Abstract
The paxillin family of intracellular scaffold proteins includes paxillin, Hic-5, and leupaxin, and all have been identified as key regulators of the cellular migration machinery in both 2- and 3-dimensional microenvironments. Herein, we provide insight into the roles of these proteins during tumorigenesis and metastasis, highlighting their functions in cancer initiation as well as tumor cell dissemination and survival. Furthermore, we speculate on the potential of paxillin family proteins as both future prognostic and therapeutic targets.
Keywords: invasion, epithelial-mesenchymal transition (EMT), cell survival, cell adhesion, extracellular matrix (ECM)
Introduction
Cell adhesion either to neighboring cells or to the surrounding extracellular matrix (ECM) or stroma plays a critical role in tumor progression by regulating proliferation and survival as well as enabling tumor cell dissemination and metastasis.1 The local tumor microenvironment, including the molecular composition and organization, density, and rigidity of the stromal ECM, has been shown to influence tumor malignancy. This information is relayed to the tumor cells through ligation of transmembrane integrin molecules on the tumor cell surface. Integrins lack enzymatic activity and thus communicate with the intracellular signal transduction pathways to regulate cell migration and/or changes in gene expression via the recruitment of multiple structural and signaling proteins to their cytoplasmic tails.2 Scaffold proteins, including members of the paxillin family,3-5 perform critical functions in coordinating these signaling events downstream of integrin-ECM ligation.
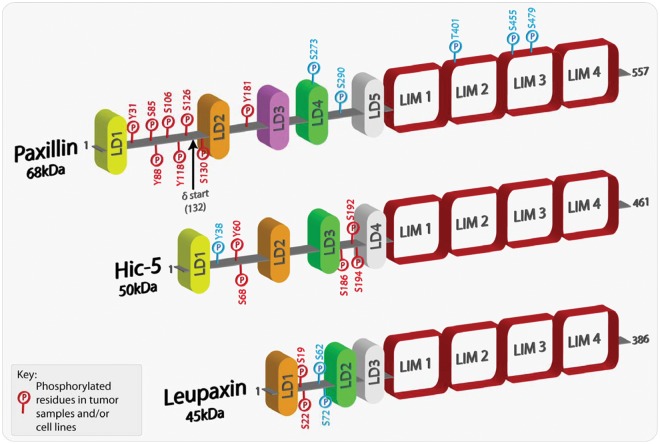
The paxillin family comprises 3 proteins: paxillin, Hic-5, and leupaxin (Fig. 1). Paxillin was first identified in a screen for substrates for the oncogene tyrosine kinase v-src in Rous sarcoma virus (RSV)–transformed fibroblasts.6,7 Consistent with its function as a molecular adaptor/scaffold protein, paxillin comprises multiple discrete protein binding modules, including numerous tyrosine as well as Ser/Thr phosphorylation sites that, when modified, contribute further to the complexity of the protein interactome.5 Many of these phosphorylated residues have been detected in tumor samples and/or cancer cell lines (Fig. 1). The amino terminal LD motifs and the C-terminal LIM domains, are highly conserved in the 2 other family members, Hic-5 and leupaxin (Fig. 1). As a result, the 3 proteins share many of the same binding partners,8-17 although their interactions can be spatiotemporally regulated by the balance of Rho family GTPase activity.8 However, it is important to note that each family member has its own unique set of interactions, for instance, the association of Hic-5 with members of the Smad family of transcription factors18,19 and paxillin with bcl-2.20 In-depth functional analysis of domain mutants has revealed a critical role for the paxillin family in coordinating cell migration by acting primarily as a hub for the regulation of Rho GTPase signaling.3,4 In addition, roles for paxillin in cell survival, mRNA processing,21,22 and Hic-5 in regulating gene expression through its function as a steroid receptor coactivator have also been reported.
Figure 1.
Schematic representation of paxillin family members highlighting phosphorylated residues. Paxillin and its family members comprise a variable number of amino-terminal LD domains, which are short leucine-rich motifs that are responsible for mediating protein-protein interactions. At the carboxyl-terminus, each family member contains 4 highly homologous LIM domains, which also serve as protein-protein binding moieties as well as being responsible for appropriate protein subcellular localization, including sites of integrin-mediated adhesion and the nucleus. Paxillin family members can also be phosphorylated at multiple sites, with the majority located in the less conserved regions between the LD domains. Furthermore, a number of the phosphorylated residues have been revealed as mediating and regulating paxillin family member protein interactions. The phospho-residues highlighted in red have been shown in multiple studies to be phosphorylated in both tumor samples and/or cancer cell lines. The amino acids highlighted in blue have been shown to be phosphorylated in a variety of studies but thus far have not been identified in cancer cells or tissues.
Paxillin is ubiquitously expressed, although protein levels in the nervous system are low.23-25 In contrast, Hic-5 is enriched in smooth muscle tissues, in particular the vasculature,26,27 and is upregulated in response to transforming growth factor (TGF)–β during epithelial-mesenchymal transition (EMT).27,28 Leupaxin, the least well-characterized member of the family, was originally thought to be restricted to cells of the leukocyte lineage,29 but more recent studies suggest a broader expression profile.30-32 Gene ablation studies in mice have generated surprisingly diverse phenotypes, with paxillin ablation causing early embryonic lethality,10 while the Hic-5 knockout mouse is viable and exhibits only very mild vascular defects.33 Importantly, paxillin, Hic-5 and leupaxin expression and/or phosphorylation changes have been linked to a number of human malignancies. In this review, we will describe some of the more recent studies that have begun to explore how disruption of paxillin family function contributes to increased cancer malignancy and speculate on their potential as therapeutic targets.
Paxillin Family Member Expression and Mutation in Human Cancers
Paxillin, Hic-5, and leupaxin have all been linked to malignant progression and have been shown, through both tissue microarray and histologic analyses, to be differentially expressed in a variety of invasive/metastatic cancers, including but not limited to breast, lung, and prostate tumors (Fig. 2).34-51 As there is a range of expression profiles of paxillin family members observed in different cancers (Fig. 2) and cancer subtypes, it precludes the classification of any individual protein as a traditional oncogene or tumor suppressor. However, given the extensive crosstalk and synergy between paxillin family members,8,13,28,52,53 it is likely that variations in the expression of any one protein will influence the downstream signaling of the other closely related family members to dictate tumor progression, invasion, and malignancy. For example, enhanced ectopic expression of either Hic-5 or leupaxin prevents paxillin phosphorylation and direct protein interactions to alter downstream signaling and cell function.13,53
Figure 2.
