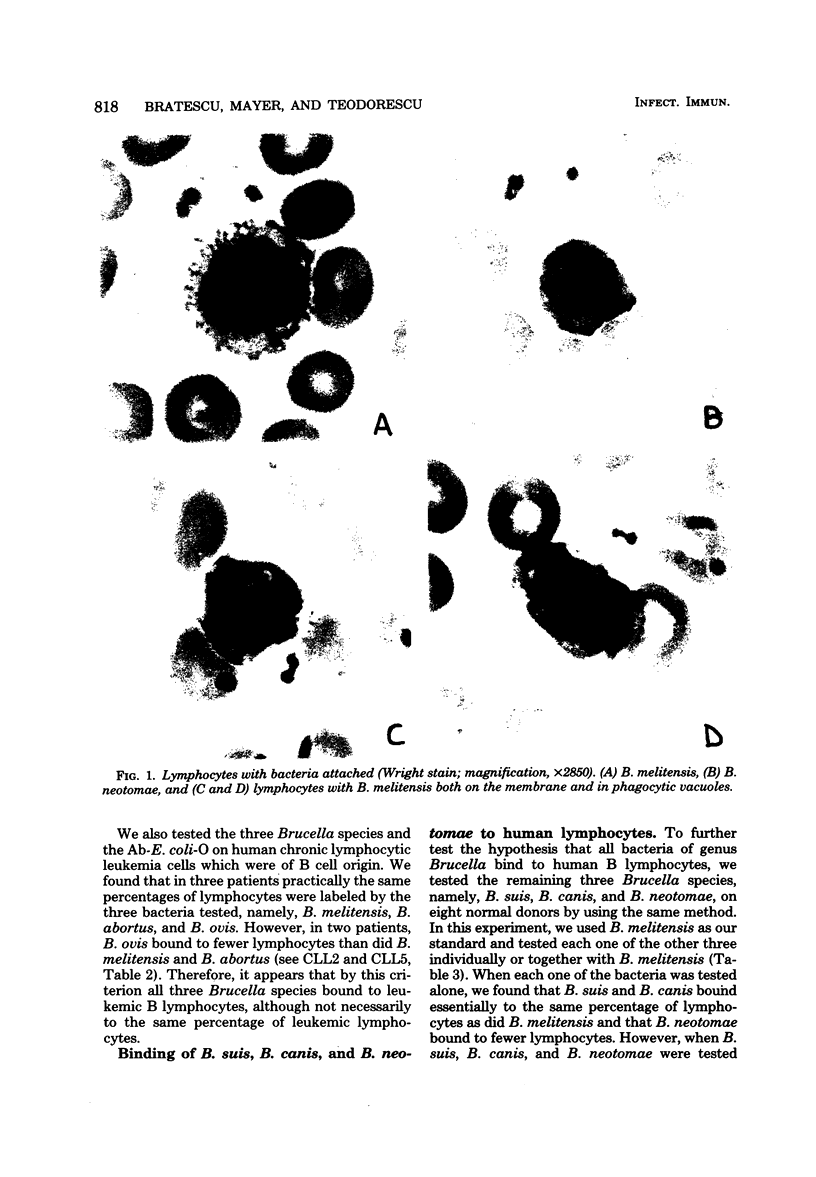
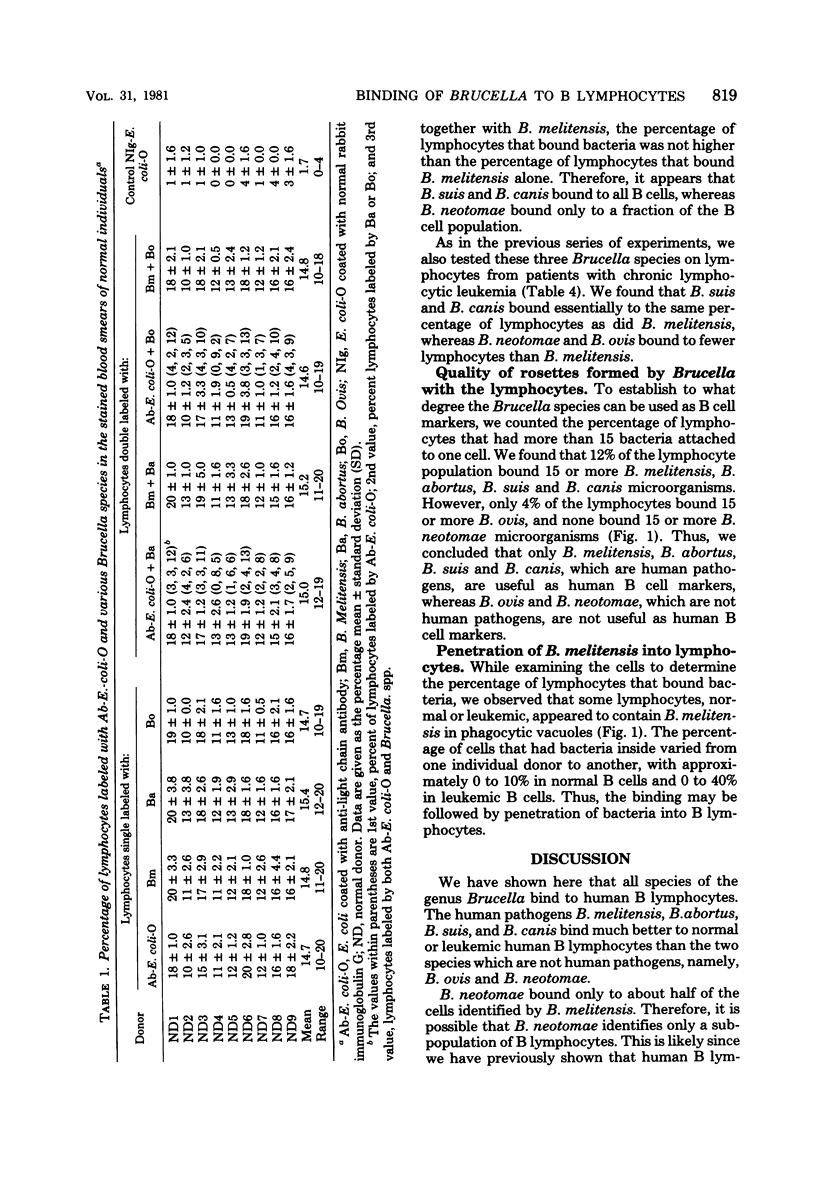
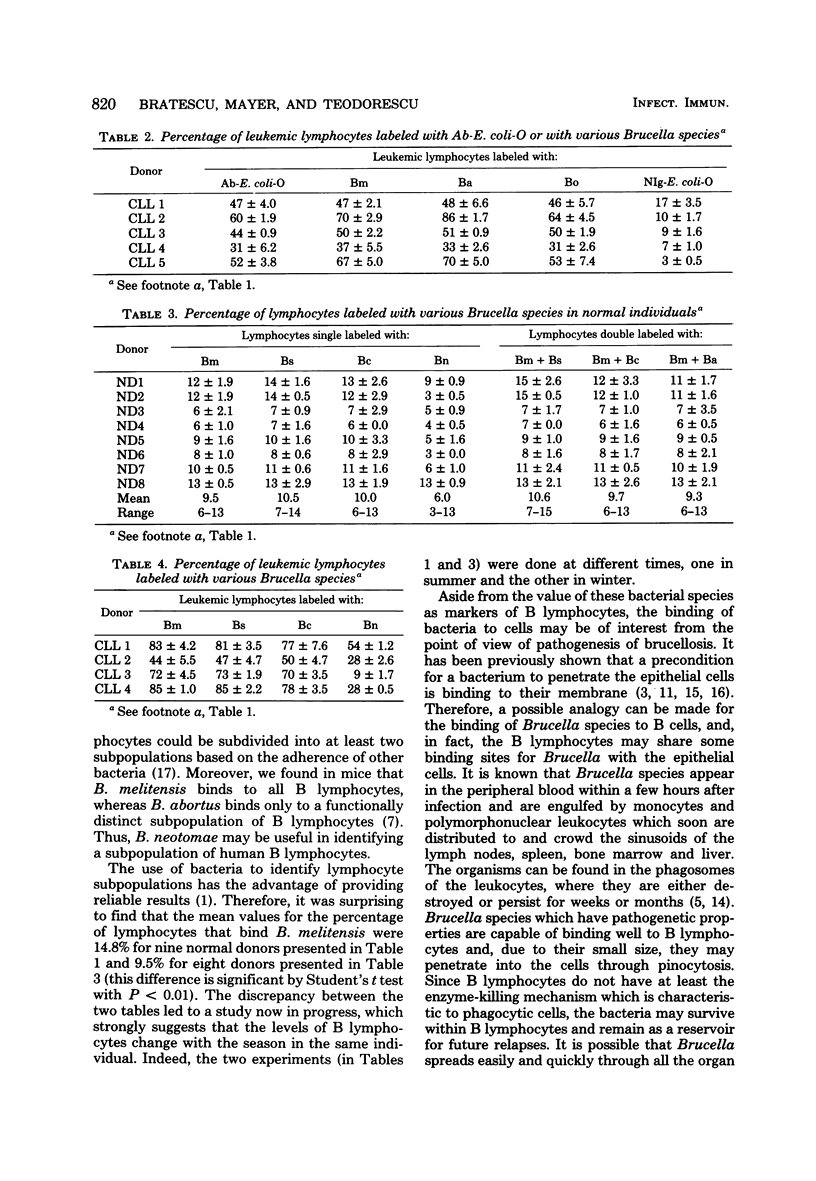
Abstract
In previous studies, we have shown that various lymphocyte subpopulations bind different strains of bacteria of different genera and species. Among these bacteria was a strain of Brucella melitensis which bound to all human B lymphocytes. To determine whether the binding of B. melitensis to human B lymphocytes was strain, species, or genus characteristic, we tested the binding of B. melitensis, Brucella abortus, Brucella ovis, Brucella suis, Brucella canis and Brucella neotomae to human normal and leukemic B lymphocytes. The binding of different Brucella species to B lymphocytes was determined by single- and double-labeling experiments in which a strain of Escherichia coli, coated with anti-light chain antibodies, was used as a marker for B cells. As in previous experiments, we found that B. melitensis and antibody-coated E. coli bound to the same cells. Also, we found that all the other species of bacteria tested bound to the B lymphocytes, normal or leukemic. B. ovis and B. neotomae, which are not human pathogens, bound to fewer B lymphocytes than did the human pathogens B. abortus, B. melitensis, B. suis, and B. canis. Furthermore, we found that the quality of rosettes formed by the nonpathogenic bacteria with the lymphocytes, i.e., the number of bacteria per lymphocytes, was lower than that of pathogenic Brucella species. We conclude that all of the Brucella species tested have the ability