Abstract
Many studies have been published in the last 10 years on the efficacy and safety of montelukast in asthma since this drug entered the market. Experimental studies, in vitro and in vivo, and clinical studies on large numbers of patients with asthma of different severity have clearly demonstrated that montelukast is able to modify the pathophysiological mechanisms of the disease, and to improve to some extent the clinical and functional manifestations of asthma. Studies of montelukast as monotherapy or in combination with other drugs, mainly inhaled corticosteroids (ICS), versus different comparator drugs have contributed to the positioning of montelukast in the different levels of asthma treatment, according to the Global Initiative for Asthma Guidelines. Montelukast may be used as monotherapy as an alternative to low-dose ICS (particularly in a step-down strategy) or in addition to ICS for improving clinical manifestations by an increase in anti-inflammatory effects and a sparing of corticosteroids. The heterogeneity of asthma has received a large amount of attention in the last few years in order to better tailor treatment according to the different clinical and biological phenotypes of asthma. Montelukast has proven to be particularly effective in exercise-induced asthma and in asthma associated with allergic rhinitis. Other phenotypes where montelukast is effective include asthma in obese patients, asthma in smokers, aspirin-induced asthma and viral-induced wheezing episodes. The safety profile of montelukast is very good, and the suspicions of increased risk of Churg—Strauss syndrome or suicide have not been confirmed.
Keywords: asthma, montelukast, phenotype, remodelling
Introduction
Leukotriene receptor antagonists (LTRAs), apart from anti-immunogloblin (Ig) E monoclonal antibodies, are the only new category of anti-asthma drugs to enter the market in the last 10 years. Up to the end of the 1990s, asthma treatment was based only on beta2-agonists and corticosteroids, with a minimal role for other older drugs such as theophylline and cromones. The novelty of LTRAs was that they target a specific mechanism, the binding of leukotrienes to their receptors, which is part of the complex pathway involved in asthma. Distinct from inhibitors of 5-lipoxygenase or other enzymes involved in the generation of leukotrienes, some competitive antagonists of the cysteinyl-leukotrienes (Cys-LTs) have been developed and launched in the market, that is, zafirlukast, pranlukast and montelukast. Among them, montelukast has shown the best efficacy and safety profile, and it has become the most widely studied anti-leukotriene compound.
Role of leukotriene in asthma pathophysiology
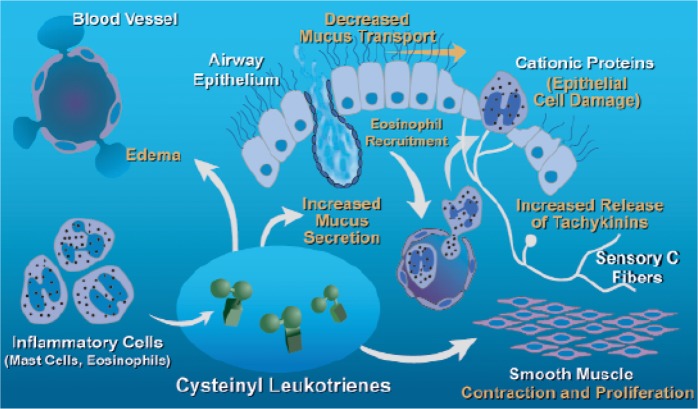
Leukotrienes are produced by many cells of the body and mediate many aspects of the inflammatory response. In the lung, the leukotriene cascade, due to the activation of intracellular 5-lipoxygenase with subsequent release of sulphidopeptide leukotrienes (LTC4, LTD4 and LTE4: Cys-LTs), is activated by different stimuli acting on many inflammatory cells, either resident (such as mast cells) or recruited in the airways (eosinophils, macrophages, etc.), but also on epithelial cells [Holgate et al. 2003]. Cys-LTs mediate several different effects on airway cells and structures. In particular, LTD4 is the most potent bronchoconstricting agent on a molar basis, but Cys-LTs also have chemoattractive properties for many inflammatory cells (mainly eosinophils), effects on vascular permeability, mucous secretions and sensory nerve activation, and are responsible for part of the pathophysiology of asthma (Figure 1) [Hamid et al. 2003].
Figure 1.
Different targets of cysteinyl leukotrienes on the resident and recruited cells in the airways in asthma. (Reproduced with permission from Hamid et al. [2003].).
In addition to the manifestations of the acute phase of airway inflammation, Cys-LTs play a role in the remodelling process of the airways leading to the progressive decline in pulmonary function observed in some asthmatic patients. Some experimental studies have demonstrated the role of Cys-LTs in inducing the proliferation and activation of mucosal fibroblasts [Asakura et al. 2004] and the secretion and deposition of some component of the extracellular matrix [Altraja et al. 2008].
For all these reasons, LTRAs held the potential to be effective as anti-asthma drugs, with the possibility of hindering both the acute phase and the long-term consequences of allergic inflammation of asthma.
Effects of LTRAs in allergic airway inflammation
In preliminary studies, LTRAs have been shown to decrease the recruitment and activation of eosinophils in the airways [Laitinen et al. 2005], and to blunt the release of pro-inflammatory cytokines from airway cells in some in vitro studies, although at relatively high doses [Maeba et al. 2005]. The anti-inflammatory effect of montelukast has been demonstrated in vivo by the decrease in sputum eosinophilia [Pizzichini et al. 1999], exhaled nitric oxide concentration [Sandrini et al. 2003] and inflammatory cells in the airway mucosa [Ramsay et al. 2009] of asthmatic subjects, as well as by the improvement of indirect markers of inflammation and remodelling, such as bronchial hyperresponsiveness [Currie and Lipworth, 2002].
