Abstract
Intravenous iron has been considered dangerous by many clinicians. In the last two decades, considerable experience has been gained with new formulations in different clinical settings. Data from clinical trials, observational studies, and postmarketing surveillance studies demonstrate that intravenous iron is safe and effective to treat iron deficiency and iron deficiency anaemia. Iron deficiency is particularly common in many digestive diseases: oral iron often fails while transfusions are not without considerable risks. In particular, in inflammatory bowel diseases, there is enough evidence to recommend intravenous iron in moderate-to-severe iron deficiency anaemia, in intolerance to oral iron, and in patients needing quick recovery (pre-operative setting). New formulations make treatment even easier and more convenient. Recent guidelines are available for inflammatory bowel diseases, and new guidelines in acute and chronic gastrointestinal bleeding are needed.
Keywords: colon cancer, digestive diseases, gastrointestinal bleeding, inflammatory bowel disease, iron deficiency, iron deficiency anaemia
Introduction
Iron is a key element for life and competition for iron is a well-known topic in biology [Rouault, 2004]. Iron may also have a role in climate change [Jickells et al. 2005]. Removing iron through bloodletting has been, perhaps with some rationale after all, one of the oldest medical treatments throughout history [Rouault, 2004]. Both excess iron [Brewer, 2001] and iron starvation [Lozoff et al. 2006] may cause disease in humans. As we lack an iron excretory mechanism, iron absorption is the key regulatory process in iron metabolism in humans [Muñoz et al. 2009c], and digestive diseases are among the most common causes of iron deficit and iron deficiency anaemia (IDA) at least in developing societies (in underdeveloped areas iron deficiency is most often caused by nutritional deficit [Zimmermann and Hurrell, 2007]). Oral and parenteral formulations have been used to treat iron deficiency for many years. However, intravenous (IV) iron has been considered a relatively dangerous therapy by many practicing clinicians for many decades. The scenario has now changed with the availability of several new compounds that are easy and safe to use, as Auerbach and colleagues' recent and excellent review summarizes [Auerbach et al. 2008a].
Our goal in this short review is to summarize the evidence of the effectiveness of IV iron in digestive diseases, especially in inflammatory bowel diseases (IBDs), and to give the most important clues for its use in everyday practice. The reader can grasp a broader perspective of iron metabolism and therapeutics from several recent extensive reviews [Muñoz et al. 2009b; Auerbach et al. 2008b]. If specific and recent reviews are available, we only abstract the most relevant information for the clinician.
Intravenous iron in digestive diseases: clinical experience
The intestine is the key organ for iron absorption, and this is the key for the control of iron metabolism in humans [Muñoz et al. 2009c]. Malabsorption of iron is a common feature in many important and frequent digestive diseases such as Helicobacter pylori infection, gastric atrophy, coeliac disease, previous gastrectomy, or tropical parasitosis [Fernández-Bañares et al. 2009]. Moreover, in many digestive diseases acute and/ or chronic bleeding, and the consequent loss of iron, is a frequent complication. This chronic loss is enhanced in patients who are taking platelet anti-aggregant or anti-inflammatory drugs. Furthermore, inflammation has a very negative impact on iron metabolism and in the availability of iron for erythropoiesis, which results in the socalled anaemia of chronic disease [Weiss and Goodnough, 2005]. In fact, Crohn's disease is the paradigm of all of these mechanisms together: blood loss, iron malabsorption because of direct muco-sal damage or bowel resection, and disturbed use by the reticuloendothelial system through systemic inflammation [Gomollón and Gisbert, 2009; Theurl et al. 2009].
