Abstract
The nonreceptor tyrosine kinases Abl and Arg are among the most well-characterized tyrosine kinases in the human genome. The activation of Abl by N-terminal fusions with Bcr (Bcr-Abl) or Gag (v-Abl) is responsible for chronic myeloid leukemia or Ph+ acute lymphoblastic leukemia and mouse leukemia virus, respectively. In addition, aberrant Abl and Arg activation downstream of several oncogenic growth factor receptors contributes to the development and progression of a variety of human cancers, often associated with poor clinical outcome, drug resistance, and tumor invasion and metastasis. Abl activation can occur by a variety of mechanisms that include domain interactions involving structural remodeling of autoinhibited conformations as well as direct phosphorylation by upstream kinases and phosphatases. Constitutive activation of Abl plays a significant role in regulating the actin cytoskeleton by modulating cell adhesion, motility, and invadopodia. This review addresses the role of Abl and Arg in tumor progression with particular emphasis on the roles of Crk and Abi1 adapter proteins as distinct molecular switches for Abl transactivation. These insights, combined with new insights into the structure of these kinases, provide the rationale to envision that Crk and Abi1 fine-tune Abl regulation to control signaling to the cytoskeleton.
Keywords: Crk, Abi1, Abl kinase, cytoskeleton, WAVE complex
Introduction
Since the discovery of adaptor proteins more than 20 years ago, there have been remarkable advances in the field of signal transduction, most notably from the realization that signaling proteins possess protein-protein and protein-lipid interacting domains that permit the assembly of large multiprotein complexes.1-3 Indeed, the “protein-protein interactome” has taken center stage in signal transduction, with the daunting challenges now to understand how complex signaling assemblages are regulated in time and space.4,5 Many of these assemblages are further regulated by protein posttranslational interactions, most notably by phosphorylation, adding complexity with respect to the regulation of interactomes but also clinical relevance in cancer as both the level of tyrosine phosphorylation and the expression of adaptor proteins are dysregulated in cancer.6,7 In this review, we discuss how the Crk and Abi1 adaptor proteins interact with Abl and propose a binary mechanism for Abl regulation under both physiological conditions and during cancer progression.
Abl and Arg Kinases
The nonreceptor tyrosine kinases, Abl and Abl-related gene (Arg), control several physiological processes for both development and tissue homeostasis.8,9 Homozygous deletions of both Abl and Arg (Abl/Arg DKO) die embryonically at late gestation accompanied by cardiovascular lesions and hemorrhages, and neurulation defects both linked to alterations in the actin cytoskeleton.10 The knockout of Abl in an Arg-sufficient background has a milder phenotype than the Abl/Arg DKO but displays defects associated with deficient T-cell receptor signaling, manifesting thymic atrophy, leukopenia, and sensitivity to infections.11,12 In addition to controlling actin-dependent morphogenetic programs during embryogenesis and physiological responses in somatic cells, Abl is important for the pathogenesis and virulence determinants of Helicobacter pylori 13,14 and Shigella flexneri.15,16 On the other hand, gain-of-function–activated species of Abl and Arg are implicated in tumor invasion and progression in a variety of cancers, particularly to the progression to metastatic disease, making them attractive targets for selective anticancer therapies.
Oncogenic Roles for Abl in Leukemia and Solid Cancer
Abl was first recognized as an oncogene encoded from the acutely transforming Abelson murine leukemia virus (v-Abl or Gag-Abl) that could directly transform both hematopoietic cells and NIH3T3 cells in culture.17 Animals infected with Mo-MuLV developed multiple tumors of the lymph nodes with bone marrow infiltration.18 The disease phenotype and progression of MuLV are reminiscent, but not identical, of the disease phenotype in chronic myeloid leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL), which is mediated by an independent N-terminal fusion of Bcr to Abl.19
In contrast to CML and Ph+ ALL where Abl is a driver mutation, in solid cancers such as melanoma,20,21 non–small cell lung cancer,22 colorectal cancer,23 and breast cancer,24-26 Abl is activated downstream of activated growth factor receptors, as outlined by Plattner in this monograph. The mechanisms by which growth factor receptors activate Abl are multifactorial. In the case of PDGFRα and ErbB2, Abl can bind directly to the phosphorylated receptors via the Abl SH2 domain, causing SH2 domain replacement, that results in phosphorylation of the activation loop of the Abl kinase domain (see below).27 Abl can reciprocally phosphorylate receptor tyrosine kinases in their cytoplasmic tails, leading to reinforced downstream signaling from pre-existing activated RTKs.28,29 A second and perhaps more physiological manner for growth factor–induced activation of Abl occurs indirectly and requires at least 2 types of receptor-activated signaling molecules. First, Src kinases that directly associate with PDGFR and become activated30, phosphorylate the activation loop of Abl and at a second site in the linker between the SH2 domain and the kinase domain to activate Abl. Second, Abl activation requires PLC-γ–dependent hydrolysis of PIP2; the latter serves as an inhibitor for Abl.31,32 The importance of Abl in PDGFa signaling is highlighted in that PDGF-inducible dorsal ruffling is defective in Abl/Arg DKO fibroblasts and can be rescued by re-expression of either Abl or Arg.27,33,34 This latter model is also reminiscent of the model of Abl activation downstream of TCR signaling in lymphocytes, where Abl activation requires Lck and its substrate ZAP70.35,36 While the necessity of Src for RTK-mediated Abl activation diversifies the signaling paradigm of RTK activation, the contribution of direct activation and indirect activation is not fully understood.
Dual Structures of Abl and Complex Interplay between Closed and Open Configurations
Although the regulation of Abl is clearly complex, X-ray crystallography models elegantly show that under physiological conditions, Abl activity is negatively regulated by multiple structural-dependent autoinhibitory mechanisms. In fact, autoinhibition has emerged as the mechanism of regulation for most, if not all, nonreceptor tyrosine kinases and particularly well described for c-Src and c-Abl, which are noted at the level of X-ray crystallography analysis37,38 and confirmed by rationale mutagenesis that unlocks key regulatory elements (see Hantschel in this issue). Nonreceptor tyrosine kinases share considerable structural homology conferred by the presence of the highly conserved structural domains of SH3 and SH2, which are positioned to tether against the catalytic kinase domain. Such 3-dimensional structures of c-Src39,40 and c-Abl41,42 revealed that the SH3 and SH2 domains bind to the catalytic domain, inducing an autoinhibitory conformation, which provides the basic mechanism of regulation of these kinases.