These effects seem to be complementary to those obtained with the use of other anti-inflammatory and anti-allergic compounds, such as antihistamines (which, however, have no role in asthma treatment) and corticosteroids. The administration of montelukast and desloratadine before allergen challenge resulted in a greater protective effect than that obtained by administering the drugs singly, on both the immediate and the late airway responses in a group of asthmatic subjects [Davis et al. 2009]. Some papers suggested that corticosteroids could not prevent allergen-induced increase in LTE4 urinary excretion [Dworski et al. 1994; O'sShaugnessy et al. 1993]. This was not confirmed by our recent study, in which 1-week inhaled beclomethasone significantly blunted the early increase in urinary LTE4 after allergen challenge [Bartoli et al. 2010]. In the same experimental model of allergen challenge, montelukast plus inhaled corticosteroids (ICS) determined a greater bronchoprotection than ICS alone on the immediate airway response [Leigh et al. 2002].
Thus, there is no doubt that LTRAs have shown anti-inflammatory properties, with complementary effects to antihistamine and ICS, and may be active in the prevention of bronchial remodelling.
Clinical studies of montelukast in asthma
The first demonstrations of the efficacy of montelukast in asthma were obtained in the mid-1990s, when the results of both comparative studies of montelukast versus placebo and studies of the protective effect of montelukast on bronchoconstriction induced by exercise or other nonspecific stimuli were published [Leff et al. 1998; Reiss et al. 1998]. Montelukast improved symptoms, rescue medication use and pulmonary function, and reduced the rate of exacerbation and the level of blood eosinophils, in mild-to-moderate asthmatics not treated with ICS. Montelukast also protected against bronchocon-striction induced by exercise better than long-acting beta2-agonists (LABAs) [Villaran et al. 1999]. These data led to the introduction of montelukast into the market at the end of the 1990s.
At the same time, the efficacy of montelukast in rhinitis was evaluated in other studies, which showed that montelukast was effective and well tolerated with additional benefits over antihistamines, although still less effective than intranasal corticosteroids [Nayak and Langdon, 2007].
The following studies were conducted in an attempt to determine the place of montelukast in asthma treatment.
Comparison with ICS
As ICS are the ‘gold standard’ for the treatment of asthma, the first evaluation was the comparison between montelukast and low-dose ICS. This point is important because monotherapy is recommended in patients with mild asthma, and the decision on which should be the ‘first option’ (ICS or montelukast) is therefore crucial. The many studies published on this point, using different study protocols for different time periods, depending on the specific outcome considered (i.e. pulmonary function, symptoms or rate of exacerbations) have largely confirmed the greater efficacy of low-dose ICS in comparison with montelukast [Busse et al. 2001; Malmstrom et al. 1999]. For this reason, all international guidelines state that ICS are more effective than LTRAs in the monotherapy of asthma, and recommend montelukast as second choice in patients with mild asthma [Global Initiative for Asthma, 2009].
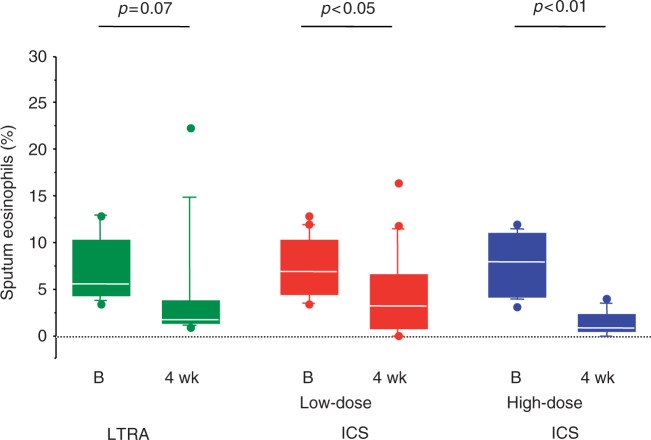
However, the majority of these studies included subjects that, at randomization, had frequent symptoms and poor pulmonary function, thus representing a sample of patients with moderate asthma in whom ICS are expected to be more effective than montelukast. When patients with mild asthma were selected, montelukast was still more effective than placebo [Barnes et al. 2001], but few studies have compared low-dose ICS with montelukast, and with controversial results. At doses equivalent to 500 μg daily of beclomethasone, LTRAs were less effective than ICS on symptoms, pulmonary function and sputum eosinophils [Bacci et al. 2010; Jayaram et al. 2005] (Figure 2). However, in a recent study of 534 patients with mild asthma well controlled by low-dose ICS, replacing ICS with montelukast was associated with good asthma control in more than 75% of patients after 6 weeks, with an increase in compliance to treatment [McIvor et al. 2009].
Figure 2.
Sputum eosinophils before (B) and after 4-weeks' treatment with zafirlukast (LTRA), low-dose ICS or high-dose ICS [Bacci et al. 2010]. ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonists. (Reproduced with permission from Price et al. [2006].).
Another argument in the decision between ICS and montelukast might be the concern for possible side effects in long-term treatment, particularly in children. Although many studies have confirmed the lack of a consistent effect of low-dose ICS on the long-term growth in children (despite a mild delay in growth in the first year) [Doull, 2004], treatment with montelukast might be used as first choice for avoiding this side effect, and changing to ICS might be considered as an alternative.
Montelukast as add-on therapy
After the results of montelukast in monotherapy and its potential additive effect to ICS, many studies have been performed in order to assess the efficacy of montelukast as add-on therapy. When added to ICS, montelukast induced further improvement in symptoms and pulmonary function, particularly in patients still symptomatic despite treatment with ICS [Vaquerizo et al. 2003; Laviolette et al. 1999].
