Abstract
Background:
Intricate relationship between epithelium and connective tissue is believed to play a significant role in pathogenesis of odontogenic lesions. Role of epithelium in its pathogenesis is well established and at the same time role of mesenchyme cannot be underestimated.
Aim:
To investigate, compare and correlate different types and pattern of collagen fibers in odontogenic cysts using picrosiriusred stain under polarizing microscopy in order to delineate its exact role in biological behavior of these lesions.
Materials and Methods:
The color and orientation of collagen fibers in the wall of 15 odontogenickeratocysts, 15 dentigerous cysts and 15 radicular cysts and 5 progressive stages of odontogenesis was studied histochemically by staining sections with picrosirius red and examining under polarizing microscope. Results were statistically analyzed using SPSS software.
Results:
In OKC, DC and progressive stages of odontogenesis the predominant color of thin collagen fibers birefringence was found to be greenish yellow and in radicular cyst it was orange red whereas thick fibers in OKC showed yellow orange color whereas DC and RC showed orange red color. OKC showed principally parallel orientation of collagen fibers in loosely packed stroma in contrast to dentigerous and radicular cyst.
Conclusion:
Quality, organization and packing of collagen fibers is different in 3 cystic lesions which accounts for difference in biological behavior of these lesions and it justifies that neoplastic growth requires a functional stroma and the ability of neoplastic cells to induce the formation of such a stroma is of great importance.
Keywords: Cyst, neoplasm, odontogenic, picrosiriusred
INTRODUCTION
Many attempts have been made to understand the pathogenesis and biological behavior of various processes which has helped to render best health care measures to mankind. It is now well accepted that the coordinated activity of epithelial cells with their stroma is fundamental in controlling growth and differentiation in normal and pathological situations.[1]
Epithelial mesenchymal interactions occurring during odontogenesis give rise to teeth at one end and bewildering varieties of pathologies in the form of odontogenic cysts and tumors at another.[2] Despite the fact that proliferation of epithelial cells is an indispensable ingredient for cyst formation, connective tissue may be regarded as a functional part of cyst and not just a structural support. Interplay between the epithelium and connective tissue can be assumed to play a significant role in the pathogenesis of odontogenic cysts.[3] Experimental studies have shown that the transplanted epithelium formed new cysts only if supported by its own stroma.[4]
Collagen which constitutes 34% of the total extracellular matrix (ECM) proteins forms the integral part of connective tissue stroma and plays a vital role in maintaining structural integrity and in determining tissue function.[5,6] Albeit, immunohistochemistry and molecular techniques have a wide application in investigative/diagnostic purposes, they have diminutive impact in demonstrating the cystic fibers and conventional histopathology along with histochemical stains continues to be the mainstay for various investigative procedures. Collagen has natural birefringence which is attributed to the arrangement of its fibers which is enhanced by special stains like van gieson, masson's trichrome and picrosiriusred.[7] Van Gieson and trichrome stains may not be ideal for detection of collagen fibers as both these methods fail to reveal thin collagen fibers, a disadvantage which can lead to a under estimation of collagen content. This perplexing issue incited Puchtler and colleagues to seek a better method and they found that Sirius red F3BA (Colour Index 35780) dissolved in a saturated picric acid solution (picrosiriusred) consistently stained thin collagen fibers, did not fade, and was appropriate for use with polarized light microscopy.[6] Examination of collagen fibers by picrosirius red in conjunction with polarizing microscope can serve as a procedure to differentiate procollagens, intermediate and pathological collagen fibers, which are not tightly packed, from normal packed fibers.[8]
Alterations in staining capacity of collagen have been observed in oral submucous fibrosis, by many authors who indicated the changes at biochemical level that, in turn, may account for the connective tissue based alterations thought to contribute to the atypical changes that often occur in overlying epithelium.[9] The role of the ECM in the pathogenesis and behavior of epithelial odontogenic lesions has been demonstrated in some publications, suggesting intricate relationship between epithelium and mesenchyme[10] but still there is lack of understanding in the precise role of mesenchyme in behavior of odontogenic lesions. So this study aimed to investigate, compare and correlate different types of collagen fibers in odontogenic cysts using a special stain (picrosiriusred stain) under polarizing microscopy and to further correlate the different color patterns and orientation of collagen fibers to the biological behavior of these lesions.
