Abstract
Dyslipidaemia is a major risk factor for cardiovascular (CV) disease. Despite the widespread availability of effective lipid-lowering agents, an unacceptably large proportion of patients fail to attain their target low-density lipoprotein cholesterol (LDL-C) level in clinical practice. Reasons for this include undertreatment, poor adherence/persistence with therapy and failure to address non-LDL-C residual risk factors such as high levels of triglycerides, low high-density lipoprotein cholesterol (HDL-C) concentrations and raised apolipoprotein B: apolipoprotein A1 ratios. Pitavastatin is a novel, well-tolerated statin with a noninferior or superior lipid-lowering efficacy to comparable doses of atorvastatin, simvastatin, and prava-statin in a wide range of patients with hypercholesterolemia or combined dyslipidaemia. Compared with other statins, pitavastatin produces consistently greater increases in HDL-C levels that are sustained over the long term. In addition to pravastatin's potent effects on lipid profiles, a number of pleiotropic benefits have been identified that may contribute to a reduction in residual cardiovascular risk in people with dyslipidaemia and could partly account for pitavastatin's ability to regress coronary plaques in patients with acute coronary syndrome. Pitavastatin's unique metabolic profile results in a high efficacy at low (1-4 mg) doses and minimal drug interactions with cytochrome CYP3A4 substrates, making it an excellent choice for people requiring multiple medications. Although future trials are required to assess the impact of pitavastatin treatment on CV morbidity and mortality, studies to date suggest that pitavastatin will play an important role in the future management of dyslipidaemia and in the overall reduction of CV risk.
Keywords: cardiovascular, dyslipidaemia, hypercholesterolemia, lipid, pitavastatin, statin
Introduction
Cardiovascular diseases (CVDs) are the most common causes of death, worldwide [World Health Organization (WHO), 2010]. According to the WHO, around 30% of the population (17.1 million people) died from CVD in 2004; of these, approximately 7.2 million were caused by coronary heart disease (CHD) and 5.7 million were caused by stroke. Major risk factors for CVD include dyslipidaemia, smoking, hypertension, diabetes, increasing age and obesity [O'Donnell et al. 2010; Graham et al. 2007; Grundy et al. 2004; Yusuf et al. 2004; National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), 2002]. As the global population ages and the incidence of obesity and diabetes continues to escalate, the annual number of deaths from CVD is set to rise. The WHO estimates that, by 2030, 23.6 million people will die from CVD every year [WHO, 2010]. The effective management of modifiable cardiovascular (CV) risk factors through lifestyle changes and pharmaceutical treatment is therefore essential if the incidence of CVD morbidity and mortality is to be reduced.
Of the nine common risk factors for CVD, dyslipidaemia is one of the most significant, accounting for 50% of the population-attributable risk for myocardial infarction (MI) [Yusuf et al. 2004], and 25% of the population-attributable risk for stroke [O'Donnell et al. 2010]. Numerous clinical studies show that statins can significantly lower low-density lipoprotein cholesterol (LDL-C) and reduce the risk of CV morbidity and mortality, with no lower limit beyond which lipid lowering is not beneficial [Baigent et al. 2005; LaRosa et al. 2005; Sever et al. 2003; ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group, 2002; Heart Protection Study Collaborative Group, 2002; Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group, 1998; Shepherd et al. 1995; Scandinavian Simvastatin Survival Study Group, 1994]. In a meta-analysis of data from 90,056 individuals in 14 randomized trials, mean reductions in LDL-C after 1 year of statin treatment ranged from 0.35 to 1.77 mmol/l (mean 1.09) [Baigent et al. 2005]. In this analysis, each mmol/l reduction in LDL-C was associated with a 12% reduction in all-cause mortality, a 19% reduction in coronary mortality [0.81, 95% confidence interval (CI) 0.76-0.85; p < 0.0001), and a 21% reduction in the total risk of major vascular events (0.79, 95% CI 0.77-0.81; p < 0.0001).