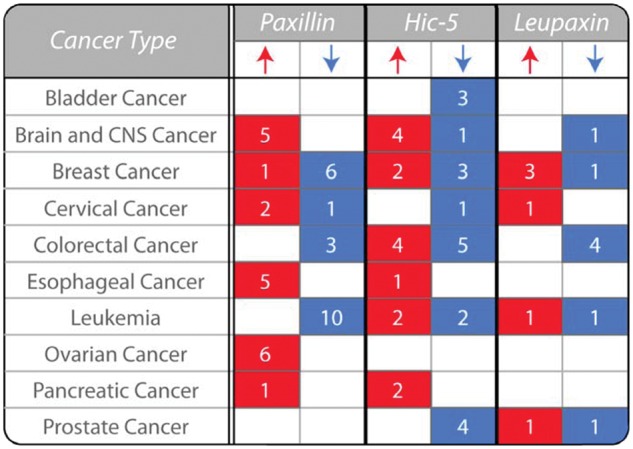
Paxillin family expression is variable in human cancers. The table highlights the variability in the expression of paxillin, Hic-5, and leupaxin in human cancers as determined by analysis of their mRNA levels in tumor samples relative to normal tissue (P < 0.00001). The number of studies that reveal a significant change in expression in tumor samples relative to normal tissues is indicated. Expression data are collated from the Oncomine cancer gene microarray database (Compendia Biosciences; Ann Arbor, MI, USA; www.oncomine.org). CNS, central nervous system.
To date, no somatic mutations in Hic-5 or leupaxin have been reported in human cancers. In contrast, analysis of lung adenocarcinoma samples identified a number of paxillin gene sequence aberrations, which were located between the LD1 and 2 motifs, the most common being an A127T mutation, which was linked to increased tumor growth and invasion.40,54 However, it is important to note that the presence of somatic mutations of paxillin in human cancers remains controversial55,56 and thus requires further study in a variety of different cancers.
Paxillin, Hic-5, and leupaxin are all downstream targets of activated hormone and growth factor receptors associated with aggressive cancers and decreased patient survival.27,28,30,57-61 For example, Hic-5 is phosphorylated in response to epidermal growth factor (EGF) receptor activation.62 Furthermore, Hic-5 is expressed in prostate stroma, where it functions as a TGF-β–induced coactivator of the androgen receptor and is linked to prostate tumorigenesis.63 Likewise, paxillin is phosphorylated downstream of EGF, TGF-β, platelet-derived growth factor (PDGF), and androgen receptor activation in a variety of cancers and affects both tumor formation and invasion.61,64-67 Furthermore, paxillin expression levels have also been shown to correlate with HER2 levels in breast cancer cells and patient samples and thus may be a predictor of therapeutic efficacy.58,60
The Roles of Paxillin and Hic-5 in Epithelial-Mesenchymal Transition (EMT)
Epithelial-mesenchymal transition is an evolutionarily conserved process that is essential for normal organism development and involves the dissolution of cell-cell adhesions, induction of cell-matrix adhesions, loss of apical-basolateral polarity, and a reorganization of the cytoskeleton to promote cell motility. Although currently controversial,68,69 the process of EMT is widely believed to be highly analogous to the changes observed during cancer initiation, in which there is a loss of cell cohesion, enhanced migration, tissue stroma invasion and subsequent metastasis.70 The conversion of epithelial cells to the more malignant mesenchymal phenotype can be stimulated by numerous factors, including activation of receptor tyrosine kinases and TGF-β–mediated signaling pathways. Cells that have undergone an EMT share various properties with a population of multipotent, self-renewing tumor cells termed cancer stem cells (CSCs), which have tumor-initiating potential.71-73 For example, cells stimulated to undergo a TGF-β–mediated EMT exhibit CD44+/CD24+ cell surface markers, upregulation of transcription factors such as Twist and Snail,74 and resistance to chemotherapy treatments,75 all of which are properties of CSCs.76
Importantly, paxillin family members appear to be differentially regulated during EMT. The paxillin gene contains an internal translation initiation site that can produce a truncated form, termed paxillin δ, which is preferentially expressed in epithelial cells but is downregulated upon TGF-β–stimulated EMT.28 Although no change in paxillin expression is observed, paxillin tyrosine phosphorylation is increased in response to TGF-β.28 In contrast to paxillin, Hic-5 is not expressed in epithelial cells but is upregulated in response to EMT, whereby TGF-β promotes both Hic-5 expression and its Src-dependent phosphorylation.66,77 Furthermore, ectopic expression of Hic-5 is sufficient to promote normal mammary cells to undergo an EMT in the absence of TGF-β.66,77 In contrast, the overexpression of paxillin is unable to induce a transition to the mesenchymal phenotype.78 It is also noteworthy that Hic-5 can stimulate the production of TGF-β through an autocrine feed-forward loop in fibroblasts,79 which may enable more epithelial cells to undergo a malignant transition and thus promote tumor growth and dissemination. It will be important to determine if, similar to EMT, paxillin family members are disregulated in CSCs at the level of protein expression and/or posttranslational modifications or indeed if they play a role in the development of stem cell–like properties in tumor cells.
Paxillin and Hic-5 Regulate Invadopodia Function
In order for tumor cells to disseminate from the primary solid tumor, they must degrade the surrounding ECM-rich basement membrane barrier to gain access to the surrounding tissues and subsequently metastasize. Analysis of invasive tumor cells in vitro and in vivo has revealed the presence of ECM-degrading protrusions, termed invadopodia.80-82 These structures aid in the process of invasion by focally disrupting the dense meshwork of ECM barriers through localized activity of membrane-bound and soluble matrix metalloproteinases (MMPs) to promote tumor cell dissemination.83,84
Invadopodia comprise an actin core that contains actin-binding and nucleating proteins as well as a surrounding ring of scaffold and adhesion proteins, including paxillin and Hic-5 (Fig. 3).85 Paxillin was identified as a component of invadopodia in both cancer cells and RSV-transformed fibroblasts,86,87 where it forms a complex with cortactin, protein kinase C (PKC) µ,86 and the ArfGAP AMAP1.88 In addition, paxillin tyrosine phosphorylation is necessary for promoting invadopodia dynamics.89
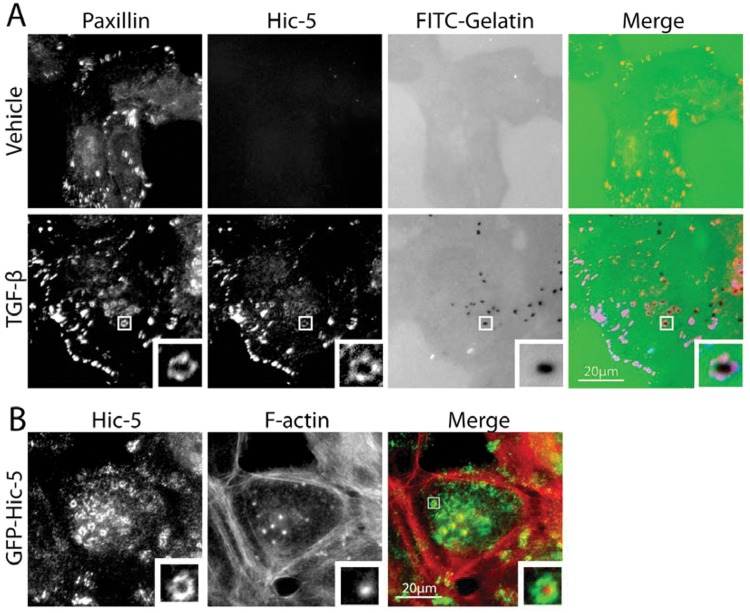
Figure 3.