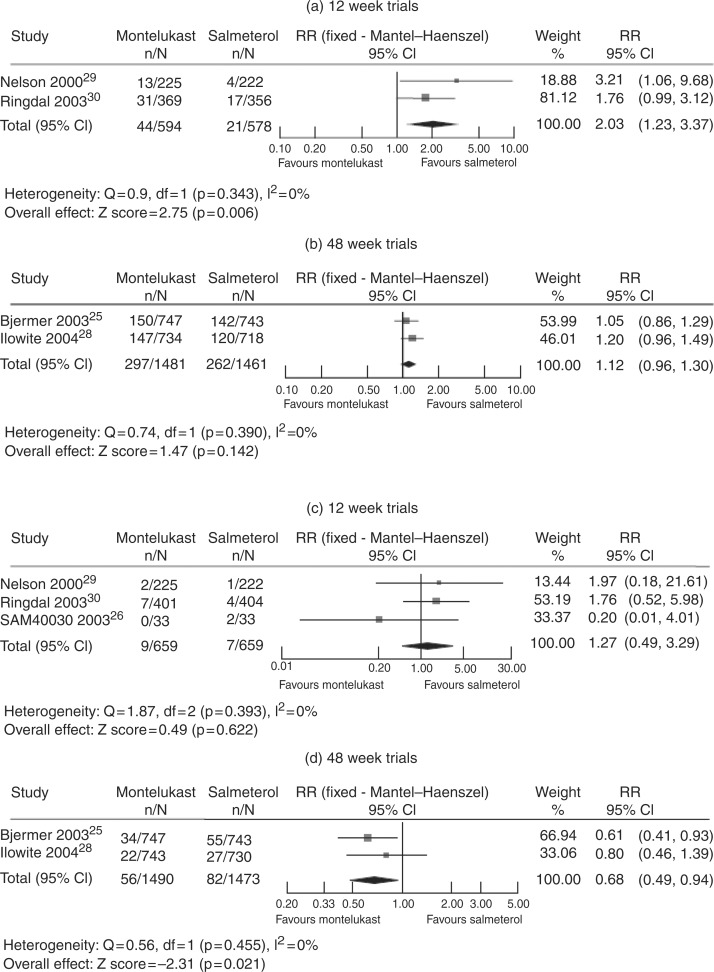
The positioning of montelukast at the step 3 level of asthma management has been studied in patients not controlled by ICS monotherapy; in these patients, the addition of a second drug (a LABA or LTRA) is recommended. Although in short-term studies the combination of ICS plus LABA was more effective on symptoms and pulmonary function than ICS plus montelukast [Deykin et al. 2007; Fish et al. 2001], in a 1-year study especially designed to assess efficacy with regard to rate of severe exacerbations, fluticasone plus montelukast provided equivalent clinical control to fluticasone plus salmeterol, and was associated with a greater reduction in blood eosinophilia [Bjermer et al. 2003]. A recent systematic review suggests that different conclusions may be drawn when either short-term or long-term trials are considered: in 12-week trials, efficacy with regard to rate of exacerbations is higher for salmeterol plus ICS than montelukast plus ICS, with a similar safety profile, whereas in 48-week trials, the two treatments are similar, with a lower rate of adverse events for montelukast plus ICS (Figure 3) [Joos et al. 2008].
Figure 3.
Meta-analysis of the addition of montelukast or salmeterol to inhaled corticosteroids on the rate of exacerbations (a and b) and adverse events (c and d) in short-term (a and c) and in long-term (b and d) studies. (Reproduced with permission from Joos et al. [2008].).
A controversial point is the efficacy of montelukast in patients with severe asthma already treated with maximal therapy (high-dose ICS plus LABAs). In these patients, we demonstrated that a subgroup showed a positive response to a LTRA added on top of maximal therapy [Tonelli et al. 2003], whereas other authors did not find any positive effects [Robinson et al. 2001].
However, the possibility of reducing the dose of ICS or oral steroids in patients with moderate-to-severe asthma by the addition of montelukast, without relevant loss of asthma control, has been suggested by some studies [Tohda et al. 2002; Löfdahl et al. 1999], and confirmed by a systematic review [Ducharme, 2002].
Montelukast in real life
Some observational studies have been performed in order to confirm the clinical effectiveness of montelukast in treating asthma in daily practice. In a large study in the UK, involving 56 centres that recruited more than 1300 asthmatic patients, 66% reported great improvement in asthma control after the addition of montelukast, and in 8.2% this improvement was dramatic [Barnes et al. 2005]. In another study performed in Belgium on 5769 patients, 89% reported a global improvement of their asthma with significant change in the asthma control questionnaire, and also an improvement of rhinitis symptoms [Korn et al. 2009]. A recent article from an observational study on 1681 patients not controlled by ICS, or ICS plus LABA, showed that the addition of montelukast improved both asthma control and asthma-related quality of life [Virchow et al. 2010].
Safety profile of montelukast
The safety profile of montelukast has been well evaluated in the many long-term studies on large groups of adult and paediatric patients, and no significant or considerable side effects have been reported [Virchow et al. 2010; Joos et al. 2008; Nayak and Langdon, 2007; Ducharme, 2002]. A recent review on more than 2700 children and adolescents concluded that the clinical and laboratory safety profile for montelukast was similar to that observed for placebo or active control/usual care therapies, and that the safety profile of montelukast did not change with long-term use [Bisgaard et al. 2009].
Since the beginning of the use of montelukast, several case reports on the occurrence of a Churg-Strauss syndrome (CSS) have been reported. These cases have been considered as being due to the loss of some ‘masking’ effect of high-dose ICS or oral corticosteroids, which were reduced after the addition of montelukast, with subsequent manifestation of full clinical features of the systemic vasculitis. A report on a large number of patients with CSS has been described in a recent paper, showing that montelukast use was associated with a 4.5-fold risk of CSS onset within 3 months, although this effect might be confounded by the escalation in asthma therapy before CSS onset [Hauser et al. 2008].
More recently, some concern was raised regarding the possibility of greater suicide rate in patients taking montelukast, but the revision of 116 clinical studies showed that the frequency of behaviour-related adverse experience and suicides in patients treated with montelukast was no different from control groups [Philip et al. 2009a, 2009b].
Montelukast: Which role in the heterogeneity of asthma?