Given the relationship between lipid lowering and CV risk, current treatment guidelines recommend lowering LDL-C to less than 100 mg/dl in high-risk patients and to less than 70-80 mg/dl in those with very high CV risk (CVD plus diabetes, smoking, poorly controlled hypertension, metabolic syndrome, or previous MI) [Graham et al. 2007; Grundy et al. 2004; NCEP Adult Treatment Panel III, 2002]. Although the use of lipid-lowering therapy has become more successful over the past decade, LDL-C target attainment rates generally remain low, ranging from 47% to 84%, worldwide [Kotseva et al. 2009; Waters et al. 2009]. A patient's ability to attain LDL-C targets can arise from pharmacogenomic factors, such as genetic variations in organic anion-transporting polypeptide (OATP) transporters [Couvert et al. 2008; Igel et al. 2006; Thompson et al. 2005], LDL receptors [Polisecki et al. 2008], multidrug resistance (MDR)1 transporter [Mega et al. 2009], apolipoprotein E (apoE) [Mega et al. 2009], breast cancer resistance protein (BCRP) [Bailey et al. 2010] and 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase [Chasman, 2004]. However, more common reasons include failure to attain LDL-C targets, which has largely been attributed to underdosing/failure to uptitrate dosages where necessary and poor compliance/persistence with treatment [Shalev et al. 2009; Meade, 2007]. Although these factors could be improved by better physician/patient education and more frequent follow-up visits, increasing demands on physician's time and insufficient funding mean this is not always possible. In the absence of regular follow-up visits, adequate dosing and good compliance/persistence rates largely depend on the safety, tolerability and efficacy of the starting dose [Meade, 2007]. It therefore follows that LDL-C target attainment rates can potentially be improved by a greater use of high-efficacy statins as first-line treatments.
Despite the widespread availability of effective statins, patients at high CV risk frequently require additional lipid-lowering agents, such as ezetimibe, niacin, and bile acid sequestrants to attain their recommended LDL-C targets. Furthermore, risk factors for CVD frequently coexist, which means patients with dyslipidaemia often require multiple medications for conditions such as hypertension, diabetes, and renal disease. The need for polypharmacy is confounded by the correlation between CV risk and increasing age; 75-80% of people aged 80 years and over have atherosclerosis [Packard et al. 2005] and two thirds of first major CV events occur in people aged 65 years and over [NCEP Adult Treatment Panel III, 2002]. Given that most patients with dyslipidaemia are likely to require some degree of polypharmacy, there is a clear need for statins with a low drug-drug interaction potential.
Patients that are fully adherent to statin treatment can attain an LDL-C reduction of at least 1.5 mmol/l [Baigent et al. 2005]. However, even in this group of patients, the risk of major vascular events is only reduced by around one third, which leaves a substantial residual risk that can partly be attributed to overall lipid profile [Sharma et al. 2009]. Based on reports from a number of studies [Ridker et al. 2010; Ray et al. 2009; Miller et al. 2008; Barter et al. 2007; Gordon et al. 1977] the NCEP Adult Treatment Panel III guideline states that low high-density lipoprotein cholesterol (HDL-C) and high triglycerides (TGs) are significant and independent risk factors for CHD [NCEP Adult Treatment Panel III, 2002]. It is therefore important that treatment strategies for dyslipidaemia should target non-LDL-C risk factors, such as TGs, HDL-C, and apoB: apoA1 ratios as well as LDL-C. Although the most commonly used high-efficacy statins (atorvastatin, simvastatin, pravastatin, fluvastatin, and rosuvastatin) have comparable LDL-C lowering efficacy, it is becoming increasing clear that some statins have greater pleiotropic benefits than others. In some cases, these include anti-inflammatory effects, benefits on endothelial function, and inhibition of smooth muscle cell proliferation that can potentially lead to coronary plaque stabilization and even regression [Ridker et al. 2009; Barter et al. 2007; Crouse et al. 2007; Nissen et al. 2006, 2004; de Lemos et al. 2004; Pasterkamp et al. 1999; Giroud et al. 1992; Little, 1990]. In this review, we examine the role for pitavastatin in the treatment of a wide range of patients with dyslipidaemia; we compare its lipid-lowering efficacy with that of other statins and discuss the pleiotropic benefits that could potentially lead to the reduction of residual CV risk.
Pitavastatin: Structure, mechanism of action and pharmacokinetic profile
Pitavastatin was launched by Kowa in Japan (September 2003) for the treatment of hypercholesterolemia and familial hypercholesterolemia. Regulatory approval followed in Korea (June 2008), Thailand (June 2008), the USA and China (August 2009) and the drug is currently under evaluation in Europe.