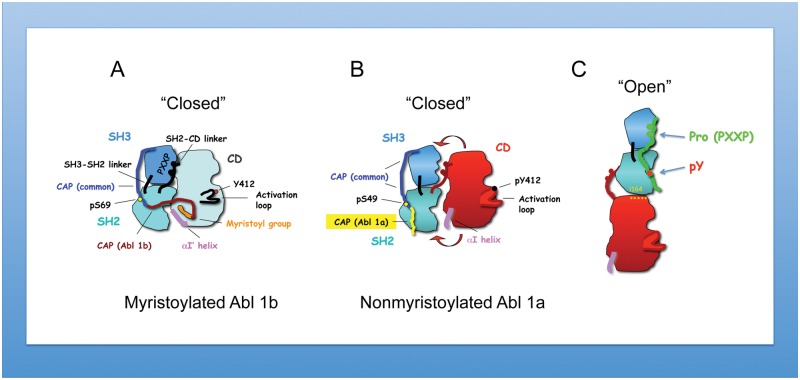
While both Src and Abl kinases are controlled by autoinhibition, they in fact differ from each other in their inhibitory mechanisms. For c-Src, inhibition is achieved by intramolecular interaction of the SH2 domain with the phosphorylated tyrosine 527 located in the C-terminal region.43 In contrast, there is no internal phosphotyrosine–SH2 domain interaction in Abl, precluding this inhibitory mechanism. Instead, important inhibitory constraints are imposed on c-Abl both by the myristoylated cap and SH3 linker interactions (the latter are shared with Src). Adding complexity to this theme, through alternative splicing of exon 1, 2 N-terminal Abl variants can arise (myristoylated Abl 1b v. nonmyristoylated Abl 1a that lacks the sequence required for myristoylation through alternative splicing) (Fig. 1A and 1B). Differences in kinase regulation arise as the myristate moiety binds directly to the C-terminal lobe of the kinase domain and by the cap region phosphoserine 69, which binds to the SH2 domain.44 These interactions further serve to lock the SH3-SH2 “clamp” onto the catalytic domain, predicting that Abl 1a is partially active or more activatable by SH2 ligands, which might lead to the “open” conformation44,45 more easily in Abl 1a than Abl 1b (Fig. 1C). Importantly, based on structural studies, in the elongated structure, integrity of the Abl SH2 domain–catalytic domain interaction is critical for maintaining Abl kinase activity.45 In the “closed” Abl 1b form, SH2 domain binding to phosphopeptides prevents Abl kinase inhibition by the myristoyl group in cis.41 The myristoyl group binding in trans,41,42 or small compounds mimicking its action,46,47 stabilizes the position of the C-terminal helix of the catalytic domain, αI, resulting in the inhibited conformation of the kinase. Therapeutically, this observation may have clinical value. An improved CML therapeutic compound called GNF-5 (or its subsequent generations) acts synergistically with imatinib or nilotinib to inhibit the Abl mutation, T315I, thus offering treatment of this imatinib-resistant mutation of BCR-Abl.48 Taking into consideration the increasing role of Abl dysregulation in solid cancers, it will be important to understand the clinical significance of Abl kinase isoforms and their sensitivities to anti-Abl compounds.
Figure 1.
Structural differences of myristoylated versus nonmyristoylated Abl kinase. (A) Regulated structure of myristoylated c-Abl with major elements of autoinhibition as determined by the crystal structure41,42: The SH3 domain interacts with the SH2–catalytic domain (CD) linker and CD; the SH2 domain interacts with CD. In the myristoylated form of the kinase, the C-terminal helix of CD, αI′, forms a binding pocket for myristates. Activation of c-Abl by several mechanisms, which include the disassociation of myristates,41,42 leads to phosphorylation of the SH2–CD linker and activation loop. These events result in uncoupling of SH2 and SH3 domains from the backside of CD. The C-terminal helix (αI) partially occludes the SH2 phosphotyrosine binding site. In this regard, Hantschel et al.41 propose the activation of myristoylated Abl by phosphotyrosine peptides binding to the SH2 domain. The “closed” structure of myristoylated Abl is incompatible with inhibition by Abi1. (B and C) Nonmyristoylated Abl 1a is likely to be less stable than myristoylated Abl (depicted by red arrows) because of the lack of the stabilizing interaction of the myristate with the CD (as this interaction provides an additional “lock,” maintaining the autoinhibitory interactions of SH3-SH2 domains with CD). Hence, the nonmyristoylated Abl is more prone than the myristoylated Abl isoform to assume the “open” (C) elongated conformation proposed by Nagar et al.44 and later studied by Filippakopoulos et al.45 The possibility of a ligand binding (such as containing proline motif consensus, PXXP, and phosphotyrosine -pY) to the open confirmation is indicated.
Modulation of Abl Activity by Abi1 and Crk
As described above, the SH2 and SH3 domains of Abl either maintain the autoinhibitory unit or facilitate their interaction with a wide range of adaptors and signaling proteins. When engaged to their respective ligands, this essentially unlocks the autoinhibited conformations, altering kinase activity and downstream tyrosine phosphorylation. While the nature of these interactions is complex and has been described in other excellent reviews in this monograph, here, we focus on the Crk and Abi1 proteins as an example of complexity in the regulation of Abl kinases and illustrate how stoichiometry may be controlled by substrate availability. As noted below, both Abi1 and Crk possess SH3 domains that bind to the Abl proline-rich domain (PRD) and appear to compete with each other to bind Abl.
Abi1
Abi1, together with Abi2 and Abi3 (also known as NESH), are a family of adaptor/scaffolding proteins that are originally characterized as Abl interactor proteins.49-52 The structure of Abi proteins is also generally conserved, characterized by 1 well-distinguished domain, the SH3 domain located in the C-terminus that binds type II proline peptides, including the PRD of Abl (Fig. 2). Juxtaposed to the SH3 domain as well as throughout the middle of Abi1, several proline-rich sequences including PEST and PXXP sequences are intercepted by tyrosine residues that are subject to posttranslational modifications. Relevant to the discussions here, several of these tyrosine residues are phosphorylated by Abl, which can potentially bind proteins with SH2 domains, including the Abl SH2 itself (Fig. 2)53 and SH2 domains of other kinases such as p85–PI3 kinase and Src, which indirectly transmit signals downstream from Abl.54
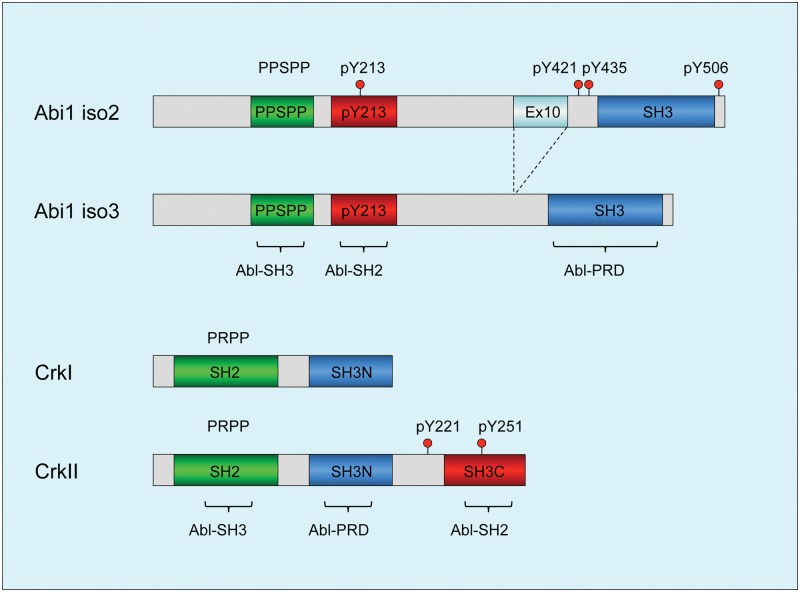
Figure 2.