Recently, great attention has been given to the different asthma phenotypes. They may be classified according to clinical presentations (such as causal or trigger factors, or comorbidities), and also to different biological mechanisms (such as eosinophilic and noneosinophilic asthma) (Table 1) [Wenzel, 2006]. These different phenotypes may require a different approach to treatment, sometimes different from that recommended by current guidelines. Some of these phenotypes seem particularly sensitive to LTRAs.
Table 1.
Different asthma phenotypes and relevance in asthma management.
| Clinical or physiological phenotypes |
| Severity-defined |
| Exacerbation-prone |
| Defined by chronic restriction |
| Treatment-resistant |
| Defined by age at onset |
| Phenotypes related to the following triggers |
| Aspirin nonsteroidal anti-inflammatory drugs |
| Environmental allergens |
| Occupational allergens or irritants |
| Menses |
| Exercise |
| Inflammatory phenotypes |
| Eosinophilic |
| Neutrophilic |
| Pauci-granulocytic |
(Reproduced with permission from Wenzel [2006].)
Exercise-induced bronchospasm is frequent in children and in young patients with mild asthma, and is often associated with other markers of uncontrolled asthma, such as symptoms induced by other nonspecific triggers or frequent exacerbations; in these cases, the patient should be managed according to general recommendations. Sometimes, however, bronchoconstriction is induced almost exclusively by exercise, particularly in elite athletes, thus representing a true clinical phenotype. In these patients, montelukast has demonstrated greater efficacy than beta2-agonists, both as regular and occasional treatment, in preventing exercise-induced asthma, with the advantage of no loss of efficacy over time [Raissy et al. 2008; Wenzel, 2006; Villaran et al. 1999].
Another trigger of asthma attacks is aspirin and other related chemicals (often present in some food as additives or preservatives). Aspirin-sensitive patients often have severe asthma, and may have-greater activation of the leukotriene cascade, as demonstrated by high levels of urinary LTE4 [Gaber et al. 2008]. Some studies tried to assess whether aspirin-sensitive patients are particularly responsive to LTRA treatment, with some positive results [Dahlén et al. 2002]. However, these data have not been confirmed by other studies.
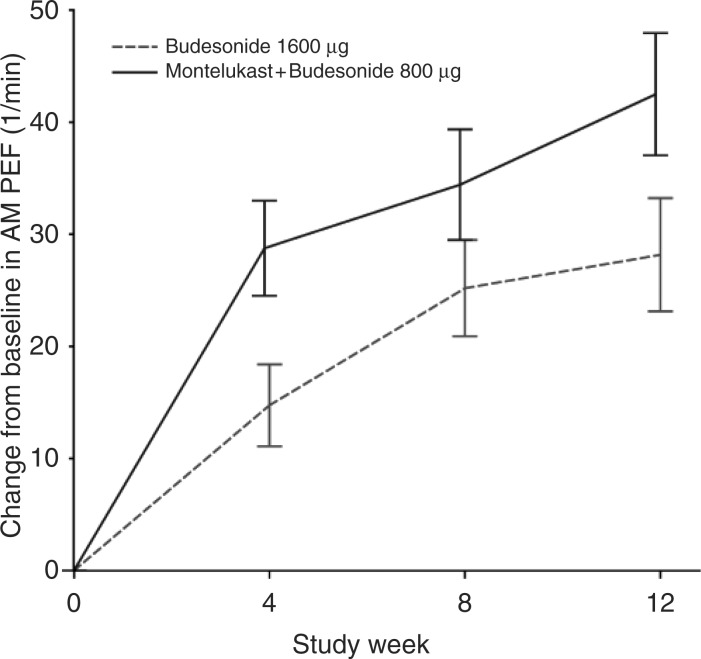
Allergic rhinitis is frequently associated with asthma both in allergic and nonallergic patients, and untreated upper airway disease represents a frequent cause of uncontrolled asthma [Bousquet et al. 2005]. As montelukast is effective on both upper and lower airways, its use might be particularly useful in patients with both asthma and rhinitis. A post hoc analysis of a subgroup of patients enrolled in a study comparing budesonide plus montelukast with a doubling dose of budesonide showed that patients with asthma and rhinitis reported a greater improvement in symptoms and pulmonary function with budesonide plus montelukast (Figure 4) [Price et al. 2006]. After that, many other clinical and observational studies have confirmed that the addition of montelukast to current treatment induced a considerable and long-lasting improvement in asthma control in patients with both asthma and rhinitis [Borderias et al. 2007; Philip et al. 2004].
Figure 4.
Effect of montelukast on pulmonary function in subjects with asthma and rhinitis: post hoc analysis of the COMPACT study. PEF, peak expiratory flow rate. (Reproduced with permission from Price et al. [2006].).
Obesity is now considered an additional risk for asthma, and also as a factor resulting in poor asthma control through different mechanisms [Lugogo et al. 2010]. Obese asthmatic patients often show a low degree of airway inflammation and seem poorly responsive to ICS. In these patients, preliminary data suggest that montelukast may be more effective than ICS [Peters-Golden et al. 2006], probably because it may more easily reach small airways. This point has also been considered in other kinds of uncontrolled asthma, and the involvement of small airways, which might be hardly reached by inhaled drugs, may explain the persistence of symptoms and exacerbations despite a normal or near normal forced expiratory volume. As small airways have a high density of Cys-LTs receptors [Mechiche et al. 2003], and montelukast may reach small airways due to its systemic distribution, this drug may have some potential in treating small airway involvement in asthma.
Smoking is a strong risk factor that may influence the severity of asthma and the response to treatment, and it is associated with a more prominent neutrophilic pattern of airway inflammation. A comparative analysis between ICS and montelukast suggests that patients with asthma who smoke may show a similar response to montelukast in comparison with ICS, which is different from their nonsmoking counterparts who show a better response to ICS [Lazarus et al. 2007].