For many years, anaemia (and, of course, the more subtle concept of iron deficiency) has been the ‘overlooked villain’ in the words of Christoph Gasché, one of the champions of this topic in recent years [Gasché, 2000]. This is now changing and, in the last decade, the extreme importance of anaemia has been recognized from pathogenesis (bench) to clinic (bedside). This ‘iron revolution’ has been supported by the discovery of new genes and proteins, including the small peptide hepcidin. The relationships between disease status and iron metabolism are rather complex in Crohn's disease: inflammation has a major role in iron regulation through hepcidin and other molecules [Weiss and Gaschel, 2010] and inflammation can block iron absorption as has clinically been proved [Semrin et al. 2006]. From a clinical point of view, several studies have demonstrated that haemoglobin levels do (independently) correlate with quality of life and even cognitive function [Wells et al. 2006]. Moreover, in a particular study designed to prove the deleterious effects of steroids in postsurgical evolution in Crohn's disease patients, steroids could not be blamed and anaemia emerged through multivariant analysis as the only significant risk factor for complications [Bruewer et al. 2003]. Thus, anaemia is a serious issue in all our patients and should not be overlooked.
Intravenous iron in inflammatory bowel diseases
One common error in the management of anaemia in IBDs is the omission or underuse of IV iron [Gisbert and Gomollón, 2008]. Although there are relatively few published studies, IV iron has been shown to be effective in several observational and controlled studies as recently reviewed by Kulnigg and Gasche [2006]. In this review, the authors emphasized several important clinical facts:
anaemia is frequent in IBD;
iron deficiency is the most common cause;
oral iron is not always efficient and could be toxic;
IV iron has been found to be useful and safe in these populations.
Free iron could be toxic not only in the long term as suggested by several authors [Kell, 2009; Brewer, 2001], but acute high levels of mucosal iron could also directly damage the mucosa through the variants of Fenton's reaction and the release of free radicals [Kulnigg and Gasche, 2006].
After the appearance of Kulnigg and Gasche's review, at least four further studies merit specific citation. Lindgren and colleagues have very recently reported on a randomized, controlled, and evaluator-blind trial comparing IV with oral iron in IBD patients. Iron sucrose (see later) was used and IV iron was better tolerated, IV iron reached the clinical goal faster than oral iron (correction of anaemia or an improvement of 2g/dl of haemoglobin values), and showed greater overall effectiveness than oral iron [Lindgren et al. 2009]. A different formulation, ferric carboxymaltose [Lyseng-Williamson and Keating, 2009], has been shown to be at least as effective as oral iron, and clearly faster in obtaining a clear response (a rise of 2 g/dl of haemoglobin values) in a randomized clinical trial [Kulnigg et al. 2007]. Based on these experiences, and the possible toxicity of oral iron, some authors have even suggested that oral iron should be abandoned in the treatment of patients with IBD.
However, in our direct experience, we have been able to demonstrate under real practice conditions that both oral and IV iron can be very effective in treating anaemia [Gisbert et al. 2009]. In this observational, multicentric, and large trial, a low daily dose (<100 mg of elemental iron) was chosen, because no convincing evidence of higher effectiveness (but evidence of higher toxicity, at least in tolerability) could be found in the literature when designing the study. In fact, oral iron was very well tolerated in this trial; however, we did acknowledge that nonblinded and uncontrolled data are always difficult to interpret. It is clear that compliance cannot be very sound if some adverse effects are so common as to affect 20–50% of patients.
A further report that underlines an important clinical issue is a follow up of patients previously included in different clinical trials of IV iron. The results show that iron deficiency and IDA do recur very commonly (more than half the time) even after previous successful replenishment of iron stores in these patients [Kulnigg et al. 2009]. IBDs are chronic conditions and follow up should always include anaemia evaluation, as this is probably the most common and important extra-intestinal manifestation of these diseases [Gomollón and Gisbert, 2009; Gisbert and Gomollón, 2008].
