Abstract
The aim of this study was to determine the incidence and describe the factors influencing ictal cardiac arrhythmias in children with epilepsy. A 2-year review within a pediatric epilepsy monitoring unit revealed 2066 electrographically confirmed seizures in 139 patients. Demographic, seizure, and cardiac variables were collected for each patient. Fisher’s exact test, Wilcoxon rank-sum test, and Spearman’s rank correlation coefficient were used to identify significant differences and associations at the seizure and patient levels. In 244 seizures meeting inclusion criteria, ictal cardiac arrhythmias were seen in 45% of the seizures (40% of the patients). The most common arrhythmia was benign respiratory sinus arrhythmia (78% of seizures with arrhythmias, 70% of patients with arrhythmias). Potentially serious arrhythmias included irregular variable arrhythmias, and abnormal QRS intervals were seen in 12% of all the patients. In seizures with ictal arrhythmias, 64% occurred in male patients (P = 0.016) and 78% occurred in white patients (P = 0.013). This study estimates the incidence of ictal arrhythmias within the pediatric population that need further medical attention and management.
Introduction
Cardiac rate and electrocardiographic (ECG) rhythm changes during seizures have been increasingly considered and studied over the last few decades, primarily because of the hypothesis that cardiorespiratory disturbances may be a factor in sudden unexpected death in epilepsy; however, the relationship between ictal autonomic changes and sudden unexpected death in epilepsy is not clearly understood. Cardiac changes are commonly seen during seizures in adults [1–5], and the most common cardiac change is tachycardia. In one study, 93% of the adult patients had an increase of 10 beats/minute and 80% had an increase of 20 beats/minute during ictal events [3]. Another study reported similar findings in their adult population with sinus tachycardia of more than 100 beats/minute in 97% [5].
Although variations in cardiac rate are the most common ictal change, changes in cardiac rhythm, repolarizations, and conduction abnormalities have also been described. In a retrospective study, approximately 22% of the adults who were analyzed had ECG abnormalities other than sinus tachycardia [2]. In another study, 21% of the adults required permanent pacemaker insertion because of severe bradycardia or periods of asystole consistently associated with their seizures [6]. In pediatric patients, the prevalence of cardiac rate changes is lower. In one pediatric study, 26% of focal seizures and 48% of generalized seizures were associated with changes in heart rate [7]; in another study, sinus tachycardia was observed in 35/42 seizures (83%) [8].
The aim of the present study was to determine the incidence of ictal cardiac arrhythmias in children with epilepsy and to define the associated factors, in order to help identify those at a higher risk and to provide knowledge vital to improving and tailoring medical care appropriately.
Study Design and Methods
Subjects
The Institutional Review Board approved this retrospective study involving patients admitted to the video electroencephalographic (video-EEG) monitoring unit at Cincinnati Children’s Hospital Medical Center between January 2005 and December 2006. The International 10–20 system was used for electrode placement. Both EEG and video data were recorded on Biologic Systems equipment (Natus Medical, Mundelein, IL). The duration of the video-EEG recording ranged from 1 to 8 days. To identify subjects, an EEG database was searched for electrographically confirmed seizures. Studies containing only nonepileptic seizures, seizures less than 30 seconds in duration, bedside recordings without video, or recordings without an ECG tracing were excluded. Each patient’s record was searched for age at time of the study, sex, and race (white or nonwhite).
Cardiac Rate
Several different time frames were analyzed to better understand the temporal relationship between cardiac rate changes and seizures. To allow for characterizing of cardiac rate changes, only seizures lasting 30 seconds or longer were included. The baseline cardiac rate was determined by counting consecutive RR intervals for 1 minute at the beginning of the video-EEG recording. The periictal cardiac rate was determined by counting consecutive RR intervals in two 15-second epochs immediately preceding the electrographic onset. The postictal cardiac rate was determined by counting consecutive RR intervals in two 15-second epochs immediately following the electrographic end of the seizure. Tachycardia or bradycardia was determined if there was at least a 10% increase or decrease in the baseline cardiac rate [7].