Biological phenotypes have been described, with the noneosinophilic phenotype being less responsive to ICS [Bacci et al. 2006]. Although LTRAs are believed to be effective mainly on the eosinophilic component of airway inflammation, the efficacy of LTRAs in viral-induced bronchoconstriction (which is often not associated with eosinophilic inflammation) may suggest that these compounds might be recommended in this biological phenotype [Fitzgerald and Mellis, 2006]. This point has not been adequately assessed and requires further well-designed prospective studies.
A similar issue has been considered in the episodes of wheezing in children. It is well known that viral infections are frequently responsible for wheezing in both atopic and nonatopic young children, and leukotrienes have been demonstrated to be released in large amounts from the airways during these episodes [Oommen and Grigg, 2003]. Occasional or regular treatment with montelukast in preschool children resulted in a more rapid resolution of the wheezing episodes and in a better management of these events [Han et al. 2010; Bisgaard et al. 2003].
Montelukast: Recommendations for asthma management
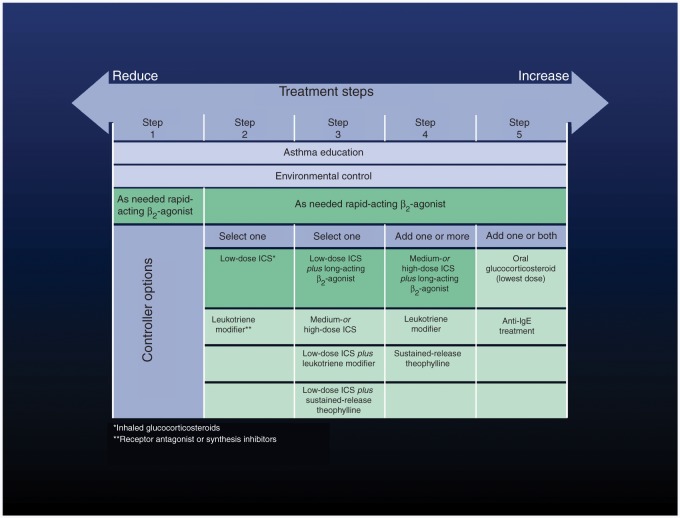
After 10 years of clinical practice and hundreds of clinical and experimental studies, the position of montelukast in the management of asthma is now well defined. According to Global Initiative for Asthma guidelines, montelukast is the recommended alternative monotherapy to low-dose ICS, especially in a step-down strategy (step 2), and also as an add-on treatment to ICS plus LABA combination, in order to improve control and reduce the dose of ICS (steps 3 and 4) (Figure 5). Some studies have evaluated the response to ICS or to montelukast in a crossover study design; in this way, it was possible to assess for potential determinants of a better response to either ICS or montelukast. Using this model, it has been observed that only about 15% of patients showed a greater response to montelukast than to ICS. These patients were younger, with better baseline pulmonary function, lower levels of airway inflammation and higher LTE4 urinary excretion [Szefler et al. 2005]. The percentage of subjects with greater response to montelukast than to ICS corresponds fairly well to the well-known percentage of patients responsive to LTRAs in clinical practice, and also to the percentage of polymorphism of some genes related to the activity of enzymes involved in the leukotriene pathway [Klotsman et al. 2007]. A more recent study compared, in a crossover design, the addition of LABA or montelukast to low-dose ICS in children not controlled under ICS, showing that many patients reported a better response to montelukast plus ICS than to LABA plus ICS [Lemanske et al. 2010]. This observation confirms the heterogeneity in response to treatment (may be related to some genetic background) and suggests that therapeutic options might be tailored to the specific response of the individual patient.
Figure 5.
Treatment steps for asthma management according to the Global Initiative for Asthma guidelines [Global Initiative for Asthma, 2009]. ICS, inhaled corticosteroids; Ig, immunoglobulin.
Some asthma phenotypes seem particularly sensitive to montelukast, such as asthma predominantly induced by exercise or asthma associated with allergic rhinitis (Table 2). Children and adults with low levels of airway inflammation seem more sensitive to montelukast than to ICS. Other potential phenotypes where montelukast might be particularly useful are represented by obese asthmatics, patients with predominant small airway involvement and patients (especially young children) with virus-induced wheezing episodes. In the future, pharmacogenetics might be of some help in selecting patients genetically predisposed to have a good response to LTRAs.
Table 2.
Recommendations for montelukast in different asthma phenotypes.
| Montelukast is recommended in: |
| exercise-induced asthma |
| asthma with allergic rhinitis |
| Montelukast may be recommended in: |
| asthma in obese patients |
| asthma in smokers |
| aspirin-sensitive asthmatics |
| viral-induced wheezing episodes |
Conclusion
In summary, montelukast (the most widely used of the LTRAs) is effective on many biological and pathophysiological mechanisms involved in asthma, and on which ICS are only partially effective (Table 3). It represents a good alternative to ICS as monotherapy, and it is a particularly good additional treatment to ICS in large groups of patients, with the aim of reaching and maintaining control of asthma with the minimal possible dose of ICS.
Table 3.
Montelukast summary of knowledge.
| Montelukast is a potent antagonist of cysteinyl leukotrienes and represents the first category of drugs that targets this inflammatory pathway in asthma |
| Montelukast as monotherapy is a good alternative to inhaled corticosteroids (ICS) for a minority of patients who respond poorly to ICS |
| Added to ICS, montelukast increases the long-term control of asthma in patients with moderate-to-severe asthma, with some ICS-sparing effect |
| Specific asthma phenotypes, such as exercise-induced asthma, asthma associated with rhinitis, aspirin-induced asthma, seem particularly responsive to montelukast, with some evidence that it is effective in asthma in obese patients, asthma in smokers, and viral-induced wheezing episodes in children |
| The effects of montelukast on some markers of airway inflammation (particularly in subjects with noneosinophilic asthma) or on airway remodelling in vivo need to be better investigated |
| The safety profile of montelukast is very good, with no confirmation of the suspected increase in the risk of Churg—Strauss syndrome or suicide |
According to the heterogeneity of asthma, montelukast has been proven particularly effective in exercise-induced asthma and in asthma associated with allergic rhinitis. Other phenotypes where montelukast is effective are: asthma in obese patients; asthma in smokers; aspirin-induced asthma; viral-induced wheezing episodes.