Paxillin and Hic-5 localize to invadopodia. (A) Immunofluorescence images of endogenous paxillin and Hic-5 highlighting their transforming growth factor (TGF)–β–dependent localization to the ring structure of invadopodia in MCF-10A, normal mammary epithelial cells. Importantly, TGF-β treatment induces Hic-5 expression and the formation of matrix-degrading invadopodia in this cell type. (B) Immunofluorescence images of MCF-10A cells indicating that ectopic expression of green fluorescent protein (GFP)–tagged Hic-5 is sufficient to promote invadopodia formation in the absence of TGF-β.
We have recently identified Hic-5 as an essential component of invadopodia formed in cells that have undergone a TGF-β–mediated EMT (Fig. 3A).77 Interestingly, overexpression of Hic-5 in epithelial cells in the absence of TGF-β can also induce the development of invadopodia (Fig. 3B), suggesting that it is both necessary and sufficient for their formation and for subsequent cell invasion.77 Interestingly, in this system, paxillin expression is dispensable for invadopodia formation.77 Although a role for leupaxin in cancer cell invadopodia has not been assessed, leupaxin has been shown to be associated with invadopodia-like podosomes at the sealing zone of osteoclasts,90 where it associates with several proteins, including the non–receptor tyrosine kinases Pyk291 and Src,31 and thus it is plausible that leupaxin plays a similar role in tumor cells.
The regulation of the formation of invadopodia and their maturation into matrix-degrading structures has been shown to require a fine balance of Rho GTPase signaling.92,93 Furthermore, Rho GTPase family proteins have been shown to be upregulated in a variety of cancers where they regulate various aspects of tumorigenesis, including invasion and cell proliferation.93 RhoC, in particular, has been shown to be upregulated during EMT94,95 and overexpressed in multiple invasive cancer types96-99 to enhance malignancy.100-103 Multiple Rho GTPase family members, along with their regulatory proteins, guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), have been shown to localize to invadopodia. Indeed, RhoC has recently been shown to be a component of invadopodia, where the discrete localization of p190RhoGEF and p190RhoGAP regulates its activity.104 Interestingly, invadopodia formation in TGF-β–treated cells also requires RhoC activity, which in turn is regulated by the upregulation of Hic-5.77 Thus, the localization of paxillin family members to the ring compartment of invadopodia positions them to function in their capacity as hubs for GTPase regulation4 through interactions with multiple GAPs and GEFs, including cdGAP105 and βPIX,17 to spatiotemporally integrate RhoGTPase activity and so coordinate invadopodia structure and function.
Paxillin and Hic-5 Regulate Tumor Cell Invasion, Plasticity, and Metastasis
The ability of paxillin and, to a lesser extent, Hic-5 and leupaxin to regulate 2-dimensional (2D) cell motility through coordinating the migration machinery is well established.3,4 However, in vivo, migrating cells rarely meet a 2D ECM substrate but instead encounter a wide array of structurally and compositionally diverse 3-dimensional (3D) microenvironments. In vitro models of 3D cancer cell invasion have revealed important roles for paxillin,52 Hic-5,63,77 and leupaxin106 in regulating motility in this more in vivo–like environment.
Intravital and in vitro imaging studies have revealed that cancer cells are able to invade through the tissue ECM microenvironment either as collective multicellular sheets or as individual cells, contributing to lymphatic and hematogenous infiltration, respectively.107-117 In addition, cancer cells can employ 2 distinct and interchangeable modes of single-cell motility, referred to as either mesenchymal or amoeboid migration,115,118 that are determined by numerous factors ranging from ECM architecture and rigidity to the balance of intracellular Rho GTPase family signaling.109 Mesenchymal migration is characterized by an elongated cell morphology requiring extracellular proteolysis and dynamic integrin-mediated interactions with the ECM. In contrast, amoeboid motility is independent of protease activity and is associated with a rounded or ellipsoid morphology.113,115 Cells exhibiting amoeboid migration squeeze through preexisting gaps in the heterogeneous ECM milieu using actin-rich membrane blebs or filopodial-like projections and display highly transient, weak interactions with their surroundings.113-115,119 The spontaneous interconversion between the 2 forms of migration, known as plasticity, is considered one of the primary reasons for cancer cell evasion of current invasion-targeted therapeutics.120-123
Paxillin and Hic-5 have been identified as critical determinants of breast cancer cell morphology and plasticity during invasion.52 The balance of paxillin and Hic-5 expression and/or signaling is required for efficient invasion and plasticity, with paxillin depletion promoting the mesenchymal mode of invasion and Hic-5 RNAi inducing an amoeboid phenotype (Fig. 4), while their respective overexpression has the reciprocal effect.52 Importantly, depletion or overexpression of either protein was found to inhibit plasticity, invasive migration, and transendothelial migration, culminating in a lack of metastasis.52 Furthermore, analysis of 3D cell-ECM adhesion contacts revealed an absolute requirement for Hic-5 for their formation and identified paxillin as a regulator of adhesion dynamics during 3D invasive migration.52 These data suggest that for optimal, efficient invasion and metastasis, cancer cells must balance the signaling and/or expression of these paxillin family members.
Figure 4.