Intravenous iron in digestive (mostly colon) cancer
In cancer patients, the presence of anaemia is common, and is not only a factor that influences quality of life, but is also clearly associated with mortality, even in the long term in several studies [Knight et al. 2004]. The management of anaemia can be an important clue because both transfusions [Jensen et al. 2005] and excess erythropoietin (EPO) have been blamed for adversely affecting overall prognosis [Muñoz et al. 2006]. Blood transfusion has been associated with a higher morbidity and mortality, increase of postsurgical infection, relapse and distant metastasis, all with a dose-dependent relationship. Oral iron has been shown to be effective in some studies, but IV iron (with or without EPO) should not be forgotten in these clinical settings because oral tolerability may be difficult and a quick response is needed (especially in the pre-operative patient). Also, IV iron permits the avoidance of transfusions and makes a lower dose of EPO effective. Several trials have shown that the use of iron sucrose is safe in these patients, and retrospective and prospective reports suggest high effectiveness, as recently reviewed by Muñoz et al. [2009a]. However, not all data confirm the effectiveness of IV iron in this setting, and the topic is controversial: some authors have shown no differences between placebo and iron sucrose for the pre-operative treatment of anaemia in a randomized pilot trial [Edwards et al. 2009]. In this (small) randomized trial, however, the sample size was not calculated to analyse their secondary outcome (transfusion rate) so observed differences did not reach statistical significance (5.9% versus 19.2% for the whole study sample) but showed an apparently important trend for anaemic patients (22% versus 55%). They had also excluded those patients with the most severe anaemias (those previously requiring transfusion), the subgroup with most potential benefit from IV iron. Of course, larger and better-controlled trials are required to evaluate not only the goal of short-term treatment of anaemia, but also the possible influence of IV iron in postoperative course and long-term prognosis.
Intravenous iron in gastrointestinal bleeding
In most gastrointestinal departments and emergency services, acute bleeding is one of the most common clinical problems: although upper gastrointestinal bleeding is becoming less frequent, lower gastrointestinal bleeding is reaching higher prevalence and total numbers remain practically the same [Lanas et al. 2009]. There is much literature on endoscopic management, the use of proton pump inhibitors, or even the need for transfusion, but it is difficult to find specific reports on IV iron use in this clinical scenario. In fact, we were only able to find abstracts presented in different gastroenterology meetings, but not any fully published articles. Curiously, the risks of allogenic blood transfusion are rarely mentioned either in textbooks or in reviews. This somewhat risky cell transplant is a scarce resource with increasing costs, so in not always available in underdeveloped areas. In our view, the experience with Jehovah's Witness patients is not widely known, and blood transfusions are overused [García-Erce et al. 2009]. We think that these patients present a clear opportunity for the use of IV iron as therapy: in many cases they are hospitalized and with IV lines, and anaemia is moderate to severe. IV iron can both treat iron deficiency and facilitate a quick response with a rapid return to normal life. Clinical trials and cost-effectiveness evaluations are required in these clinical situations to establish indications and normalized protocols. IV iron is also widely used in chronic gastrointestinal blood loss caused by vascular malformations, a rather common scenario in those patients on anticoagulant treatment, particularly because of prosthetic heart valves. It is also relatively common in clinical gastroenterological practice to find anaemia and iron deficiency in patients treated with nonsteroidal anti-inflammatory agents and/or antiplatelet drugs. These drugs can damage the upper and lower gastrointestinal tract, and recent endoscopic studies using new devices (endoscopic capsule, enteroscopes) have proven that small intestinal erosions and ulcers are very common in these patients [Sostres et al. 2009]. In many cases, there is no effective measure to treat these lesions and the need for the offending drug is clear (for instance, in those patients with ischaemic heart disease and a recently placed coronary stent). Sometimes, periodical IV iron infusions may be the best practical way to avoid anaemia in those cases. We also need controlled (or at least well-designed observational) studies in these patients.
Intravenous iron: practical issues
Why not IV iron?