to bind to human B lymphocytes, but that only those which are human pathogens bind firmly to all B lymphocytes and may be used as reliable markers for these cells. We also suggest that the binding of Brucella species to B lymphocytes may have some bearing on the pathogenesis of brucellosis in humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratescu A., Mayer E., Teodorescu M. Factors affecting the differential counting of human lymphocyte subpopulations in blood smears. Clin Exp Immunol. 1980 Sep;41(3):547–558. [PMC free article] [PubMed] [Google Scholar]
- Chao W., Yokoyama M. M. Determination of B lymphocyte population using antibody-coated polyacrylamide beads. Clin Chim Acta. 1977 Jul 1;78(1):79–84. doi: 10.1016/0009-8981(77)90339-4. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Forbes I. J., Zalewski P. D. A subpopulation of human B lymphocytes that rosette with mouse erythrocytes. Clin Exp Immunol. 1976 Oct;26(1):99–107. [PMC free article] [PubMed] [Google Scholar]
- Mayer E. P., Chen W. Y., Dray S., Teodorescu M. The identification of six mouse lymphocyte subpopulations by their natural binding of bacteria. J Immunol. 1978 Jan;120(1):167–173. [PubMed] [Google Scholar]
- Mayer E. P., Teodorescu M. Bacterial adherence to eucaryotic cells: isolation of lymphocyte-binding mutants. Infect Immun. 1980 Jul;29(1):66–69. doi: 10.1128/iai.29.1.66-69.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M. Evidence that specific oligosaccharides block early events necessary for the expression of antigen-specific proliferation by human lymphocytes. J Immunol. 1980 Sep;125(3):1306–1311. [PubMed] [Google Scholar]