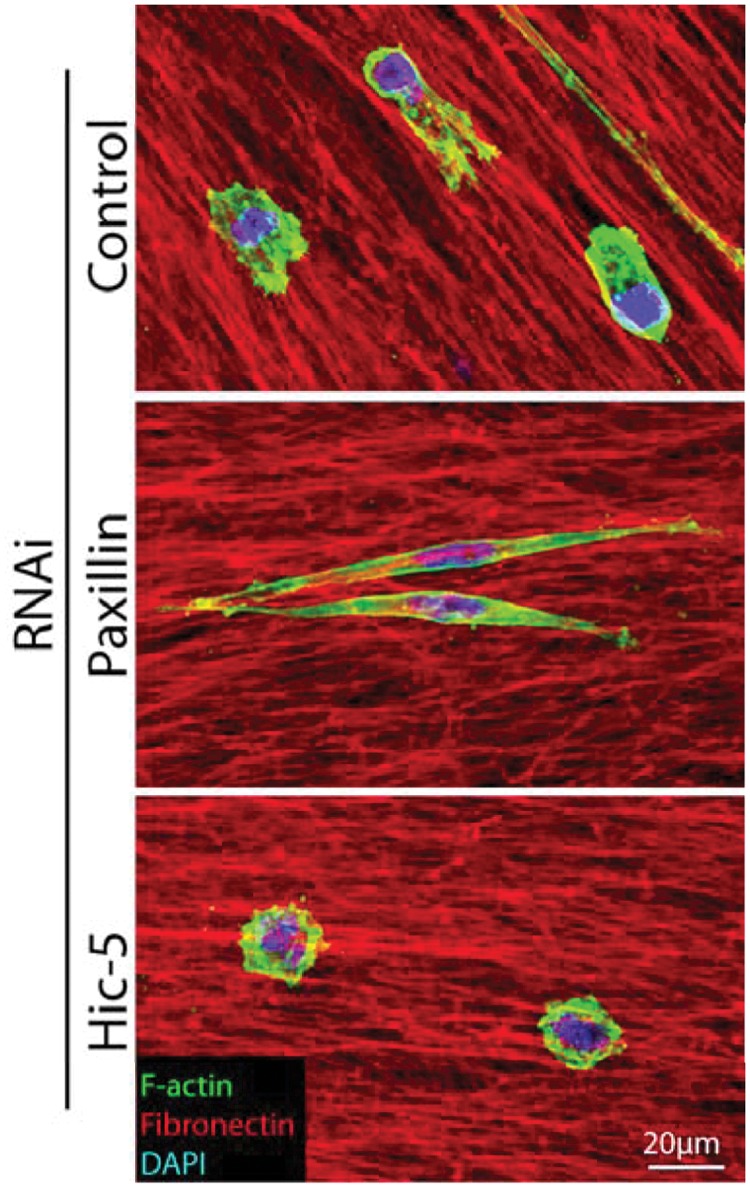
Paxillin and Hic-5 regulate breast cancer cell morphology in 3D environments to coordinate plasticity during invasion. Representative immunofluorescence images of MDA-MB-231 breast cancer cells invading through a 3D cell-derived matrix (CDM). Cells were fixed and stained for F-actin (green), fibronectin (red), and DAPI (blue). RNAi of paxillin promotes a highly elongated mesenchymal phenotype, whereas Hic-5 depletion induces the amoeboid phenotype. Importantly, depletion of either paxillin or Hic-5 prevents invasion, plasticity, and metastasis.
Paxillin is widely expressed in normal tissue, whereas Hic-5 and leupaxin expression appears more spatially restricted.24,25,27,29,32 A balance of Hic-5 and paxillin expression by tumor cells may be achieved through TGF-β–mediated upregulation of Hic-5.28,77 Intravital imaging has revealed that cells exhibiting constitutive TGF-β activation, and thus presumably elevated Hic-5 expression, invade readily as single cells and intravasate to promote distant metastasis.111 Therefore, it is plausible that the efficiency of invasion and plasticity in vivo may be determined by spatiotemporally localized TGF-β–driven fluctuations in Hic-5 expression.
Control of Cell Survival and Apoptosis
In order for solid tumor cells to metastasize to distant sites, they must survive and proliferate outside their normal tissue microenvironment. These normally adhesive cells must also endure the lack of cell adhesion–related survival signals during the process of lymphovascular infiltration.124,125 Indeed, the ability of tumor cells to exhibit anchorage-independent growth correlates with their metastatic potential and thus malignancy.126 Paxillin has been found to promote transformation and facilitate anchorage-independent cell growth through its interaction with the bovine form of the human papillomavirus (HPV) E6 protein.127 The HPV virus is known to cause multiple malignancies, including cervical, oropharyngeal, and lung cancers,128-130 through promoting the degradation of the tumor suppressor p53.131 Indeed, it has been suggested that nearly all cervical cancer patients are positive for HPV infection.132
Interestingly, paxillin has also been implicated in regulating anchorage-independent growth and cell survival through its regulated tyrosine phosphorylation as well as by its direct interactions with p210BCR/ABL, FAK, and vinculin.67,133-136 Furthermore, paxillin is able to promote cell survival signaling through its direct interaction with the pro-survival, proto-oncogene bcl-2,20 which is known to function to prevent apoptosis in the absence of cell adhesion in both normal and cancerous cells, in part through maintaining adhesion-related signaling through FAK.137,138 Importantly, neither Hic-5 nor leupaxin interacts with bcl-2 family members, although the former may regulate cell survival by other mechanisms, for example, through regulating the nuclear function of the pro-survival protein cyclin D1.139 Furthermore, Hic-5 interacts with the heat shock protein 27 (hsp27) to increase cell death in response to heat shock.140 Interestingly, the role of paxillin and Hic-5 in regulating cell survival represents another example of their reciprocal relationship, with paxillin predominantly functioning to promote cell survival, whereas increased Hic-5 expression appears to correlate with cell death in cancer and normal cell populations.63,140-143
Prognostic Marker and Therapeutic Potential
The elevated phosphorylation and/or expression of paxillin has been implicated as a prognostic marker for long-term patient survival in ovarian cancer,144 breast carcinoma,58,145 hepatocellular carcinoma,146 and lung adenocarcinoma.39 However, it is noteworthy that elevated paxillin expression has also been shown to exhibit no, or a negative correlation with patient outcome and recurrence-free survival.147,148 In contrast to paxillin, the potential for Hic-5 and leupaxin as prognostic indicators is enigmatic, although it is important to note that increased leupaxin and reduced Hic-5 expression have been observed in prostate cancers and that Hic-5 may be linked to treatment responsiveness and recurrence-free survival.30,41,42,63,149Importantly, none of the above analyses concurrently assessed the relative levels of the other paxillin family members. Indeed, cell-based studies suggest that a balance of paxillin family members is critical for tumor cell survival, proliferation, and invasion. Taken together, these studies indicate the possibility that the expression of paxillin and Hic-5 is variable in different cancers and that their prognostic value may depend on their relative, rather than individual, levels of expression.
The single worst prognostic indicator in cancer is the presence of distant metastases, which is associated with decreased therapeutic effectiveness and the vast majority of cancer-related deaths. Therefore, paxillin and Hic-5 represent attractive targets for anti-metastatic drug development as their inhibition not only prevents breast cancer cell plasticity but also impairs invasion, transendothelial migration, and metastasis.52 Furthermore, malignant cancer cells can often survive for extended time periods in a dormant state at distant secondary sites, only to begin proliferating long after treatment and removal of the primary tumor.150 Given the ability of paxillin family members to regulate cell survival, as well as invasion mechanisms, they may also represent viable candidates for therapeutics targeting against these elusive dormant tumor cell populations and thereby improve patient long-term survival.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Studies from the authors’ laboratory are supported by grants from the National Institute of Health, (NIH) GM47607 and CA163296 (C.E.T), as well as a Susan G. Komen for the Cure Postdoctoral Fellowship (N.O.D).