MATERIALS AND METHODS
The material for the study included 50 formalin-fixed paraffin-embedded tissue blocks retrieved from the Department of Oral Pathology and Microbiology, ITS Dental College, Ghaziabad. Histopathologically confirmed 15 cases of OKC, Dentigerous cysts, Radicular cysts each and 5 tissues of progressive stages of odontogenesis as control were selected according to criteria proposed by WHO in 1992.[11] Sectioned of 4-μm thickness were taken and floated on to the slides and incubated at 47°C on slide warmer for ensuring adhesion of the sections to the slides. Following deparaffinization and hydration in distilled water; the sections were incubated in 0.1% (w/v) Sirius red F3B in saturated Picric acid solution for 1 hour at room temperature. This was followed by rinsing with distilled water, staining with Mayer's haematoxylin, differentiation in 1% HCl, alkalinization with tap water, dehydration and mounting. The sections were then examined under bright field and polarizing microscope. Collagen fibers appear uniformly dark Pink under Bright field microscope and birefringence colors under polarized light microscopy. Polarization colors were determined separately for thin and thick fibers. With the application of image analyzer software (Biowizard; Dewinter) fiber thickness measuring 0.8μm or less was grouped under thin fibers and thickness between 1.4μm and 2.4μm were grouped under thick fibers. In each section, three separate high power fields with at least 50 fibers of each size were examined. The connective tissue in these slides showed polarization colors varying from greenish-yellow to yellow-orange to orange red. The color of the collagen fibers was noted by two independent observers to eliminate the subjective bias.
Collagen Fiber orientation in relation to the epithelial component was also observed. The analysis was performed at ×100 and ×400 magnifications. Each case was classified according to the fiber orientation pattern as 1: Not parallel; or 2: Parallel. The organization of collagen fibers was also evaluated in areas near and distant from the epithelial component. Each case was classified as 1: Loose bundles of collagen fibers (loosely arranged and interwoven in all directions); or 2: Dense (well-defined organization with orderly organized collagen fibers forming collagen lamellae). Statistical analysis was done using SPSS version 14 (Statistical Package for Social Sciences, Chicago, Illinois, USA) and findings obtained were subjected to F-test, one-way ANOVA and Chi-square test.
RESULTS
In the present study, picrosirius red stained tissues under polarizing microscopy showed different birefringence which were recorded and statistically analyzed by using one way ANOVA test. The progressive stages of embryonic odontogenic tissue was taken as control which showed prodominantly greenish-yellow birefringence interspersed with some amount of yellowish-orange birefringence towards basement membrane [Figure 1].
Figure 1.
