Abstract
Follicular dendritic cells (FDCs) are non-phagocytic, non-lymphoid cells of immune system, which are necessary for antigen presentation and regulation of the reactions in the germinal centers of lymph node. Follicular dendritic cell sarcoma (FDCS) is unusual and those with an extranodal origin in the head and neck region are extremely rare. Here, we report a case of FDCS of the left tonsil in a 27-year-old male patient. The patient presented with swelling of the left tonsil and resultant difficulty in swallowing for last three months. The tumor was excised and was sent for histopathologic examination. Microscopic examination and immunohistochemical analysis proved the case to be FDCS. After the diagnosis, the patient received post-operative radiotherapy. The patient is on six months follow-up which is uneventful.
Keywords: Follicular dendritic cell sarcoma, immunohistochemistry, tonsil
INTRODUCTION
Dendritic cells in the lymph node are of four types: follicular, interdigitating, Langerhans and histiocytic/fibroblastic cells (FDCs). Different forms of follicular dendritic cell proliferation and neoplastic conditions include reactive follicular hyperplasia, follicular lymphoma, mantle cell lymphoma, nodular lymphocyte predominant Hodgkin's lymphoma and angioimmunoblastic T cell lymphoma.[1]
The existence of a primary neoplasm of the FDCs in the lymph node was first observed by Monda et al. in 1986.[2] Till date most of the reported cases of FDCS are known to be of lymph node origin. Tumors arising from the FDCs are placed along with the histiocytic and dendritic cell neoplasms in the World Health Organization (WHO) classification of tumors. Apart from FDCS, this group also includes Langerhans cell histiocytosis, histiocytic sarcoma, Langerhans cell sarcoma, interdigitating dendritic cell sarcoma and dendritic cell sarcoma not otherwise specified. FDCS is a rare neoplasm and extranodal involvement especially in the head neck region is extremely rare.
CASE REPORT
A 27-year-old male patient presented with difficulty in swallowing for the last three months. There was no history of hoarseness of voice. Intraoral examination revealed a red swelling over the left tonsil [Figure 1a]. Tonsil of the opposite side was normal. General systemic examination was normal and also no lymphadenopathy or neck mass was detected. On indirect laryngoscopy, the vocal cord apparatus was absolutely normal. Routine biochemical and hematological investigations were within normal limits. Chest X-ray also revealed no abnormality. Computed Tomography (CT) scan showed a growth arising from the left tonsil [Figure 1b]. The clinical impression was tonsillar carcinoma or a lymphoma. The patient underwent tonsillectomy under general anesthesia.
Figure 1.
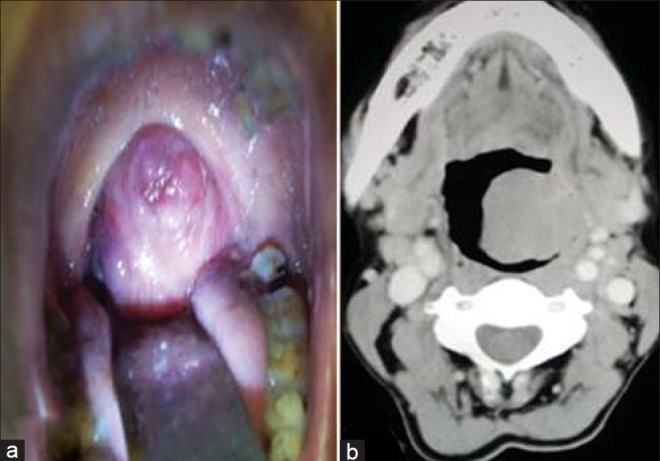
(a) The growth over the left tonsil. (b) CT scan shows the growth on the left tonsil
Histologic findings
Grossly, the resected mass measured 2.8 cm × 2.6 cm × 2.3 cm. It weighed 32 g. It was soft in consistency. Cut section revealed a homogenous solid mass with a smooth surface. Multiple sections were taken from different areas of the tumor. Microscopical examination revealed a tumor situated underneath the squamous epithelial lining and lymphoid follicles of the tonsil [Figure 2a]. The tumor was composed of continuous sheets of spindle cells with elongated plump-shaped nuclei arranged in fascicles and in storiform pattern [Figure 2b]. Most strikingly, there were frequent distinct whorling of the tumor cells with 360 degree rotation [Figure 2b (Black bordered box)]. Occasional multinucleated giant cells [Figure 2b (Inset)] and mitotic figures were also noted along with evidence of lymphocytic infiltration. Based on morphology, a histologic diagnosis of FDCS was given and immunohistochemistry was suggested to confirm the case.
Figure 2.
