Abstract
Increasing evidence suggests that changes in cytosolic Ca2+ levels and phosphorylation play important roles in the regulation of stomatal aperture and as ion transporters of guard cells. However, protein kinases responsible for Ca2+ signaling in guard cells remain to be identified. Using biochemical approaches, we have identified a Ca2+-dependent protein kinase with a calmodulin-like domain (CDPK) in guard cell protoplasts of Vicia faba. Both autophosphorylation and catalytic activity of CDPK are Ca2+ dependent. CDPK exhibits a Ca2+-induced electrophoretic mobility shift and its Ca2+-dependent catalytic activity can be inhibited by the calmodulin antagonists trifluoperazine and N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide. Antibodies to soybean CDPKα cross-react with CDPK. Micromolar Ca2+ concentrations stimulate phosphorylation of several proteins from guard cells; cyclosporin A, a specific inhibitor of the Ca2+-dependent protein phosphatase calcineurin enhances the Ca2+-dependent phosphorylation of several soluble proteins. CDPK from guard cells phosphorylates the K+ channel KAT1 protein in a Ca2+-dependent manner. These results suggest that CDPK may be an important component of Ca2+ signaling in guard cells.
Guard cells define and control stomatal aperture by osmotic swelling and shrinking. Stomatal opening involves hyperpolarization of the plasma membrane by a H+-ATPase, uptake of K+ and Cl−, and production of organic solutes (Assmann, 1993). Stomatal closure requires depolarization of the plasma membrane and efflux of anions and K+ (Assmann, 1993). Increases in cytosolic Ca2+ regulate several ion transporters that are essential in the control of stomatal aperture (MacRobbie, 1997; McAinsh et al., 1997). The plasma membrane proton pump of Vicia faba guard cells, which hyperpolarizes the plasma membrane and thus provides the driving force for K+ and Cl− uptake, is inhibited by increased cytosolic Ca2+ (Kinoshita et al., 1995). The inward K+ channels in the plasma membrane of V. faba guard cells, which are responsible for K+ influx, are also inhibited by elevated cytosolic Ca2+ (Schroeder and Hagiwara, 1989). Conversely, a type of K+ channel in the tonoplast of V. faba guard cells, which may control K+ efflux from guard cell vacuoles during stomatal closure, is activated when cytosolic Ca2+ is increased to approximately 1 μm (Ward and Schroeder, 1994). Both S-type (slow) and R-type (rapid) plasma membrane anion channels, which allow Cl− and malate efflux during stomatal closure, are also activated by elevated cytosolic Ca2+ concentrations (Schroeder and Hagiwara, 1989; Hedrich et al., 1990). Consistent with these electrophysiological data, exogenous application of Ca2+ inhibits opening of closed stomata and stimulates closure of open stomata in isolated epidermal peels of Commelina communis (De Silva et al., 1985; Schwartz, 1985; Schwartz et al., 1988); such Ca2+ application is known to increase cytosolic Ca2+ levels (Gilroy et al., 1991). In addition, a variety of stimuli such as ABA, CO2, and oxidative stress can rapidly induce increases in cytosolic Ca2+ concentrations in guard cells (McAinsh et al., 1997, and refs. therein). These data all point to increases in cytosolic Ca2+ concentrations as being critical in the inhibition of stomatal opening and promotion of stomatal closure. On the other hand, calmodulin antagonists inhibit stomatal opening and H+ pumping in V. faba, suggesting that Ca2+ may also play a role in mediating stomatal opening (Shimazaki et al., 1992).
Despite a growing body of evidence that physiological signals increase cytosolic Ca2+ levels in guard cells and that this in turn affects ion transporters, the biochemical steps between elevation of cytosolic Ca2+ concentrations and electrophysiological response are incompletely understood. Recently, elegant work from Pei et al. (1996) showed that a CDPK activated a tonoplast Cl− channel in isolated vacuoles from V. faba guard cells. However, it is still unclear whether CDPK directly phosphorylates the ion channel or phosphorylates an intermediary regulatory protein(s). In addition, the study of Pei et al. (1996) utilized recombinant Arabidopsis CDPK protein purified from Escherichia coli. There is no direct evidence that CDPK exists in guard cells, which have not only unique morphology and highly specialized metabolism but also unique responses to environmental signals.
Electrophysiological studies using general protein kinase inhibitors indicate that inward K+ channels, outward K+ channels, and anion channels of the guard cell plasma membrane may be modulated by phosphorylation (Armstrong et al., 1995; Schmidt et al., 1995). However, the kinases involved in regulation of these ion channels remain to be identified. Inward K+ channels provide a major pathway for K+ uptake into plant cells including guard cells (Schroeder et al., 1994). KAT1, a plant K+ channel gene, was initially cloned from Arabidopsis by complementation of a K+-transport-deficient strain of Saccharomyces cerevisiae (Anderson et al., 1992). Electrophysiological studies utilizing heterologous expression of KAT1 in Xenopus oocytes, S. cerevisiae, or the insect cell line Sf9 indicate that KAT1 encodes a voltage-gated inward K+ channel (Schachtman et al., 1992; Bertl et al., 1995; Hoshi, 1995; Marten et al., 1996). Moreover, the KAT1 gene is primarily expressed in guard cells of transgenic Arabidopsis plants (Nakamura et al., 1995). These results indicate that the KAT1 protein is very likely to be the guard cell inward K+ channel in which activity is inhibited by elevated cytosolic Ca2+ levels (Schroeder and Hagiwara, 1989; Blatt et al., 1990; Lemtiri-Chlieh and MacRobbie, 1994). Sequence analysis of KAT1 suggests that the deduced protein from the KAT1 gene is rich in potential phosphorylation sites for protein kinases. However, whether the KAT1 protein can be phosphorylated by protein kinases remains to be determined.
In the present study we biochemically identify and characterize a CDPK from guard cells of V. faba. We also demonstrate that this guard cell CDPK can phosphorylate the KAT1 protein in a Ca2+-dependent manner. Our data suggest that CDPK may be involved in Ca2+-regulated modulation of plasma membrane ion channels in guard cells.
MATERIALS AND METHODS
Chemicals
Acrylamide, bisacrylamide, and microsomal membranes derived from canine pancreas were purchased from Boehringer Mannheim. Ampholytes and affinity-purified goat anti-rabbit IgG conjugated with alkaline phosphatase were purchased from Bio-Rad. The 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium phosphatase substrate system was purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD). CsA was a gift from Sandoz Research Institute (East Hanover, NJ). Nitrocellulose membranes were purchased from Schleicher & Schuell. SDS, 10-kD protein ladder, and prestained protein molecular weight standards were purchased from GIBCO-BRL. Rabbit polyclonal antibodies to the calmodulin-like domain of soybean CDPKα (Bachmann et al., 1996) were immunopurified on a column of immobilized soybean CDPKα and generously provided by Dr. Alice Harmon (University of Florida, Gainesville). The single tube protein system 2 (STP2) for in vitro transcription and translation and S-tag CL-AP western-blot kit were purchased from Novagen (Madison, WI). [γ-32P]ATP and [35S]Met were obtained from Amersham. Urea was purchased from EM Science (Gibbstown, NJ). All other chemicals were obtained from Sigma.