References
- 1. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673-87 [DOI] [PubMed] [Google Scholar]
- 3. Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84(4):1315-39 [DOI] [PubMed] [Google Scholar]
- 4. Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121(Pt 15):2435-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner CE. Paxillin interactions. J Cell Sci. 2000;113(Pt 23):4139-40 [DOI] [PubMed] [Google Scholar]
- 6. Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989;108(6):2401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turner CE, Glenney JR, Jr, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111(3):1059-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deakin NO, Ballestrem C, Turner CE. Paxillin and hic-5 interaction with vinculin is differentially regulated by Rac1 and RhoA. PLoS One. 2012;7(5):e37990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denhez F, Wilcox-Adelman SA, Baciu PC, et al. Syndesmos, a syndecan-4 cytoplasmic domain interactor, binds to the focal adhesion adaptor proteins paxillin and hic-5. J Biol Chem. 2002;277(14):12270-4 [DOI] [PubMed] [Google Scholar]
- 10. Hagel M, George EL, Kim A, et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 2002;22(3):901-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishino K, Kaneyama Shibanuma M, Nose K. Specific decrease in the level of hic-5, a focal adhesion protein, during immortalization of mouse embryonic fibroblasts, and its association with focal adhesion kinase. J Cell Biochem. 2000;76(3):411-9 [DOI] [PubMed] [Google Scholar]
- 12. Jones CA, Nishiya N, London NR, et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol. 2009;11(11):1325-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishiya N, Tachibana K, Shibanuma M, Mashimo JI, Nose K. Hic-5-reduced cell spreading on fibronectin: competitive effects between paxillin and hic-5 through interaction with focal adhesion kinase. Mol Cell Biol. 2001;21(16):5332-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishiya N, Shirai T, Suzuki W, Nose K. Hic-5 interacts with GIT1 with a different binding mode from paxillin. J Biochem. 2002;132(2):279-89 [DOI] [PubMed] [Google Scholar]
- 15. Nishiya N, Iwabuchi Y, Shibanuma M, Cote JF, Tremblay ML, Nose K. Hic-5, a paxillin homologue, binds to the protein-tyrosine phosphatase PEST (PTP-PEST) through its LIM 3 domain. J Biol Chem. 1999;274(14):9847-53 [DOI] [PubMed] [Google Scholar]
- 16. Rathore VB, Okada M, Newman PJ, Newman DK. Paxillin family members function as csk-binding proteins that regulate lyn activity in human and murine platelets. Biochem J. 2007;403(2):275-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turner CE, Brown MC, Perrotta JA, et al. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J Cell Biol. 1999;145(4):851-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shola DT, Wang H, Wahdan-Alaswad R, Danielpour D. Hic-5 controls BMP4 responses in prostate cancer cells through interacting with smads 1, 5 and 8. Oncogene. 2012;31(19):2480-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H, Song K, Sponseller TL, Danielpour D. Novel function of androgen receptor–associated protein 55/Hic-5 as a negative regulator of Smad3 signaling. J Biol Chem. 2005;280(7):5154-62 [DOI] [PubMed] [Google Scholar]
- 20. Sheibani N, Tang Y, Sorenson CM. Paxillin’s LD4 motif interacts with bcl-2. J Cell Physiol. 2008;214(3):655-61 [DOI] [PubMed] [Google Scholar]
- 21. Woods AJ, Roberts MS, Choudhary J, et al. Paxillin associates with poly(A)-binding protein 1 at the dense endoplasmic reticulum and the leading edge of migrating cells. J Biol Chem. 2002;277(8):6428-37 [DOI] [PubMed] [Google Scholar]
- 22. Woods AJ, Kantidakis T, Sabe H, Critchley DR, Norman JC. Interaction of paxillin with poly(A)-binding protein 1 and its role in focal adhesion turnover and cell migration. Mol Cell Biol. 2005;25(9):3763-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazaki Y, Hashimoto S, Sabe H. Monocyte cells and cancer cells express novel paxillin isoforms with different binding properties to focal adhesion proteins. J Biol Chem. 1997;272(11):7437-44 [DOI] [PubMed] [Google Scholar]
- 24. Salgia R, Li JL, Lo SH, et al. Molecular cloning of human paxillin, a focal adhesion protein phosphorylated by P210BCR/ABL. J Biol Chem. 1995;270(10):5039-47 [DOI] [PubMed] [Google Scholar]
- 25. Yuminamochi T, Yatomi Y, Osada M, et al. Expression of the LIM proteins paxillin and hic-5 in human tissues. J Histochem Cytochem. 2003;51(4):513-21 [DOI] [PubMed] [Google Scholar]
- 26. Kim-Kaneyama JR, Suzuki W, Ichikawa K, et al. Uni-axial stretching regulates intracellular localization of hic-5 expressed in smooth-muscle cells in vivo. J Cell Sci. 2005;118(Pt 5):937-49 [DOI] [PubMed] [Google Scholar]
- 27. Shibanuma M, Mashimo J, Kuroki T, Nose K. Characterization of the TGF beta 1–inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J Biol Chem. 1994;269(43):26767-74 [PubMed] [Google Scholar]
- 28. Tumbarello DA, Brown MC, Hetey SE, Turner CE. Regulation of paxillin family members during epithelial-mesenchymal transformation: a putative role for paxillin delta. J Cell Sci. 2005;118(Pt 20):4849-63 [DOI] [PubMed] [Google Scholar]
- 29. Lipsky BP, Beals CR, Staunton DE. Leupaxin is a novel LIM domain protein that forms a complex with PYK2. J Biol Chem. 1998;273(19):11709-13 [DOI] [PubMed] [Google Scholar]
- 30. Kaulfuss S, Grzmil M, Hemmerlein B, et al. Leupaxin, a novel coactivator of the androgen receptor, is expressed in prostate cancer and plays a role in adhesion and invasion of prostate carcinoma cells. Mol Endocrinol. 2008;22(7):1606-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sahu SN, Khadeer MA, Robertson BW, Nunez SM, Bai G, Gupta A. Association of leupaxin with src in osteoclasts. Am J Physiol Cell Physiol. 2007;292(1):C581-90 [DOI] [PubMed] [Google Scholar]
- 32. Sundberg-Smith LJ, DiMichele LA, Sayers RL, Mack CP, Taylor JM. The LIM protein leupaxin is enriched in smooth muscle and functions as a serum response factor cofactor to induce smooth muscle cell gene transcription. Circ Res. 2008;102(12):1502-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim-Kaneyama JR, Takeda N, Sasai A, et al. Hic-5 deficiency enhances mechanosensitive apoptosis and modulates vascular remodeling. J Mol Cell Cardiol. 2011;50(1):77-86 [DOI] [PubMed] [Google Scholar]
- 34. Azuma K, Tanaka M, Uekita T, et al. Tyrosine phosphorylation of paxillin affects the metastatic potential of human osteosarcoma. Oncogene. 2005;24(30):4754-64 [DOI] [PubMed] [Google Scholar]