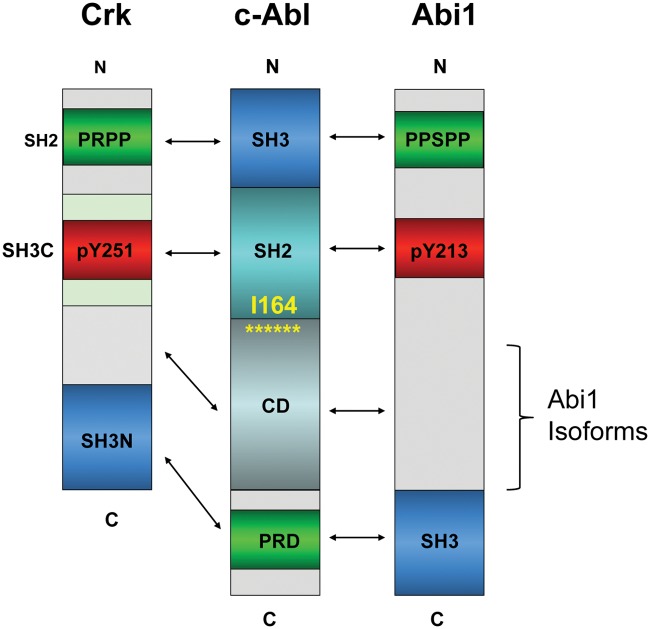
Diagrams depicting structural determinants of Abi1 and Crk: functional domains of Abi1 and Crk that regulate c-Abl tyrosine kinase. (Top) Two major isoforms of Abi1, isoform 2 and isoform 3,52,92 and key determinants for Abl kinase regulation are depicted within the highlighted boxes: the c-Abl–SH3 domain binding region 185-PPSPP-189; pY213 that binds to the SH2 domain,53 and the SH3 domain that binds to the Abl proline-rich linker. Phosphotyrosine residues pY421, pY435, and pY506 that bind to the p85 regulatory subunit of PI3 kinase through SH2 domain binding of p8554 are depicted above the boxes. pY421 also targets Src family tyrosine kinases, and pY213 targets the C-terminal SH2 domain of p85.54 (Bottom) Primary structure of CrkI and Crk II, with the SH2, SH3N, and SH3C domains depicted within boxes. Phosphotyrosine residues implicated in Abl kinase regulation: pY221 and pY251.61,93 Phosphotyrosine pY207 that binds to the Crk SH2 domain to form an autoinhibited structure in CrkL59 (not shown). Abl kinase regulatory domain binding regions are indicated in brackets.
Intriguingly, the proximity of pY213 (Abl SH2) and PPSPP (Abl SH3) sequences on Abi1, along with SH3 domain binding to the PRD of Abl (this possibility was demonstrated for the conserved Abi2–SH3 domain),55 predicts that Abi1 can interact with Abl using 3 independent motifs and when tyrosine phosphorylated would comprise a consolidated ligand-Abl SH3-SH2 domain binding surface possibly to act as a co-regulator of Abl function at multiple levels that operate cooperatively (Fig. 2). Cooperativity of the Abl SH3-SH2 interaction with ligands was previously suggested by Cowburn et al.56
CT10 Regulator of Kinase: Crk
Crk, together with CrkL, is a family of adaptor proteins that lack tyrosine kinase activity but transmit intracellular signals downstream of tyrosine kinases. In vertebrates, Crk is alternatively spliced to produce 2 variants, crk I, composed of a SH2 and a SH3 domain, and a more abundant variant called crk II, which has an additional C-terminal SH3 domain and a 50–amino acid linker between the SH3 domains.57 Crk-like (crk-L) is encoded by a distinct gene as crk and is structurally most similar to Crk II, although despite approximately 80% homology in the protein sequence in the SH domains, they have distinct nonoverlapping roles in development, reflected by the fact that knockouts are either embryonic (CrkL) or perinatal (Crk) lethal. Recent studies by Kalodimos and Inagaki58 revealed fascinating distinctions in the SH2 and SH3 domain organizations between Crk II and CrkL, the latter acting as a preferred substrate to the Bcr-Abl oncoprotein.59 Unexpectedly, these differences can be attributed to the distinct folding properties of Crk II and CrkL, mediated in large part by the interdomain regulatory elements.
At the functional level, the tandem SH2 and SH3 domains in Crk and CrkL recruit tyrosine-phosphorylated proteins and proline-rich proteins, respectively, and relevant to the arguments here, like Abi1, the first Crk SH3 domain (SH3N) binds to the PRD of Abl. While Abl has multiple PXXP motifs in its PRD, Antoku and Mayer60 elegantly showed that they each contain sequence-specific information to bind distinct subsets of SH3 domains, although a comparison of the sequences for Abi1 and Crk was not directly investigated. Interestingly, in the study by Jung et al.,55 these investigators found that phosphorylation of Abl by PAK2 stimulated the tyrosine kinase activity of Abl in part by blocking the interaction with Abi1 and enhancing the interaction with Crk. In agreement with this model, as shown in Figure 3, Crk acts as a bona fide competitive inhibitor for Abi1 binding to Abl. This clearly supports the idea that Crk and Abi1 are in dynamic equilibrium for Abl, which will likely depend on 1) the concentration of Crk versus Abi1 in the cell, 2) their posttranslational modifications, and 3) the subcellular localization of Abl.
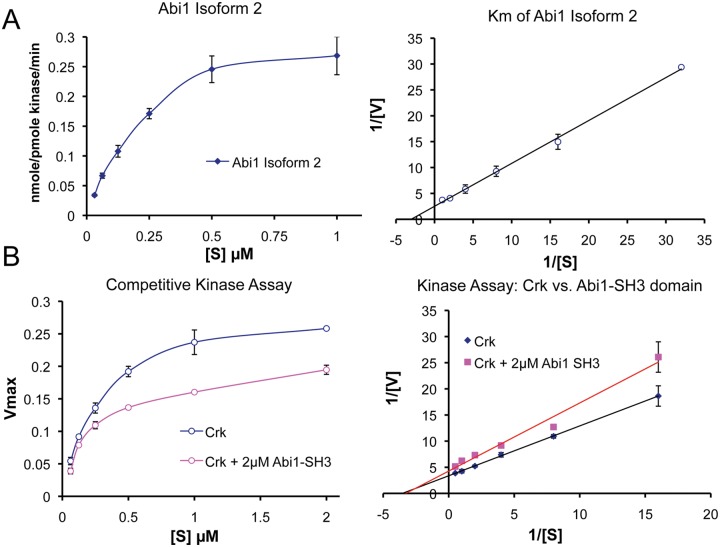
Figure 3.
Abi1 competes with Crk at the SH3 domain interaction site. (A) Determination of Abi1 Km for Abl kinases. Km is an important value that characterizes the relative substrate affinity for a given enzyme. Moreover, Km allows the comparison of different substrates for the same enzyme. Km of purified GST Abi1 isoform 292 was determined using recombinant c-Abl (47aa-end) purified from baculovirus culture treated with Gleevec (STI-571, Novartis Pharmaceuticals, East Hanover, NJ) as described53 and using reaction conditions as described by Tanis et al.94 The Km of GST Abi1 was 0.33 ± 0.5 µM, and the Km of GST Crk was 0.32 ± 0.02 µM (see below); hence, they are comparable. (B) Abi1 competes with Crk at its Abl SH3 domain binding site. For the competition assay with GST Crk, the purified Abi1 SH3 domain was cleaved from GST and used at 2 µM. Reduction of the Km from 0.32 ± 0.02 µM to 0.17 ± 0.01 µM and reduction of the Vmax by 24% indicated mixed competition. Crk associates with Abl through binding of its SH3 domain to the Abl proline-rich domain (PRD); the Abl PRD also binds Abi149 and Abi2.55 (Left) Kinase activity plots. (Right) Double reciprocal Lineweaver-Burk plots of the kinase activity data.
In addition to an apparent direct competition of Abi1 and Crk to 1 or more of the PXXP motifs in the PRD of Abl, Crk also appears to comprise a similar consolidated ligand for the Abl SH3/SH2 surface as Abi1. In this capacity, Sriram et al.61 showed that EGFR induces phosphorylation of Crk on pY251, which in turn induces binding to the Abl SH2 domain. In combination with this observation, Anafi et al.62 showed that Crk also binds to the Abl SH3 domain via PRPPVP within an extended loop in the SH2 domain. As shown in the model in Figure 4, these data suggest that Abi1 and Crk interact with conserved motifs, acting synergistically and possible cooperatively. These interactions include both Abi1 and Crk binding to the PRD of Abl, pY213 (Abi1) and pY251 (Crk) binding to the Abl SH2 domain, and PPSPP (Abi1) and PRPPVP (Crk) binding to the Abl SH3 domain.