ECG Data
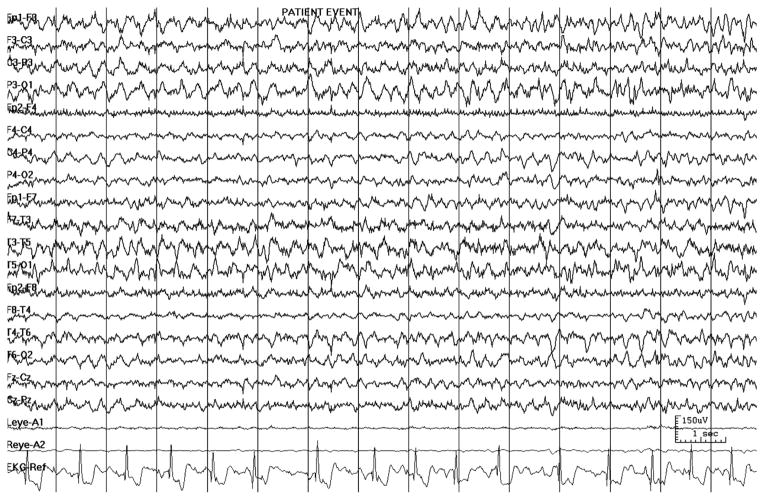
The ECG data was obtained from one ECG channel. As part of routine care during the EEG, one or two ECG electrodes are placed on the anterior left side of the chest, although exact positioning was not standardized, and referenced to the ground electrode on the head. The ECG was analyzed during the seizures for ictal rhythm abnormalities. The ECG abnormalities were reviewed by a board certified cardiologist. The arrhythmias were categorized into two groups: benign respiratory sinus arrhythmia and potentially serious arrhythmias. Respiratory sinus arrhythmia is defined as ≥50% increase in heart rate during inspiration, compared with expiration. The Johns Hopkins Hospital Harriet Lane Handbook was used for further classification of the categories of potentially serious arrhythmias [9]. The potentially serious arrhythmias included supraventricular, ventricular, conduction abnormalities, irregular variable rhythm, and asystole. An irregular variable rhythm was confirmed by the cardiologist when a nodal escape or junctional rhythm or a severe fluctuation between sinus bradycardia and sinus tachycardia could not be determined with the one-channel ECG used with video-EEG (Fig 1).
Figure 1.
An example of an irregular variable rhythm in an EEG recording at 150 microvolts per millimeter, with the one-channel ECG typical of video-EEG monitoring.
Seizure Description
Each patient’s EEG report was reviewed for the following: type of seizure (partial, generalized, or both), location of ictal onset, and electrographic duration of at least 30 seconds. If the EEG report did not specifically list each seizure, the patient’s recording was further reviewed for individual sequential seizures lasting at least 30 seconds. The beginning and the ending of the seizure was based on electrographic changes.
Statistical Analyses
Statistical analysis was performed using nonparametric statistical measures (Fisher’s exact test, Wilcoxon rank-sum test, and Spearman’s rank correlation coefficient). Differences were considered statistically significant at P < 0.05.
Results
The study group consisted of 58 children (29 male and 29 female; mean age, 9.3 years; standard deviation S.D. = 4.7 years; range, 1–18.3 years) (Table 1). In the 58 patients, there were 244 seizures that were at least 30 seconds in duration included in the study for analysis. Out of the 244 seizures, 187 were focal in onset (77%) and 57 were primary generalized (23%). The mean number of seizures per patient was 5.2 (S.D. = 4.85; range, 1–22).
Table 1.