Plant Material
Plants of Vicia faba L. cv Long Pod were grown in growth chambers with a 10-h light (200 μmol m−2 s−1 white light):14-h dark regime. Temperature was maintained at 21°C during the light period and 18°C during the dark period. First fully expanded leaves from 3-week-old plants were used in all experiments.
Preparation of Proteins
Guard cell protoplasts were isolated and purified as described by Ling and Assmann (1992). The purity of guard cell protoplasts was 99.9% based on counting a sample of about 6000 cells. Soluble and microsomal membrane proteins from guard cell protoplasts were prepared as described previously (Li and Assmann, 1996). Protein concentrations were measured by the method of Bradford (1976) using the Bio-Rad protein assay kit and BSA (catalog no. P7656, Sigma) as the standard.
Gel Electrophoresis
SDS-PAGE was carried out according to the method of Laemmli (1970). To detect Ca2+-induced electrophoretic mobility shifts, CaCl2 or EGTA was added to protein samples in SDS-PAGE sample buffer to a final concentration of 2 mm. The protein samples were boiled for 2 min and then analyzed on a 12% SDS-polyacrylamide gel. Two-dimensional electrophoresis was performed according to the method of Hochstrasser et al. (1988). Proteins (50 μg) were subjected to IEF with pH 3.0 to 10.0 ampholytes for 12 h at 500 V and then for 3 h at 800 V. After IEF the proteins were separated in the second dimension using a 12% SDS-polyacrylamide gel.
In-Gel Autophosphorylation and Kinase Assays
Autophosphorylation of proteins in polyacrylamide SDS gels was carried out as described by Li and Chollet (1993) based on the method of Kameshita and Fujisawa (1989), except that 8 m urea was used to denature the proteins in the gels, and the subsequently renatured gels were incubated with 40 mm Hepes-NaOH, pH 7.5, 10 mm MgCl2, 0.45 mm EGTA, and 2 mm DTT (buffer A) containing 10 μCi mL−1 [γ-32P]ATP (3000 Ci mmol−1) in the absence or presence of 0.55 mm CaCl2 for 1 h at room temperature. The gels were air dried between two sheets of cellophane and exposed to Kodak X-Omat AR film for 3 d at room temperature. The in-gel kinase activity assay was performed as described above, except that the separating gel was polymerized in the presence of 0.5 mg mL−1 histone III-S as a substrate for kinases.
In Vitro Protein Kinase Activity Assay
Protein kinase activity was determined by phosphorylation of histone III-S (Harmon et al., 1987) using the method of Yao et al. (1995). Briefly, proteins in SDS-polyacrylamide gels were denatured and renatured (Li and Chollet, 1993). Portions of the gel containing the autophosphorylating 57-kD band (see Results) or blank gel (negative control) were excised and crushed with pestles in microcentrifuge tubes. After the sample was centrifuged, the supernatant from the gel slurry was removed and histone III-S was added to 0.02 mg mL−1. The phosphorylation reaction (100 μL) was initiated by addition of 50 μCi mL−1 [γ-32P]ATP. After 5 min at room temperature, the reaction was stopped by addition of 10% (w/v) TCA. After the sample was centrifuged for 10 min in a microfuge, the pellets were rinsed twice with ice-cold acetone. Precipitated proteins were dissolved in SDS-PAGE sample buffer and boiled for 2 min. The samples were then electrophoresed on a 12% SDS-polyacrylamide gel. Phosphorylated histone III-S was detected by autoradiography.
Immunoblotting
Following SDS-PAGE, proteins on one- or two-dimensional gels were electrophoretically transferred to 0.2-μm nitrocellulose membranes at 30 V and 8°C overnight (Towbin et al., 1979). The membranes were blocked with 5% (w/v) nonfat dry milk in TBS (20 mm Tris-HCl, pH 7.5, and 500 mm NaCl) for 2 h and then incubated with either affinity-purified rabbit polyclonal antibodies to the calmodulin-like domain of soybean CDPKα or nonimmune rabbit serum for 2 h at room temperature. The membranes were washed for 30 min with 4 × 100 mL of TBS containing 0.05% (v/v) Tween 20 and incubated for 1 h with goat anti-rabbit IgG conjugated with alkaline phosphatase (1:3000 dilution). The membranes were washed as described above and developed using a 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium substrate system according to the manufacturer's protocol.
Phosphorylation of Guard Cell Proteins
Proteins (30 μg) from guard cell protoplasts were added to a phosphorylation buffer containing 25 mm Tris-HCl (pH 7.0), 5 mm MgCl2, 0.1 mm DTT, 0.25 mm EGTA, and appropriate amounts of CaCl2 to give desired free Ca2+ concentrations in a final volume of 50 μL. Free Ca2+ concentrations were calculated by the computer program Calcium (Chang et al., 1988). To solubilize membranes, microsome membranes were incubated in the phosphorylation buffer containing 0.2% (w/v) Triton X-100 for 10 min on ice (Short et al., 1992). The phosphorylation reaction was initiated by addition of [γ-32P]ATP. After 5 min at room temperature, the reaction was stopped by addition of 10% (w/v) TCA. After the sample was centrifuged for 10 min in a microfuge, the pellets were rinsed twice with ice-cold acetone. To determine the effect of CsA on protein phosphorylation, CsA from a 5 mm stock in 100% ethanol was added to the reaction mixture to a final concentration of 10 μm 2 min after the initiation of the phosphorylation reaction. The reaction was then terminated with TCA after incubation for 5 min at room temperature. The phosphoproteins were resolved on 5 to 20% gradient acrylamide gels. The gels were dried and subjected to autoradiography as described above.
Transcription and Translation of KAT1
KAT1 was subcloned into the pCITE-4c(+) vector (Novagen) and the pCITE-KAT1 plasmid was generated. The KAT1 protein was produced using an in vitro transcription and translation system (STP2, T7 rabbit reticulocyte system, Novagen) according to the manufacturer's protocol. Briefly, transcription of KAT1 was performed by incubating 0.5 μg of pCITE-KAT1 plasmid DNA with the transcription mixture for 15 min at 30°C. Translation of KAT1 was then carried out by adding [35S]Met, canine pancreatic microsomes, and the translation mixture to a final volume of 50 μL and incubating for 60 min at 30°C. The purpose of adding microsome membranes is to examine membrane insertion of the translated KAT1 protein, since the deduced protein from the KAT1 gene contains six putative transmembrane domains (Anderson et al., 1992). Immediately after translation, the translation mixture was placed on ice for 10 min and then centrifuged at 100,000g for 40 min at 4°C. The supernatants and pellets (membrane fraction) were collected. Translated protein from the KAT1 gene was identified by detecting 35S-labeled protein by autoradiography. Since pCITE-KAT1 contains an S-tag sequence, translated proteins were also detected on blots using the S-protein-alkaline phosphatase conjugate (Novagen) according to the manufacturer's protocol. For phosphorylation experiments, the transcription/translation reaction was scaled up and nonradioactive Met instead of [35S]Met was used.