Oral iron can, in many cases, be effective [Gisbert et al. 2009]; however, time is very important for our patients, and with oral iron it can take 2–2.5 weeks for haemoglobin levels to increase, as long as 2 months to reach normal values, and at least 6 months to supply enough iron to fill iron stores [Gisbert et al. 2009]. For years IV iron has been considered dangerous, and even today many practitioners feel uncomfortable with its use, mainly because of safety concerns [Chertow et al. 2005]. Most of the fears come from the use of the older pharmacological preparations of IV iron. All IV iron agents are colloids with spheroidal iron—carbohydrate particles, which in some way are artificial versions of the very old and successful natural design of ferritin [Marchetti et al. 2009]. In recent years, high molecular weight dextran (HMWID), iron gluconate, iron sucrose, low molecular weight iron dextran (LMWID), and ferric carboxymaltose have become available for clinical use. Their diverse pharmacological properties give clinicians a choice for different clinical scenarios: for instance, the extreme chemical stability of dextrans makes it possible to use them for giving the total dose in only one administration. On the other hand, most of the negative previous experiences were reported with HMWID, which was associated with variable frequency to anaphylactic reactions that sometimes proved to be fatal. In the two last decades, extensive experience has been gained with the general use of iron gluconate and iron sucrose in chronic renal failure, especially in dialysis patients. IV iron seems to be rather safe, even with very chronic use. Postmarketing observational data show that the incidence of life-threatening adverse effects is 0.9 per million doses with iron gluconate, 0.6 per million doses with iron sucrose, and 11.3 per million doses with LMWID; the death rate attributed to IV iron is 0.25, 0.11 and 0.78 per million, respectively. It seems that even in this very fragile population, the incidence of severe side effects is rather uncommon with IV iron. Most of the clinical trials and observational studies reported in digestive diseases have been using iron sucrose, with less than one severe adverse effect per million patients, and one death every 10 million doses to be expected even in the high-risk population of chronic dialysis patients [Chertow et al. 2005]. For comparison, in Spain between 2006 and 2008, 15 cases of death have been notified out of 5,725,064 units transfused, at least 25 times more frequent fatal events than with IV iron.
Recent studies, however, have highlighted the association of mortality with the treatment of anaemia with EPO in some populations, when the target haemoglobin reaches normal or high values, both in renal and oncologic patients [Phrommintikul et al. 2007]. However, most data signal the use of high-dose EPO as the agent to blame, and IV iron, in spite of theoretical concerns [Kell, 2009] shows no direct correlation with mortality in those trials [Singh et al. 2009]. Of course, these data confirm that the correction of anaemia should be done judiciously, and that EPO agents should be used when clinically indicated, not as a routine.
Definitions of anaemia and iron deficiency
IV iron costs money, needs an IV line, and is not without risks. So, we need clear definitions of the indications for its use in clinical practice. Iron (oral or IV) should be used to treat iron deficiency and/or IDA. In every particular patient we need to know the exact iron status. In the absence of inflammation and/or liver or haematological diseases, the task is rather easy: serum ferritin levels do show a nice correlation with body iron stores [Bermejo and Garcia-López, 2009]. However, in daily clinical practice things are considerably more complex. Inflammation can influence serum ferritin levels, as ferritin is a well-recognized acute-phase reactant [Bermejo and Garcia-López, 2009]. To define iron stores we do need to determine other acute-phase reactants (in IBD, C-reactive protein is commonly used), and measure transferrin, free iron, transferrin saturation and, if available, soluble transferrin receptor (Table 1). Several haematological analyzer parameters (hypochromic red cell percentage or reticulocyte haemoglobin continent), available with no increase in cost, are very effective at identifying iron functional deficiency.
Table 1.
Differential diagnosis of anaemia according to laboratory parameters.
| Serum iron | TIBC | Haemoglobin | Ferritin | TS (%) | TsR (mg/l) | EPO | |
|---|---|---|---|---|---|---|---|
| Ferropaenia | ↓ | ↑ | N | ↓ | ↓ | ↑ | ↑ ↑ |
| Ferropaenic anaemia | ↓ | ↑ | ↓ | ↓ | ↓ | ↑ ↑ | ↑ ↑ ↑ |
| Chronic disease anaemia | ↓ | ↓ | ↓ | ↑ | N | ↓N | ↑* |
| Chronic disease anaemia and ferropaenia | ↓ | ↓ | ↓ | N | N | ↑ | ↑* |
EPO, erythropoietin; N, normal; TIBC, total iron binding capacity; TS, transferrin saturation; TsR, transferrin soluble receptor; *Inappropriate low levels.