- 35. Bhattacharjee A, Richards WG, Staunton J, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A. 2001;98(24):13790-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cui W, Wang X, Liu YC, Wan YL, Guo HJ, Zhu J. Expression of HIC-5/ARA55 in colonrectal cancer and its mechanisms of action. Beijing Da Xue Xue Bao. 2006;38(3):280-3 [PubMed] [Google Scholar]
- 37. Farmer P, Bonnefoi H, Becette V, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24(29):4660-71 [DOI] [PubMed] [Google Scholar]
- 38. Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518-27 [DOI] [PubMed] [Google Scholar]
- 39. Kanteti R, Yala S, Ferguson MK, Salgia R. MET, HGF, EGFR, and PXN gene copy number in lung cancer using DNA extracts from FFPE archival samples and prognostic significance. J Environ Pathol Toxicol Oncol. 2009;28(2):89-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mackinnon AC, Tretiakova M, Henderson L, et al. Paxillin expression and amplification in early lung lesions of high-risk patients, lung adenocarcinoma and metastatic disease. J Clin Pathol. 2011;64(1):16-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mestayer C, Blanchere M, Jaubert F, Dufour B, Mowszowicz I. Expression of androgen receptor coactivators in normal and cancer prostate tissues and cultured cell lines. Prostate. 2003;56(3):192-200 [DOI] [PubMed] [Google Scholar]
- 42. Miyoshi Y, Ishiguro H, Uemura H, et al. Expression of AR associated protein 55 (ARA55) and androgen receptor in prostate cancer. Prostate. 2003;56(4):280-6 [DOI] [PubMed] [Google Scholar]
- 43. Nagata M, Fujita H, Ida H, et al. Identification of potential biomarkers of lymph node metastasis in oral squamous cell carcinoma by cDNA microarray analysis. Int J Cancer. 2003;106(5):683-9 [DOI] [PubMed] [Google Scholar]
- 44. Radvanyi L, Singh-Sandhu D, Gallichan S, et al. The gene associated with trichorhinophalangeal syndrome in humans is overexpressed in breast cancer. Proc Natl Acad Sci U S A. 2005;102(31):11005-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Richardson AL, Wang ZC, De Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121-32 [DOI] [PubMed] [Google Scholar]
- 46. Rodina A, Schramm K, Musatkina E, Kreuser ED, Tavitian A, Tatosyan A. Phosphorylation of p125FAK and paxillin focal adhesion proteins in src-transformed cells with different metastatic capacity. FEBS Lett. 1999;455(1-2):145-8 [DOI] [PubMed] [Google Scholar]
- 47. Salgia R, Li JL, Ewaniuk DS, et al. Expression of the focal adhesion protein paxillin in lung cancer and its relation to cell motility. Oncogene. 1999;18(1):67-77 [DOI] [PubMed] [Google Scholar]
- 48. Shi J, Wang S, Zhao E, Shi L, Xu X, Fang M. Paxillin expression levels are correlated with clinical stage and metastasis in salivary adenoid cystic carcinoma. J Oral Pathol Med. 2010;39(7):548-51 [DOI] [PubMed] [Google Scholar]
- 49. Turashvili G, Bouchal J, Baumforth K, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang HJ, Chen JZ, Zhang WL, Ding YQ. Focal adhesion plaque associated cytoskeletons are involved in the invasion and metastasis of human colorectal carcinoma. Cancer Invest. 2010;28(2):127-34 [DOI] [PubMed] [Google Scholar]
- 51. Zhao H, Langerod A, Ji Y, et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15(6):2523-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deakin NO, Turner CE. Distinct roles for paxillin and hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol Biol Cell. 2011;22(3):327-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanaka T, Moriwaki K, Murata S, Miyasaka M. LIM domain-containing adaptor, leupaxin, localizes in focal adhesion and suppresses the integrin-induced tyrosine phosphorylation of paxillin. Cancer Sci. 2010;101(2):363-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jagadeeswaran R, Surawska H, Krishnaswamy S, et al. Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res. 2008;68(1):132-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim MS, Yoo NJ, Lee SH. Absence of paxillin gene mutation in lung cancer and other common solid cancers. Tumori. 2011;97(2):211-3 [DOI] [PubMed] [Google Scholar]
- 56. Pallier K, Houllier AM, Le Corre D, Cazes A, Laurent-Puig P, Blons H. No somatic genetic change in the paxillin gene in nonsmall-cell lung cancer. Mol Carcinog. 2009;48(7):581-5 [DOI] [PubMed] [Google Scholar]
- 57. Fujimoto N, Mizokami A, Harada S, Matsumoto T. Different expression of androgen receptor coactivators in human prostate. Urology. 2001;58(2):289-94 [DOI] [PubMed] [Google Scholar]
- 58. Short SM, Yoder BJ, Tarr SM, et al. The expression of the cytoskeletal focal adhesion protein paxillin in breast cancer correlates with HER2 overexpression and may help predict response to chemotherapy: a retrospective immunohistochemical study. Breast J. 2007;13(2):130-9 [DOI] [PubMed] [Google Scholar]
- 59. Vadlamudi R, Adam L, Talukder A, Mendelsohn J, Kumar R. Serine phosphorylation of paxillin by heregulin-beta1: role of p38 mitogen activated protein kinase. Oncogene. 1999;18(51):7253-64 [DOI] [PubMed] [Google Scholar]
- 60. Vadlamudi R, Adam L, Tseng B, Costa L, Kumar R. Transcriptional up-regulation of paxillin expression by heregulin in human breast cancer cells. Cancer Res. 1999;59(12):2843-6 [PubMed] [Google Scholar]
- 61. Yu JA, Deakin NO, Turner CE. Paxillin-kinase-linker tyrosine phosphorylation regulates directional cell migration. Mol Biol Cell. 2009;20(22):4706-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hetey SE, Lalonde DP, Turner CE. Tyrosine-phosphorylated hic-5 inhibits epidermal growth factor-induced lamellipodia formation. Exp Cell Res. 2005;311(1):147-56 [DOI] [PubMed] [Google Scholar]
- 63. Li X, Martinez-Ferrer M, Botta V, Uwamariya C, Banerjee J, Bhowmick NA. Epithelial hic-5/ARA55 expression contributes to prostate tumorigenesis and castrate responsiveness. Oncogene. 2011;30(2):167-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ren Y, Meng S, Mei L, Zhao ZJ, Jove R, Wu J. Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and src activation in response to epidermal growth factor. J Biol Chem. 2004;279(9):8497-505 [DOI] [PubMed] [Google Scholar]
- 65. Tatsumi Y, Cho YY, He Z, et al. Involvement of the paxillin pathway in JB6 Cl41 cell transformation. Cancer Res. 2006;66(11):5968-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tumbarello DA, Turner CE. Hic-5 contributes to epithelial-mesenchymal transformation through a RhoA/ROCK-dependent pathway. J Cell Physiol. 2007;211(3):736-47 [DOI] [PubMed] [Google Scholar]
