Abstract
Objective
The aim of this study was to compare the pharmacokinetic parameters between two brands of pregabalin in healthy Chilean volunteers.
Methods
A randomized, single-dose, two-period, two-sequence, crossover study design with a 2-week washout period was conducted in healthy Chilean males. Plasma samples were collected over a 12-hour period after administration of 150 mg pregabalin in each period. A validated ultra-performance liquid chromatography with positive ionization mass spectrometric detection method was used to analyze pregabalin concentration in plasma. Pharmacokinetic parameters were determined using a noncompartmental method. Bioequivalence between the test and reference products was determined when the ratio for the 90% confidence intervals (CIs) of the difference in the means of the log-transformed area under the curve (AUC)0—t, AUC0—∞, and maximum concentration (Cmax) of the two products were within 0.80 and 1.25.
Results
The study was carried out on 22 healthy Chilean volunteers. The mean (SD) Cmax, AUC0—t and AUC0—∞ of the test formulation (Pregobin™) of pregabalin were 2.10 (0.56) μg/ml, 10.35 (2.00) μgxh/ml and 13.92 (2.74) μgxh/ml, respectively. The mean (SD) Cmax, AUC0—t and AUC0—∞ of the reference formulation (Lyrica™) of pregabalin were 2.15 (0.52) μg/ml, 10.31 (1.85) μgxh/ml and 13.78 (2.25) μgxh/ml, respectively. The parametric 90% CIs for Cmax, AUC0—t, and AUC0—∞ were 0.97–1.13, 1.01–1.04, and 0.98–1.02, respectively.
Conclusions
These results suggest that both products are bioequivalent and can be used as interchangeable options in the clinical setting.
Keywords: bioavailability, bioequivalence, gabapentin, pharmacokinetics, pregabalin
Introduction
Pregabalin (PGB), (S)-3-(aminomethyl)-5-methylhexanoic acid, is an anticonvulsant drug structurally related to the inhibitory neurotransmitter of the central nervous system, γ-aminobutyric acid (GABA). PGB is used in combination with other anticonvulsant agents in the management of partial seizures in adult patients presenting postherpetic neuralgia (PHN), pain associated with diabetic peripheral neuropathy (DPN), and in generalized anxiety disorder [Shneker and McAuley, 2005; Zareba, 2005]. It was designed as a more potent successor to gabapentin (GBP) and was first marketed by Pfizer under the trade name Lyrica®. Recent studies have shown that PGB is also effective in treating chronic pain in disorders such as fibromyalgia [Crofford et al. 2005] and spinal cord injury [Siddall et al. 2006].
Although this active pharmaceutical ingredient was developed as a GABA analog it does not bind GABA or benzodiazepine receptors; therefore, it does not increase GABA-like responses nor its uptake or degradation. However, it can increase the GABA transporter protein. Like GBP, PGB binds to the α2δ subunit of the voltage-dependent calcium channel [Rogawski and Taylor, 2006] in the central nervous system. However, the exact mechanism of action is still unknown. In vitro, PGB reduces calcium-dependent release of several neurotransmitters, e.g. glutamate, norepinephrine and substance P, probably due to modulation of calcium channels [Fink et al. 2002; Dooley et al. 2000a, 2000b].
PGB does not bind to proteins in plasma and it is not substantially metabolized in humans. Its only N-methylated metabolite is found in urine at 0.9% of the dose. Bioavailability studies with PGB single doses in healthy volunteers showed proportional values of maximum concentration (Cmax) and area under the curve (AUC), a time to maximum concentration (Tmax) of about 1 hour, a half life (t1/2) of about 5–7 hours, and an oral bioavailability of 90%, with an apparent volume of distribution following oral administration of approximately 0.5l/kg [Busch et al. 1998]. Although the mechanism of PGB absorption is unknown, it has been proposed that, as for GBP, PGB should be a substrate for the L-aminoacid transport system [Stewart et al. 1993].
The simultaneous consumption of food with PGB can reduce Cmax by 25–30%, and increase the Tmax to 3 hours [Blommel and Blommel, 2007]. The serum concentrations for healthy volunteers obtained with a single dose of 200 mg of PGB was 5.96μg/ml [Bockbrader et al. 2010]. The drug does not bind to proteins and is not expected to be an inducer of liver enzymes [Kwan, 2006].