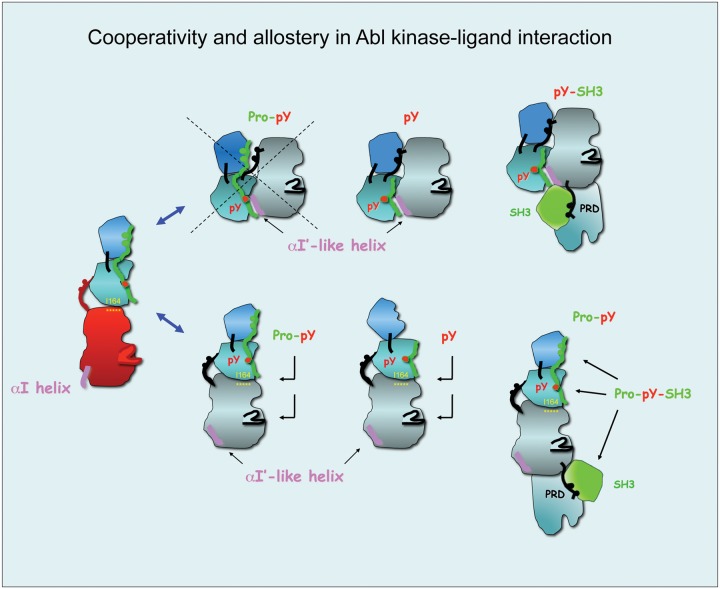
Figure 4.
Cooperativity and allostery in Abl kinase-ligand interactions: candidate binding modes of Abi1 and Crk. Protein modules of Abi1 and Crk have the potential to interact with different Abl kinase conformations and to engage all 3 regulatory domains of Abl. (Top) Potential interactions with the Abl autoinhibitory/closed structure. PXXP-pY peptides cannot engage the Abl SH3-SH2 dual domain in the closed conformation because the Abl SH3 domain interacts tightly with the proline consensus of the SH2–catalytic domain (CD) linker and with the CD interface (left). The SH2 domain, however, might bind phosphopeptides as suggested by modeling data using the autoinhibited Abl structure and Src structure pY527 (not shown). Abi1 pY213 and published Crk pY221/207 data (REF) (middle). In addition, the closed structure might engage the proline-rich region in Abl (PRD) and an SH3 domain in a ligand such as Crk and Abi1 (far right). (Bottom) Potential interactions of ligands with an open/active structure of Abl. PXXP-pY–containing peptides such as Abi1 181-PPSPP-185; pY213 might interact with the Abl SH3-SH2 dual domain (left) and with the SH2 domain (middle). It is not yet known what the effect of such interactions is on the Abl SH2 interface residue I116, which is critical in maintaining Abl kinase activity.45 Moreover, proteins such as Crk or Abi1 might in addition engage the proline-rich linker (PRD) that immediately follows the CD of Abl. In such cases, there is a potential for an additional effect on the CD itself through a possible steric effect.
Modes of Abl kinase activity regulation by Crk and Abi1 and their functional consequences: can adaptor proteins play a dual role in Abl activation and inhibition?
In the above arguments, we posit that Crk and Abi1 can compete for binding PRD motifs in Abl. Furthermore, once bound, important distinctions arise with respect to pY213 (Abl) and pY251 (Crk) binding to the SH2 domain of Abl. This is because the SH2 domain interaction with the Abl catalytic domain is proposed to play a dual role in Abl kinase activity: The SH2 domain inhibits Abl activity in the “closed” autoinhibited structure but promotes Abl activation in the “open” active conformation. By similar argument, ligand-mediated phosphotyrosine–Abl SH2 interaction has the potential to activate Abl activity by unlocking the closed conformation by disrupting the SH2–catalytic domain interaction, as proposed by Hantschel et al.41 or by disrupting the active open “elongated structure,” as demonstrated by the loss-of-function mutation I164E in Abl45 or by the anti-Abl SH2 domain antibody.63,64
Therefore, despite that both Abi1 and Crk each interact with Abl and appear to use overlapping regulatory mechanisms to engage the SH2 and SH3 domains of Abl, the biological consequences of Abi1 and Crk may be different, the former inducing Abl inhibition and the latter inducing Abl transactivation. For example, we have shown that Abl 1a can be inhibited when Abi1 binds in trans to the proline-rich region of Abl. As a consequence of this interaction, Abi1 becomes phosphorylated on Tyr213, which in turn induces an interaction with the Abl SH2 domain. What is perplexing from this finding is why Abl SH2 domain engagement by pTyr213 Abi1 does not result in Abl transactivation. As noted above, an analogous interaction between Abl and Crk causes Tyr251 phosphorylation; the latter also binds the Abl SH2 domain but, unlike Abi1, causes Abl activation. One possible scenario, which at present remains speculative, is that the Abl-pTyr213 Abi1 complex adopts an autoinhibitory complex in trans, similar to the Src-pTyr527 intramolecular interaction. This is consistent with our observations that Y213F Abi1 is much less effective in the transinhibition of Abl by Abi1. Under this model, the Abi1 PPSPP would not be able to engage the SH3 domain interacting with the Abl catalytic domain in the autoinhibited/closed conformation (Fig. 4).
However, another intriguing possibility for how and why Abi1 inhibits Abl may also result if indeed Abl is in further equilibrium with an open Abl conformation and includes an effect on the integrity of the SH2–catalytic domain interaction (Fig. 5). Here, there might be a role for the earlier mentioned alternatively spliced region of Abi1. Clearly, the use of solution phase nuclear magnetic resonance spectroscopy could be used to resolve these questions and provide more formal proof for the proposed snap-lock structure alluded to above or for the effect on the I164 residue interaction with the catalytic domain. Destabilizing mutations in the SH2–catalytic domain interaction were found in several kinases to associate with various human cancers including lung cancer (Fer kinase), X-linked agammaglobulinemia (Btk), and severe combined immunodeficiency (Zap70 and Jak3).65
Figure 5.
Abi1 and Crk might regulate Abl kinase activity by affecting the stability of the SH2–catalytic domain (CD) interaction in the open conformation of Abl kinase. In our article,53 we propose that Abi1 Pro-pY213 peptide inhibits Abl kinase activity through binding to Abl SH3-SH2 in an elongated conformation as proposed by small-angle X-ray scattering measurements44 and affecting the integrity of the SH2-CD interaction. pY213 peptide might exert its inhibition by binding to the Abl SH2 domain only. Based on our cell studies53 indicating that Abi1 inhibits c-Abl kinase, we propose that the mechanism involves multiple interactions of Abi1 and c-Abl at multiple domains starting from Abi1 PPSPP and pY213 sequences through the middle region of the Abi1 protein and including the SH3 domain at the C-terminus. The Abi1 SH3 domain was previously demonstrated to interact with the Abl proline-rich domain (PRD)49; homologous Abi2 SH3 was also demonstrated to interact with the Abl PRD.51,55 In fact, the “open” elongated conformation of Abl is compatible with the positioning of Abl and Abi1 binding sites alongside each other with the SH3 domain of Abi1 binding to the proline-rich region of Abl located C-terminally to the CD. Crk is proposed to interact in a similar fashion. The role of Abi1 or Crk on Abl kinase activity will depend on the effect on SH2-CD interaction. The critical I164 is indicated in yellow. Brackets indicate the alternatively spliced region of Abi1.
Interestingly, in the extended open conformation, the Abl catalytic domain is located in between the SH3 domain and PXXP-pY binding sites; thus, it is reasonable to hypothesize that Abi1 or Crk binding might also involve interactions with the Abl catalytic domain. It will be interesting to learn whether these sequences have any effect on Abl kinase activity. In the case of Abi1, the sequences located between pY213 and the SH3 domain represent the differentially spliced region of Abi1.52
What is the biological significance of the binary regulation of Abl by Abi1 and Crk in human cancers, and what are the consequences for actin cytoskeleton regulation?