Pitavastatin is a synthetic HMG-CoA reductase inhibitor with heptenoate as the basic structure, a quinoline ring at the core and novel fluorophenyl and cyclopropyl side chains [Yamazaki et al. 2004]. In vitro studies show that, compared with other statins, pitavastatin has a greater HMG-CoA reductase inhibitor activity, induces greater LDL receptor expression and LDL uptake, and more effectively inhibits very-low-density lipoprotein (VLDL) secretion from HepG2 cells [Ose et al. 2010; Saito, 2009; Yamazaki et al. 2004; Morikawa et al. 2000; Aoki et al. 1997]. Other in vitro studies show that pitavastatin stimulates secretion of apoA1 more strongly than atorvastatin, and simvastatin promotes ATP-binding cassette transporter A1 (ABCA1) expression in HepG2 cells and facilitates HDL neogenesis by apoA1-dependent cholesterol efflux in hepatocytes [Ose et al. 2010; Suzuki et al. 2001].
Although a statin's ability to inhibit HMG-CoA reductase is largely independent of its physicochemical properties, some of these properties can affect the ways in which statins are transported, distributed, metabolized, and eliminated [Ose et al. 2010; Shitara and Sugiyama, 2006]. Unlike other statins, the cyclopropyl group on the pitavastatin molecule appears to divert the drug away from metabolism by cytochrome P450 (CYP) 3A4 and allows only a small degree of clinically insignificant metabolism by CYP2C9 [Ose et al. 2010; Saito, 2009; Nakaya et al. 2001; Fujino et al. 1999]. As a result, pitavastatin is minimally metabolized; most of the bioavailable fraction of an oral dose is excreted unchanged in the bile and is then ready for entero-hepatic recirculation by reabsorption in the small bowel (Table 1). In contrast, lovastatin, simvastatin and atorvastatin are predominantly metabolized in the liver by CYP3A4, whereas fluvastatin, pravastatin and rosuvastatin are metabolized by CYP2C9 [Ose et al. 2010; Saito, 2009; Mukhtar et al. 2005; Fujino et al. 2004, 2003]. Pitavastatin's lack of metabolism in the gut wall and the liver probably account for its high bioavailability, which at 51-60% is high compared with other statins (<5% for lovastatin and simvastatin, 12% for atorvastatin, 17% for pravastatin, 10-35% for fluvastatin, 20% for rosuvastatin and 60% for cerivastatin) [Pitavastatin Prescribing Information, 2010, 2007; Mukhtar et al. 2005].
Table 1.
Long-term treatment with pitavastatin is well tolerated in subgroups of patients with primary hypercholesterolemia or combined dyslipidaemia (phase III extension studies).
| Pitavastatin 4 mg/day for 52 weeks (n = 1353) [Ose et al. 2010] N (%) | Pitavastatin 4 mg/day for 44 weeks (n=121) [Hounslow et al. 2010] N (%) | Pitavastatin 2 mg/day for 60 weeks (n = 537) [Stender and Hounslow, 2009] N (%) | Pitavastatin 4 mg/day* for 60 weeks (n = 95) [Stender and Hounslow, 2009] N (%) | |
|---|---|---|---|---|
| Patient population | Hypercholesterolemia or combined dyslipidaemia | Hypercholesterolemia or combined dyslipidaemia and at least 2 additional CHD risk factors | Hypercholesterolemia or combined dyslipidaemia and age ≥ 65 years | |
| Any TEAE | 741 (54.8) | 92 (76) | 407 (75.8) | 57 (60.0) |
| Serious TEAE | 49 (3.6) | 4 (3.3) | 51 (9.5) | 7 (7.4) |
| Treatment-related TEAE | 162 (12.0) | 13 (10.7) | 72 (13.4) | 4 (4.2) |
| Discontinued caused by TEAE | 55 (4.1) | 7 (5.8) | 29 (5.4) | 7 (7.4) |
| Death caused by TEAE | 2 (0.1) | 1 (0.8) | 2 (0.4) | 0 (0) |
| TEAE severity | ||||
| Mild | 368 (27.2) | 38 (31.4) | 151 (28.1) | 23 (24.2) |
| Moderate | 337 (24.9) | 45 (37.2) | 216 (40.2) | 28 (29.5) |
| Severe | 36 (2.7) | 9 (7.4) | 40 (7.4) | 6 (6.3) |
4 mg group includes patients who had been uptitrated from 2 to 4 mg after week 8.