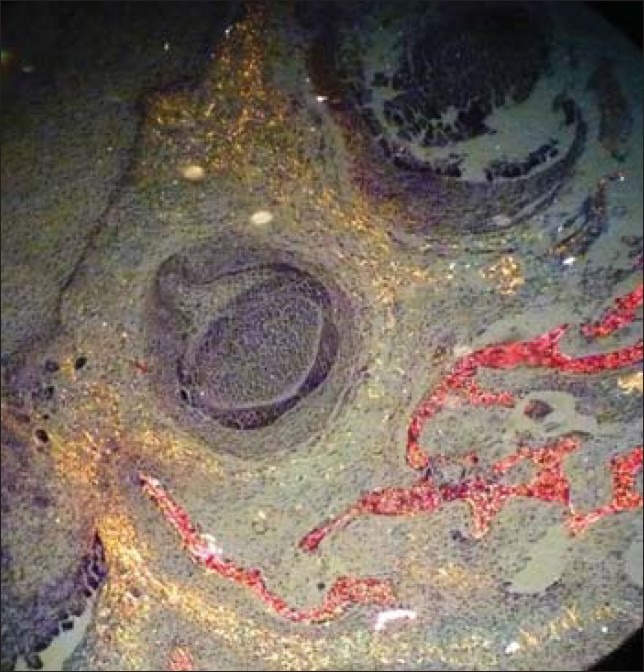
Embryonic odontogenic tissue showed predominantly greenish yellow colored fibers (PSR; ×10)
25.745±1.718 thin fibers of OKC showed greenish yellow birefringence [Figures 2 and 3] which was significantly higher than dentigerouscyst [Figures 4 and 5] and radicular cyst [Figures 6 and 7] which showed 20.638±2.116 and 15.598±1.6, respectively. Yellow orange birefringence in OKC was observed in 22.28±2.602 thick fibers which were statistically non-significant with dentigerous cyst and radicular cyst which revealed 20.325±2.371 and 16.85±2.047 thick fibers respectively whereas orange red birefringence of 19.86±1.321 thick fibers of radicular cyst was significantly higher than 13.203±1.382 of dentigerous cyst and 4.78±1.071 of radicular cyst [Table 1].
Figure 2.
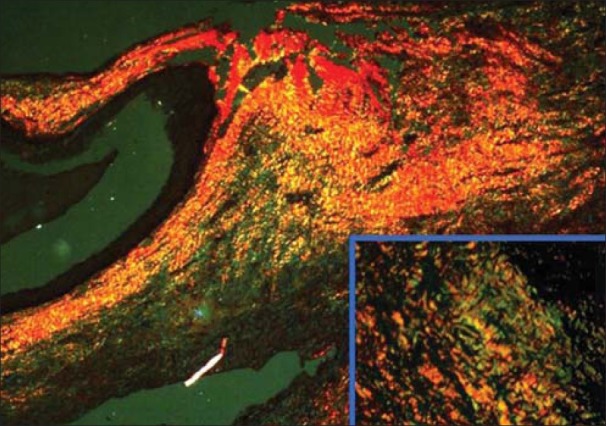
Odontogenickeratocyst showing greenish yellow and yellowish orange collagen fibers (PSR; ×10) (inset; ×40)
Figure 3.
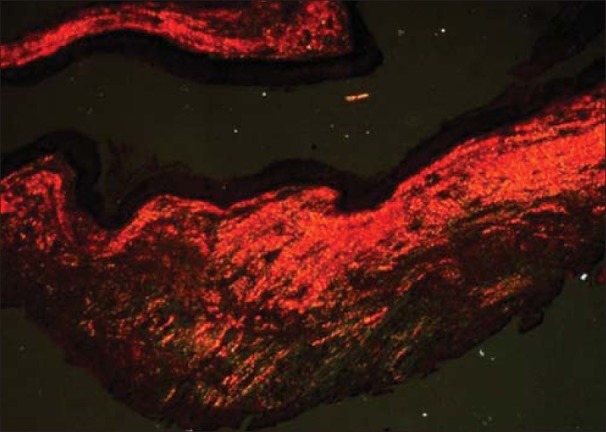
Odontogenickeratocyst showing yellowish orange collagen fibers (PSR; ×10)
Figure 4.
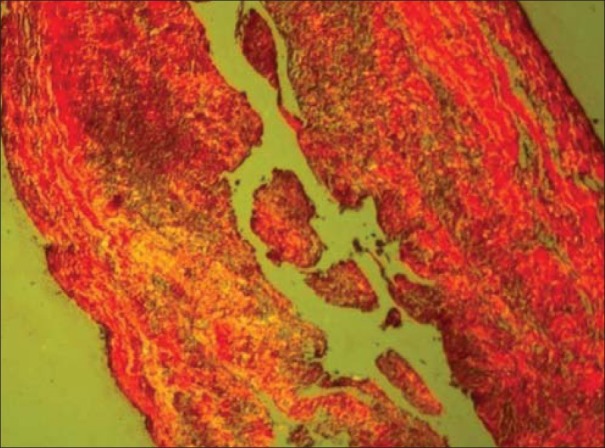
Dentigerous cyst showing yellowish orange and orange red collagen fibers (PSR; ×10)
Figure 5.