Phosphorylation of KAT1 Protein
The supernatant or microsome membrane fractions of the in vitro-translated products containing the KAT1 protein were incubated with kinase (CDPK or AAPK) and 20 μCi [γ-32P]ATP in a final volume of 100 μL of phosphorylation buffer (40 mm Hepes, pH 7.5, 10 mm MgCl2, 5 mm DTT, 100 μm PMSF, 10 μg mL−1 leupeptin and pepstatin, and 0.2% [w/v] Triton X-100 in the presence of 1 mm CaCl2 or 1 mm EGTA) for 15 min at 22°C. The reaction was stopped by addition of 10% TCA as described in “Phosphorylation of Guard Cell Proteins.” The protein samples were then resolved on 9% SDS-polyacrylamide gels.
RESULTS
Identification of a 57-kD Kinase from Guard Cells as a CDPK
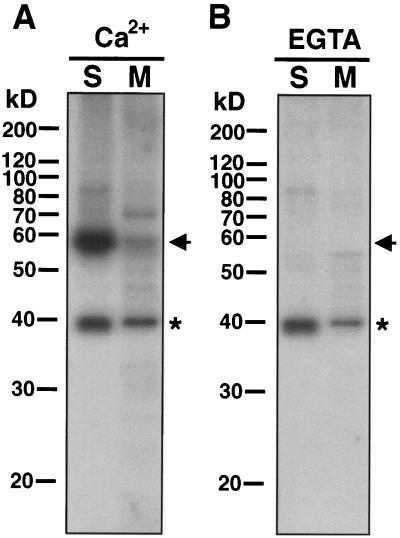
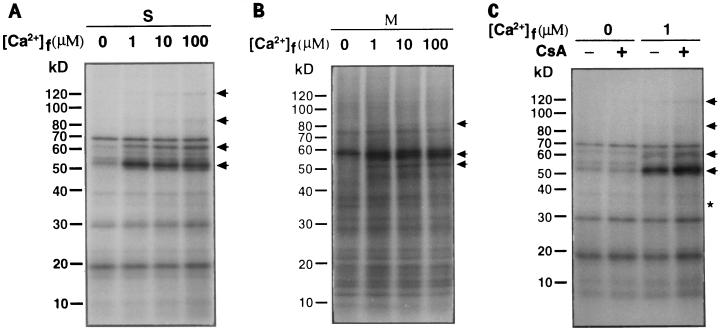
Since most protein kinases have autophosphorylating properties, i.e. protein kinases can phosphorylate themselves in the presence of ATP (Smith et al., 1993), we utilized this as a means of identifying protein kinases in the limited amount of protein available from guard cells (Li and Assmann, 1996). When proteins extracted from purified guard cell protoplasts were assayed for autophosphorylation activity in the presence of 100 μm Ca2+, a 57-kD 32P-labeled band was found in both the soluble and membrane protein samples (Fig. 1A, arrow). This 57-kD band was no longer detected when the autophosphorylation assay was performed in the presence of 450 μm EGTA (Fig. 1B). These results indicate that the autophosphorylation of the 57-kD protein is Ca2+ dependent. In contrast, a 38-kD 32P-labeled band was found in the presence of either Ca2+ or EGTA (Fig. 1, asterisks).
Figure 1.
Detection of Ca2+-dependent autophosphorylation activity in guard cells. Soluble fractions (lanes S, 40 μg of protein) and membrane fractions (lanes M, 20 μg of protein) from GCPs were separated on a 12% SDS-polyacrylamide gel. After proteins in the gels were denatured and renatured, autophosphorylation activity was detected in gel in the presence of 100 μm Ca2+ (A) or 450 μm EGTA (B). The molecular masses of protein standards (10-kD protein ladder) are shown at the left of each panel in kilodaltons. The arrows and asterisks at the right of each panel indicate the positions of the 57- and 38-kD radiolabeled proteins, respectively.
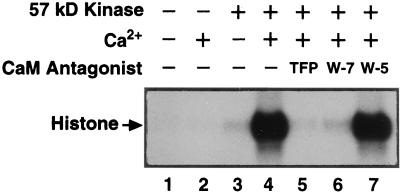
We next examined the catalytic activity of the 57-kD protein using histone III-S as a substrate. The 57-kD protein with Ca2+-dependent autophosphorylation was gel purified (see Methods) and then incubated with histone III-S and [γ-32P]ATP in the presence of Ca2+ or EGTA. The proteins were then separated by SDS-PAGE and 32P-labeled polypeptides were detected by autoradiography. In the presence of 450 μm EGTA, only minor phosphorylation of histone III-S was detected (Fig. 2, lane 3). In contrast, histone III-S was strongly phosphorylated in the presence of 20 μm Ca2+ (Fig. 2, lane 4). When the 57-kD protein was omitted from the incubation system (histone III-S and [γ-32P]ATP only), no phosphorylation of histone III-S was observed (Fig. 2, lanes 1 and 2). These results show that the catalytic activity of the 57-kD kinase is also Ca2+ dependent. In addition, the Ca2+-dependent phosphorylation of histone III-S by the 57-kD kinase could be inhibited by the calmodulin antagonists TFP and W7 (Fig. 2, lanes 5 and 6). By contrast, W-5, an inactive analog of W-7, had no apparent effect on histone III-S phosphorylation catalyzed by the 57-kD kinase in the presence of Ca2+ (Fig. 2, lane 7).
Figure 2.
Ca2+-dependent catalytic activity of the 57-kD kinase and its inhibition by calmodulin (CaM) antagonists. The catalytic activity of the gel-purified 57-kD kinase was assayed using histone type III-S as a substrate in the presence of 20 μm Ca2+ or 450 μm EGTA as described in Methods. TFP, W-7, or W-5 each was used at 250 μm. The phosphorylated histone III-S was resolved on a 12% polyacrylamide gel. −, Absent; +, present.