On the other hand, the use of generic antiepileptic drugs has increased in Chile as well as globally during the past few years. Although these less-expensive products may represent an important alternative for many patients, it is not clear at this point whether the generic forms are comparable with the standards of their more expensive counterparts. The Chilean medical community has shown concerns about this issue indicating the need for new data and tight application of regulations. In this context, the main goal of this study was to evaluate bioavailability of two sources of PGB in healthy males and females Chilean volunteers. The goal was to determine bioequivalence of a test formulation of 150mg (capsules) of PGB, Pregobin™ (Drugtech-Recalcine S.A., Santiago, Chile) and another commercial formulation of 150mg (capsules) of PGB, Lyrica™ (Pfizer GMBH, Freiburg, Germany, imported to Chile by Pfizer Chile S.A.) used as a reference formulation.
Subjects, materials and methods
Subjects
Twenty-two healthy adult male and female volunteers between 21 and 50 years old with normal body mass indexes were selected. All subjects were considered healthy as determined by screening tests including medical history, physical examination, and laboratory analyses (Table 1).
Table 1.
Demographic characteristics and baseline data of hematological and biochemical parameters of healthy Chilean volunteers subjects (n = 422).
| Mean | SD | Normal range | |
|---|---|---|---|
| Age (years) | 28.5 | 8.26 | NA |
| Weight (kg) | 69.38 | 13.24 | NA |
| Height (cm) | 169.04 | 9.43 | NA |
| BMI (kg/m2) | 24.27 | 3.51 | 19–27 |
| Creatinine (mg/dl) | 0.88 | 0.14 | 0.8–1.5 |
| Alkaline phosphatase (UI) | 70.40 | 16.91 | 38.0–126.0 |
| Glucose (mg/dl) | 85.13 | 9.08 | 60.0–100.0 |
| Uremia (mg/dl) | 27.81 | 8.66 | 0.0–50.0 |
| AST (UI) | 20.40 | 6.17 | 5.0–40.0 |
| ALT (UI) | 23.18 | 10.76 | 7.0–56.0 |
| Hematocrit | 40.03 | 6.55 | 40.0–54.0 |
| Leukocytes (x μL) | 6226.36 | 1433.19 | 5000–10,000 |
| Hemoglobin (mg/dl) | 13.94 | 1.27 | 13.0–16.0 |
| Total bilirubin (mg/dl) | 0.75 | 0.34 | 0.2–1.3 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; NA, not applicable; SD, standard deviation.
Before enrollment and at the end of the study, each subject underwent a physical examination and clinical laboratory testing (blood chemistry, hematology, and urinalysis). Baseline clinical laboratory tests were also performed before drug administration.
The enrollment criteria excluded subjects who had taken any prescription drug within 2 weeks prior to entering the study, those with clinical history of drug hypersensitivity, pregnant women or those presently using steroidal contraception and postmenopausal women.
The experimental protocol was designed in accordance with the general ethical principles outlined in the Declaration of Helsinki and under US Food and Drug Administration and World Health Organization guidelines [Food and Drug Administration, 2003; World Health Organization, 1998; Nuremberg Doctors' Trial, 1964]. The protocol for this study, as well as the protocol amendments, and the informed consent documents were reviewed and approved by the Ethics Committee of the Faculty of Medicine of the University of Chile (protocol ID PREG-03-2007, approved with number 2435 on 2006/12/15).
Study design
The study was conducted in a double-blind, randomized, single-dose, two-period, crossover design with a 1-week washout period between doses. A single dose of 150mg PGB of either formulation was administered after overnight fasting. After dosing, serial blood samples were collected during a period of 12 hours. Blood samples (5ml) were drawn at 0, 0.08, 0.16, 0.30, 0.50, 1.0, 1.5, 2.0, 2.5, 4.0, 6.0, 8.0, and 12 hours after administration. The subjects were housed for 14 hours postdose and were monitored for safety and adverse effects throughout the study. There was an interval of 2 weeks between two clinical phases: the first period was 15 January 2007; the second period was 29 January 2007.
Pregabalin sources
The commercially available test product, Pregobin™ (Drugtech-Recalcine S.A.) from batch number 100106 (expiry date September 2008), and the innovator product, Lyrica™ (Pfizer) from batch number 0175125 (expiry date November 2008), contained 150mg of PGB per capsule and were characterized with regard to content and in vitro solubility profile.