Dysregulation of Crk in cancers is well established 66 Lung,67,68 breast,69,70 and gastric cancers71 as well as glioblastoma72 have demonstrated mostly elevated levels of Crk66 (see Bell and Park in this monograph). At the mechanistic level, both transcriptional and posttranscriptional mechanisms may contribute to Crk expression. In the latter scenario, a Crk-specific miRNA (miR-126) is downregulated in several cancers, which leads to elevated Crk levels71 (see Tsuda and Tanaka in this issue).
However, in the case of Abi1, the dual role of Abi1 function is observed in human cancer (Table 1). Increased Abi1 is linked to enhanced oncogenesis, for example, in invasive breast cancer, ovarian cancer, and leukemia and to tumor suppression in gastric73 and prostate cancers.74,75 Low levels of Abi1 are consistent with the tumor suppressor hypothesis in prostate and gastric cancers. The proposed binary regulation of c-Abl tyrosine kinase by Crk competing with Abi1 for c-Abl might help to explain the dysregulation of actin cytoskeleton dynamics.
Table 1.
Role of Abi1 in Cancer
| Primary tumor studies | ||||
|---|---|---|---|---|
| Cancer origin | Molecular pathway | Mutation | Role | Reference |
| Prostate | ND | Yes | Suppressor | Macoska et al.74 |
| β-catenin/E-cadherin/Akt | Yes | Suppressor | Xiong et al.75 | |
| Stomach | ND | ND | Suppressor | Cui et al.73 |
| ND | ND | Suppressor | Baba et al.95 | |
| Colon | kRas | ND | Oncogene | Steinestel et al.96 |
| Colon/rectum | ND | Yes | ND | COSMIC |
| Liver | ND | Yes | ND | ICGC |
| Leukemia | MLL-Abi1 fusion | Yes | Oncogene | Taki et al.97 |
| Shibuya et al.98 | ||||
| Breast | PI3 kinase/AKT | ND | Oncogene | Wang et al.85 |
| Lung | ND | Yes | ND | ICGG |
| Ovary | SOS1/Eps8 | ND | Oncogene | Chen et al.99 |
| Pancreas | ND | Yes | Suppressor | ICGC |
| Skin/melanoma | ND | Yes | ND | ICGC |
| Brain/glioblastoma | ND | Yes | ND | COSMIC |
| Head and neck/mouth | ND | Yes | ND | COSMIC |
| WAVE/NWASP complex studies | ||||
| Primary gene | Cancer origin | Change | Role | Reference |
| WAVE1 | Prostate | Upregulation | Oncogene | Fernando et al.88 |
| Leukemia | Upregulation | Oncogene | Kang et al.100 | |
| WAVE2 | Breast | Upregulation | Oncogene | Fernando et al.101 |
| Wang et al.86 | ||||
| Lung | Upregulation | Oncogene | Semba et al.102 | |
| Colon | Upregulation | Oncogene | Iwaya et al.103 | |
| Melanoma | Upregulation | Oncogene | Kurisu et al.104 | |
| Prostate | Downregulation | Suppressor | Xiong et al.75 | |
| Neuroblastoma | Upregulation | Oncogene | Sossey-Alaoui et al.105 | |
| WAVE3 | Breast | Upregulation | Oncogene | Sossey-Alaoui et al.89 |
| Prostate | Upregulation | Oncogene | Fernando et al.90 | |
| NWASP | Colon | Upregulation | Oncogene | Yanagawa et al.106 |
| Esophagus | Upregulation | Oncogene | Chattopadhyay et al.107 | |
| Breast | Downregulation | Suppressor | Martin et al.108 | |
Note: ND = not determined; COSMIC = Catalogue of Somatic Mutations in Cancer; ICGC = International Cancer Genome Consortium.
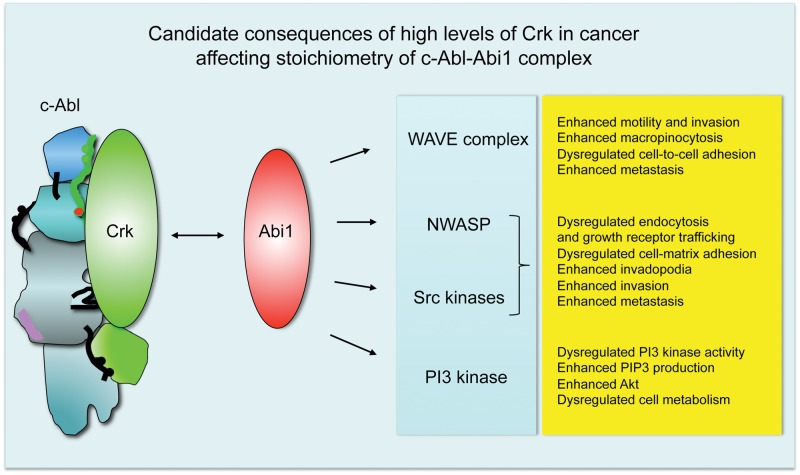
But what could be the consequences of altered Crk/Abi1 ratios in cancer? Abi1 is an established component of actin regulatory complexes WAVE and NWASP; it also enters the p85 regulatory subunit of PI3 kinase54,76 and can target the Src family of tyrosine kinases through pY421–SH2 domain interaction.54 High levels of Crk opposite Abi1 would likely compete off Abi1 from Abl, thus leading to higher levels of Abi1 in its actin regulatory complexes and leading to dysregulation of the actin cytoskeleton and resulting in tumorigenic phenotypes in cancer. These consequences of Abi1 level dysregulation are listed in Figure 6.
Figure 6.
Dysregulated stoichiometry of Crk-Abl and Abi1-Abl complexes leads to an invasive phenotype of the tumor-associated actin cytoskeleton. Biochemical data49,53,61,62 indicate that Abi1 and Crk target the same regulatory domains of c-Abl, thus suggesting binary regulation. We propose that Crk binding to Abl would lead to increased levels of Abi1 in actin regulatory complexes such as WAVE and/or NWASP, which would likely result in a tumorigenic phenotype (far right). Abi1 is also capable of association with the Src SH2 domain and p85 SH2 domain.54 The latter association would likely result in an enhanced76,86 or dysregulated PI3 kinase activity, as suggested by Abi1 knockout cell studies.75
Furthermore, enhanced WAVE complex activity leads to enhanced cellular motility and invasiveness. Dysregulation of WAVE complexes might lead to abnormal cellular adhesion and dysregulation of adherens junction formation, as suggested by the loss of CYFIP in several epithelial cancers77 and our mouse Abi1 knockout studies.75 Enhanced levels of Abi1 WAVE can also explain the enhanced cell–extracellular matrix adhesion, leading to the progression of leukemia78,79 and breast cancer.80 WAVE complex dysregulation in cancer has been elegantly covered in a recent review by Kurisu and Takenawa.81 Enhanced invadopodia formation is a feature of breast cancer in vivo and in vitro through activation of Src80,82 and NWASP.83,84
Higher levels of Abi1 might lead to PI3 kinase upregulation through SH2 domain interaction as suggested54,76 and subsequent metabolic changes as a consequence of enhanced PIP3 and Akt signaling as described, for example, for invasive breast cancer.85,86 In prostate cancer, downregulation of the PI3 kinase α isoform but upregulation of the PI3 kinase β isoform are associated with invasive changes.87 The role of Abi1 in PI3 kinase isoform regulation is yet to be determined, but it might be important from the clinical point of view of the widespread evaluation of PI3 kinase inhibitors in solid cancer trials and the critical role of Abi1/WAVE/NWASP complexes in tumor invasion.
High levels of Crk versus high levels of Abi1 in some cancers can also cause higher on and off rates of the proteins interacting with Abl, PI3 kinase, and Abi1-containing actin regulatory complexes. For example, enhanced Abi1 and Crk could lead to an enhanced turnover of invadopodia, breaking up the matrix and leading to very invasive changes.