A combination of these data indicate whether there is real iron deficiency, and guides treatment [Goddard et al. 2000]. In most chronic IBD patients, for instance, a combination of iron and drugs to control inflammation should be used. While studying these patients, it is important not to forget that other factors can contribute to anaemia, such as folic acid deficiency or low levels of cyanocobalamin, hypothyroidism or renal insufficiency and/or the use of some drugs; the clinical picture is not complete without knowing these data [Theurl et al. 2009].
IV iron formulations
In gastrointestinal daily practice in Europe, given the availability, experience and convenience, we can use as many as five different IV iron preparations (Table 2), and we comment here specifically on the three most commonly used preparations in our setting.
Table 2.
Comparative analysis of intravenous iron formulations available in Europe.
| Product | CosmoFer® (low mol wt iron dextran) | Ferrlecit® (iron gluconate) | Venofer® (iron sucrose) | Ferinject® (iron carboxymaltose) | Monofer® (iron isomaltoside 1000) |
|---|---|---|---|---|---|
| Manufacturer | Pharmacosmos | Sanofi Aventis | Vifor | Vifor | Pharmacosmos |
| Carbohydrate | Dextran (Branched polysaccharides) | Gluconate (Monosaccharides) | Sucrose (Disaccharides) | Carboxymaltose (Branched polysaccharide) | Isomaltoside 1000 (Unbranched linear oligosaccharides) |
| Maximum single dose | 20mg/kg | 125mg | 200mg | 15mg/kg Single dose limit:1000mg | 20mg/kg |
| Maximum single dose administration in a 80 kg man | 1600mg | 125mg | 200mg | 1000mg | 1600mg |
| Maximum single dose administration in a 60 kg woman | 1200mg | 125mg | 200mg | 900mg | 1200mg |
| One dose iron repletion (TDI) | Yes | No | No | No | Yes |
| Infusion within 1 hour | No | NA | NA | Yes | Yes |
| Test dose required | Yes | No | Yes/No | No | No |
| Iron concentration (mg/ml) | 50 | 12.5 | 20 | 50 | 100 |
| Vial volume (ml) | 2 | 5 | 5 | 2 and 10 | 1, 5 or 10 |
TID, three times daily.
Iron sucrose
The most commonly used preparation to date in digestive patients has been iron sucrose. Iron sucrose is very safe, and published experience is extensive in clinical trials and in observational studies. Single doses of up to 500 mg have been used with apparent safety, but given its pharmacological properties, single, 1-hour, 100–200-mg doses are the standard, with a maximal weekly dose of 600 mg. In many patients, this means that to treat all of the iron deficiency and replenish iron stores, at least 2–4 weeks are required.
LMWID
While LMWID does not show as favourable a safety record as iron sucrose, it allows the administration of the total calculated iron deficit in only one dose, in 3–4 hours. Some authors [Auerbach et al. 2008a] with very extensive experience claim that this approach is safe, and use it in a wide variety of clinical settings. However, a testing dose is needed, anaphylactoid reactions, although rare, do occur, and a long stay in day-care facilities is required.
Ferric carboxymaltose
This is a relatively new formulation and seems to have several advantages in practice: a single 1000-mg dose is possible (most patients can be fully treated with one or two doses) and a very short (10–15 minutes) period of infusion is required, which is very convenient for our (almost always) saturated facilities. Experience with this formulation has been reported in diverse diseases, and specifically in IBDs [Kulnigg et al. 2007]. However, there are also limitations: it is more expensive (if one only considers direct costs, but remember that blood transfusion is even more expensive), real-life experience is limited and safety concerns will remain until long-term data become available.
With those three formulations now available in Europe, the most convenient can be chosen in each case according to local considerations.