Analytical method
Ultra-performance liquid chromatography (UPLC) with positive ionization mass spectrometric detection was used to determine PGB plasma concentrations. This method was a modified version of that developed by Ji and colleagues [Ji et al. 2006] and was validated (for specificity, sensitivity, linearity, recovery, precision, and accuracy) in our laboratory. GBP was used in this study as an internal standard (Merck AG).
Chromatographic and MS/MS conditions
For LC/MS/MS analysis, the chromatographic system consisted of an UPLC Acquity unit (Waters Corporation), a Quatro Micro API detector ESCI Multimode-Ionization. The separation was performed on an Acquity UPLC BEH-Hilic column 1.7 μm, 2.1 mm × 50 mm (Waters Corporation), using a mobile phase of acetonitrile/ammonium formate 100mM pH 3.0 (85/15 v/v) with a flow rate of 0.4ml/min. The total injected volume was 5 μl of each sample.
The temperatures of the column and autosampler tray were 30°C and 4°C, respectively. The optimum collision energy was 15 eV for both, using argon as the collision gas and multiple-reaction monitoring (MRM) mode was used for the quantification m/z 160.3→97.3 for PGB and m/z 172.4→154.3 for GBP. Peak areas were integrated using Masslynx version 4.1 software (Micromass, UK).
Blood sample preparation
Sample preparation involved a simple protein precipitation with acetonitrile. The plasma samples were filtered through 0.22 μM Millex-GV Millipore filters, then to 10 μl of plasma was added 10 μl of internal standard (1 μg/ml in methanol) and 100 μl of acetonitrile. The mixture was vortex mixed for 30 s and centrifuged at 8000 rpm for 5 min at 4°C. Finally, 5 μl of supernatant was injected into the UPLC/MS/MS system.
Validation procedure
Standard stock solutions of PGB and GBP (IS) were prepared in methanol to 1 μg/ml and stored at 4°C. Working standard solutions for calibration and controls were prepared from the stock solution by adequate dilution using acetonitrile. A calibration curve includes the following concentration points: 0.1, 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, and 8.0 μg/ml.
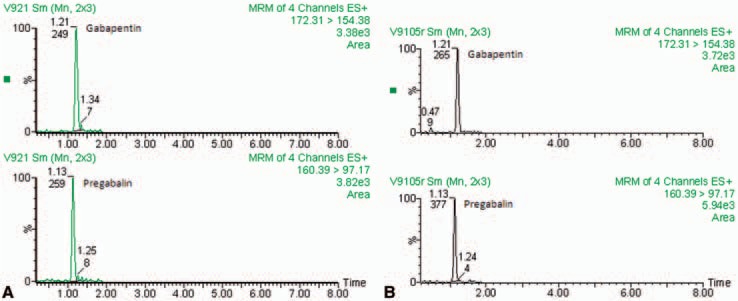
The quality control samples (QC) were prepared with human plasma and appropriate amounts of drug to obtain concentrations of 0.5, 1.0, and 4.0 μg/ml. The retention time was 1.05 min for PGB and 1.12 min for GBP. Standard plots of the ratio of PGB/GBP concentrations versus PGB plasma concentration were linear over a range of 0.1– 8.0 μg/ml (r = 0.99916). Accuracy from QC samples at 0.5, 1.0, and 4.0 μg/ml concentrations were 3.8%, 4.5%, and 1.8%, respectively. The intra-assay coefficient of variation was 4.7%, 6.1%, and 4.1%, respectively, and the inter-assay coefficient of variation was 1.1%, 0.7%, and 2.0%, respectively. No significant degradation of PGB during storage and processing conditions was noted. A representative chromatogram is presented in Figure 1.
Figure 1.
Representative chromatographic profiles from one volunteer for A (Pregobin™) and B (Lyrica™) formulations.
Pharmacokinetic and statistical analyses
The sample size (n) was calculated on the basis of a crossover design with log-transformed data, considering an intra-individual variation coefficient of 20%, a power of 80% and a significance level of 5%, according to Chow and Wang [2001]. Under these conditions, the calculated sample size was 18 volunteers; thus, taking into consideration potential withdrawals and dropouts we enrolled 24 volunteers. PGB has no reported intra-individual variation coefficient; however, because it is a high solubility/high permeability drug with low hepatic metabolism we can conclude it should have very low variability.