- Nelson R., Bratescu A., Teodorescu M. Relationship between bacterial binding to lymphocytes and clinical features in chronic lymphocytic leukemia. Cancer. 1979 Nov;44(5):1665–1670. doi: 10.1002/1097-0142(197911)44:5<1665::aid-cncr2820440520>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Rabellino E. M., Polley M. J., Grey H. M. Combined studies of complement receptor and surface immunoglobulin-bearing cells and sheep erythrocyte rosette-forming cells in normal and leukemic human lymphocytes. J Clin Invest. 1973 Feb;52(2):377–385. doi: 10.1172/JCI107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. L., Thorne D. R., Loh H. H. Lectins: endogenous carbohydrate-binding proteins from vertebrate tissues: functional role in recognition processes? Life Sci. 1978 Mar;22(9):727–748. doi: 10.1016/0024-3205(78)90242-4. [DOI] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., King G., Zeligs B. Studies on gonococcus infection. VIII. 125Iodine labeling of gonococci and studies on their in vitro interactions with eukaryotic cells. Infect Immun. 1975 Mar;11(3):453–459. doi: 10.1128/iai.11.3.453-459.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodorescu M., Bratescu A., Mayer E. P. The use of the natural binding of bacteria for the identification of human T- and B-lymphocyte subpopulations in blood smears. Clin Immunol Immunopathol. 1979 Jun;13(2):194–210. doi: 10.1016/0090-1229(79)90064-3. [DOI] [PubMed] [Google Scholar]