Guidelines and protocol for use and monitoring
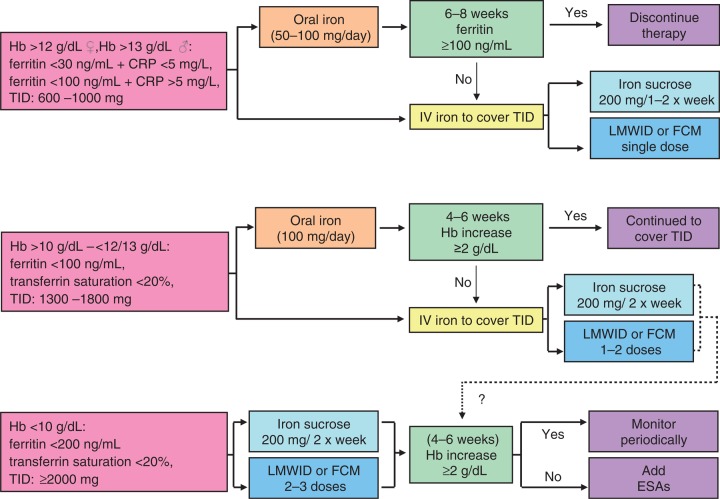
In the recent literature, there are two recent guidelines on the management of IDA in digestive diseases. The guidelines from the British Society of Gastroenterology on the management of IDA [Goddard et al. 2000] review all aspects (diagnosis, causes and treatment) and provide excellent clues to the practicing clinician. However, the role of IV iron is limited to its use in those patients intolerant to oral iron, and no discussion of its possible applications in severe anaemia or avoiding transfusions can be found. More recently, an international group of physicians with expertise in IBD developed a consensus-guided document for the management of anaemia in IBD, based on experiences reported in the last decade [Gasché et al. 2007]. Although these recommendations were developed specifically for IBD patients, we feel that most of them can be applied to other anaemic patients, especially with chronic diseases. A suggested algorithm is given in Figure 1.
Figure 1.
A tentative algorithm for iron replacement therapy in patients with inflammatory bowel disease and anemia (could be applied to any digestive anemia). CRP, C-reactive protein; ESAs, erythropoiesis-stimulating agents; FCM, ferric carboxymaltose; Hb, haemoglobin; IV, intravenous; LMWID, low molecular weight iron dextran; TID, three times daily. Courtesy of Professor Manuel Muñoz.
Practical issues: a short guide
The practical protocol to use IV iron should follow several steps:
Define the iron stores in the context of the specific disease of the patient. Specifically, the presence of inflammation, cyanocobalamin and folic acid levels, and other clues of haematological or liver diseases should be investigated.
Treat the cause of the anaemia simultaneously: there is no chance of complete improvement if the underlying disease is not controlled. This is important to remember in IBDs where some inflammatory activity is not always easy to exclude.
Use IV iron if anaemia is severe (haemoglobin <10.5g/dl), a quick response clinically is needed (planned surgery, for instance), or intolerance to oral iron is present.
-
Calculate the total dose of iron using Ganzoni's formula:
Total iron deficit (mg) = weight (kg) × (ideal haemoglobin — actual haemoglobin) (g/dl) × 0.24 +depot iron (500 mg)
In the specific case of IBD patients, add 500 mg to the calculated deficit, as several studies have suggested that Ganzoni's formula underestimates iron deficiency in these patients, and in all circumstances in male patients.
Use the IV formulation you are more familiar with. Consider convenience, costs, availability, experience and safety. Patients' views are to be taken into account too: iron sucrose could be marginally safer but several doses, IV lines and visits to facilities are required in contrast to LMWID or ferric carboxymaltose.
Plan follow up. If you use IV iron, a reasonable goal is to raise haemoglobin values by 2 g/dl in 1 month. The same goal is reasonable in 2–3 months if you use oral iron. Oral therapy should last 6 months if stores are to be replenished.
Most patients have chronic illness: anaemia will recur, so plan periodical follow up and design a long-term strategy.
If both the treatment of underlying disease and iron fail to correct anaemia, add EPO agents, in this case with the goal not of normal, but sufficient haemoglobin as a target.
Do not underuse IV iron: it is an extraordinary weapon to help some of your patients.
Conclusion
In summary, IV iron is a useful and effective tool to treat iron deficiency in digestive diseases. Although we need more and larger trials to further define indications, protocols, and follow-up rules in different pathologies, we think that available guidelines for IBD patients are a good start, and a very convenient reference for the practicing clinician.