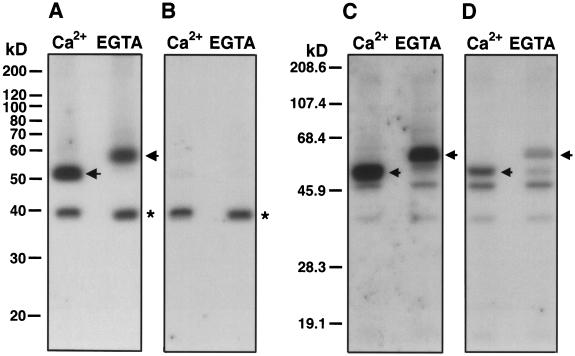
Ca2+-binding proteins such as CDPK migrate in gels at different rates in the Ca2+-bound versus Ca2+-free state (Roberts and Harmon, 1992). To investigate this phenomenon for the 57-kD kinase, Ca2+ or EGTA was added to guard cell protein samples just before electrophoresis and then the proteins in the gel were assayed for autophosphorylation (Fig. 3, A and B) or kinase activity (Fig. 3, C and D). When the autophosphorylation assay was performed in the presence of Ca2+, a 32P-labeled polypeptide with different mobilities was observed (Fig. 3A, arrows). The apparent molecular mass of this polypeptide was 52 kD when the sample buffer contained Ca2+ and 57 kD when the sample buffer contained EGTA. When the autophosphorylation assay was performed in the presence of EGTA, the 32P-labeled polypeptide with a Ca2+-induced mobility shift was no longer detected (Fig. 3B). In contrast, the 38-kD band that showed autophosphorylation that was not Ca2+ dependent (Fig. 1, asterisks) did not exhibit a Ca2+-dependent electrophoretic mobility shift (Fig. 3, A and B, asterisks). In accord with the autophosphorylation assay, a major 32P-labeled band with a Ca2+-induced mobility shift (52 or 57 kD in the presence of Ca2+ or EGTA, respectively) was also observed by an in-gel kinase assay in which histone III-S was included in the gel as a substrate (Fig. 3, C and D, arrows). The activity of this kinase as determined by the in-gel assay was strongly enhanced by Ca2+ (Fig. 3, C and D, arrows), which is consistent with the in vitro kinase activity assay (Fig. 2, lanes 3 and 4). In addition to the 57-kD band with a Ca2+-induced mobility shift, several faint 32P-labeled bands were found, but neither the intensities nor the mobilities of these bands seemed to be affected by Ca2+ (Fig. 3, C and D). These results demonstrate that the 57-kD kinase that has Ca2+-dependent autophosphorylation and catalytic activities exhibits a Ca2+-induced electrophoretic mobility shift.
Figure 3.
The 57-kD kinase exhibits a Ca2+-dependent electrophoretic mobility shift in both autophosphorylation and in-gel activity assays. Ca2+ or EGTA to a final concentration of 2 mm was added to the GCP-soluble proteins dissolved in SDS-PAGE sample buffer. The Ca2+- and EGTA-treated samples were loaded (A and B, 40 μg of protein per lane; C and D, 20 μg of protein per lane) with a blank lane between the two samples and resolved on 12% polyacrylamide gels. Autophosphorylation (A and B) and in-gel kinase activity (C and D) assays were performed by incubating the renatured gels with [γ-32P]ATP in the presence of 100 μm free Ca2+ (A and C) or 450 μm EGTA (B and D). The presence of histone III-S (0.5 mg mL−1) in the polyacrylamide separating gel precludes protein staining; therefore, prestained protein molecular mass standards are used in the kinase activity assay (C and D). The arrows indicate the positions of the 57-kD kinase with a Ca2+-induced electrophoretic mobility shift. The asterisks indicate the positions of the 38-kD kinase that does not exhibit a Ca2+-induced electrophoretic mobility shift.
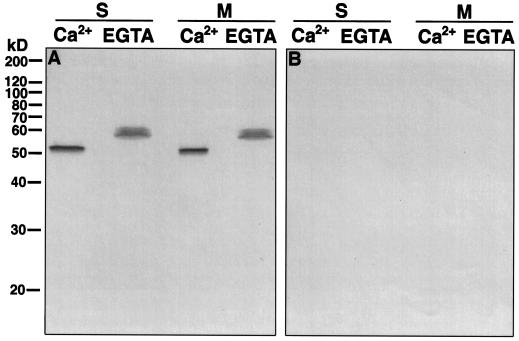
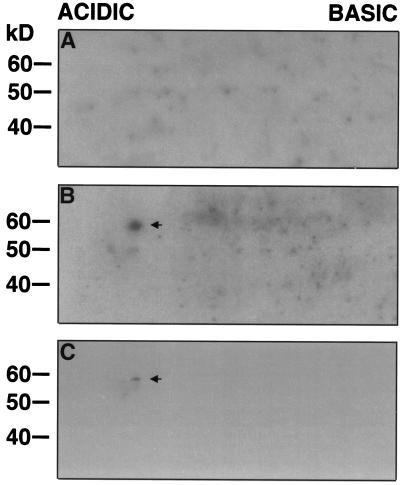
We further assessed the relatedness of the 57-kD Ca2+-dependent kinase from GCPs to CDPKs. As shown in Figure 4A, affinity-purified antibodies to the calmodulin-like domain of soybean CDPKα cross-reacted with a single band at 57 or 52 kD from EGTA- or Ca2+-treated protein samples, respectively. When the CDPK antibodies were replaced by nonimmune serum, no bands were detected (Fig. 4B). These results demonstrated that the 57-kD kinase that exhibited a Ca2+-induced electrophoretic mobility shift can be recognized by CDPK antibodies. The identity of the 57-kD kinase and the 57-kD band recognized by affinity-purified CDPK antibodies was further confirmed by two-dimensional electrophoretic analysis. The autophosphorylation assay was performed on a portion of the two-dimensional gel between the 30- and 70-kD molecular mass standards (Fig. 5, A and B). Although the background in the two-dimensional autophosphorylation assay was higher than that of the one-dimensional autophosphorylation assay, as also observed by other researchers (Keen et al., 1987), a 32P-labeled 57-kD protein spot was consistently found when the autophosphorylation assay was carried out in the presence of 100 μm Ca2+ (n = 5, Fig. 5B). However, this 32P-labeled 57-kD protein spot was no longer detected when the autophosphorylation assay was performed in the presence of 450 μm EGTA (n = 5, Fig. 5A), indicating that the autophosphorylation of the 57-kD protein spot is Ca2+ dependent. Furthermore, on two-dimensional immunoblots, the affinity-purified CDPK antibodies detected a 57-kD protein spot with a position identical to that of the 57-kD protein spot with Ca2+-dependent autophosphorylation (n = 2, Fig. 5C), confirming that they are the same protein. Taken together, all of the results consistently indicate that the 57-kD kinase from guard cells is a CDPK.
Figure 4.
Affinity-purified CDPK antibodies cross-react with the 57-kD kinase. The soluble (S) or membrane (M) proteins (80 μg per lane) were treated with Ca2+ or EGTA as described in Figure 3 and resolved on a 12% polyacrylamide gel. The proteins were then transferred to nitrocellulose membranes and subjected to immunostaining with affinity-purified CDPK antibodies (A) or nonimmune rabbit serum (B) as described in Methods.
Figure 5.
Detection of the 57-kD kinase from GCPs on two-dimensional gels. Soluble proteins (50 μg) from GCPs were resolved by IEF in the horizontal dimension and then by SDS-PAGE in the vertical dimension. Proteins on the two-dimensional gels were subjected to the autophosphorylation assay in the presence of 450 μm EGTA (n = 5, A) or 100 μm Ca2+ (n = 5, B) or transferred to a nitrocellulose membrane that was then probed with affinity-purified CDPK antibodies (n = 2, C). The arrows indicate the position of the protein spot showing Ca2+-dependent autophosphorylation (B) or the identical position of the protein spot recognized by the CDPK antibody (C).