Plasma concentrations of PGB versus time were evaluated by standard noncompartmental analysis methods. The highest plasma concentration observed and the corresponding time was defined as the Cmax and Tmax values, respectively. The elimination rate constant (Kel) was obtained by linear regression from the best-fit slope of the terminal log-linear decline in plasma concentrations versus time profile. The half-life (t1/2) was obtained as 0.693/Kel. The area under the plasma concentration curve to the last quantifiable concentration (Ct) at time t (AUC0-t) was determined by linear trapezoidal integration. The AUC extrapolated to infinity (AUC0-∞) was calculated as AUC0-t + Ct/Kel. The apparent total body clearance after oral administration (Cl/F) was estimated as dose/AUC0-∞. It was assumed that the terminal t1/2 was the elimination half life, thus the apparent volume of distribution after oral dosing (V/F) was calculated as (Cl/F)/Kel. The area under the first moment of the plasma concentration versus time curve (AUMC0-∞) was obtained by applying the linear trapezoidal method to the product of concentration × time versus time up to time t and adding the extrapolated area of Ct × t/Kel + Ct/(Kel)2. Mean residence time (MRT), reflecting the average time that a molecule remains in the body, was calculated from AUMC0-∞/AUC0-∞. The pharmacokinetic parameters were generated using the software AUC-RPP [Ritschel, 1986].
Bioequivalence analysis
STATA 10.0 and SPSS 11 were used to generate statistical outputs. STATA pk command was used to evaluate average bioequivalence, specifically pkcross and pkequiv, with the former performing a crossover analysis using an analysis of variance (ANOVA) model considering sequence, period, treatment effects and using a 90% confidence interval (CI), the latter using different approaches to evaluate bioequivalence. In this study we use a standard classic CI and two one-sided Schuirmann hypothesis test [Schuirmann, 1987; Ritschel, 1986].
Results
Two of the 24 selected volunteers did not complete the study due to their absence at the first session, while the remaining 22 individuals participated in the study.
Table 1 provides the anthropometric and biochemical characteristics and the identification of the 22 volunteers finally included in this study. Mean age of the group was 28.5 ± 8.26 years; mean body weight was 69.38 ± 13.24 kg; mean height was 169.04 ± 9.43 cm and mean of body mass index was 24.27 ± 3.51 kg/m2. The values for glycemia, uremia, creatinine, bilirubin, hematocrit, hemoglobin, leukocytes, alkaline phosphatase, and aminotransferases ranged between normal values for all volunteers; thus verifying their healthy condition.
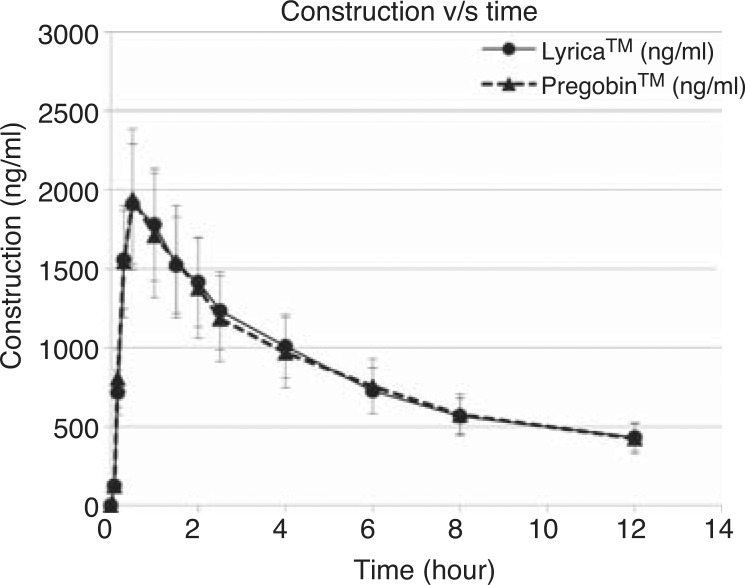
Figure 2 (average plasmatic concentrations of PGB versus time), shows that plasma concentration diminished in a multiexponential mode after the peak concentration time.
Figure 2.
Changes in pregabalin concentrations in plasma (mean ± SD) after ingestion of 150 mg of either Pregobin™ or Lyrica™ formulations.