While the preceding discussion mainly focused on the stoichiometry of Abi1 and Crk, more recently, it has also been shown that mutations can occur in Abi1, adding complexity to the aforementioned themes relating to stoichiometry. Mutations in Abi174,75 (Table 1) can also affect the dynamics of the binary Abi1-Crk regulation of Abl. The prostate tumor LNCaP cell line contains the loss-of-function mutation in the Abl SH2 domain binding site of Abi1, which lacks pY213,74 leading to dysregulation of Abl kinase activity and enhanced phosphorylation of Crk.53 Loss of Abi1 in prostate cancer mouse models leads to decreased cell-to-cell adhesion through downregulation of β-catenin and E- cadherin downstream from the WAVE2 complex.75 In humans, upregulation of the other WAVE complexes such as associated with WAVE188 or WAVE389,90 underlies invasive prostate tumors. Hence, depending on the ability of mutated Abi1 to reconstitute WAVE complexes, and to compete with Crk for Abl as proposed here, invasiveness might be associated with WAVE complex dysregulation, as observed in cells lacking Abi1.91 Dysregulation of WAVE complexes is not only specific to prostate cancer: elevated levels of WAVE3 are found in invasive breast cancer. This is also true for WAVE2, which is also upregulated in invasive lung and colon cancer as well as in melanoma (Table 1).
The devil is in the detail: complexity versus simplification
Over the past several decades, much effort has been delineated in the role of tyrosine kinases in cancer. Now, it is equally apparent that analogous to kinases, modulation of adaptor proteins can have equally important roles in cancer and may do so in part by modulating tyrosine kinase signaling. As in many forums in biology, information learned from one area is often inexplicably linked to other systems. As we turn to explain the role of Abl regulators such as Abi1 and Crk in a simple analysis, one might require more detailed information from this model system. This might lead to better explanations of tumor progression mechanisms, drug action mechanisms, and their differential efficacies in different types of cancers.
Acknowledgments
The authors thank Stephan Knapp (Oxford University, Oxford, UK), David Cowburn (Albert Einstein College of Medicine, Bronx, NY), and Tony Koleske (Yale University, New Haven, CT) for helpful discussions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: L.K. was supported by grants from the Department of Defense Prostate Cancer Research Program (W81XWH-08-1-0320), the National Institutes of Health (R01 NS044968), and the FM Kirby Foundation Inc. R.B.B. was supported by grant R01-CA165077 from the National Cancer Institute.
References
- 1. Miller ML, Jensen LJ, Diella F, et al. Linear motif atlas for phosphorylation-dependent signaling. Sci Signal. 2008;1:ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075-80 [DOI] [PubMed] [Google Scholar]
- 3. Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326:1220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Machida K, Mayer BJ, Nollau P. Profiling the global tyrosine phosphorylation state. Mol Cell Proteomics. 2003;2:215-33 [DOI] [PubMed] [Google Scholar]
- 5. Machida K, Thompson CM, Dierck K, et al. High-throughput phosphotyrosine profiling using SH2 domains. Mol Cell. 2007;26:899-915 [DOI] [PubMed] [Google Scholar]
- 6. Obenauer JC, Yaffe MB. Computational prediction of protein-protein interactions. Methods Mol Biol. 2004;261:445-68 [DOI] [PubMed] [Google Scholar]
- 7. Yaffe MB, Cantley LC. Signal transduction: grabbing phosphoproteins. Nature. 1999;402:30-1 [DOI] [PubMed] [Google Scholar]
- 8. Schwartzberg PL, Stall AM, Hardin JD, et al. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991;65:1165-75 [DOI] [PubMed] [Google Scholar]
- 9. Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153-63 [DOI] [PubMed] [Google Scholar]
- 10. Koleske AJ, Gifford AM, Scott ML, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259-72 [DOI] [PubMed] [Google Scholar]
- 11. Gu JJ, Ryu JR, Pendergast AM. Abl tyrosine kinases in T-cell signaling. Immunol Rev. 2009;228:170-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. Defective T cell development and function in the absence of Abelson kinases. J Immunol. 2007;179:7334-43 [DOI] [PubMed] [Google Scholar]
- 13. Tammer I, Brandt S, Hartig R, Konig W, Backert S. Activation of Abl by Helicobacter pylori: a novel kinase for CagA and crucial mediator of host cell scattering. Gastroenterology. 2007;132:1309-19 [DOI] [PubMed] [Google Scholar]
- 14. Tegtmeyer N, Backert S. Role of Abl and Src family kinases in actin-cytoskeletal rearrangements induced by the Helicobacter pylori CagA protein. Eur J Cell Biol. 2011;90:880-90 [DOI] [PubMed] [Google Scholar]
- 15. Burton EA, Plattner R, Pendergast AM. Abl tyrosine kinases are required for infection by Shigella flexneri. EMBO J. 2003;22:5471-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nhieu GT, Enninga J, Sansonetti P, Grompone G. Tyrosine kinase signaling and type III effectors orchestrating Shigella invasion. Curr Opin Microbiol. 2005;8:16-20 [DOI] [PubMed] [Google Scholar]
- 17. Abelson HT, Rabstein LS. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970;30:2213-22 [PubMed] [Google Scholar]
- 18. Scott ML, Van Etten RA, Daley GQ, Baltimore D. v-abl causes hematopoietic disease distinct from that caused by bcr-abl. Proc Natl Acad Sci U S A. 1991;88:6506-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038-42 [DOI] [PubMed] [Google Scholar]
- 20. Ganguly SS, Fiore LS, Sims JT, et al. c-Abl and Arg are activated in human primary melanomas, promote melanoma cell invasion via distinct pathways, and drive metastatic progression. Oncogene. 2012;31:1804-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin J, Arlinghaus R. Activated c-Abl tyrosine kinase in malignant solid tumors. Oncogene. 2008;27:4385-91 [DOI] [PubMed] [Google Scholar]
- 22. Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190-203 [DOI] [PubMed] [Google Scholar]
- 23. Chen WS, Kung HJ, Yang WK, Lin W. Comparative tyrosine-kinase profiles in colorectal cancers: enhanced arg expression in carcinoma as compared with adenoma and normal mucosa. Int J Cancer. 1999;83:579-84 [DOI] [PubMed] [Google Scholar]
- 24. Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648-55 [DOI] [PubMed] [Google Scholar]
- 25. Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008;27:1095-105 [DOI] [PubMed] [Google Scholar]
- 26. Gil-Henn H, Patsialou A, Wang Y, Warren MS, Condeelis JS, Koleske AJ. Arg/Abl2 promotes invasion and attenuates proliferation of breast cancer in vivo. Oncogene. Epub 2012. July 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plattner R, Koleske AJ, Kazlauskas A, Pendergast AM. Bidirectional signaling links the Abelson kinases to the platelet-derived growth factor receptor. Mol Cell Biol. 2004;24:2573-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srinivasan D, Kaetzel DM, Plattner R. Reciprocal regulation of Abl and receptor tyrosine kinases. Cell Signal. 2009;21:1143-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furstoss O, Dorey K, Simon V, Barila D, Superti-Furga G, Roche S. c-Abl is an effector of Src for growth factor-induced c-myc expression and DNA synthesis. EMBO J. 2002;21:514-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Plattner R, Irvin BJ, Guo S, et al. A new link between the c-Abl tyrosine kinase and phosphoinositide signalling through PLC-gamma1. Nat Cell Biol. 2003;5:309-19 [DOI] [PubMed] [Google Scholar]