CHD, coronary heart disease; TEAE, treatment emergent adverse event. Discontinuation means permanent discontinuation of study treatment.
Given its unique metabolic profile, pitavastatin has a lower potential for drug-drug interactions than other statins and provides a useful advantage for the large group of dyslipidaemic patients that require multiple medications. A detailed review of pitavastatin-drug interactions was published by Ose and colleagues [Ose et al. 2010]. Briefly, no significant pharmacokinetic interactions have been reported with bezafibrate or ezetimibe and the minimal interactions with fenofibrate and gemfibrozil are not considered to be clinically significant [Mathew et al. 2004]. No pharmacokinetic interactions have been reported for digoxin [Neuvonen et al. 2006] or warfarin [Inagaki et al. 2009], and although the concomitant use of ciclosporin and pitavastatin is associated with a 4.6-fold increase in pitavastatin exposure [Hasunuma et al. 2003], the contraindication is largely due to lack of safety data in patients receiving combination therapy. Importantly, the interaction is less marked than that between ciclosporin and atorvastatin (8.7-fold increase) or rosuvastatin (7-fold increase), the concomitant use of which is either contraindicated (atorvastatin) or restricted (rosuvastatin).
Dosage
The LDL-C lowering efficacy of statins increases in a dose-dependent manner; however, the dosage is also inversely proportional to drug safety and tolerability [Jacobson, 2006]. It therefore follows that the use of low-dose, highefficacy agents has the potential to improve LDL-C target attainment rates by reducing the risk of undertreatment and by improving persistence rates through the minimization of adverse events. To simplify treatment choices, statins are classified into ‘strong’ and ‘weak’ categories in Japan [Hayashi et al. 2007]. The ‘strong statins’ include atorvastatin, rosuvastatin and, more recently, pitavastatin [Saito, 2009].
In a 12-week dose-finding study in Japanese patients with hypercholesterolemia, the LDL-C lowering effect of pitavastatin was 34% (n = 81) with pitavastatin 1 mg, 42% (n = 75) with 2 mg, and 47% (n = 76) with 4 mg [Saito et al. 2002]. This compares to 37%, 42%, and 47% with atorvastatin 10 mg, 20 mg and 40 mg and 28%, 35%, and 39% with simvastatin 10 mg, 20 mg and 40 mg [Mukhtar et al. 2005]. Based on this and similar studies, the usual starting dose of pitavastatin is 1 mg once daily, adjusted at 4-weekly intervals to a maximum dose of 4 mg, according to LDL-C levels [Pitavastatin Prescribing Information, 2007].
Lipid-lowering efficacy of pitavastatin in patients with hypercholesterolemia and mixed dyslipidaemia
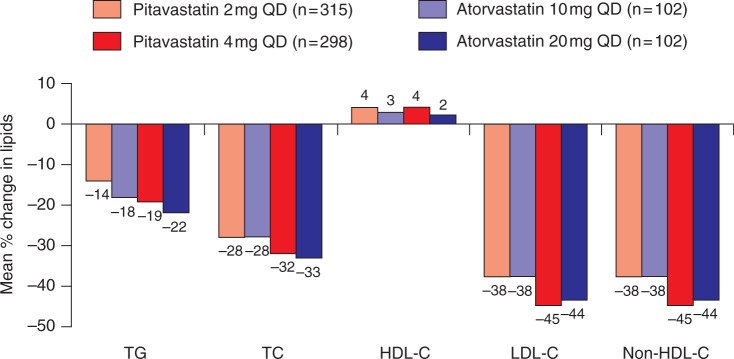