The safety profile of montelukast is very good, and the suspicions of an increased risk of CSS or suicide have not been confirmed.
Footnotes
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
PLP has received in the last 5 years funds from AstraZeneca, Abbott, Boehringer Ingelheim, Chiesi Pharmaceutical, GlaxoSmithKline, and MerckSharp&Dohme, Novartis, Nycomed and Valeas for teaching and research activities. EB has no competing interest to declare.
References
- Altraja S., Kadai M., Rekker E., Altraja A. (2008) Synthesis of tenascin and laminin beta2 chain in human bronchial epithelial cells is enhanced by cysteinyl leukotrienes via CysLT1 receptor. Respir Res 9: 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T., Ishii Y., Chibana K., Fukuda T. (2004) Leukotriene D4 stimulates collagen production from myofibroblasts transformed by TGF-beta. J Allergy Clin Immunol 114: 310–315 [DOI] [PubMed] [Google Scholar]
- Bacci E., Cianchetti S., Bartoli M., Dente F.L., Di Franco A., Vagaggini B., et al. (2006) Low sputum eosinophils predict the lack of response to beclomethasone in symptomatic asthmatic patients. Chest 129: 565–572 [DOI] [PubMed] [Google Scholar]
- Bacci E., Di Franco A., Dente F.L., Bartoli M.L., Cianchetti S., Vagaggini B., et al. (2010) Short-term effects of oral zafirlukast on sputum eosinophilia in mild asthmatics: Comparison with inhaled corticosteroids (submitted).
- Barnes N., Thomas M., Price D., Tate H. (2005) The national montelukast survey. J Allergy Clin Immunol 115: 47–54 [DOI] [PubMed] [Google Scholar]
- Barnes N., Wei L.X., Reiss T.F., Leff J.A., Shingo S., Yu C., et al. (2001) Analysis of montelukast in mild persistent asthmatic patients with near-normal lung function. Respir Med 95: 379–386 [DOI] [PubMed] [Google Scholar]
- Bartoli M.L., Dente F.L., Bancalari L., Bacci E., Cianchetti S., Di Franco A., et al. (2010) Beclomethasone propionate blunts allergen-induced early increase in urinary LTE4. Eur J Clin Invest 40: 566–569 [DOI] [PubMed] [Google Scholar]
- Bisgaard H., Skoner D., Boza M.L., Tozzi C.A., Newcomb K., Reiss T.F., et al. (2009) Safety and tolerability of montelukast in placebo-controlled pediatric studies and their open-label extensions. Pediatr Pulmonol 44: 568–579 [DOI] [PubMed] [Google Scholar]
- Bisgaard H. Study Group on Montelukast Respiratory Syncytial Virus (2003) A randomized trial of montelukast in respiratory syncytial virus post-bronchiolitis. Am J Respir Crit Care Med 167: 379–383 [DOI] [PubMed] [Google Scholar]
- Bjermer L., Bisgaard H., Bousquet J., Fabbri L.M., Greening A.P., Haahtela T., et al. (2003) Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: One year, double blind, randomised, comparative trial. BMJ 327: 891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borderias L., Mincewicz G., Paggiaro P.L., Guilera M., Kocevar V. Sazonov, Taylor S.D., et al. (2007) Asthma control in patients with asthma and allergic rhinitis receiving add-on montelukast therapy for 12 months: A retrospective observational study. Curr Med Res Opin 23: 721–730 [DOI] [PubMed] [Google Scholar]
- Bousquet J., Gaugris S., Kocevar V.S., Zhang Q., Yin D.D., Polos P.G., et al. (2005) Increased risk of asthma attacks and emergency visits among asthma patients with allergic rhinitis: A subgroup analysis of the investigation of montelukast as a partner agent for complementary therapy. Clin Exp Allergy 35: 723–727 [DOI] [PubMed] [Google Scholar]
- Busse W., Raphael G., Galant S., Kalberg C., Goode-Sellers S., Srebro S., et al. (2001) Low-dose fluticasone propionate compared with montelukast for first-line treatment of persistent asthma: A randomized clinical trial. J Allergy Clin Immunol 107: 461–468 [DOI] [PubMed] [Google Scholar]
- Currie G.P., Lipworth B.J. (2002) Bronchoprotective effects of leukotriene receptor antagonists in asthma. A meta-analysis. Chest 122: 146–150 [DOI] [PubMed] [Google Scholar]
- Dahlén S.E., Malmström K., Nizankowska E., Dahlén B., Kuna P., Kowalski M., et al. (2002) Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med 165: 9–14 [DOI] [PubMed] [Google Scholar]
- Davis B.E., Illamperuma C., Gauvreau G.M., Watson R.M., O'Byrne P.M., Deschesnes F., et al. (2009) Single-dose desloratadine and montelukast and allergen-induced late airway responses. Eur Respir J 33: 1302–1308 [DOI] [PubMed] [Google Scholar]
- Deykin A., Wechsler M.E., Boushey H.A., Chinchilli V.M., Kunselman S.J., Craig T.J., et al. (2007) Combination therapy with a long-acting beta-agonist and a leukotriene antagonist in moderate asthma. Am J Respir Crit Care Med 175: 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doull I.J. (2004) The effect of asthma and its treatment on growth. Arch Dis Child 89: 60–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme F.M. (2002) Anti-leukotrienes as add-on therapy to inhaled glucocorticoids in patients with asthma: Systematic review of current evidence. BMJ 324: 1545–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworski R., Fitzgerald G.A., Oates J.A., Sheller J.R. (1994) Effect of oral prednisone on airway inflammatory mediators in atopic asthma. Am J Respir Crit Care Med 149: 953–959 [DOI] [PubMed] [Google Scholar]