- 32. Van Etten RA. c-Abl regulation: a tail of two lipids. Curr Biol. 2003;13:R608-10 [DOI] [PubMed] [Google Scholar]
- 33. Campa F, Machuy N, Klein A, Rudel T. A new interaction between Abi-1 and betaPIX involved in PDGF-activated actin cytoskeleton reorganisation. Cell Res. 2006;16:759-70 [DOI] [PubMed] [Google Scholar]
- 34. Michael M, Vehlow A, Navarro C, Krause M. c-Abl, Lamellipodin, and Ena/VASP proteins cooperate in dorsal ruffling of fibroblasts and axonal morphogenesis. Curr Biol. 2010;20:783-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neumeister EN, Zhu Y, Richard S, Terhorst C, Chan AC, Shaw AS. Binding of ZAP-70 to phosphorylated T-cell receptor zeta and eta enhances its autophosphorylation and generates specific binding sites for SH2 domain-containing proteins. Mol Cell Biol. 1995;15:3171-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zipfel PA, Zhang W, Quiroz M, Pendergast AM. Requirement for Abl kinases in T cell receptor signaling. Curr Biol. 2004;14:1222-31 [DOI] [PubMed] [Google Scholar]
- 37. Courtneidge SA. Cancer: escape from inhibition. Nature. 2003;422:827-8 [DOI] [PubMed] [Google Scholar]
- 38. Wang JY. Controlling Abl: auto-inhibition and co-inhibition? Nat Cell Biol. 2004;6:3-7 [DOI] [PubMed] [Google Scholar]
- 39. Williams JC, Weijland A, Gonfloni S, et al. The 2.35 A crystal structure of the inactivated form of chicken Src: a dynamic molecule with multiple regulatory interactions. J Mol Biol. 1997;274:757-75 [DOI] [PubMed] [Google Scholar]
- 40. Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629-38 [DOI] [PubMed] [Google Scholar]
- 41. Hantschel O, Nagar B, Guettler S, et al. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell. 2003;112:845-57 [DOI] [PubMed] [Google Scholar]
- 42. Nagar B, Hantschel O, Young MA, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112:859-71 [DOI] [PubMed] [Google Scholar]
- 43. Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513-609 [DOI] [PubMed] [Google Scholar]
- 44. Nagar B, Hantschel O, Seeliger M, et al. Organization of the SH3-SH2 unit in active and inactive forms of the c-Abl tyrosine kinase. Mol Cell. 2006;21:787-98 [DOI] [PubMed] [Google Scholar]
- 45. Filippakopoulos P, Kofler M, Hantschel O, et al. Structural coupling of SH2-kinase domains links Fes and Abl substrate recognition and kinase activation. Cell. 2008;134:793-803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adrian FJ, Ding Q, Sim T, et al. Allosteric inhibitors of Bcr-abl-dependent cell proliferation. Nat Chem Biol. 2006;2:95-102 [DOI] [PubMed] [Google Scholar]
- 47. Choi Y, Seeliger MA, Panjarian SB, et al. N-myristoylated c-Abl tyrosine kinase localizes to the endoplasmic reticulum upon binding to an allosteric inhibitor. J Biol Chem. 2009;284:29005-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang J, Adrian FJ, Jahnke W, et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010;463:501-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shi Y, Alin K, Goff SP. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 1995;9:2583-97 [DOI] [PubMed] [Google Scholar]
- 50. Miyazaki K, Matsuda S, Ichigotani Y, et al. Isolation and characterization of a novel human gene (NESH) which encodes a putative signaling molecule similar to e3B1 protein. Biochim Biophys Acta. 2000;1493:237-41 [DOI] [PubMed] [Google Scholar]
- 51. Dai Z, Pendergast AM. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995;9:2569-82 [DOI] [PubMed] [Google Scholar]
- 52. Ziemnicka-Kotula D, Xu J, Gu H, et al. Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton. J Biol Chem. 1998;273:13681-92 [DOI] [PubMed] [Google Scholar]
- 53. Xiong X, Cui P, Hossain S, et al. Allosteric inhibition of the nonmyristoylated c-Abl tyrosine kinase by phosphopeptides derived from Abi1/Hssh3bp1. Biochim Biophys Acta. 2008;1783:737-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dubielecka PM, Machida K, Xiong X, et al. Abi1/Hssh3bp1 pY213 links Abl kinase signaling to p85 regulatory subunit of PI-3 kinase in regulation of macropinocytosis in LNCaP cells. FEBS Lett. 2010;584:3279-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jung JH, Pendergast AM, Zipfel PA, Traugh JA. Phosphorylation of c-Abl by protein kinase Pak2 regulates differential binding of ABI2 and CRK. Biochemistry. 2008;47:1094-104 [DOI] [PubMed] [Google Scholar]
- 56. Cowburn D, Zheng J, Xu Q, Barany G. Enhanced affinities and specificities of consolidated ligands for the Src homology (SH) 3 and SH2 domains of Abelson protein-tyrosine kinase. J Biol Chem. 1995;270:26738-41 [DOI] [PubMed] [Google Scholar]
- 57. Birge RB, Kalodimos C, Inagaki F, Tanaka S. Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell Commun Signal. 2009;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kobashigawa Y, Sakai M, Naito M, et al. Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK. Nat Struct Mol Biol. 2007;14:503-10 [DOI] [PubMed] [Google Scholar]
- 59. Jankowski W, Saleh T, Pai MT, Sriram G, Birge RB, Kalodimos CG. Domain organization differences explain Bcr-Abl’s preference for CrkL over CrkII. Nat Chem Biol. 2012;8:590-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Antoku S, Mayer BJ. Distinct roles for Crk adaptor isoforms in actin reorganization induced by extracellular signals. J Cell Sci. 2009;122: 4228-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sriram G, Reichman C, Tunceroglu A, et al. Phosphorylation of Crk on tyrosine 251 in the RT loop of the SH3C domain promotes Abl kinase transactivation. Oncogene. 2011;30:4645-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anafi M, Rosen MK, Gish GD, Kay LE, Pawson T. A potential SH3 domain-binding site in the Crk SH2 domain. J Biol Chem. 1996;271:21365-74 [DOI] [PubMed] [Google Scholar]
- 63. Wojcik J, Hantschel O, Grebien F, et al. A potent and highly specific FN3 monobody inhibitor of the Abl SH2 domain. Nat Struct Mol Biol. 2010;17:519-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grebien F, Hantschel O, Wojcik J, et al. Targeting the SH2-kinase interface in Bcr-Abl inhibits leukemogenesis. Cell. 2011;147:306-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Filippakopoulos P, Muller S, Knapp S. SH2 domains: modulators of nonreceptor tyrosine kinase activity. Curr Opin Struct Biol. 2009;19:643-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sriram G, Birge RB. Emerging roles for crk in human cancer. Genes Cancer. 2010;1:1132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miller CT, Chen G, Gharib TG, et al. Increased C-CRK proto-oncogene expression is associated with an aggressive phenotype in lung adenocarcinomas. Oncogene. 2003;22:7950-7 [DOI] [PubMed] [Google Scholar]
- 68. Kim YH, Kwei KA, Girard L, et al. Genomic and functional analysis identifies CRKL as an oncogene amplified in lung cancer. Oncogene. 2010;29:1421-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rodrigues SP, Fathers KE, Chan G, et al. CrkI and CrkII function as key signaling integrators for migration and invasion of cancer cells. Mol Cancer Res. 2005;3:183-94 [DOI] [PubMed] [Google Scholar]
- 70. Fathers KE, Rodrigues S, Zuo D, et al. CrkII transgene induces atypical mammary gland development and tumorigenesis. Am J Pathol. 2010;176:446-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Feng R, Chen X, Yu Y, et al. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298:50-63 [DOI] [PubMed] [Google Scholar]
- 72. Wang L, Tabu K, Kimura T, et al. Signaling adaptor protein Crk is indispensable for malignant feature of glioblastoma cell line KMG4. Biochem Biophys Res Commun. 2007;362:976-81 [DOI] [PubMed] [Google Scholar]