Micromolar Levels of Ca2+ Stimulate Protein Phosphorylation
We next examined the effects of Ca2+ on the phosphorylation of guard cell proteins. As shown in Figure 6, which exemplifies four replicate experiments, micromolar concentrations of Ca2+ markedly enhanced the phosphorylation of several soluble proteins, e.g. the 120-, 85-, 63-, 57-, and 52-kD polypeptides (Fig. 6A). Micromolar concentrations of free Ca2+ also enhanced the phosphorylation of several membrane proteins, e.g. the 80-, 57-, and 52-kD polypeptides (Fig. 6B, arrows).
Figure 6.
Ca2+ stimulates phosphorylation of certain guard cell proteins. Protein phosphorylation was performed by incubating 30 μg of soluble (S) proteins (A) or membrane (M) proteins (B) with [γ-32P]ATP in the presence of various concentrations of free Ca2+ ([Ca2+]f). Phosphorylation of soluble proteins (30 μg) in the presence of 10 μm CsA (lanes +) or 0.2% ethanol (lanes −) at nominally 0 or 1 μm free Ca2+ was performed as described in Methods. The phosphoproteins were resolved on 5 to 20% gradient polyacrylamide gels. The arrows indicate the positions of proteins with Ca2+-stimulated phosphorylation. The asterisk indicates the position of a protein with CsA-enhanced phosphorylation. The 120- and 52-kD proteins that exhibited Ca2+-stimulated phosphorylation (indicated by the highest and the lowest arrows) also exhibited CsA-enhanced phosphorylation.
Since the phosphorylation status of a given protein is determined by the balance of activities of protein kinases and protein phosphatases, we further examined the effect of CsA (a specific inhibitor of the Ca2+-dependent protein phosphatase calcineurin) on phosphorylation of guard cell proteins. We did not see any effect of CsA on phosphorylation of membrane proteins from GCPs (data not shown). However, in the presence of 1 μm Ca2+, 10 μm CsA did increase the phosphorylation of the 120-, 52-, and 35-kD polypeptides from the soluble fraction of GCPs (Fig. 6C, the highest and the lowest arrows and the asterisk). In the absence of Ca2+, CsA had no apparent effect on the phosphorylation of these proteins (Fig. 6C). Although the CsA-induced changes in protein phosphorylation were not as marked as those induced by Ca2+, they were consistently observed in four replicate experiments. These results suggest that a Ca2+-dependent protein phosphatase may be involved in regulating the Ca2+-dependent phosphorylation status of guard cell proteins.
Guard Cell CDPK Phosphorylates KAT1 Protein in a Ca2+-Dependent Manner
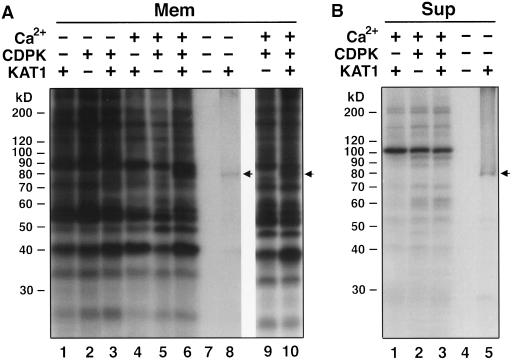
To determine whether the guard cell CDPK can phosphorylate the KAT1 protein, a DNA construct, pCITE-KAT1, which contains the KAT1 cDNA, was generated and verified by DNA sequencing. The KAT1 DNA was transcribed and translated using an in vitro transcription/translation system. A single 35S-labeled band at 79 kD, which is close to the calculated molecular mass of the KAT1 protein based on its sequence (Anderson et al., 1992), was detected in the membrane and supernatant fractions of the translated product when the KAT1 DNA was present as the template (Fig. 7, A, lane 8, and B, lane 5, and Fig. 8, lane 2). When the KAT1 DNA was omitted from the transcription/translation system, no 35S-labeled band was detected (Fig. 7, A, lane 7, and B, lane 4, and Fig. 8, lane 1). The 5′ end of the coding region of pCITE-KAT1 contains an S-tag; therefore, the translated products from pCITE-KAT1 were also detected on blots using the S-protein-alkaline phosphatase conjugate based on the specific, high-affinity interaction between the S-tag peptide and S-protein (Kim and Raines, 1993). A single band at 79 kD was identified by the S-protein probe on blots containing the translated product from pCITE-KAT1 (data not shown). These results indicate that the 79-kD protein is indeed the translated KAT1 protein from the KAT1 gene.
Figure 7.
CDPK phosphorylates KAT1 protein in a Ca2+-dependent manner. The membrane (Mem; A, lanes 1–6, and 10) or supernatant (Sup; B, lanes 1–3) fractions of the product translated in the presence of microsome membranes were subjected to phosphorylation by CDPK in the presence of [γ-32P]ATP and Ca2+ (A, lanes 4–6, 9 and 10; B, lanes 1–3) or EGTA (A, lanes 1–3) as described in Methods. A, Lane 8, and B, lane 5, [35S]Met-labeled translation product from the KAT1 cDNA template. A, Lane 7 and B, lane 4, [35S]Met-labeled translation product without DNA template. In lane 9, the microsome membranes were added to the translation system just after translation and then phosphorylated by CDPK in the presence of Ca2+. The arrows indicate the position of the 35S-labeled KAT1 protein; note the corresponding phosphorylated band in lanes 6 and 10. The protein samples (20 μg of protein per lane) were resolved on 9% polyacrylamide gels. −, Absent; +, present.
Figure 8.
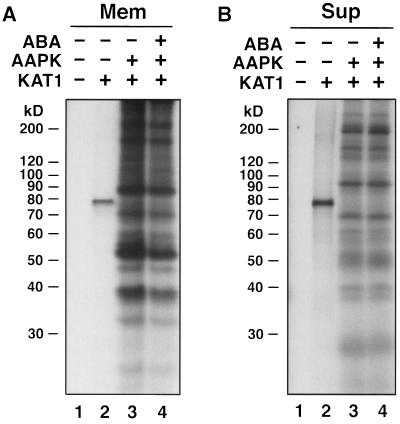
AAPK does not phosphorylate the KAT1 protein. The membrane (Mem, A, lanes 3 and 4) or supernatant (Sup, B, lanes 3 and 4) fractions of the product translated in the presence of microsome membranes were subjected to phosphorylation by AAPK purified from GCPs treated with ABA (lanes 4) or without ABA (lanes 3). Lanes 1, [35S]Met-labeled translation product without KAT1 cDNA template. Lanes 2, [35S]Met-labeled translation product from the KAT1 cDNA template. The arrows indicate the position of the 35S-labeled KAT1 protein. The protein samples (20 μg of protein per lane) were resolved on 9% polyacrylamide gels. −, Absent; +, present.