An increasing number of studies have shown that standard doses of pitavastatin have a comparable lipid-lowering efficacy to well established statins in a wide range of patients with primary hypercholesterolemia or combined dyslipidaemia (Figures 1–3) [Hounslow et al. 2010; Watson 2010; Ose et al. 2010, 2009; Budinski et al. 2009; Stender and Hounslow, 2009; Kurihara et al. 2008; Yokote et al. 2008]. In a pivotal phase III study, 821 Caucasian patients with primary hypercholesterolemia or combined dyslipidaemia underwent a 6- to 8-week dietary lead-in period before being randomized to one of four treatment regimens for 12 weeks [Budinski et al. 2009]. Two groups received once-daily pitavastatin 2 mg or atorvastatin 10 mg and two groups received the same treatment for 4 weeks followed by forced titration to pitavastatin 4 mg or atorvastatin 20 mg. After 12 weeks, pitavastatin produced a noninferior reduction from baseline in LDL-C and total cholesterol (TC) concentrations compared with atorvastatin (Figure 1). The mean reduction in LDL-C was 37.9% and 37.8% for pitavastatin 2 mg and atorvastatin 10 mg respectively and 44.6% and 43.5% for pitavastatin 4 mg and atorvastatin 20 mg respectively. Pitavastatin and atorvastatin were not significantly different in terms of changes in nonHDL-C, TG, apoB or apoA1. Most patients reached NCEP LDL-C targets (pitavastatin 4 mg, 77.9%; atorvastatin 20 mg, 70.6%; pitavastatin 2 mg, 56.8%; atorvastatin 10 mg, 65.7%) and European Atherosclerosis Society (EAS) LDL-C targets (pitavastatin 4 mg, 78.5%; atorvastatin 20 mg, 76.5%; pitavastatin 2 mg, 56.8%; atorvastatin 10 mg, 59.8%), with no significant difference between treatment groups. HDL-C levels increased from baseline by 4% and 5% with pitavastatin 2 mg and 4 mg respectively compared with 3% and 2.5% with atorvastatin 10 mg and 20 mg respectively (Figure 1).
Figure 1.
Phase III comparison of pitavastatin and atorvastatin in patients with primary hypercholesterolemia or combined dyslipidaemia [Budinski et al. 2009]. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; QD, every day; TC, total cholesterol; TG, triglyceride.
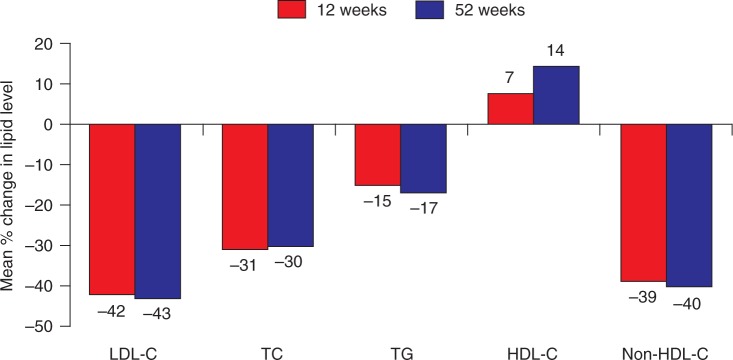
Figure 3.
Open-label study of patients in the phase III studies who elected to receive pitavastatin 4 mg/day for up to 52 weeks [Ose et al. 2010]. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Similar results were reported by the Collaborative study on Hypercholesterolemia drug Intervention and their Benefits for Atherosclerosis prevention (CHIBA) study [Yokote et al. 2008]. In this phase IV Japanese trial, patients with TC ≥ 220 mg/dl were randomized to receive once-daily pitavastatin 2 mg (n = 126) or atorvastatin 10 mg (n = 125) for 12 weeks. The percentage change from baseline in non-HDL-C level (the primary endpoint) was 39.0% with pitavastatin versus 40.3% with atorvastatin (p = 0.456). Both pitavastatin and atorvastatin significantly reduced LDL-C by 42.6% and 44.1%, TC by 29.7% and 31.1%, and TG by 17.3% and 10.7% respectively (p = NS between treatments). HDL-C showed a significant increase at 12 weeks with pitavastatin (3.2%, p = 0.033 versus baseline) but not with atorvastatin (1.7%, p = 0.221 versus baseline).