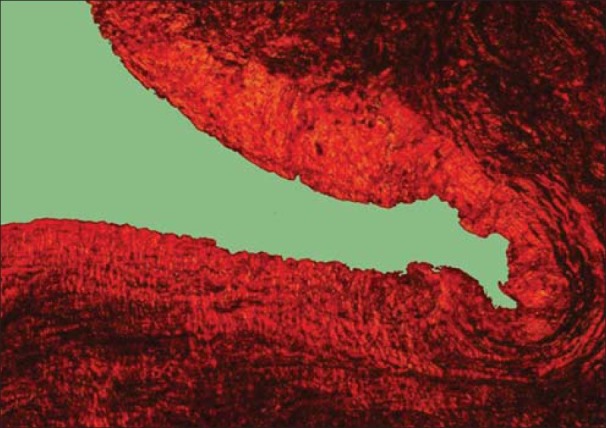
Dentigerous cyst showing orange red collagen fibers (PSR; ×10)
Figure 6.
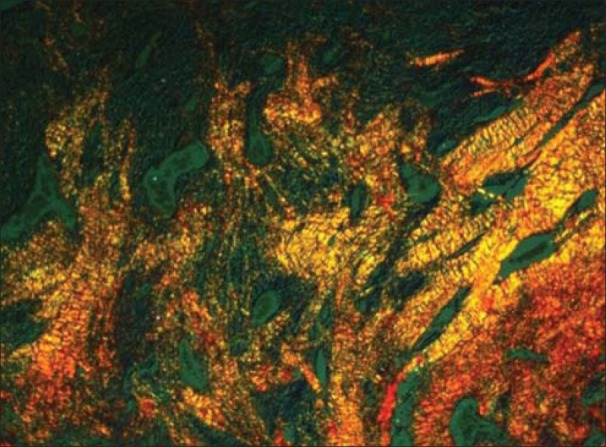
Radicular cyst showing greenish yellow and yellowish orange collagen fibers (PSR; ×40)
Figure 7.
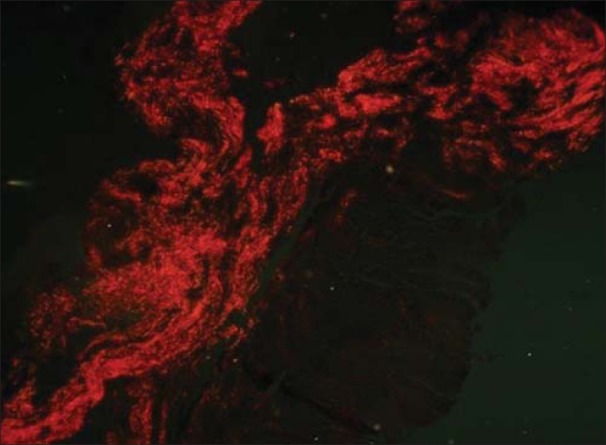
Radicular cyst showing orange red collagen fibers (PSR; ×40)
Table 1.
Distribution of polarization colors of thin collagen fibers in different groups

Greenish yellow birefringence observed in 11.05±2.946 thin fibers of OKC were statistically non-significant in relation to dentigerous cyst and radicular cyst which showed 9.750±1.708 and 8.625±1.797 respectively. Yellowish orange birefringence of thin fibers also showed statistically non-significant difference among all the three groups whereas radicular cyst showed statistically significantly high number of orange red birefringence of thick fibers, i.e. 28.24±1.500 than DC and OKC which showed 23.84±1.915 and 13.25±1.88 [Figures 2–7, Table 2].
Table 2.
Distribution of polarization colors of thick collagen fibers in different groups

Orientation and packing of collagen fibers in relation to epithelial surface were also recorded. It was revealed that 12/15 (80%) cases of OKC and 6/15 (40%) cases of DC and 2/15 (13.33%) cases of RC showed parallel arrangement of collagen fibers. This different pattern of collagen orientation was found out to be statistically significant (P<0.05) [Table 3].