- 73. Cui M, Yu W, Dong J, Chen J, Zhang X, Liu Y. Downregulation of ABI1 expression affects the progression and prognosis of human gastric carcinoma. Med Oncol. 2010;27:632-9 [DOI] [PubMed] [Google Scholar]
- 74. Macoska JA, Xu J, Ziemnicka D, Schwab TS, Rubin MA, Kotula L. Loss of expression of human spectrin src homology domain binding protein 1 is associated with 10p loss in human prostatic adenocarcinoma. Neoplasia. 2001;3:99-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xiong X, Chorzalska A, Dubielecka PM, et al. Disruption of Abi1/Hssh3bp1 expression induces prostatic epithelial neoplasia in conditional Abi1/Hssh3bp1 mice. Oncogenesis. 2012;1:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kotula L. Abi1, a critical molecule coordinating actin cytoskeleton reorganization with PI-3 kinase and growth signaling. FEBS Lett. 2012;586:2790-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Silva JM, Ezhkova E, Silva J, et al. Cyfip1 is a putative invasion suppressor in epithelial cancers. Cell. 2009;137:1047-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yu W, Sun X, Clough N, Cobos E, Tao Y, Dai Z. Abi1 gene silencing by short hairpin RNA impairs Bcr-Abl-induced cell adhesion and migration in vitro and leukemogenesis in vivo. Carcinogenesis. 2008;29:1717-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li Y, Clough N, Sun X, et al. Bcr-Abl induces abnormal cytoskeleton remodeling, beta1 integrin clustering and increased cell adhesion to fibronectin through the Abl interactor 1 pathway. J Cell Sci. 2007;120:1436-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sun X, Li C, Zhuang C, et al. Abl interactor 1 regulates Src-Id1-matrix metalloproteinase 9 axis and is required for invadopodia formation, extracellular matrix degradation and tumor growth of human breast cancer cells. Carcinogenesis. 2009;30:2109-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kurisu S, Takenawa T. WASP and WAVE family proteins: friends or foes in cancer invasion? Cancer Sci. 2010;101:2093-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gu JJ, Lavau CP, Pugacheva E, Soderblom EJ, Moseley MA, Pendergast AM. Abl family kinases modulate T cell-mediated inflammation and chemokine-induced migration through the adaptor HEF1 and the GTPase Rap1. Sci Signal. 2012;5:ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125:724-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev. 2007;8:37-48 [DOI] [PubMed] [Google Scholar]
- 85. Wang C, Tran-Thanh D, Moreno JC, et al. Expression of Abl interactor 1 and its prognostic significance in breast cancer: a tissue-array-based investigation. Breast Cancer Res Treat. 2011;129:373-86 [DOI] [PubMed] [Google Scholar]
- 86. Wang C, Navab R, Iakovlev V, et al. Abelson interactor protein-1 positively regulates breast cancer cell proliferation, migration, and invasion. Mol Cancer Res. 2007;5:1031-9 [DOI] [PubMed] [Google Scholar]
- 87. Jia S, Liu Z, Zhang S, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fernando HS, Sanders AJ, Kynaston HG, Jiang WG. WAVE1 is associated with invasiveness and growth of prostate cancer cells. J Urol. 2008;180:1515-21 [DOI] [PubMed] [Google Scholar]
- 89. Sossey-Alaoui K, Safina A, Li X, et al. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am J Pathol. 2007;170:2112-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fernando HS, Sanders AJ, Kynaston HG, Jiang WG. WAVE3 is associated with invasiveness in prostate cancer cells. Urol Oncol. 2010;28:320-7 [DOI] [PubMed] [Google Scholar]
- 91. Dubielecka PM, Ladwein KI, Xiong X, et al. Essential role for Abi1 in embryonic survival and WAVE2 complex integrity. Proc Natl Acad Sci U S A. 2011;108:7022-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dubielecka PM, Cui P, Xiong X, et al. Differential regulation of macropinocytosis by Abi1/Hssh3bp1 isoforms. PloS One. 2010;5: e10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sriram G, Birge RB. Commentary. The carboxyl-terminal Crk SH3 domain: regulatory strategies and new perspectives. FEBS Lett. 2012;586: 2615-8 [DOI] [PubMed] [Google Scholar]
- 94. Tanis KQ, Veach D, Duewel HS, Bornmann WG, Koleske AJ. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol Cell Biol. 2003;23:3884-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Baba RA, Bhat HF, Wani LA, et al. E3B1/ABI-1 isoforms are down-regulated in cancers of human gastrointestinal tract. Dis Markers. 2012;32:273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Steinestel K, Bruderlein S, Steinestel J, et al. Expression of Abelson interactor 1 (Abi1) correlates with inflammation, KRAS mutation and adenomatous change during colonic carcinogenesis. PloS One. 2012;7:e40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Taki T, Shibuya N, Taniwaki M, et al. ABI-1, a human homolog to mouse Abl-interactor 1, fuses the MLL gene in acute myeloid leukemia with t(10;11)(p11.2;q23). Blood. 1998;92:1125-30 [PubMed] [Google Scholar]
- 98. Shibuya N, Taki T, Mugishima H, et al. t(10;11)-acute leukemias with MLL-AF10 and MLL-ABI1 chimeric transcripts: specific expression patterns of ABI1 gene in leukemia and solid tumor cell lines. Genes Chromosomes Cancer. 2001;32:1-10 [DOI] [PubMed] [Google Scholar]
- 99. Chen H, Wu X, Pan ZK, Huang S. Integrity of SOS1/EPS8/ABI1 tri-complex determines ovarian cancer metastasis. Cancer Res. 2010;70:9979-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kang R, Tang D, Yu Y, et al. WAVE1 regulates Bcl-2 localization and phosphorylation in leukemia cells. Leukemia. 2010;24:177-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fernando HS, Davies SR, Chhabra A, et al. Expression of the WASP verprolin-homologues (WAVE members) in human breast cancer. Oncology. 2007;73:376-83 [DOI] [PubMed] [Google Scholar]
- 102. Semba S, Iwaya K, Matsubayashi J, et al. Coexpression of actin-related protein 2 and Wiskott-Aldrich syndrome family verproline-homologous protein 2 in adenocarcinoma of the lung. Clin Cancer Res. 2006;12:2449-54 [DOI] [PubMed] [Google Scholar]
- 103. Iwaya K, Oikawa K, Semba S, et al. Correlation between liver metastasis of the colocalization of actin-related protein 2 and 3 complex and WAVE2 in colorectal carcinoma. Cancer Sci. 2007;98:992-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kurisu S, Suetsugu S, Yamazaki D, Yamaguchi H, Takenawa T. Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene. 2005;24:1309-19 [DOI] [PubMed] [Google Scholar]
- 105. Sossey-Alaoui K, Su G, Malaj E, Roe B, Cowell JK. WAVE3, an actin-polymerization gene, is truncated and inactivated as a result of a constitutional t(1;13)(q21;q12) chromosome translocation in a patient with ganglioneuroblastoma. Oncogene. 2002;21:5967-74 [DOI] [PubMed] [Google Scholar]
- 106. Yanagawa R, Furukawa Y, Tsunoda T, et al. Genome-wide screening of genes showing altered expression in liver metastases of human colorectal cancers by cDNA microarray. Neoplasia. 2001;3:395-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chattopadhyay I, Phukan R, Singh A, et al. Molecular profiling to identify molecular mechanism in esophageal cancer with familial clustering. Oncol Rep. 2009;21:1135-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Martin TA, Pereira G, Watkins G, Mansel RE, Jiang WG. N-WASP is a putative tumour suppressor in breast cancer cells, in vitro and in vivo, and is associated with clinical outcome in patients with breast cancer. Clin Exp Metastasis. 2008;25:97-108 [DOI] [PubMed] [Google Scholar]