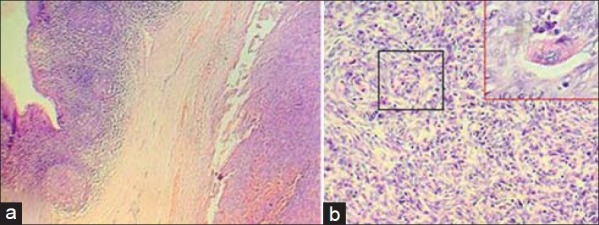
(a) Photomicrograph showing a tumor situated underneath the squamous epithelial lining and lymphoid follicles of the tonsil. (Hematoxylin and Eosin, ×100). (b) Photomicrograph showing continuous sheet of spindle-shaped tumor cells with elongated plump-shaped nuclei arranged in fascicles and storiform pattern with distinct whorling with 360 degree rotation of the tumor cells [Black bordered box] and multinucleated giant cells. [Inset] (Hematoxylin and Eosin, ×400)
Immunohistochemical findings
On immunostaining, the tumor cells expressed strong membrane positivity for all the three FDCs markers e.g. CD21 [Figure 3a], CD35 [Figure 3b] and CD23. It was negative for CD1a, CD45 and desmin.
Figure 3.
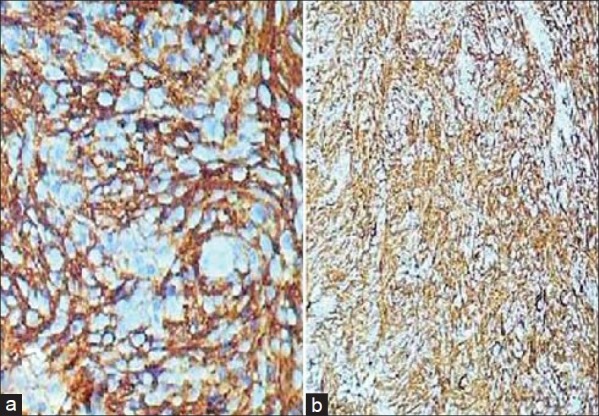
(a) Tumor cells showing strong membrane positivity for CD21. The 360 degree whorling pattern can be easily appreciated. (b) Tumor cells showing strong membrane positivity for CD3
Patient received postoperative radiotherapy. A total radiation dose of 60 Gray in 30 equal fractions was given over six weeks. The patient is on six months follow-up, which is uneventful and he is now maintaining normal life and daily activities.
DISCUSSION
Monda et al. first described the malignant neoplasm arising from these FDCs of lymph node in 1986.[2] Till date some cases all over the world have been reported, but most of those cases happened in the lymph nodes. But these FDCs are also present in very few numbers outside the lymph nodes. Extranodal FDCS are extremely rare. Very few such cases involving the extranodal sites have been reported worldwide. Duan et al. reported FDCSs involving soft palate, retromolar trigone, nasopharynx and parapharyngeal space.[3] Zhou et al. described a single case arising from the parotid.[4] Regarding other parts of body, Hollowood et al. described two cases of FDCS involving small intestine and periduodenal retroperitoneal soft tissue.[5] An et al. reported an inflammatory pseudotumor variant of FDCS involving the liver.[6] In addition, De Pas et al. described pancreas and mesocolon also.[7] Nayler et al. reported the first case of FDCS of tonsil.[8]
Due to its extreme rare occurrence wrong diagnosis have been documented in several occasions.[9–11] First case of extranodal FDCS (of palate) described by Chan et al. was initially misdiagnosed as an acinic cell carcinoma.[9]
Choi et al. reported two cases of extranodal FDCS, both of which were wrongly diagnosed as malignant ectopic thymic tumor consistent with CASTLE (carcinoma showing a thymus-like element).[10] The correct diagnoses were delayed by 11 years in one case and 18 months in the other one.
Extranodal FDCS of palate reported by Araujo et al. was first clinically taken to be a benign salivary gland tumor, histologically first diagnosed as benign fibrous histiocytoma.[11] Two out of three cases were misdiagnosed initially by Biddle et al. too with one impression of malignant schwannoma in biopsy and the other with inflammatory pseudotumor or low-grade malignancy in fine needle aspiration cytology.[12] Almost a year elapsed for the correct tissue diagnosis, meanwhile one of them developed metastatic masses in lung and over the chest.
In most of the cases two causes were responsible for initial wrong diagnoses. First, during the diagnosis, possibility of FDCS was not kept in mind as a differential diagnosis primarily for its rare occurrence and secondly because of its extremely rare extranodal involvement. Apart from this reason, routine immunohistochemical panels did not include FDC markers. CD21 and CD35 are the most sensitive and specific markers for diagnosis of FDCS. Though these antibodies are usually used separately; Dominguez-Malagon et al. states that their sensitivity is enhanced in a cocktail preparation.[13] Variable non-specific positivity was reported for CD23, CD68, Ki-FDC1p, Ki-M4p and S-100. Our case was positive for CD21, CD35 and CD23. It was negative for CD1a, CD45 and desmin, thus nullifying the possibilities of Langerhans cell histocytosis, lymphomas and interdigitating dendritic cell tumors, respectively.
Epstein-Barr Virus (EBV) seems to play no role in the causation of both nodal and extranodal FDCS. So in situ hybridization for EBV-encoded RNAs (EBER) is not advised.