Table 3.
Orientation of collagen fibers in odontogenic cysts

DISCUSSION
Most studies have focused on the evaluation of the proliferative activity in the epithelial component of the odontogeniclesions[12–14] but role of mesenchyme cannot be underestimated. Only few studies have focused on the importance of epithelial-mesenchymalinteractions for the expansion of odontogenic cysts. Stroma is essential for maintaining the epithelial tissues and both these make up an ecosystem with continuous molecular interactions. Stromal changes in cystic lesions can be depicted by picrosirius red stain and this stain imparts birefringence to collagen fibers only.[7] PSR impart different colors in shades of green-yellow to orange-red to collagen fibers in various cystic lesions.
Greenish yellow birefringence of thin fibers was significantly higher in OKC whereas thick fibers showed non-significant difference among 3 groups. Similar pattern of birefringence is observed in collagen fibers in progressive stages of odontogenesis. Hence, the greenish-yellow birefringence imparted in OKC can be attributed to the young and immature collagen fibers. According to Hirshberg [15] these greenish yellow collagen fibers in OKC probably represents procollagen, intermediate or pathological collagen. This could also probably partially validate the aggressive behavior of OKC.
Orange red birefringence of thin and thick fibers was significantly higher in radicular cyst whereas non-significant difference was observed between yellowish orange colored fibers. This difference in color patterns of collagen fibers in radicular cyst represents an accumulation of mature closely packed fibers and can be attributed to an altered microenvironment. Inflammation in radicular cyst releases various cytokines and growth factors which cause proliferation of fibroblasts and extra-cellular matrix which results in formation of thick mature collagen. During maturation of collagen fibers, there is change in proteoglycan content of fibers causing dehydration of fibers resulting in increase in diameter of collagen fibers and intensity of birefringence. Hence, the change in polarizing colors.[16]
Junqueira et al. stated that under pathological conditions birefringence shows a different pattern in comparison with collagen in normal tissue and proved that type I collagen were thick, strongly birefringent red fibers while type III collagen appeared as thin weakly birefringent green fibers.[17] So, we probably feel that cystic lesions have different types of collagen fibers as they show different pattern of birefringence. Recently Moure et al.[10] quantitatively analyzed the collagen component by confocal laser microscopy and revealed that all the 3 cystic lesions had similar collagen content. However, they were unable to separate the types of collagen and they hypothesized that different pattern of collagen fibers obtained in different cystic lesions in their study could be due to different types of collagen besides the alteration in local microenvironment. But our present study was able to differentiate the types of collagen fibers; however, further investigations on the biochemical and molecular basis are warranted to exactly delineate the role of collagen and mesenchyme in behavior of odontogenic lesions.
Majority of our cases of OKC (80%) showed parallel arrangement of collagen fibers in relation to epithelial surface whereas dentigerous (40%) and radicular cyst (10%) showed lesser percentage of it. This loose and parallel arrangement of collagen fibers observed in OKC may act as an additional factor in facilitating the separation of the epithelial lining from the underlying connective tissue wall. However, collagen fibers showed different morphological features regarding orientation and arrangement. The quality and organization of collagen fibers significantly affect the tensile strength of a connective tissue and consequently its capacity to support tissues and organs.[10] Dentigerous connective tissue with non-parallel fibers is a reactive tissue inducing an expansile growth associated with fluid accumulation while OKC with its loosely and parallel arranged collagen fibers may be associated with behavior of neoplastic epithelium.