To examine phosphorylation of the KAT1 protein by the guard cell CDPK, the translated proteins were incubated with CDPK in the presence of [γ-32P]ATP. In the presence of Ca2+ and in the absence of CDPK, a number of phosphoproteins were found in the translation product in the membrane fraction (Fig. 7A, lane 4), indicating the presence of endogenous kinase activities in the in vitro transcription/translation system. However, in the presence of CDPK and Ca2+, a new phosphoprotein at 79 kD was detected in the translated product when the KAT1 cDNA was used as the template (Fig. 7A, lanes 6 and 10), but was not detected when the KAT1 DNA template was omitted (Fig. 7A, lane 5). In addition, when canine pancreatic microsomes were added to the translation system just after translation rather than concomitantly, the 79-kD phosphoprotein was not found in the membrane fraction of the translation product (Fig. 7A, lane 9), suggesting that the 79-kD phosphoprotein found in the membrane fraction (Fig. 7A, lanes 6 and 10) is not an endogenous membrane protein but rather the translation product that translocated or inserted into the membrane during translation. In contrast, in the presence of EGTA, no 79-kD phosphoprotein was observed in any of the three treatments (Fig. 7A, lanes 1–3). These data show that CDPK can, in a Ca2+-dependent fashion, phosphorylate the translated KAT1 protein in the membrane fraction. When the translated products in the supernatant fraction were analyzed for phosphorylation, no 79-kD phosphoprotein was found despite the fact that 35S labeling confirmed the presence of KAT1 protein in the supernatant as well as the membrane fraction (Fig. 7B). In sum, these results suggest that KAT1 can be phosphorylated by CDPK only when it is membrane localized.
We next examined whether the KAT1 protein can be phosphorylated by another guard cell protein kinase, AAPK, which is Ca2+ independent and ABA activated (Li and Assmann, 1996). As shown in Figure 8, no 79-kD phosphoprotein was found in either the membrane or the supernatant fractions of the translated products, suggesting that AAPK could not phosphorylate the KAT1 protein.
DISCUSSION
A 57-kD protein kinase has been identified in V. faba GCPs. Autophosphorylation of this kinase, like CDPKs (Harmon et al., 1987; Binder et al., 1994), is Ca2+ dependent (Figs. 1 and 3, A and B). Both in solution and in gel, activity assays show that the catalytic activity of this kinase is Ca2+ dependent (Fig. 2 and Fig. 3, C and D). Furthermore, this kinase, like soybean CDPK (one of the best-characterized CDPKs), exhibits a Ca2+-induced electrophoretic mobility shift on SDS-PAGE gels (Figs. 3 and 4). The Ca2+-induced electrophoretic mobility shift of this kinase suggests that it may contain a calmodulin-like domain (Harper et al., 1991; Roberts and Harmon, 1992). Evidence further supporting this notion comes from the following observations: (a) this kinase can be specifically recognized by affinity-purified antibodies to the calmodulin-like domain of soybean CDPKα on both one- and two-dimensional blots (Figs. 3 and 5); (b) the Ca2+-dependent catalytic activity can be inhibited by the calmodulin antagonists TFP and W-7 but not by the inactive analog W-5 (Fig. 2). Taken together, all of the data indicate that the 57-kD protein kinase from V. faba GCPs is a Ca2+-dependent protein kinase with a calmodulin-like domain (CDPK).
It has been well documented from physiological studies of stomata that Ca2+ plays very important roles in mediating stomatal closure and opening (for review, see McAinsh et al., 1997). However, signaling intermediaries between Ca2+ and stomatal responses have not been identified. CDPKs are encoded by a large gene family and members of the CDPK family expressed in different cell types may have distinct roles in signal transduction (Estruch et al., 1994; Abo-El-Saad and Wu, 1995; Hrabak et al., 1996). Biochemical identification of a CDPK in guard cells, one of the most specialized cell types in plants, strengthens the notion that CDPK mediates at least a subset of Ca2+-regulated stomatal responses (Assmann, 1993).
To examine potential targets of CDPK in guard cells, phosphorylation of guard cell proteins was performed at various Ca2+ concentrations. Physiological levels of free Ca2+ (1 μm) promote the phosphorylation of a number of soluble and membrane proteins from GCPs (Fig. 6, A and B). A similar experiment was carried out by Kinoshita and Shimazaki (1995). However, in contrast to their observations, we did not see Ca2+-stimulated phosphorylation of proteins at 41, 31, and 25 kD in either the soluble or membrane fractions (Fig. 6, A and B). Instead, we found that 1 μm free Ca2+ stimulated phosphorylation of several proteins with molecular masses different from those that they observed (Fig. 6, A and B). The differences may be due to different plant growth environments (Kinoshita and Shimazaki [1995] used greenhouse grown plants) or different methods used for isolating GCPs. Differences in guard cell Cl− content from V. faba plants grown in a growth chamber versus a greenhouse have been reported (Talbott and Zeiger, 1996), suggesting that different growth environments may also affect other cellular processes, such as protein phosphorylation.
The level of phosphorylation is determined by the relative activities of protein kinases and protein phosphatases. In this light, it is interesting to note that the Ca2+-stimulated phosphorylation of the 120- and 52-kD soluble polypeptides from GCPs is enhanced by CsA (Fig. 6C), a specific inhibitor of the Ca2+-dependent protein phosphatase calcineurin (Liu et al., 1992). A Ca2+-dependent phosphatase activity that is inhibited by CsA has been detected in epidermal peels of V. faba (Luan et al., 1993). In addition, CsA has been recently shown to antagonize ABA-induced stomatal closure and ABA-inhibited stomatal opening (Hey et al., 1997). Our biochemical data suggest that CsA-sensitive Ca2+-dependent protein phosphatase(s) as well as CDPK are present not only in the epidermis but specifically in guard cells, and that they are involved in the Ca2+-regulated phosphorylation of guard cell proteins and therefore Ca2+-regulated stomatal responses.
The phosphorylation of several proteins was enhanced by micromolar levels of Ca2+ (Fig. 6), suggesting that these proteins are potential targets of the guard cell CDPK. However, without other information, the identities of these proteins are very difficult to determine because of the limited amount of proteins that can be obtained from GCPs. In particular, ion channel proteins, which are key players in osmotic regulation of stomatal aperture, are present at very low concentrations (Sussman and Harper, 1989). The low abundance and water-insoluble nature of channel proteins make biochemical analysis of ion channels very difficult. Sequence analysis of KAT1, the inward K+ channel gene primarily expressed in guard cells (Nakamura et al., 1995), suggests that the KAT1 protein contains a number of potential phosphorylation sites for protein kinases. Therefore, it would be reasonable to examine whether the KAT1 protein can be phosphorylated by the CDPK from guard cells.