Very recently, Lan et al. proposed a prognostic assessment system for FDCS. Tumor size ≥5 cm, mitotic count ≥5/10 HPF and high-grade histology were related to tumor recurrence.[14] Depending upon five histological parameters (architecture, cellular features, mitotic count, necrosis and lymphocytic infiltration) and one immunohistochemical parameter (Ki-67 proliferative index), they divided FDCS into low-grade and high-grade varieties.
Our objective of this case report is to highlight the fact that without high degree of suspicion on behalf of the histopathologist, it can easily be missed despite some distinctive histopathological features. Thus we opine that for any tonsillar mass, possibility of FDCS should be consciously kept in mind. It may mimic a variety of diverse histopathological entities like extracranial meningioma, peripheral nerve sheath tumor, ectopic thymoma, malignant fibrous histiocytoma, interstitial reticulum cell sarcoma. Therefore to achieve a conclusive and correct diagnosis amidst a lot of differential diagnoses, we stress that the final diagnosis can be rendered only after proper immunohistochemical examination with reliable FDC markers e.g. CD21 and CD35 because morphologic criteria are not alone enough to designate a tumor as FDCS particularly when it is extranodal in location.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Chan JK, Banks PM, Clearly ML, Delsol G, de Wolf Peters C, Falini B, et al. A revised European-American classification of lymphoid neoplasms proposed by the International Lymphoma Study Group. A summary vision. Am J Pathol. 1995;103:543–60. doi: 10.1093/ajcp/103.5.543. [DOI] [PubMed] [Google Scholar]
- 2.Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562–72. [PMC free article] [PubMed] [Google Scholar]
- 3.Duan GJ, Wu F, Zhu J, Guo D, Zhang R, Shen L, et al. Extranodal follicular dendritic cell sarcoma of the pharyngeal region. A potential diagnostic pitfall with literature review. Am J Clin Pathol. 2010;133:49–58. doi: 10.1309/AJCP7U8YISBUAVNW. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Z, Zheng LY, Wang JD, Liu Q. Follicular dendritic cell sarcoma of parotid gland: 1 case report. Chin Arch Otolaryngol Head Neck Surg. 2005;12:138. [Google Scholar]
- 5.Hollowood K, Stamp G, Zouvani I, Fletcher CD. Extranodal follicular dendritic cell sarcoma of the gastrointestinal tract. Morphologic, immunohistochemical and ultrastructural analysis of two cases. Am J Clin Pathol. 1995;103:90–7. doi: 10.1093/ajcp/103.1.90. [DOI] [PubMed] [Google Scholar]
- 6.An XJ, Zhang ZX, Shi QL, Wu B, Ma J, Zhou HB, et al. Hepatic inflammatory pseudotumor like follicular dendritic cell tumor report of one case and review of literature. J Diagn Concepts Pract. 2009;8:63–6. [Google Scholar]
- 7.De Pas T, Spitaleri G, Pruneri G, Curigliano G, Noberasco C, Luini A, et al. Dendritic cell sarcoma: An analytic overview of the literature and presentation of original five cases. Crit Rev Oncol Hematol. 2008;65:1–7. doi: 10.1016/j.critrevonc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Nayler SJ, Verhaart MJ, Cooper K. Follicular dendritic cell tumour of the tonsil. Histopathology. 1996;28:89–92. doi: 10.1046/j.1365-2559.1996.t01-3-258289.x. [DOI] [PubMed] [Google Scholar]
- 9.Chan JK, Tsang WY, Ng CS, Tang SK, Yu HC, Lee AW. Follicular dendritic cell tumors of the oral cavity. Am J Surg Pathol. 1994;18:148–57. doi: 10.1097/00000478-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Choi PC, To KF, Lai FM, Lee TW, Yim AP, Chan JK. Follicular dendritic cell sarcoma of the neck. Cancer. 2000;89:664–72. [PubMed] [Google Scholar]
- 11.Araujo VC, Martins MT, Salmen FS, Araujo NS. Extranodal follicular dendritic cell sarcoma of the palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:209–14. doi: 10.1016/s1079-2104(99)70274-x. [DOI] [PubMed] [Google Scholar]
- 12.Biddle DA, Ro JY, Yoon GS, Yong YW, Ayala AG, Ordonez NG, et al. Extranodal follicular dendritic cell sarcoma of the head and neck region: Three new cases, with a review of the literature. Mod Pathol. 2002;15:50–8. doi: 10.1038/modpathol.3880489. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez-Malagon H, Cano-Valdez AM, Mosqueda-Taylor A, Hes O. Follicular dendritic cell sarcoma of the pharyngeal region: Histolgic, cytologic, immunohistochemical and ultrastructural study of three cases. Ann Diagn Pathol. 2004;8:325–32. doi: 10.1053/j.anndiagpath.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Shi YH, Guo ZJ, Qiu T, Guo L, Yang HY, et al. Clinicopathological features and prognosis assessment of extranodal follicular dendritic cell sarcoma. World J Gastroenterol. 2010;16:2504–19. doi: 10.3748/wjg.v16.i20.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]