Demographic and seizure data
| Variable | Value |
|---|---|
| Total sample, no. | 58 |
| Sex, no. (%) | |
| Male | 29 (50) |
| Female | 29 (50) |
| Race, no. (%) | |
| White | 45 (78) |
| Nonwhite | 13 (22) |
| Seizure type, no. (%) | |
| Total | 244 |
| Focal | 187 (77) |
| Generalized | 57 (23) |
| Age, yr, mean ± S.D. (range) | 9.28 ± 4.72 (1–18.3) |
| Seizures, no. | 5.2 ± 4.9 (1–22) |
Abbreviations: S.D. = Standard deviation
Ictal arrhythmias were seen in 109 (45%) of the 244 seizures. Of the 109 seizures with ictal arrhythmias, 85 (78%) were benign respiratory sinus arrhythmia and 24 (22%) were potentially serious arrhythmias, such as an abnormal QRS complex or an irregular variable rhythm. Of the 58 patients, 23 (40%) had ictal arrhythmias with their seizures; 7 patients (12% of the total) had potentially serious arrhythmias. The frequency of seizures with any ictal arrhythmia per patient averaged 0.411, or 0.07 when only potentially serious arrhythmias were considered.
There were significant associations of the seizures with arrhythmias and both sex and race. Ictal arrhythmias were more common in male patients (P = 0.016). Of the 24 seizures with potentially serious arrhythmias, all seizures with an abnormal QRS complex (2/2) and 16/22 with irregular variable rhythm (73%) occurred in male patients. In addition, 52/85 with a benign respiratory sinus arrhythmia occurred in male patients (61%). Of the 109 seizures involving an ictal arrhythmia, 85 occurred in white patients (78%). Of the 24 seizures with potentially serious arrhythmias, all seizures with an abnormal QRS complex (2/2), 21/22 with irregular variable rhythm (95%), and 62/85 with a benign respiratory sinus arrhythmia (73%) (P = 0.013) occurred in white patients.
Both periictal and postictal cardiac rate changes occurred most often in female patients (P = 0.003 and P < 0.0003, respectively). For example, 5 of the 6 seizures involving periictal sinus bradycardia (83%) and 14 of the 18 involving periictal sinus tachycardia (78%) occurred in female patients. All seizures with postictal sinus bradycardia (100%) and 33/49 with postictal sinus tachycardia (67%) occurred in female patients.
The duration of the seizure seemed to be affected by the cardiac rate. When sinus tachycardia was present in the first 15 seconds of the seizure, the average duration was 189 seconds, compared with 52.2 seconds with sinus bradycardia (P = 0.031). When sinus tachycardia was present in the last 15 seconds of the seizure, the average duration was 171 seconds, compared with 54 seconds with sinus bradycardia (P = 0.018). When postictal sinus tachycardia was present, the average duration of the seizure was 252.7 seconds, compared with 84.6 seconds when the heart rate remained within the normal range (P = 0.005).
There were significant associations between cardiac rate changes and localization and lateralization of the seizures. Regarding localization, all six seizures with periictal sinus bradycardia were temporal in onset (P = 0.043).
Although ictal tachycardia has been associated with localizing temporal lobe seizures [10–12], the present study did not reveal a relationship. There was, however, an association between laterality of the temporal lobe seizure and ictal tachycardia. Of the 18 temporal lobe seizures with ictal sinus tachycardia, 14 (78%) lateralized to the right temporal lobe (P = 0.022).
Discussion
The specific aim of this study was to determine the incidence of ictal cardiac arrhythmias in children with epilepsy and to define the associated factors. The importance of knowing if a patient has a cardiac arrhythmia is not difficult to understand, especially if the arrhythmia is potentially serious. However, understanding how and when the cardiac rate changes in relation to a seizure is also important and useful for the physician.
According to studies of adults, up to 45% of patients with refractory epilepsy have ictal cardiac arrhythmias [2,3,13]. Similarly, in the present pediatric study, ictal arrhythmias were seen in 40% of the patients. Note that this proportion includes all arrhythmias, including such as benign respiratory sinus arrhythmia. Nonetheless, 22% of seizures with ictal arrhythmias in the video-EEG unit were potentially serious, involving an abnormal QRS complex or an irregular variable rhythm. This alarmingly high proportion is similar to that reported form other studies, with a mixture of adults and pediatric patients [2,6]. In the present study, 7/58 patients (12%) had a potentially serious ictal arrhythmia, compared with 24% reported from another pediatric study [7].