Table 2 shows the pharmacokinetic profiles for Pregobin™ and Lyrica™. The 90% CIs for Cmax, AUC0-t and AUC0-∞ were 96.76–112.90%, 100.38–103.69% and 97.22–102.22%, respectively (Table 3). Other pharmacokinetic parameters, not considered for determining bioequivalence tmax, t1/2, and MRT and clearance (Cl) were also analyzed (Table 2).
Table 2.
Pharmacokinetic parameters of test and reference 150mg pregabalin capsules of two different pharmaceutical forms after single-dose administration in healthy Chilean volunteers.
| Parameters | Pregobin™ | Lyrica™ |
|---|---|---|
| AUC0-t (μg/ml × h−1) | ||
| Mean (SD) | 10.35 (2.0) | 10.31 (1.85) |
| %CV | 19.32 | 17.94 |
| AUC0-∞ (μg/ml × h−1) | ||
| Mean (SD) | 13.92 (2.74) | 13.78 (2.25) |
| %CV | 19.68 | 16.33 |
| Cmax (μg/ml) | ||
| Mean (SD) | 2.10 (0.56) | 2.15 (0.52) |
| %CV | 26.67 | 24.19 |
| Tmax (h) | ||
| Mean (SD) | 0.75 (0.43) | 0.63 (0.4) |
| %CV | 57.33 | 63.49 |
| t1/2, (h) | ||
| Mean (SD) | 5.67 (1.12) | 5.56 (0.98) |
| %CV | 19.75 | 17.63 |
| MRT (h) | ||
| Mean (SD) | 8.60 (1.77) | 8.49 (1.55) |
| %CV | 20.58 | 18.26 |
| Cltot/F (ml/min) | ||
| Mean (SD) | 185.77 | 186.92 |
| (34.1) | (35.87) | |
| %CV | 18.36 | 19.19 |
| Vdbeta/F (l/kg) | ||
| Mean (SD) | 1.30 (0.24) | 1.29 (0.22) |
| %CV | 18.46 | 17.05 |
AUC, area under the curve; Cltot/F, apparent total body clearance; Cmax, maximum concentration; CV, coefficient of variation; MRT, mean residence time; SD, standard deviation; t1/2, half life; Tmax, time to maximum concentrations; Vdbeta/F, apparent terminal volume of distribution.
Table 3.
Classic confidence interval for bioequivalence and Schuirmann's test to compare Lyrica™ (reference) and Pregobin™ (test) parameters in healthy volunteers.
| Classic confidence interval for bioequivalence | Schuirmann's test | |||||||
|---|---|---|---|---|---|---|---|---|
| Equivalence limits reference (%) | Test limits (%) | Bioequivalence probability (%)* | Two one-side test | p-value | ||||
| Ln Cmax | ||||||||
| difference | −0.141 | 0.141 | −0.023 | 0.091 | 96.00 | Upper side | −3.243 | 0.004 |
| Ratio | 80 | 120 | 96.791 | 112.905 | Lower side | 5.319 | >0.0001 | |
| Ln AUC0→t | ||||||||
| difference | −0.463 | 0.463 | −0.039 | 0.037 | 100.00 | Upper side | −1.141 | >0.0001 |
| ratio | 80 | 120 | 100.384 | 103.686 | Lower side | 21.077 | >0.0001 | |
| LnAUC0→∞ | ||||||||
| difference | −0.523 | 0.523 | −0.073 | 0.058 | 100.00 | Upper side | −13.997 | >0.0001 |
| ratio | 80 | 120 | 97.222 | 102.220 | Lower side | −13.612 | >0.0001 | |
AUC, area under the curve; Cmax, maximum concentration; Ln, log normal.
Probability test limits are within equivalence limits.
ANOVA analysis is shown in Table 4. For inter-subjects, there was evidence of variability to Cmax and AUC. On the other hand, there were not sequence effects in pharmacokinetic parameters. Moreover, for intrasubjects no significant variability was observed, with the exception of AUC0-t (p = 0.0462).
Table 4.