- 67. Zhao Y, Zhang X, Guda K, et al. Identification and functional characterization of paxillin as a target of protein tyrosine phosphatase receptor T. Proc Natl Acad Sci U S A. 2010;107(6):2592-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65(14):5996-6000; discussion 6000-1. [DOI] [PubMed] [Google Scholar]
- 69. Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65(14):5991-5; discussion 5995. [DOI] [PubMed] [Google Scholar]
- 70. Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818-29 [DOI] [PubMed] [Google Scholar]
- 71. Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23(43):7274-82 [DOI] [PubMed] [Google Scholar]
- 72. Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313-23 [DOI] [PubMed] [Google Scholar]
- 73. Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396-401 [DOI] [PubMed] [Google Scholar]
- 74. Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764-74 [DOI] [PubMed] [Google Scholar]
- 75. Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. Epub 2012. April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-beta-induced epithelial-mesenchymal transition. J Cell Biol. 2012;197(3):421-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nakamura K, Yano H, Uchida H, Hashimoto S, Schaefer E, Sabe H. Tyrosine phosphorylation of paxillin alpha is involved in temporospatial regulation of paxillin-containing focal adhesion formation and F-actin organization in motile cells. J Biol Chem. 2000;275(35):27155-64 [DOI] [PubMed] [Google Scholar]
- 79. Dabiri G, Tumbarello DA, Turner CE, Van de, Water L. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop. J Invest Dermatol. 2008;128(10):2518-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125(Pt 3):724-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kelly T, Mueller SC, Yeh Y, Chen WT. Invadopodia promote proteolysis of a wide variety of extracellular matrix proteins. J Cell Physiol. 1994;158(2):299-308 [DOI] [PubMed] [Google Scholar]
- 82. Tolde O, Rosel D, Vesely P, Folk P, Brabek J. The structure of invadopodia in a complex 3D environment. Eur J Cell Biol. 2010;89(9):674-80 [DOI] [PubMed] [Google Scholar]
- 83. Kelly T, Yan Y, Osborne RL, et al. Proteolysis of extracellular matrix by invadopodia facilitates human breast cancer cell invasion and is mediated by matrix metalloproteinases. Clin Exp Metastasis. 1998;16(6):501-12 [DOI] [PubMed] [Google Scholar]
- 84. Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12(7):413-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu Rev Cell Dev Biol. 2011;27:185-211 [DOI] [PubMed] [Google Scholar]
- 86. Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18(31):4440-9 [DOI] [PubMed] [Google Scholar]
- 87. Mueller SC, Yeh Y, Chen WT. Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J Cell Biol. 1992;119(5):1309-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Onodera Y, Hashimoto S, Hashimoto A, et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005;24(5):963-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Badowski C, Pawlak G, Grichine A, et al. Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization. Mol Biol Cell. 2008;19(2):633-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gupta A, Lee BS, Khadeer MA, et al. Leupaxin is a critical adaptor protein in the adhesion zone of the osteoclast. J Bone Miner Res. 2003;18(4):669-85 [DOI] [PubMed] [Google Scholar]
- 91. Sahu SN, Nunez S, Bai G, Gupta A. Interaction of Pyk2 and PTP-PEST with leupaxin in prostate cancer cells. Am J Physiol Cell Physiol. 2007;292(6):C2288-96 [DOI] [PubMed] [Google Scholar]
- 92. Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28(1-2):137-49 [DOI] [PubMed] [Google Scholar]
- 93. Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582(14):2093-101 [DOI] [PubMed] [Google Scholar]
- 94. Bellovin DI, Simpson KJ, Danilov T, et al. Reciprocal regulation of RhoA and RhoC characterizes the EMT and identifies RhoC as a prognostic marker of colon carcinoma. Oncogene. 2006;25(52):6959-67 [DOI] [PubMed] [Google Scholar]
- 95. Dietrich KA, Schwarz R, Liska M, et al. Specific induction of migration and invasion of pancreatic carcinoma cells by RhoC, which differs from RhoA in its localisation and activity. Biol Chem. 2009;390(10):1063-77 [DOI] [PubMed] [Google Scholar]
- 96. Kleer CG, Griffith KA, Sabel MS, et al. RhoC-GTPase is a novel tissue biomarker associated with biologically aggressive carcinomas of the breast. Breast Cancer Res Treat. 2005;93(2):101-10 [DOI] [PubMed] [Google Scholar]
- 97. Kleer CG, Teknos TN, Islam M, et al. RhoC GTPase expression as a potential marker of lymph node metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2006;12(15):4485-90 [DOI] [PubMed] [Google Scholar]
- 98. Shikada Y, Yoshino I, Okamoto T, Fukuyama S, Kameyama T, Maehara Y. Higher expression of RhoC is related to invasiveness in non-small cell lung carcinoma. Clin Cancer Res. 2003;9(14):5282-6 [PubMed] [Google Scholar]
- 99. Wang W, Yang LY, Huang GW, et al. Genomic analysis reveals RhoC as a potential marker in hepatocellular carcinoma with poor prognosis. Br J Cancer. 2004;90(12):2349-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406(6795):532-5 [DOI] [PubMed] [Google Scholar]
- 101. Hakem A, Sanchez-Sweatman O, You-Ten A, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19(17):1974-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Simpson KJ, Dugan AS, Mercurio AM. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 2004;64(23):8694-701 [DOI] [PubMed] [Google Scholar]
- 103. Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193(4):655-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol. 2011;21(8):635-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. LaLonde DP, Grubinger M, Lamarche-Vane N, Turner CE. CdGAP associates with actopaxin to regulate integrin-dependent changes in cell morphology and motility. Curr Biol. 2006;16(14):1375-85 [DOI] [PubMed] [Google Scholar]
- 106. Kaulfuss S, von Hardenberg S, Schweyer S, et al. Leupaxin acts as a mediator in prostate carcinoma progression through deregulation of p120catenin expression. Oncogene. 2009;28(45):3971-82 [DOI] [PubMed] [Google Scholar]
- 107. Carragher NO, Walker SM, Scott Carragher LA, et al. Calpain 2 and src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene. 2006;25(42):5726-40 [DOI] [PubMed] [Google Scholar]
- 108. Friedl P, Noble PB, Zanker KS. T lymphocyte locomotion in a three-dimensional collagen matrix: expression and function of cell adhesion molecules. J Immunol. 1995;154(10):4973-85 [PubMed] [Google Scholar]