In the present study, 78 (32%) of the 244 seizures involved ictal sinus tachycardia. This finding is similar to other pediatric studies and lower in comparison to adult studies. Reports of several adult studies analyzing ECG and EEG recordings reliably indicate a higher proportion of adult patients with ictal sinus tachycardia [2,13,14]. The present study may further support the hypothesis that a sympathetic discharge associated with seizures is more commonly seen in adults [7]. Nonetheless, it is challenging to make comparisons when reviewing the literature on cardiac rhythm and rate changes associated with seizures, because of the different methodologies. There are inconsistencies regarding the definition and duration of time frames analyzed within various studies. For example, some reports combine as the ictal time frame what here is separately defined as periictal or postictal [3,13]. Additionally, some studies define a longer duration of time to measure periictal or postictal cardiac rate changes [2,3,13]. These different definitions of time frames could lead to a greater frequency of cardiac rate changes than evidenced in the present study.
A lower proportion of temporal lobe seizures with ictal sinus tachycardia was observed in the present study (42%) than reported by Mayer et al. [10] (98%). One possible explanation for this difference could be patient selection within the studies. Mayer et al. [10] included only patients with drug-resistant unilateral symptomatic temporal lobe epilepsy, and 85% of these patients were diagnosed with mesial temporal lobe lesions on diagnostic imaging. In contrast, the present study did not differentiate among temporal lobe seizures as mesial or neocortical in localization. Ictal sinus tachycardia is more strongly associated with mesial than neocortical temporal lobe structures [10–12]. If a relatively greater proportion of patients in the present study had a neocortical temporal focus, this could have influenced the frequency of ictal sinus tachycardia observed within the present study population.
Evolving research supports a right cerebral hemispheric predominance on cardiac sympathetic regulation [15–17]. Based on observations during epilepsy surgeries, Oppenheimer et al. [15] reported an association between sympathetic cardiac changes and stimulation of the right insular cortex. Zamrini et al. [16] reported increased heart rates in patients with inactivation of the left hemisphere by intracarotid injection of amobarbital and decreased rates with inactivation of the right. Although some studies could not demonstrate a right or left predominant relationship on the cardiac sympathetic regulation, recent reports from other studies support a right hemispheric predominance [11,18,19]. In the present study, there was no statistical association between lateralization of all seizures and ictal sinus tachycardia. There was, however, a right hemispheric lateralization within temporal lobe seizures with ictal sinus tachycardia, similar to findings from other studies [10,20]. The present results add more evidence of a right hemispheric predominance on cardiac sympathetic regulation in children with temporal lobe epilepsy.
Cardiac rate changes may be the earliest clinical changes (periictal or at onset) experienced by the patient and recognized by the physician. This additional information could be helpful in identifying the ictal onset, as well as for automatic seizure detection programs [3]. In the present study, a periictal sinus bradycardia was predictive of a temporal onset. Tigaran et al. [4] similarly found that sinus bradycardic changes were associated with temporal lobe seizures. In patients for whom it is difficult to locate the ictal onset zone electrographically, detecting these early cardiac rate changes would provide the physician with a measurable sign that a focal seizure may follow. This could be useful, for example, with ictal single photon emission computed tomography (SPECT) imaging for presurgical evaluations.
The present study revealed several significant associations between cardiac rate changes and seizures that are clinically helpful. On average, when ictal or postictal sinus tachycardia is present, the seizure lasts three times longer than when sinus bradycardia occurs. The duration of the seizure was not significantly affected by preictal cardiac changes. Understanding the association between sinus tachycardia and longer seizures could lead to better management plans and expectations regarding the longer duration. For example, patients with a history of ictal sinus tachycardia should be considered for abortive seizure therapy more aggressively than those patients with ictal sinus bradycardia. Having such information may also help in counseling the parents regarding expectations of seizure duration.