Analysis of variance (ANOVA) for maximum concentration Cmax, area under the curve (AUC)0→t and AUC0→∞ (after logarithmic transformation) for parallel group design and two-treatment, two-period crossover design to pregabalin formulations.
| Sources of variation | Sum of squares | Degree of freedom | Mean sum of squares | Fisher statistics | p-value |
|---|---|---|---|---|---|
| Cmax | |||||
| Intersubjects | |||||
| Sequence effect | 0.33 | 1 | 0.33 | 2.78 | 0.1112 |
| Residuals | 2.35 | 20 | 0.12 | 9.91 | >0.0001 |
| Intrasubjects | |||||
| Treatment effect | 0.00 | 1 | 0.00 | 0.00 | 0.9455 |
| Period effect | 0.01 | 1 | 0.01 | 1.08 | 0.3118 |
| Residuals | 0.24 | 20 | 0.01 | ||
| AUC0→t | |||||
| Intersubjects | |||||
| Sequence effect | 0.04 | 1 | 0.04 | 0.46 | 0.5047 |
| Residuals | 1.55 | 20 | 0.08 | 14.62 | >0.0001 |
| Intrasubjects | |||||
| Treatment effect | 0.02 | 1 | 0.02 | 4.52 | 0.0462 |
| Period effect | 0.00 | 1 | 0.00 | 0.00 | 0.9748 |
| Residuals | 0.11 | 20 | 0.01 | ||
| AUC0→∞ | |||||
| Intersubjects | |||||
| Sequence effect | 0.00 | 1 | 0.00 | 0.07 | 0.7960 |
| Residuals | 1.01 | 20 | 0.05 | 3.20 | 0.0062 |
| Intrasubjects | |||||
| Treatment effect | 0.07 | 1 | 0.07 | 4.13 | 0.0555 |
| Period effect | 0.00 | 1 | 0.00 | 0.04 | 0.8491 |
| Residuals | 0.32 | 20 | 0.02 |
Following administration of the PGB capsules, four of the subjects reported mild or moderate adverse effects; the most frequent were somnolence (two volunteers), nausea (one volunteer) and xerostomia (one volunteer). There were no serious adverse effects observed throughout the study. During the study there were no significant changes in clinical laboratory values, vital signs, physical findings, or other observations related to safety.
Discussion
This study was designed to measure all relevant aspects underlying the pharmacokinetics of PGB that may be used to establish the degree of similarity between the two formulations indicated above. Almost perfectly overlapped curves of drug concentration and time were observed (Figure 1; the arithmetic differences are irrelevant due to the 90% CI). The clinical implications of these results allow us to have an anticonvulsant objective point of reference to choose an anticonvulsant medication.
In general terms, the magnitude of the absorption of a drug is reflected in the value of the parameter AUC related to the time postadministration. In the present study, the AUC between 0 and 12 hours (AUC0→t) and between zero and infinite time (AUC0→∞) were analyzed. For these periods of time, reliable measurements of the bioavailability of the drug were obtained from each individual.
Here, the pharmacokinetics results showed conclusive data with regards to therapeutic equivalence. The comparison between the test (formulation A) and the reference (formulation B) of Cmax, Tmax, AUC0→t and AUC0→∞, showed percentages that fall in the rank of equivalence (according to the FDA and ISP, Chile) (Table 3).
The similar pharmacokinetic profile of both formulations is also reflected in the MRT with a ratio of 1.013 between Lyrica™ and Pregobin™. This should not mean a bigger difference with respect to Lyrica™ due to the similar behavior of Pregobin™ in the body reflected in AUC0→t and AUC0→∞.
The pharmacokinetic values are similar to those reported in scientific literature [Blommel and Blommel, 2007]. The results indicate similarity regarding pharmacokinetics of the drug in the body for both formulations.
According to the biopharmaceutical classification system (BCS) of the FDA, PGB has a high solubility/high permeability profile; thus, it is a class 1 drug which can be bioexempted for in vivo bio-equivalence studies. However, it is highly convenient to make in vivo studies which reflect, in a better way, the bioavailability profiles.
In summary, according to the analyses of results, we conclude that both formulations of PGB are bioequivalent. Therefore, following FDA guidelines and Chilean ISP criteria, we recommend that, for clinical usage, Pregobin™ 150 mg be used as interchangeable with Lyrica™ 150 mg.
