. In a series of 28 children with unilateral hemispheric lesions, 75% had bilateral ictal or interictal findings on scalp EEG.8 Another published series identified 50 patients with generalized or bilateral EEG abnormalities; the majority of these patients had hemispherectomy and were seizure free.11 Our series was consistent with these reports, showing that contralateral or unlocalizing ictal onset is common and does not portend poor seizure outcome.
Conversely, a series of 12 patients found that there is an additive group of risks identifiable on EEG which may predict outcome: in this report, the three patients with contralateral hemispheric slowing, absence of ipsilateral slowing, bilateral independent spikes, and generalized discharges all had poor seizure outcome.7 Smith et al. found that five of 25 children had bilateral independent epileptogenic foci, and three of these five (60%) were seizure free postoperatively, considered a poor outcome in the overall sample.12 We report on a larger sample size of 24 patients with nonlateralizing discharges, and find no statistical difference in seizure outcome. It should be noted that we did not consider background frequency slowing in our analysis, which was factored in one of the studies cited.7 In our experience, background slowing was quite variable depending on frequency of seizures, patient’s age, AEDs, and the location of lesions. In our sample, when EEGs were re-classified considering both interictal and ictal EEG, 41/54 (76%) could be categorized as lateralizing. In this group of 41, nine were categorized on the basis of interictal lateralization in combination with ictal nonlateralization. Interestingly, the lateralizing group as a whole and the nonlateralizing/contralateral group had similar seizure outcomes. However, within the lateralizing group only 5/9 (56%) categorized on the basis of interictal abnormalities were seizure free, showing a trend toward poor seizure outcome. This finding serves to caution against using interictal scalp EEG for lateralization and surgical planning.
Our seizure outcome data (78% seizure free) and distribution of etiologies are similar to other published series.6,13–15 In previous reports, unihemispheric pathology such as RS and CVA had better outcomes after hemispherectomy compared to MCD.16 Cortical abnormalities in MCD are often bilateral or diffuse, resulting in the potential for multifocal sites of epileptogenesis. In a series reporting on 105 patients, 38% of patients with MCD had findings of bilateral abnormality on MRI, EEG, or both.6 Since unihemispheric pathologies have better outcomes after hemispherectomy, and MCDs have been reported to have a higher prevalence of bilateral EEG abnormalities, this might suggest that EEG non-lateralization in a patient with a likely MCD implies a less favorable pre-operative prognosis. In our series, the MCD subgroup of 21 patients had a similar seizure outcome and prevalence of nonlateralization compared to other etiologies. No difference in seizure outcome was seen between unihemispheric (RS, CVA and SWS) and MCD etiologies. Nine of 21 (43%) MCD patients had bilateral abnormalities on EEG, which was consistent with 24/54 (44%) for the entire population. Surprisingly, in the CVA subgroup, 13/20 (65%) had EEG nonlateralization.
Malformations of cortical development include a large spectrum, ranging from pathologies with typically bilateral imaging and pathological findings such as polymicrogyria17 to focal cortical dysplasia (FCD). Even in FCD, there is evidence of multifocal and bilateral sites of epileptogenesis in many cases.18 This fact is reflected by the care taken prior to offering respective surgery in MCD: there may be a higher burden of proof associated with the decision to proceed with hemispherectomy in MCD compared to unihemispheric pathologies. The prevalence of nonlateralization in MCD is consistent with published series, but the CVA group in our series demonstrated a high incidence of nonlateralization. We could speculate that the large number of nonlateralizing patients in the CVA group compared to the MCD group reflects either longer duration of epilepsy in the CVA group before surgery or perhaps a selection bias. For example, cases presenting with MCD as a likely preoperative etiology and nonlateralizing EEG findings may be less likely to proceed to hemispherectomy, and hence not included in this postsurgical analysis. The finding of MRI abnormalities consistent with prior CVA increases confidence in the potential benefit of hemispherectomy, and therefore nonlateralizing EEG may not be weighted very heavily in determining surgical candidacy in these cases. Further, there appears to be a relationship between duration of epilepsy and prevalence of nonlateralization. In our series, this relationship was statistically significant. The CVA group had a longer duration of epilepsy than the other etiology groups, making these patients more likely to have nonlateralizing findings on EEG. We emphasize that this finding is merely associated with duration of epilepsy and not predictive of prognosis.
Progressive disorders such as RS may worsen to involve both hemispheres, but whether scalp EEG nonlateralization is a sensitive indicator of this worsening is unknown. Nine of 10 (90%) of the RS patients in our analysis were seizure free and had lateralizing EEGs. Only one patient with RS had a nonlateralizing EEG, and this patient also had a poor surgical outcome. This child suffered from epilepsy for a much longer duration (11 years) than the typical RS patient, and consequently developed seizures from the other hemisphere. Due to small sample size, it is not clear whether the nonlateralizing EEG in our RS group is truly a prognostic indicator.