Conflict of interest statement
The authors declare they have no conflicts of interest.
References
- Auerbach M., Coyne D., Ballard H. (2008a) Intravenous iron: from anathema to standard of care. Am J Hematol 83: 580–588 [DOI] [PubMed] [Google Scholar]
- Auerbach M., Goodnough L.T., Picard D., Maniatis A. (2008b) The role of intravenous iron in anemia management and transfusion avoidance. Transfusion 48: 988–1000 [DOI] [PubMed] [Google Scholar]
- Bermejo F., García-López S. (2009) A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol 15: 4638–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer G.J. (2001) Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer's disease. Exp Biol Med (Maywood) 232: 323–335 [PubMed] [Google Scholar]
- Bruewer M., Utech M., Rijcken E.J.M., Antohni C., Laukoetter M.G., Kersting S., et al. (2003) Preoperative steroid administration: effect on morbidity among patients undergoing intestinal bowel resection for Crohn's disease. World J Surg 27: 1306–1310 [DOI] [PubMed] [Google Scholar]
- Chertow G.M., Mason P.D., Vaage-Nielsen O., Ahlmén J. (2005) Update on adverse drug events associated with parenteral iron. Neprhorl Dial Transpant 21: 378–382 [DOI] [PubMed] [Google Scholar]
- Edwards T.J., Noble E.J., Durran A. (2009) Randomized clinical trial of preoperative intravenous iron sucrose to reduce blood transfusion in anaemic patients alter colorrectal cancer surgery. Br J Surg 96: 1122–1128 [DOI] [PubMed] [Google Scholar]
- Fernández-Bañares F., Monzón H., Forné M. (2009) A short review of malabsorption and anemia. World J Gastroenterol 15: 4644–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Erce J.A., Gomollón F., Muñoz M. (2009) Blood transfusion for the treatment of acute anemia in inflammatory bowel disease and other digestive diseases. World J Gastroenterol 15: 4686–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasché C. (2000) Anemia in IBD: the overlooked villain. Inflamm Bowel Dis 6: 142–150 [DOI] [PubMed] [Google Scholar]
- Gasché C., Berstad A., Befrits R., Beglinger C., Dignass A., Erichsen K., et al. (2007) Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis 13: 1545–1553 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Bermejo F., Pajares R., Pérez-Calle J.L., Rodríguez M., Algaba A., et al. (2009) Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis 15: 1485–1491 [DOI] [PubMed] [Google Scholar]
- Gisbert J.P., Gomollón F. (2008) Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol 103: 1299–1307 [DOI] [PubMed] [Google Scholar]
- Goddard A.F., McIntyre A.S., Scott B.B. (2000) Guidelines for the Management of iron deficiency anaemia. British Society of Gastroenterology. Gut 46(Suppl 3–4): IV1–IV5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomollón F., Gisbert J.P. (2009) Anemia and inflammatory bowel diseases. World J Gastroenterol 15: 4659–4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L.S., Puho E., Pedersen L., Mortensen F.V., Sorensen H.T. (2005) Long-term survival after colorectal surgery associated with buffy-coat-poor and leucocyte-depleted blood transfusion: a follow-up study. Lancet 365: 681–682 [DOI] [PubMed] [Google Scholar]
- Jickells T.D., An Z.S., Andersen K.K., Baker A.R., Bergametti G., Brooks N., et al. (2005) Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 308: 67–71 [DOI] [PubMed] [Google Scholar]
- Kell D.B. (2009) Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genom 8: 1–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K., Wade S., Balducci L. (2004) Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 116 Suppl 7: 11S–26S [DOI] [PubMed] [Google Scholar]
- Kulnigg S., Gasche C. (2006) Systematic review: managing anaemia in Crohn's disease. Aliment Pharmacol Ther 24: 1507–1523 [DOI] [PubMed] [Google Scholar]