CONCLUSION
Neoplastic growth requires a functional stroma, the ability of neoplastic cells to induce the formation of such a stroma is of great importance. Dentigerous connective tissue with non-parallel fibers is a reactive tissue inducing an expansile growth associated with fluid accumulation while OKC with its loosely and parallel arranged collagen fibers may be associated with behavior of neoplastic epithelium.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: A key player in the control of tumor cell behavior. Int J DevBiol. 2004;48:509–17. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 2.Prabhu SR, Wilson DF, Daftary DK, Johnson NW. Oral diseases in the tropics. 1st ed. Oxford: Oxford University Press; 1992. p. 367. [Google Scholar]
- 3.Pindborg JJ, Hansen J. Studies on odontogenic cyst epithelium. 2. Clinical and roentgenologic aspects of odontogenic keratocysts. Acta Pathol Microbiol Scand. 1963;58:283–94. [PubMed] [Google Scholar]
- 4.Vedtofte P, Holmstrup P, Dabelsteen E. Human odontogenic keratocyst transplants in nude mice. Scand J Dent Res. 1982;90:306–14. doi: 10.1111/j.1600-0722.1982.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal P, Saxena S. Stromal differences in odontogenic cysts of a common histopathogenesis but with different biological behavior: A study with picrosirius red and polarizing microscopy. Indian J Cancer. 2011;48:211–5. doi: 10.4103/0019-509X.82897. [DOI] [PubMed] [Google Scholar]
- 6.Whittaker P, Rich L. Collagen and picrosirius red staining: A polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22:97–104. [Google Scholar]
- 7.Constantine VS, Mowry RW. Selective staining of human dermal collagen. II. The use of picrosirius red F3BA with polarization microscopy. J Invest Dermatol. 1968;50:419–23. doi: 10.1038/jid.1968.68. [DOI] [PubMed] [Google Scholar]
- 8.Vij R, Vij H, Rao NN. Evaluation of collagen in connective tissue walls of odontogenic cysts–A histochemical study. J Oral Pathol Med. 2011;40:257–62. doi: 10.1111/j.1600-0714.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamner JE, 3rd, Mehta FS, Pindborg JJ, Daftry DK. Altered staining reaction of connective tissues in 53 submucousfibrosis patients. J Dent Res. 1971;50:388–92. doi: 10.1177/00220345710500024601. [DOI] [PubMed] [Google Scholar]
- 10.Moure SP, Carrard VC, Lauxen IS, Manso PP, Oliviera MG, Martins MD, et al. Collagen and elastic fibers in odontogenic entities: Analysis using light and confocal laser microscopic methods. Open Dent J. 2011;5:116–21. doi: 10.2174/1874210601105010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philipsen HP. Keratocystic odontogenic tumour. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Head and Neck tumours. Lyon: IARC Press; 2005. pp. 306–7. [Google Scholar]
- 12.Sudiono J, Zain RB. PCNA expression in epithelial linings of odontogenic cysts. Annal Dent Univ Malaya. 2003;10:1–5. [Google Scholar]
- 13.de Oliveira MG, Lauxen Ida S, Chaves AC, Rados PV, Sant’AnaFilho M. Immunohistochemical analysis of patterns of p53 and PCNA expression in odontogenic cystic lesions. Med Oral Pathol Oral Cir Buccal. 2008;13:E275–80. [PubMed] [Google Scholar]
- 14.Piattelli A, Fioroni M, Santinelli A, Rubini C. Expression of proliferating cell nuclear antigen in ameloblastomas and odontogenic cysts. Oral Oncol. 1998;34:408–12. doi: 10.1016/s1368-8375(98)00027-x. [DOI] [PubMed] [Google Scholar]
- 15.Hirshberg A, Sherman S, Buchner A, Dayan D. Collagen fibres in the wall of odontogenic keratocysts: A study with picrosirius red and polarizing microscopy. J Oral Pathol Med. 1999;28:410–2. doi: 10.1111/j.1600-0714.1999.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 16.Szendröi M, Vajta G, Kovács L, Schaff Z, Lapis K. Polarization colours of collagen fibres: A sign of collagen production activity in fibrotic processes. Acta Morphol Hung. 1984;32:47–55. [PubMed] [Google Scholar]
- 17.Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn. 1978;41:267–74. doi: 10.1679/aohc1950.41.267. [DOI] [PubMed] [Google Scholar]