It is not clear whether contralateral EEG findings alone represent true epileptogenesis in individual patients. In studying six children with encephaloclastic lesions and paradoxical ictal onset, Garzon et al. presented three hypotheses to explain this phenomenon. The most plausible and often cited is that ictal low voltage fast rhythms originate in the diseased hemisphere,10 but the seizure may not be detectable by scalp EEG until propagated to the “healthy” hemisphere, at which time the more robust volume and organization of neurons in the contralateral hemisphere allow for rhythmic discharges detected on scalp EEG. In children with encephaloclastic lesions, this explanation seems sufficient; but when considering MCDs with no detectable hemispheric differences in brain volume and only subtle structural differences based on MRI, it would seem that the leading edge of the seizure should lateralize to the diseased hemisphere on scalp EEG. This hypothesis also may not explain cases where there is persistent contralateral EEG abnormalities following hemispherectomy. In our study, three patients had contralateral epileptiform discharges on postoperative EEG, but were seizure-free on the last follow-up. On the other hand, we have seen patients whose EEG abnormalities showed progression over several years from clear unilateral discharges to bilateral independent abnormalities along with worsening of cognitive development and seizure as well as MRI findings. Presumably frequent seizures starting in the diseased hemisphere but spreading to the contralateral side produce irritability in the “healthy” hemisphere. Our finding correlating long duration of seizure disorder preoperatively with nonlateralization on EEG supports this hypothesis. We believe that these cases illustrate true secondary epileptogenesis, but we are not certain when this process becomes irreversible and hemispherectomy can no longer be offered due to these concerns.
There were several limiting factors in this retrospective series. Multiple surgical techniques were used, based on institution and neurosurgeon preference. In four cases a second surgery was necessary to complete disconnection. Despite these potential confounders, previously published reports demonstrating similar efficacy between different surgical techniques make this a comparable dataset.19 With respect to EEG analysis, the patients’ records were oversimplified for ease of statistical analysis. Scalp EEGs were considered ‘nonlateralizing’ or ‘lateralizing’. In clinical practice, an epileptologist will consider the burden of abnormalities present in each hemisphere, as well as various qualitative features of the evaluation not included here, before making a recommendation for hemispherectomy. With respect to outcome analysis, this is a medium-sized sample with associated limitations. This was apparent when attempting to draw conclusions from the RS subgroup.
Seizure outcome is only one component of the patient outcome following hemispherectomy. The importance of effects on development cannot be overestimated, and a patient with occasional seizures but excellent developmental outcome would generally be considered a success. However, in the hemispherectomy literature development appears to follow seizure outcome. In a series following developmental assessments postoperatively after hemispherectomy, a reduction in seizures was associated with a constant improvement in intellectual function.20 Estimating the effect of an abnormal contralateral hemisphere on developmental outcome is difficult. Recently published data indicates that MRI—but not EEG—abnormalities in the hemisphere contralateral to the hemispherectomy have an important negative developmental prognosis. In a retrospective analysis of the developmental outcomes of 43 patients, those with contralateral MRI abnormalities had poor postoperative development and a lower incidence of seizure freedom. In this same study, 24 patients had contralateral interictal discharges prior to hemispherectomy. There was no difference in the cognitive outcomes of these patients.21 In our series methodological issues limited the acquisition of neuropsychological and developmental outcome data in the majority of cases.
In summary, hemispherectomy is effective in achieving seizure freedom in selected candidates. Our series demonstrates that patients with bilateral or contralateral EEG abnormalities have an excellent seizure outcome postoperatively. Nonlateralization on EEG was seen in a large minority of patients, in all etiologies, including CVA and MCD. It is important not to exclude hemispherectomy based on scalp EEG; candidacy should be determined based on other clinical factors and evaluations.