- 109. Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188(1):11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gadea G, Sanz-Moreno V, Self A, Godi A, Marshall CJ. DOCK10-mediated Cdc42 activation is necessary for amoeboid invasion of melanoma cells. Curr Biol. 2008;18(19):1456-65 [DOI] [PubMed] [Google Scholar]
- 111. Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11(11):1287-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Parri M, Taddei ML, Bianchini F, Calorini L, Chiarugi P. EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res. 2009;69(5):2072-81 [DOI] [PubMed] [Google Scholar]
- 113. Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5(8):711-9 [DOI] [PubMed] [Google Scholar]
- 114. Sanz-Moreno V, Gadea G, Ahn J, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135(3):510-23 [DOI] [PubMed] [Google Scholar]
- 115. Wolf K, Mazo I, Leung H, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160(2):267-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wolf K, Wu YI, Liu Y, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9(8):893-904 [DOI] [PubMed] [Google Scholar]
- 117. Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16(15):1515-23 [DOI] [PubMed] [Google Scholar]
- 118. Friedl P, Wolf K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem Soc Symp. 2003;70(70):277-85 [DOI] [PubMed] [Google Scholar]
- 119. Yoshida K, Soldati T. Dissection of amoeboid movement into two mechanically distinct modes. J Cell Sci. 2006;119(Pt 18):3833-44 [DOI] [PubMed] [Google Scholar]
- 120. Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387-92 [DOI] [PubMed] [Google Scholar]
- 121. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3(5):362-74 [DOI] [PubMed] [Google Scholar]
- 122. Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2(9):657-72 [DOI] [PubMed] [Google Scholar]
- 123. Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19(56):6642-50 [DOI] [PubMed] [Google Scholar]
- 124. Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563-72 [DOI] [PubMed] [Google Scholar]
- 125. Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45(4):773-82 [PubMed] [Google Scholar]
- 126. Mori S, Chang JT, Andrechek ER, et al. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009;28(31):2796-805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vande Pol SB, Brown MC, Turner CE. Association of bovine papillomavirus type 1 E6 oncoprotein with the focal adhesion protein paxillin through a conserved protein interaction motif. Oncogene. 1998;16(1):43-52 [DOI] [PubMed] [Google Scholar]
- 128. Chen YC, Chen JH, Richard K, Chen PY, Christiani DC. Lung adenocarcinoma and human papillomavirus infection. Cancer. 2004;101(6):1428-36 [DOI] [PubMed] [Google Scholar]
- 129. Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24(17):2606-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ganguly N, Parihar SP. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J Biosci. 2009;34(1):113-23 [DOI] [PubMed] [Google Scholar]
- 131. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129-36 [DOI] [PubMed] [Google Scholar]
- 132. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12-9 [DOI] [PubMed] [Google Scholar]
- 133. Sachdev S, Bu Y, Gelman IH. Paxillin-Y118 phosphorylation contributes to the control of src-induced anchorage-independent growth by FAK and adhesion. BMC Cancer. 2009;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Salgia R, Brunkhorst B, Pisick E, et al. Increased tyrosine phosphorylation of focal adhesion proteins in myeloid cell lines expressing p210BCR/ABL. Oncogene. 1995;11(6):1149-55 [PubMed] [Google Scholar]
- 135. Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165(3):371-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zouq NK, Keeble JA, Lindsay J, et al. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci. 2009;122(Pt 3):357-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134(3):793-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lin HM, Lee YJ, Li G, Pestell RG, Kim HR. Bcl-2 induces cyclin D1 promoter activity in human breast epithelial cells independent of cell anchorage. Cell Death Differ. 2001;8(1):44-50 [DOI] [PubMed] [Google Scholar]
- 139. Mori K, Hirao E, Toya Y, et al. Competitive nuclear export of cyclin D1 and hic-5 regulates anchorage dependence of cell growth and survival. Mol Biol Cell. 2009;20(1):218-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ, Smoyer WE. Identification and characterization of hic-5/ARA55 as an hsp27 binding protein. J Biol Chem. 2001;276(43):39911-8 [DOI] [PubMed] [Google Scholar]
- 141. Fujita H, Kamiguchi K, Cho D, Shibanuma M, Morimoto C, Tachibana K. Interaction of hic- 5, a senescence-related protein, with focal adhesion kinase. J Biol Chem. 1998;273(41):26516-21 [DOI] [PubMed] [Google Scholar]
- 142. Gao ZL, Deblis R, Glenn H, Schwartz LM. Differential roles of HIC-5 isoforms in the regulation of cell death and myotube formation during myogenesis. Exp Cell Res. 2007;313(19):4000-14 [DOI] [PubMed] [Google Scholar]
- 143. Hornigold N, Craven RA, Keen JN, Johnson T, Banks RE, Mooney AF. Upregulation of hic-5 in glomerulosclerosis and its regulation of mesangial cell apoptosis. Kidney Int. 2010;77(4):329-38 [DOI] [PubMed] [Google Scholar]
- 144. Kim G, Davidson B, Henning R, et al. Adhesion molecule protein signature in ovarian cancer effusions is prognostic of patient outcome. Cancer. 2012;118(6):1543-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Madan R, Smolkin MB, Cocker R, Fayyad R, Oktay MH. Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum Pathol. 2006;37(1):9-15 [DOI] [PubMed] [Google Scholar]
- 146. Li HG, Xie DR, Shen XM, Li HH, Zeng H, Zeng YJ. Clinicopathological significance of expression of paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World J Gastroenterol. 2005;11(10):1445-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Li BZ, Lei W, Zhang CY, et al. Increased expression of paxillin is found in human oesophageal squamous cell carcinoma: a tissue microarray study. J Int Med Res. 2008;36(2):273-8 [DOI] [PubMed] [Google Scholar]
- 148. Wu DW, Cheng YW, Wang J, Chen CY, Lee H. Paxillin predicts survival and relapse in non-small cell lung cancer by microRNA-218 targeting. Cancer Res. 2010;70(24):10392-401 [DOI] [PubMed] [Google Scholar]
- 149. Wikstrom P, Marusic J, Stattin P, Bergh A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69(8):799-809 [DOI] [PubMed] [Google Scholar]
- 150. Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proc Natl Acad Sci U S A. 2011;108(30):12396-400 [DOI] [PMC free article] [PubMed] [Google Scholar]