In the present study, there were limitations concerning the interpretation of the ECG. First, the single-channel ECG could not be interpreted for all seizures because of interference, particularly for generalized seizures. This occurrence could have led to a smaller proportion of ictal arrhythmias or cardiac rate changes then reported. Second, because only one ECG channel was used, some abnormal rhythms that are challenging to see with only one channel could be missed, such as an elevation or depression of the ST segment. This fact would most likely lead to a higher proportion of false-negative ECG interpretations. Third, the number of potentially serious ictal arrhythmias in the present study may be an overestimate. Of the 109 seizures involving ictal arrhythmias, 22 (20%) were interpreted as irregular variable rhythm. This describes a rhythm that could be either benign (fluctuation between sinus bradycardia and sinus tachycardia) or serious (a nodal escape or junctional rhythm). Without more ECG data, however, the rhythm cannot be interpreted further.
Because the outcomes vary considerably between a benign and a serious rhythm, it is imperative to promptly analyze and classify the cardiac rhythm further. The additional evaluation may include, at minimum, a consultation with cardiology to determine if more ECG channels and further cardiac evaluation are necessary. Because the traditional video-EEG recording uses only one ECG channel, some institutions are discussing or progressing toward more formal cardiac telemetry for all patients admitted for video-EEG [21].
The appropriateness of screening certain subpopulations of patients with epilepsy with more extensive ECG monitoring is currently under discussion. Recently, Johnson et al. [22] reported a possible link between long QT syndrome and epilepsy. The cardiac channelopathy associated with the long QT syndrome type 2 is caused by a mutation in the gene known as KCNH2 (previously LQT2). They reported that a seizure phenotype was present in 29% of all long QT syndrome patients and was more common in type 2 patients (47%) [22]. In addition, mutations in the sodium channel gene SCN1A have been associated with sudden unexplained death in epilepsy [23]. Thus, epileptologists may consider routine screening with formal cardiac telemetry in patients with channelopathies. Performing these studies within this subpopulation may not only lead to a better understanding if a link exists, but it may also be life saving. None of the patients in the present study had known channelopathies; however, the incidence of potentially serious ictal arrhythmias seen in the present study was higher than would be expected if channelopathies were the only factor contributing to this phenomenon.
In conclusion, potentially serious ictal cardiac arrhythmias were seen in 12% of the children. Potentially serious ictal arrhythmias were more common in white male patients. Although the relationship between ictal autonomic changes and sudden unexpected death in epilepsy is not clearly understood, it is helpful to identify the patients at risk for ictal cardiac arrhythmias and perhaps for sudden unexpected death in epilepsy. By understanding the risk factors involved with ictal cardiac arrhythmias as well as the specific time frames of cardiac rate changes, physicians can develop better medical and prognostic management for their pediatric patients.
Acknowledgments
The authors thank cardiologist Thomas Kulik, MD, for reviewing the electrocardiographic tracings of the patients in the study.
References
- 1.Stöllberger C, Finsterer J. Cardiorespiratory findings in sudden unexplained/unexpected death in epilepsy (SUDEP) Epilepsy Res. 2004;59:51–60. doi: 10.1016/j.eplepsyres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res. 2002;52:117–27. doi: 10.1016/s0920-1211(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 3.Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: prevalence and definition of an objective clinical sign. Epilepsia. 2002;43:847–54. doi: 10.1046/j.1528-1157.2002.37801.x. [DOI] [PubMed] [Google Scholar]