- Fish J.E., Israel E., Murray J.J., Emmett A., Boone R., Yancey S.W., et al. (2001) Salmeterol powder provides significantly better benefit than montelukast in asthmatic patients receiving concomitant inhaled corticosteroid therapy. Chest 120: 423–430 [DOI] [PubMed] [Google Scholar]
- Fitzgerald D.A., Mellis C.M. (2006) Leukotriene receptor antagonists in virus-induced wheezing: Evidence to date. Treat Respir Med 5: 407–417 [DOI] [PubMed] [Google Scholar]
- Gaber F., Daham K., Higashi A., Higashi N., Gülich A., Delin I., et al. (2008) Increased levels of cysteinyl-leukotrienes in saliva, induced sputum, urine and blood from patients with aspirin-intolerant asthma. Thorax 63: 1076–1082 [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma (2009) www.ginasthma.org
- Hamid Q., Tulic M.K., Liu M.C., Moqbel R. (2003) Inflammatory cells in asthma: Mechanisms and implications for therapy. J Allergy Clin Immunol 111(Suppl 1): S5–S17 [DOI] [PubMed] [Google Scholar]
- Han J., Jia Y., Takeda K., Shiraishi Y., Okamoto M., Dakhama A., et al. (2010) Montelukast during primary infection prevents airway hyperresponsiveness and inflammation following re-infection with respiratory syncytial virus. Am J Respir Crit Care Med [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser T., Mahr A., Metzler C., Coste J., Sommerstein R., Gross W.L., et al. (2008) The leukotriene receptor antagonist montelukast and the risk of Churg-Strauss syndrome: A case-crossover study. Thorax 63: 677–682 [DOI] [PubMed] [Google Scholar]
- Holgate S.T., Peters-Golden M., Panettieri R.A., Henderson W.R. (2003) Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodelling. J Allergy Clin Immunol 111(Suppl 1): S18–S34 [DOI] [PubMed] [Google Scholar]
- Jayaram I., Pizzichini E., Lemière C., Man S.F., Cartier A., Hargreave F.E., et al. (2005) Steroid naive eosinophilic asthma: Anti-inflammatory effects of fluticasone and montelukast. Thorax 60: 100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos S., Miksch A., Szemcsenyi J., Wieseler B., Grouven U., Kaiser T., et al. (2008) Montelukast as add-on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: A systematic review. Thorax 63: 453–462 [DOI] [PubMed] [Google Scholar]
- Klotsman M., York T.P., Pillai S.G., Vargas-Irwin C., Sharma S.S., van den Oord E.J., et al. (2007) Pharmacogenetics of the 5-lipoxygenase biosynthetic pathway and variable clinical response to montelukast. Pharmacogenet Genomics 17: 189–196 [DOI] [PubMed] [Google Scholar]
- Korn D., van den Brande P., Potvin E., Dramaix M., Herbots E., Peché R. (2009) Efficacy of addon montelukast in patients with non-controlled asthma: A Belgian open-label study. Curr Med Res Opin 25: 489–497 [DOI] [PubMed] [Google Scholar]
- Laitinen A., Lindqvist A., Halme M., Altraja A., Laitinen L.A. (2005) Leukotriene E(4)-induced persistent eosinophilia and airway obstruction are reversed by zafirlukast in patients with asthma. J Allergy Clin Immunol 115: 259–265 [DOI] [PubMed] [Google Scholar]
- Laviolette M., Malmstrom K., Lu S., Chervinsky P., Pujet J.C., Peszek I., et al. (1999) Montelukast added to inhaled beclomethasone in treatment of asthma. Am J Respir Crit Care Med 160: 1862–1868 [DOI] [PubMed] [Google Scholar]
- Lazarus S.C., Chinchilli V.M., Rollings N.J., Boushey H.A., Cherniack R., Craig T.J., et al. (2007) Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med 175: 783–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff J.A., Busse W.W., Pearlman D., Bronsky E.A., Kemp J., Hendeles L., et al. (1998) Montelukast, a leukotriene receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. New Engl J Med 339: 147–152 [DOI] [PubMed] [Google Scholar]
- Leigh R., Vethanayagam D., Yoshida M., Watson R.M., Rerecich T., Inman M.D., et al. (2002) Effect of montelukast and budesonide on airway responses and airway inflammation in asthma. Am J Respir Crit Care Med 166: 1212–1217 [DOI] [PubMed] [Google Scholar]
- Lemanske R.F., Jr, Mauger D.T., Sorkness C.A., Jackson D.J., Boehmer S.J., Martinez F.D., et al. (2010) Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med 362: 975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfdahl C.G., Reiss T.F., Leff J.A., Israel E., Noonan M.J., Finn A.F., et al. (1999) Randomised, placebo controlled trial of effect of a leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patients. BMJ 319: 87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugogo N.L., Kraft M., Dixon A.E. (2010) Does obesity produce a distinct asthma phenotype? J Appl Physiol 108: 729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba S., Ichiyama T., Ueno Y., Makata H., Matsubara T., Furukawa S. (2005) Effect of montelukast on nuclear factor kappaB activation and proinflammatory molecules. Ann Allergy Asthma Immunol 94: 670–674 [DOI] [PubMed] [Google Scholar]
- Malmstrom K., Rodriguez-Gomez G., Guerra J., Villaran C., Piñeiro A., Wei L.X., et al. (1999) Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. Ann Intern Med 130: 487–495 [DOI] [PubMed] [Google Scholar]