References
- 1.De Ribaupierre S, Delalande O. Hemispherotomy and other disconnective techniques. Neurosurg Focus. 2008;25(3):E14. doi: 10.3171/FOC/2008/25/9/E14. [DOI] [PubMed] [Google Scholar]
- 2.Cook SW, Nguyen ST, Hu B, Yodovin S, Shields WD, Vinters HV, et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg. 2004;100(2 Suppl):125–41. doi: 10.3171/ped.2004.100.2.0125. [DOI] [PubMed] [Google Scholar]
- 3.Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia. 2005;46(4):561–7. doi: 10.1111/j.0013-9580.2005.03504.x. [DOI] [PubMed] [Google Scholar]
- 4.Devlin AM, Cross JH, Harkness W, Chong WK, Harding B, Vargha-Khadem, et al. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain. 2003;126(Pt 3):556–66. doi: 10.1093/brain/awg052. [DOI] [PubMed] [Google Scholar]
- 5.Hallbook T, Ruggieri P, Adina C, Lachwani DK, Gupta A, Kotagal P, et al. Contralateral MRI abnormalities in candidates for hemispherectomy for refractory epilepsy. Epilepsia. 2010;51(4):556–63. doi: 10.1111/j.1528-1167.2009.02335.x. [DOI] [PubMed] [Google Scholar]
- 6.Kossoff EH, Vining EPG, Pillas DJ, Pyzik PL, Avelino AM, Carson BS, et al. Hemispherectomy for intractable unihemispheric epilepsy etiology vs. outcome. Neurology. 2003;61(7):887–90. doi: 10.1212/01.wnl.0000090107.04681.5b. [DOI] [PubMed] [Google Scholar]
- 7.Carmant L, Kramer U, Riviello JJ, Helmers SL, Mikati MA, Madsen JR, et al. EEG prior to hemispherectomy: correlation with outcome and pathology. Electroencephalogr Clin Neurophysiol. 1995;94(4):265–70. doi: 10.1016/0013-4694(95)98477-p. [DOI] [PubMed] [Google Scholar]
- 8.Doring S, Cross H, Boyd S, Harkness W, Neville B. The significance of bilateral EEG abnormalities before and after hemispherectomy in children with unilateral major hemisphere lesions. Epilepsy Res. 1999;34(1):65–73. doi: 10.1016/s0920-1211(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 9.Bingaman WE, Wyllie E, Lachwani D, Kotagal P, Gonzalez-Martinez JA, Gupta A, et al. Hemispherectomy for catastrophic epilepsy in infants. Epilepsia. 2005;46(9):1518–25. doi: 10.1111/j.1528-1167.2005.53704.x. [DOI] [PubMed] [Google Scholar]
- 10.Garzon E, Gupta A, Bingaman W, Sakamoto AC, Luders H. Paradoxical ictal EEG lateralization in children with unilateral encephaloclastic lesions. Epileptic Disord. 2009;11(3):215–21. doi: 10.1684/epd.2009.0264. [DOI] [PubMed] [Google Scholar]
- 11.Wyllie E, Lachwani DK, Gupta A, Chirla A, Cosmo G, Worley S, et al. Successful surgery for epilepsy due to early brain lesions despite generalized EEG findings. Neurology. 2007;69(4):389–97. doi: 10.1212/01.wnl.0000266386.55715.3f. [DOI] [PubMed] [Google Scholar]
- 12.Smith SJ, Andermann F, Villemure JG, Rasmussen TB, Quesney LF. Functional hemispherectomy: EEG findings, spiking from isolated brain postoperatively, and prediction of outcome. Neurology. 1991;41(11):1790–4. doi: 10.1212/wnl.41.11.1790. [DOI] [PubMed] [Google Scholar]
- 13.Duchowny M, Jayakar P, Resnick T, Harvey AS, Alvarez L, Dean P, et al. Epilepsy surgery in the first three years of life. Epilepsia. 1998;39(7):737–43. doi: 10.1111/j.1528-1157.1998.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 14.Peacock WJ, Wehby-Grant MC, Shields WD, Shewmon DA, Chugani HT, Sankar R, et al. Hemispherectomy for intractable seizures in children: a report of 58 cases. Childs Nerv Syst. 1996;12(7):376–84. doi: 10.1007/BF00395089. [DOI] [PubMed] [Google Scholar]
- 15.Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44(5):740–8. doi: 10.1002/ana.410440507. [DOI] [PubMed] [Google Scholar]
- 16.Tubbs RS, Nimjee SM, Oakes WJ. Long-term follow-up in children with functional hemispherectomy for Rasmussen’s encephalitis. Childs Nerv Syst. 2005;21(6):461–5. doi: 10.1007/s00381-005-1136-2. [DOI] [PubMed] [Google Scholar]
- 17.Leventer RJ, Jansen A, Pilz DT, Stoodley N, Marini C, Dubeau F, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010;133(Pt 5):1415–27. doi: 10.1093/brain/awq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGonigal A, Bartolomei F, Figarella-Branger D, Girard N, Peragut J, Wendling F, et al. Local and remote epileptogenicity in focal cortical dysplasias and neurodevelopmental tumours. Brain. 2009;132(Pt 11):3072–86. doi: 10.1093/brain/awp242. [DOI] [PubMed] [Google Scholar]
- 19.Basheer SN, Connolly MB, Lautzenhiser A, Sherman EMS, Hendson G, Steinbok P. Hemispheric surgery in children with refractory epilepsy: seizure outcome, complications, and adaptive function. Epilepsia. 2007;48(1):133–40. doi: 10.1111/j.1528-1167.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- 20.Maehara T, Shimizu H, Kawai K, Shigetomo R, Tamagawa K, Yamada T, et al. Postoperative development of children after hemispherotomy. Brain Dev. 2002;24(3):155–60. doi: 10.1016/s0387-7604(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 21.Boshuisen K, van Schooneveld MMJ, Leijten FSS, de Kort GAP, van Rijen PC, Gosselaar PH, et al. Contralateral MRI abnormalities affect seizure and cognitive outcome after hemispherectomy. Neurology. 2010;75(18):1623–30. doi: 10.1212/WNL.0b013e3181fb4400. [DOI] [PubMed] [Google Scholar]