Although electrophysiological techniques have been widely used to study ion channel regulation, including phosphorylation, these approaches cannot distinguish whether regulation of an ion channel by phosphorylation is due to direct phosphorylation of the channel protein or whether the regulation is due to phosphorylation of some intermediate protein(s), which in turn affects the channel activity. To circumvent these problems, we translated the cloned KAT1 gene in vitro in the presence of canine pancreatic microsomal membranes. The inclusion of microsome membranes in the in vitro translation system has been widely used to study membrane insertion and translocation of channel proteins (Miao et al., 1992; Dunlop et al., 1995). The translated 35S-labeled-KAT1 protein was found in both membrane and supernatant fractions (Figs. 7 and 8), as was also true for the expression of a rat brain K+ channel gene RCK1 in Xenopus oocytes (Ivanina et al., 1994). However, guard cell CDPK only phosphorylated the translated KAT1 protein in the membrane fraction and only if microsome membranes were provided during the translation step (Fig. 7). Since it is known that, upon insertion into the membrane, integral proteins fold into a conformation that exposes the hydrophobic residues to the lipid bilayer (Singer, 1990), our observation implies that CDPK may phosphorylate the KAT1 protein only when it is correctly configured in the membrane. The fact that phosphorylation of KAT1 by CDPK is not indiscriminate suggests that this phosphorylation will be of physiological relevance for channel regulation. Moreover, the phosphorylation of the KAT1 protein seems to be specific to CDPK, since another guard cell protein kinase, AAPK, which is Ca2+ independent and ABA activated (Li and Assmann, 1996), was not able to phosphorylate the KAT1 protein (Fig. 8). Because of the very low abundance of channel proteins in cells (Sussmann and Harper, 1989), phosphorylation of channel proteins such as the KAT1 protein homolog is not expected to be detected in the microsome membrane fraction of GCPs (Fig. 6B). Furthermore, the guard cell inward K+ channel from V. faba has not yet been purified or cloned, so it cannot be identified by molecular mass.
Preliminary studies involving expression of KAT1 in Xenopus oocytes have shown that inward K+ currents were in fact greatly reduced when CDPK was co-expressed with KAT1 (Kamasani et al., 1997). Electrophysiological studies of guard cells have shown that the inward K+ channel can be inhibited by increased cytosolic Ca2+ (Schroeder and Hagiwara, 1989; MacRobbie, 1997). However, it is not clear from the study of Kamasani et al. (1997) whether CDPK directly phosphorylates the KAT1 protein or whether it phosphorylates other proteins. Such proteins could be regulators of KAT1 activity or other ion transporters such as the H+ or Ca2+ ATPase, modulation of which could result in altered intracellular ion concentrations that might in turn affect K+ channel activity.
Our data showing that CDPK phosphorylates the KAT1 protein itself in a Ca2+-dependent manner suggests that the inhibition of the inward K+ channel in guard cells by Ca2+ could be mediated by direct Ca2+-dependent phosphorylation of the inward K+ channel by CDPK. At first sight, this result seems contradictory to the results from the study by Luan et al. (1993), whose electrophysiological data suggested that the inhibition of the inward K+ channel in guard cells by Ca2+ was mediated by a Ca2+-dependent dephosphorylation mechanism. However, it is still not known whether Ca2+ stimulates dephosphorylation of the inward K+ channel itself or whether regulatory proteins are the dephosphorylation target. Even if the channel were to be directly dephosphorylated by a Ca2+-dependent phosphatase, Ca2+-dependent dephosphorylation and Ca2+-dependent phosphorylation would not necessarily occur at the same site(s), since the KAT1 K+ channel protein is rich in potential phosphorylation sites. In addition, in vitro phosphorylation data showed that Ca2+-dependent phosphorylation of certain soluble proteins from GCPs could be enhanced by CsA, a specific inhibitor of Ca2+-dependent protein phosphatase (Fig. 6C).
Taken together, our results and those of Luan et al. (1993) imply that the effect of Ca2+ on the inward K+ channel may involve phosphorylation and dephosphorylation on multiple sites of the K+ channel protein or multiple routes of phosphorylation and dephosphorylation to achieve a fine modulation of the K+ channel in response to a variety of environmental stimuli. Further studies of KAT1 incorporated into lipid bilayers (Rosenberg and East, 1992) or expressed in Xenopus oocytes should shed new light on the regulation of this inward K+ channel.
ACKNOWLEDGMENTS
We would like to thank Dr. Alice Harmon for providing CDPK antibodies, Drs. Leon Kochian, Gerald Berkowitz, and Julian Schroeder for providing KAT1 cDNA plasmids, and Dr. Simon Gilroy for helpful discussion concerning initial experiments.
Abbreviations:
- AAPK
ABA-activated protein kinase
- CDPK
calcium-dependent protein kinase containing a calmodulin-like domain
- CsA
cyclosporin A
- GCP
guard cell protoplast
- KAT1
a potassium channel cDNA from Arabidopsis
- TFP
trifluoperazine
- W-5
N-(6-aminohexyl)-1-naphthalenesulfonamide
- W-7
N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide
Footnotes
This research was supported by National Science Foundation grant no. MCB-9316319 to S.M.A.
LITERATURE CITED
- Abo-El-Saad M, Wu R. A rice membrane calcium-dependent protein kinase is induced by gibberellin. Plant Physiol. 1995;108:787–793. doi: 10.1104/pp.108.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR. Sensitivity to abscisic acid of guard cell K+ channels is suppressed by abi-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc Natl Acad Sci USA. 1995;92:9520–9524. doi: 10.1073/pnas.92.21.9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Bachmann M, Shiraishi N, Campbell WH, Yoo B-C, Harmon AC, Huber SC. Identification of Ser-543 as the major regulatory phosphorylation site in spinach leaf nitrate reductase. Plant Cell. 1996;8:505–517. doi: 10.1105/tpc.8.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A, Anderson JA, Slayman CL, Gaber RF. Use of Saccharomyces cerevisiae for patch-clamp analysis of heterologous membrane proteins: characterization of Kat1, an inward-rectifying K+ channel from Arabidopsis thaliana, and comparison with endogenous yeast channels and carriers. Proc Natl Acad Sci USA. 1995;92:2701–2705. doi: 10.1073/pnas.92.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Harper JF, Sussman MR. Characterization of an Arabidopsis calmodulin-like domain protein kinase purified from Escherichia coli using an affinity sandwich technique. Biochemistry. 1994;33:2033–2041. doi: 10.1021/bi00174a008. [DOI] [PubMed] [Google Scholar]
- Blatt MR, Thiel G, Trentham DR. Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1990;346:766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang D, Hsieh PS, Dawson DC. Calcium: a program in BASIC for calculating the composition of solutions with specified free concentrations of calcium, magnesium and other divalent cations. Comput Biol Med. 1988;18:351–366. doi: 10.1016/0010-4825(88)90022-4. [DOI] [PubMed] [Google Scholar]
- De Silva DLR, Hetherington AM, Mansfield TA. Synergism between calcium ions and abscisic acid in preventing stomatal opening. New Phytol. 1985;100:473–482. [Google Scholar]