- McIvor R.A., Kaplan A., Koch C., Sampalis J.S. (2009) Montelukast as an aternative to low-dose inhaled corticosteroids in to management of mild asthma (the SIMPLE trial): An open-label effectiveness trial. Can Respir J 16(Suppl A): 11A–21A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechiche H., Naline E., Candenas L., Pinto F.M., Birembault P., Advenier C., et al. (2003) Effects of cysteinyl leukotrienes in small human bronchus and antagonist activity of montelukast and its metabolites. Clin Exp Allergy 33: 887–894 [DOI] [PubMed] [Google Scholar]
- Nayak A., Langdon R.B. (2007) Montelukast in the treatment of allergic rhinitis: An evidence-based review. Drugs 67: 887–901 [DOI] [PubMed] [Google Scholar]
- O'Shaugnessy K.M., Wellings R., Gillies B., Fuller R.W. (1993) Differential effects of fluticasone proprionate on allergen-evoked bronchoconstriction and increased urinary LTE4 excretion. Am Rev Respir Dis 147: 1472–1476 [DOI] [PubMed] [Google Scholar]
- Oommen A., Grigg J. (2003) Urinary leukotriene E4 in preschool children with acute clinical viral wheeze. Eur Respir J 21: 149–154 [DOI] [PubMed] [Google Scholar]
- Peters-Golden M., Swern A., Bird S.S., Hustad C.M., Grant E., Edelman J.M. (2006) Influence of body mass index on the response to asthma controller agents. Eur Respir J 27: 495–503 [DOI] [PubMed] [Google Scholar]
- Philip G., Hurstad C.M., Malice P.M., Noonan G., Ezekowitz A., Reiss T.F., et al. (2009a) Analysis of behavior-related adverse experiences in clinical trials of montelukast. J Allergy Clin Immunol 124: 699–706 [DOI] [PubMed] [Google Scholar]
- Philip G., Hustad C., Noonan G., Malice M.P., Ezekowitz A., Reiss T.F., et al. (2009b) Reports of suicidality in clinical trials of montelukast. J Allergy Clin Immunol 124: 691–696 [DOI] [PubMed] [Google Scholar]
- Philip G., Nayak A.S., Berger W.E., Leynadier F., Vrijens F., Dass S.B., et al. (2004) The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin 20: 1549–1558 [DOI] [PubMed] [Google Scholar]
- Pizzichini E., Leff J.A., Reiss T.F., Hendeles L., Boulet L.P., Wei L.X., et al. (1999) Montelukast reduces airway eosinophilic inflammation in asthma: A randomized, controlled trial. Eur Respir J 14: 12–18 [DOI] [PubMed] [Google Scholar]
- Price D.B., Swern A., Tozzi C.A., Philip G., Polos P. (2006) Effect of montelukast on lung function in asthma patients with allergic rhinitis: Analysis from the COMPACT trial. Allergy 61: 737–742 [DOI] [PubMed] [Google Scholar]
- Raissy H.H., Harkins M., Kelly F., Kelly H.W. (2008) Pretreatment with albuterol versus montelukast for exercise-induced bronchospasm in children. Pharmacotherapy 28: 287–294 [DOI] [PubMed] [Google Scholar]
- Ramsay C.F., Sullivan P., Gizycki M., Wang D., Swern A.S., Barnes N.C., et al. (2009) Montelukast and bronchial inflammation in asthma: A randomised, double-blind placebo-controlled trial. Respir Med 103: 995–1003 [DOI] [PubMed] [Google Scholar]
- Reiss T.F., Chervinsky P., Dockhorn R.J., Shingo S., Seidenberg B., Edwards T.B., et al. (1998) Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma. A multicenter, randomized, double-blnd trial. Arch Int Med (Chic) 158: 1213–1220 [DOI] [PubMed] [Google Scholar]
- Robinson D.S., Campbell D., Barnes P.J. (2001) Addition of leukotriene antagonists to therapy in chronic persistent asthma: A randomised double-blind placebo-controlled trial. Lancet 357: 2007–2011 [DOI] [PubMed] [Google Scholar]
- Sandrini A., Ferreira I.M., Gutierrez C., Jardim J.R., Zamel N., Chapman K.R. (2003) Effect of montelukast on exhaled nitric oxide and nonvolatile markers of inflammation in mild asthma. Chest 124: 1334–1340 [DOI] [PubMed] [Google Scholar]
- Szefler S.J., Phillips B.R., Martinez F.D., Chinchilli V.M., Lemanske R.F., Strunk R.C., et al. (2005) Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 115: 233–242 [DOI] [PubMed] [Google Scholar]
- Tohda Y., Fujimura M., Taniguchi H., Takagi K., Igarashi T., Yasuhara H., et al. (2002) Leukotriene receptor antagonist, montelukast, can reduce the need for inhaled steroid while maintaining the clinical stability of asthmatic patients. Clin Exp Allergy 32: 1180–1186 [DOI] [PubMed] [Google Scholar]
- Tonelli M., Zingoni M., Bacci E., Dente F.L., Di Franco A., Giannini D., et al. (2003) Short-term effect of the addition of leukotriene receptor antagonists to the current therapy in severe asthmatics. Pulm Pharmacol Ther 16: 237–240 [DOI] [PubMed] [Google Scholar]
- Vaquerizo M.J., Casan P., Castillo J., Perpiña M., Sanchis J., Sobradillo V., et al. (2003) Effect of montelukast added to inhaled budesonide on control of mild to moderate asthma. Thorax 58: 204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaran C., O'Neill S.J., Helbling A., van Noord J.A., Lee T.H., Chuchalin A.G., et al. (1999) Montelukast versus salmeterol in patients with asthma and exercise-induced bronchoconstriction. J Allergy Clin Immunol 104: 547–553 [DOI] [PubMed] [Google Scholar]
- Virchow J.C., Metha A., Ljungblad L., Mitfessel, H. the MONICA study group (2010) Add-on montelukast in inadequately controlled asthma patients in a 6-month open-label study: The MONtelukast In Chronic Asthma (MONICA) study. Respir Med 104: 644–651 [DOI] [PubMed] [Google Scholar]
- Wenzel S.E. (2006) Asthma: Defining of the persistent adult phenotypes. Lancet 368: 804–813 [DOI] [PubMed] [Google Scholar]