Acknowledgments
This manuscript is dedicated to the memory of Dr Mario Escala Zanolli, an excellent physician who left us unexpectedly. The authors wish to thank to Dr Hernán Vásquez for reviewing this manuscript, Mrs Magdalena Orellana, Ms Jeannette Hermosilla, Mr Héctor Valenzuela, Mr Ramiro Pérez, Mr Santiago Leyton and Mr Juan Rojas for their excellent technical support. We also thank Biohealth LLC Clinical Research for their collaboration.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
References
- Blommel M.L., Blommel A.L. (2007) Pregabalin: an antiepileptic agent useful for neuropathic pain. Am J Health Syst Pharm 64: 1475–1482 [DOI] [PubMed] [Google Scholar]
- Bockbrader H.N., Radulovic L.L., Posvar E.L., Strand J.C., Alvey C.W., Busch J.A., et al. (2010) Clinical pharmacokinetics of pregabalin in healthy volunteers. J Clin Pharmacol 50(8): 941–950 [DOI] [PubMed] [Google Scholar]
- Busch J.A., Strand J.A., Posvar E.L. (1998) Pregabalin (CI-1008) single-dose pharmacokinetics and safety/tolerance in healthy subjects after oral administration of pregabalin solution or capsule doses. Epilepsia 39(Suppl 6): 58 [Google Scholar]
- Chow S.-Ch., Wang H. (2001) On sample size calculation in bioequivalence trials. J Pharmacokinet Pharmacodynam 28: 155–169 [DOI] [PubMed] [Google Scholar]
- Crofford L.J., Rowbotham M.C., Mease P.J., Russell I.J., Dworkin R.H., Corbin A.E., et al. (2005) Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 52: 1264–1273 [DOI] [PubMed] [Google Scholar]
- Dooley D.J., Donovan C.M., Pugsley T.A. (2000a) Stimulus-dependent modulation of [(3)H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J Pharmacol Exp Ther 295: 1086–1093 [PubMed] [Google Scholar]
- Dooley D.J., Mieske C.A., Borosky S.A. (2000b) Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett 280: 107–110 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (2003) BA and BE Studies for Orally Administered Drug Products-General Considerations. FDA Center for Drug Evaluation and Research [Google Scholar]
- Fink K., Dooley D.J., Meder W.P., Suman-Chauhan N., Duffy S., Clusmann H., et al. (2002) Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 42: 229–236 [DOI] [PubMed] [Google Scholar]
- Ji H.Y., Jeong D.W., Kim Y.H., Kim H.H., Yoon Y.S., Lee K.C., et al. (2006) Determination of gabapentin in human plasma using hydrophilic interaction liquid chromatography with tandem mass spectrometry. Rapid Comm Mass Spectro 20: 2127–2132 [DOI] [PubMed] [Google Scholar]
- Kwan P. (2006) Pregabalin: a new drug for epilepsy, neuropathic pain and anxiety. Hong Kong Med Diary 11: 15–16 [Google Scholar]
- Nuremberg Doctors' Trial (1964) Declaration of Helsinki 7 December. BMJ 313: 1448–1449 [Google Scholar]
- Ritschel W.A. (1986) AUC-RPP: BASIC computer program for compartment model independent pharmacokinetic analysis. Methods Find Exp Clin Pharmacol 8: 633–640 [PubMed] [Google Scholar]
- Rogawski M.A., Taylor C.P. (2006) Calcium channel α2-δ subunit: a new antiepileptic drug target. Epilepsy Res 69: 183–27216835945 [Google Scholar]
- Schuirmann D.J. (1987) A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 15: 657–680 [DOI] [PubMed] [Google Scholar]
- Shneker B.F., McAuley J.W. (2005) Pregabalin: a new neuromodulator with broad therapeutic indications. Ann Pharmacother 39: 2029–2037 [DOI] [PubMed] [Google Scholar]
- Siddall P.J., Cousins M.J., Otte A., Griesing T., Chambers R., Murphy T.K. (2006) Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 67: 1792–1800 [DOI] [PubMed] [Google Scholar]
- Stewart B.H., Kugler A.R., Thompson P.R., Bockbrader H.N. (1993) A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res 10: 276–281 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1998) Guidelines on registration requirements to establish interchangeability. In Code of Federal Regulations. Title 21, part 320. “Bioavailability and Bioequivalence Requirements”. Technical Report Series N (WHO-96). Washington, DC: WHO [Google Scholar]
- Zareba G. (2005) Pregabalin: a new agent for the treatment of neuropathic pain. Drugs Today (Barc) 41: 509–516 [DOI] [PubMed] [Google Scholar]