- Dunlop J, Jones PC, Finbow ME. Membrane insertion and assembly of ductin: a polytopic channel with dual orientations. EMBO J. 1995;14:3609–3616. doi: 10.1002/j.1460-2075.1995.tb00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch JJ, Kadwell S, Merlin E, Crossland L. Cloning and characterization of a maize pollen-specific calcium-dependent calmodulin-independent protein kinase. Proc Natl Acad Sci USA. 1994;91:8837–8841. doi: 10.1073/pnas.91.19.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Fricker MD, Read ND, Trewavas AJ. Role of calcium in signal transduction of Commelina guard cells. Plant Cell. 1991;3:333–344. doi: 10.1105/tpc.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon AC, Putnam-Evans C, Cormier MJ. A calcium-dependent but calmodulin-independent protein kinase from soybean. Plant Physiol. 1987;83:830–837. doi: 10.1104/pp.83.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Sussman MR, Schaller GE, Putnam-Evans C, Charbonneau H, Harmon AC. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991;252:951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Hedrich R, Busch H, Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990;9:3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey SJ, Bacon A, Burnett E, Neill SJ. Abscisic acid signal tranduction in epidermal cells of Pisum sativum L. Argenteum: both dehydrin mRNA accumulation and stomatal responses require protein phosphorylation and dephosphorylation. Planta. 1997;202:85–92. [Google Scholar]
- Hochstrasser DF, Harrington MG, Hochstrasser A-C, Miller MJ, Merril CR. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988;173:424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak EM, Dickmann LJ, Satterlee JS, Sussmann MR. Characterization of eight new members of the calmodulin-like domain protein kinase gene family of Arabidopsis thaliana. Plant Mol Biol. 1996;31:405–412. doi: 10.1007/BF00021802. [DOI] [PubMed] [Google Scholar]
- Ivanina T, Perets T, Thornhill WB, Levin G, Dascal N, Lotan I. Phosphorylation by protein kinase A of RCK1 K+ channels expressed in Xenopus oocytes. Biochemistry. 1994;33:8786–8792. doi: 10.1021/bi00195a021. [DOI] [PubMed] [Google Scholar]
- Kamasani U, Zhang X, Lawton M, Berkowitz GA (1997) Ca2+-dependent protein kinase modulates activity of the K+ channel KAT1 (abstract no. 980). Plant Physiol 114: S-197
- Kameshita I, Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Keen JH, Chestnut MH, Beck KA. The clathrin coat assembly polypeptide complex: autophosphorylation and assembly activities. J Biol Chem. 1987;262:3864–3871. [PubMed] [Google Scholar]
- Kim JS, Raines RT. Ribonuclease S-peptide as a carrier in fusion proteins. Protein Sci. 1993;2:348–356. doi: 10.1002/pro.5560020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Shimazaki K. Cytosolic concentration of Ca2+ regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell. 1995;7:1333–1342. doi: 10.1105/tpc.7.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. Evidence for Ca2+-dependent protein phosphorylation in vitro in guard cells from Vicia faba L. Plant Sci. 1995;110:173–180. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EAC. Role of calcium in the modulation of Vicia guard cell potassium channels by abscisic acid: a patch clamp study. J Membr Biol. 1994;137:99–107. doi: 10.1007/BF00233479. [DOI] [PubMed] [Google Scholar]
- Li B, Chollet R. Resolution and identification of C4 phosphoenolpyruvate-carboxylase protein-kinase polypeptides and their reversible light activation in maize leaves. Arch Biochem Biophys. 1993;307:416–419. doi: 10.1006/abbi.1993.1609. [DOI] [PubMed] [Google Scholar]
- Li J, Assmann SM. An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell. 1996;8:2359–2368. doi: 10.1105/tpc.8.12.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V, Assmann SM. Cellular distribution of calmodulin and calmodulin-binding proteins in Vicia faba L. Plant Physiol. 1992;100:970–978. doi: 10.1104/pp.100.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Albers MW, Wandless TJ, Luan S, Alberg DG, Belsshaw PJ, Cohen P, MacKintosh C, Klee CB, Schreiber SL. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Luan S, Li W, Rusnak F, Assmann SM, Schreiber SL. Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard cells. Proc Natl Acad Sci USA. 1993;90:2202–2206. doi: 10.1073/pnas.90.6.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC. Signalling in guard cells and regulation of ion channel activity. J Exp Bot. 1997;48:515–528. doi: 10.1093/jxb/48.Special_Issue.515. [DOI] [PubMed] [Google Scholar]
- Marten I, Gaymard F, Lemaillet G, Thibaud J-B, Sentenac H, Hedrich R. Functional expression of the plant K+ channel KAT1 in insect cells. FEBS Lett. 1996;380:229–232. doi: 10.1016/0014-5793(96)00042-7. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Calcium ions as second messengers in guard cell signal transduction. Physiol Plant. 1997;100:16–29. [Google Scholar]
- Miao GH, Hong Z, Verma DPS. Topology and phosphorylation of soybean nodulin-26, an intrinsic protein of the peribacteroid membrane. J Cell Biol. 1992;118:481–490. doi: 10.1083/jcb.118.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WL, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z-M, Ward JM, Harper JF, Schroeder JI. A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 1996;15:6564–6574. [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Harmon AC. Calcium-modulated proteins: targets of intracellular calcium signals in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:375–414. [Google Scholar]
- Rosenberg RL, East JE. Cell-free expression of functional Shaker potassium channels. Nature. 1992;360:166–169. doi: 10.1038/360166a0. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schelle I, Liao Y, Schroeder JI. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA. 1995;92:9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Schroeder JI, Ward JM, Gassmann W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- Schwartz A. Role of Ca2+ and EGTA on stomatal movements in Commelina communis L. Plant Physiol. 1985;79:1003–1005. doi: 10.1104/pp.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Ilan N, Grantz DA. Calcium effects on stomatal movements in Commelina communis. Plant Physiol. 1988;87:583–587. doi: 10.1104/pp.87.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Kinoshita T, Nishimura M. Involvement of Ca2+/calmodulin-dependent myosin light chain kinase in blue light-dependent H+ pumping of guard cell protoplasts from Vicia faba L. Plant Physiol. 1992;99:1416–1421. doi: 10.1104/pp.99.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short TW, Porst M, Briggs WR. A photoreceptor system regulating in vivo and in vitro phosphorylation of a pea plasma membrane protein. Photochem Photobiol. 1992;55:773–781. [Google Scholar]
- Singer SJ. The structure and insertion of integral proteins in membranes. Annu Rev Cell Biol. 1990;6:247–296. doi: 10.1146/annurev.cb.06.110190.001335. [DOI] [PubMed] [Google Scholar]
- Smith JA, Francis SH, Corbin JD. Autophosphorylation: a salient feature of protein kinases. Mol Cell Biochem. 1993;127:51–73. doi: 10.1007/BF01076757. [DOI] [PubMed] [Google Scholar]
- Sussman MR, Harper JF. Molecular biology of the plasma membrane of higher plants. Plant Cell. 1989;1:953–960. doi: 10.1105/tpc.1.10.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. Central roles for potassium and sucrose in guard-cell osmoregulation. Plant Physiol. 1996;111:1051–1057. doi: 10.1104/pp.111.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon T. Electrophoretic transfer of proteins from polyacrylamide gel to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B, Zhang Y, Delikat S, Mathias S, Basu S, Kolesnick R. Phosphorylation of Raf by ceramide-activated protein kinase. Nature. 1995;378:307–310. doi: 10.1038/378307a0. [DOI] [PubMed] [Google Scholar]