- Kulnigg S., Stoinov S., Simanenkov V., Dudar L.V., Kamafel W., García L.C., et al. (2007) A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol 103: 1182–1192 [DOI] [PubMed] [Google Scholar]
- Kulnigg S., Teischinger L., Dejaco C., Waldhör T., Gasché C. (2009) Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol 104: 1460–1467 [DOI] [PubMed] [Google Scholar]
- Lanas A., García-Rodríguez L.A., Polo-Tomás M., Ponce M., Alonso-Abreu I., Pérez-Aisa M.A., et al. (2009) Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol 104: 1633–1641 [DOI] [PubMed] [Google Scholar]
- Lindgren S., Wikman O., Befrits R., Blom H., Eriksson A., Grännö C., et al. (2009) Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: a randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol 44: 838–845 [DOI] [PubMed] [Google Scholar]
- Lozoff B., Jimenez E., Smith J.B. (2006) Double burden of iron deficiency in infancy and low socioeconomic status. Arch Pediatr Adolesc Med 160: 1108–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyseng-Williamson K.A., Keating G.M. (2009) Ferric carboxymaltose: a review of its use in iron-deficiency anaemia. Drugs 69: 739–756 [DOI] [PubMed] [Google Scholar]
- Marchetti A., Parker M.S., Moccia L.P., Lin E.O., Arrieta A.L., Ribalet F., et al. (2009) Ferritin is used for iron storage in Bloom-forming marine pennate diatoms. Nature 457: 467–470 [DOI] [PubMed] [Google Scholar]
- Muñoz M., Campos A., García-Erce J.A. (2006) Intravenous iron in colorrectal cancer surgery. Semin Hematol 43: S36–S38 [Google Scholar]
- Muñoz M., García-Erce J.A., Díaz-Lobo A.I., Campos A., Sebastianes C., Bisbe E. (2009a) Utilidad de la administración de hierro sacarosa intravenoso para la corrección de la anemia preoperatoria en pacientes sometidos a cirugía mayor. Med Clin (Barc) 132: 303–306 [DOI] [PubMed] [Google Scholar]
- Muñoz M., Gómez-Ramírez S., García-Erce J.A. (2009b) Intravenous iron in inflammatory bowel disease. World J Gastroenterol 15: 4666–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M., Villar I., García-Erce J.A. (2009c) An update on iron physiology. World J Gastroenterol 15: 4617–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phrommintikul A., Haas S.J., Elsik M., Krum H. (2007) Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet 369: 381–388 [DOI] [PubMed] [Google Scholar]
- Rouault T.A. (2004) Pathogenic bacteria prefer heme. Science 305: 1577–1578 [DOI] [PubMed] [Google Scholar]
- Semrin G., Fishman D.S., Bousvaros A., Zholudev A., Saunders A.C., Correla C.E., et al. (2006) Impaired iron absorption in Crohn's disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis 12: 1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Himmelfarb J., Szczech L.A. (2009) Resolved: targeting a higher hemoglobin is associated with greater risk in patients with CKD anemia. J Am Soc Nephrol 20: 1436–1443 [DOI] [PubMed] [Google Scholar]
- Sostres C., Gargallo C., Lanas A. (2009) Drug-related damage of the ageing gastrointestinal tract. Best Pract Res Clin Gastroenterol 23: 849–860 [DOI] [PubMed] [Google Scholar]
- Theurl I., Aiugner E., Theurl M., Nairz M., Seifert M., Schroll A., et al. (2009) Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood 113: 5277–5286 [DOI] [PubMed] [Google Scholar]
- Weiss G., Gaschel C. (2010) Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica 95: 175–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G., Goodnough L.T. (2005) Anemia of chronic disease. N Engl J Med 352: 1011–1023 [DOI] [PubMed] [Google Scholar]
- Wells C.W., Lewis S., Barton J.R., Corbett S. (2006) Effects of changes in haemoglobin levels on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis 12: 123–130 [DOI] [PubMed] [Google Scholar]
- Zimmermann H.B., Hurrell R.F. (2007) Nutritional iron deficiency. Lancet 370: 511–520 [DOI] [PubMed] [Google Scholar]