- 4.Tigaran S, Rasmussen V, Dam M, Høgenhaven H, Friberg B. ECG changes in epilepsy patients. Acta Neurol Scand. 1997;96:72–5. doi: 10.1111/j.1600-0404.1997.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 5.Keilson MJ, Hauser WA, Magrill JP. Electrocardiographic changes during electrographic seizures. Arch Neurol. 1989;46:1169–70. doi: 10.1001/archneur.1989.00520470023018. [DOI] [PubMed] [Google Scholar]
- 6.Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR, Duncan JS. Cardiac arrhythmias in focal epilepsy: a prospective long-term study. Lancet. 2004;364:2212–9. doi: 10.1016/S0140-6736(04)17594-6. [DOI] [PubMed] [Google Scholar]
- 7.O’Regan ME, Brown JK. Abnormalities in cardiac and respiratory function observed during seizures in childhood. Dev Med Child Neurol. 2005;47:4–9. doi: 10.1017/s0012162205000022. [DOI] [PubMed] [Google Scholar]
- 8.Hewertson J, Boyd SG, Samuels MP, Neville BG, Southall DP. Hypoxaemia and cardio respiratory changes during epileptic seizures in young children. Dev Med Child Neurol. 1996;38:511–22. doi: 10.1111/j.1469-8749.1996.tb12112.x. [DOI] [PubMed] [Google Scholar]
- 9.Cardiology Gajewski K. In: Johns Hopkins Hospital: The Harriet Lane handbook. 17. Robertson J, Shilkofski N, editors. Philadelphia: Elsevier Mosby; 2005. pp. 174–9. [Google Scholar]
- 10.Mayer H, Benninger F, Urak L, Plattner B, Geldner J, Feucht M. EKG abnormalities in children and adolescents with symptomatic temporal lobe epilepsy. Neurology. 2004;63:324–8. doi: 10.1212/01.wnl.0000129830.72973.56. [DOI] [PubMed] [Google Scholar]
- 11.Leutmezer F, Schernthaner C, Lurger S, Pötzelberger K, Baumgartner C. Electrocardiographic changes at the onset of epileptic seizures. Epilepsia. 2003;44:348–54. doi: 10.1046/j.1528-1157.2003.34702.x. [DOI] [PubMed] [Google Scholar]
- 12.Garcia M, D’Giano C, Estellés S, Leiguarda R, Rabinowicz A. Ictal tachycardia: its discriminating potential between temporal and extra-temporal seizure foci. Seizure. 2001;10:415–9. doi: 10.1053/seiz.2000.0529. [DOI] [PubMed] [Google Scholar]
- 13.Nei M, Ho R, Sperling M. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia. 2000;41:542–8. doi: 10.1111/j.1528-1157.2000.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 14.Galimberti CA, Marchioni E, Barzizza F, Manni R, Sartori I, Tartara A. Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia. 1996;37:742–7. doi: 10.1111/j.1528-1157.1996.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 15.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–32. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 16.Zamrini EY, Meador KJ, Loring DW, Nichols FT, Lee GP, Figueroa RE, Thompson WO. Unilateral cerebral inactivation produces differential left/right heart rate responses. Neurology. 1990;40:1408–11. doi: 10.1212/wnl.40.9.1408. [DOI] [PubMed] [Google Scholar]
- 17.Hilz MJ, Dütsch M, Perrine K, Nelson PK, Rauhut U, Devinsky O. Hemispheric influence on autonomic modulation and baroreflex sensitivity. Ann Neurol. 2001;49:575–84. [PubMed] [Google Scholar]
- 18.Epstein MA, Sperling MR, O’Connor MJ. Cardiac rhythm during temporal lobe seizures. Neurology. 1992;42:50–3. doi: 10.1212/wnl.42.1.50. [DOI] [PubMed] [Google Scholar]
- 19.Keilson MJ, Hauser WA, Magrill JP, Goldman M. ECG abnormalities in patients with epilepsy. Neurology. 1987;37:1624–6. doi: 10.1212/wnl.37.10.1624. [DOI] [PubMed] [Google Scholar]
- 20.Kirchner A, Pauli E, Hilz MJ, Neundörfer B, Stefan H. Sex differences and lateral asymmetry in heart rate modulation in patients with temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2002;73:73–5. doi: 10.1136/jnnp.73.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noe KH, Drazkowski JF. Safety of long-term video-electroencephalographic monitoring for evaluation of epilepsy. Mayo Clin Proc. 2009;84:495–500. doi: 10.4065/84.6.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JN, Hofman N, Haglund CM, Cascino GD, Wilde AA, Ackerman MJ. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology. 2009;72:224–31. doi: 10.1212/01.wnl.0000335760.02995.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hindocha N, Nashef L, Elmslie F, Birch R, Zuberi S, Al-Chalabi A, Crotti L, Schwartz PJ, Makoff A. Two cases of sudden unexpected death in epilepsy in a GEFS+ family with an SCN1A mutation. Epilepsia. 2008;49:360–5. doi: 10.1111/j.1528-1167.2007.01439_2.x. [DOI] [PubMed] [Google Scholar]