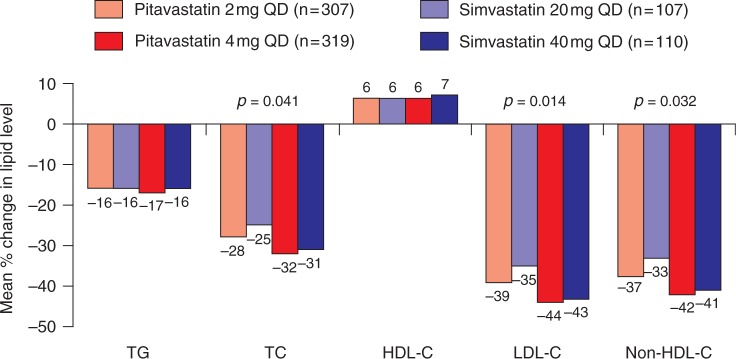
A second pivotal phase III study comparing once-daily pitavastatin 2–4 mg with simvastatin 20-40 mg in 857 Caucasian patients with primary hypercholesterolemia or combined dyslipidaemia showed that, compared with simvastatin 20 mg, pitavastatin 2 mg produced significantly greater reductions in LDL-C (35% versus 39% respectively; p = 0.014), non-HDL-C (p = 0.021) and TC (p = 0.041) (Figure 2) [Ose et al. 2009]. Furthermore, a significantly greater proportion of patients treated with pitavastatin 2 mg achieved the EAS LDL-C target than did those receiving simvastatin 20 mg (59.6% versus 48.6%; p = 0.049). For the higher dose comparison (pitavastatin 4 mg versus simvastatin 40 mg), the reductions in LDL-C concentrations were similar (44.0% and 42.8% respectively; Figure 2). Moreover, HDL-C concentrations were significantly increased from baseline by a similar extent by both treatments (6.0% and 6.2% with pitavastatin 2 mg and 4 mg respectively; 5.0% and 6.8% with simvastatin 20 mg and 40 mg respectively).
Figure 2.
Phase III comparison of pitavastatin and simvastatin in patients with primary hypercholesterolemia or combined dyslipidaemia [Ose et al. 2009]. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; QD, every day; TC, total cholesterol; TG, triglyceride.
Of the patients who completed the two phase III studies, 1353 patients elected to receive open-label pitavastatin 4 mg/day for up to 52 weeks [Ose et al. 2010]. The proportion of patients achieving NCEP and EAS LDL-C targets at week 12 was sustained over the long term, with 74.0% and 73.5% of patients respectively achieving their targets at week 52. Changes in efficacy parameters [LDL-C, TGs, TC, non-HDL-C, apoA1 and apoB, high-sensitivity C-reactive protein (CRP), oxidized LDL) and ratios (TC: HDL-C, non-HDL-C: HDL-C and apoB: apoA1) were also sustained over 52 weeks (Figure 3). However, HDL-C levels rose continually, ultimately increasing by 14.3% compared with the initial baseline.
Overall, phase III and IV studies showed that pitavastatin has a similar LDL-C lowering efficacy to atorvastatin and simvastatin in Japanese and Caucasian patients with hypercholesterolemia or combined dyslipidaemia but pitavastatin has additional benefits for the long-term elevation of HDL-C.
Lipid-lowering efficacy of pitavastatin in patients with primary hypercholesterolemia or combined dyslipidaemia and high CV risk
To assess the lipid-lowering effects of pitavastatin in ‘difficult-to-treat’ patients with dyslipidaemia and high CV risk, a double-blind, parallel-group phase III noninferiority study was carried out comparing once daily pitavastatin 2–4 mg with simvastatin 10-20 mg in 355 patients with primary hypercholesterolemia or combined dyslipidaemia and at least two additional CHD risk factors [Hounslow et al. 2010]. In this study, 12 weeks of treatment with pitavastatin 4 mg was noninferior to simvastatin 40 mg for reducing LDL-C (44.0% versus 43.8%; p = NS); however, pitavastatin produced a significantly greater reduction in TGs (19.8% versus 14.8%; p = 0.044) and a numerically greater increase in HDL-C (6.8% versus 4.5%; p = 0.083). Changes from baseline in non-HDL-C, apoB and apoA1 were similar in both treatment groups, as were the percentages of patients that achieved NCEP/EAS LDL-C targets (>80% for both).
Continuing from this study, 178 patients entered a double-blind, double dummy extension phase comparing the long-term effects of pitavastatin 4 mg with simvastatin 40-80 mg [Hounslow et al. 2010]. After 44 weeks, pitavastatin 4 mg and simvastatin 40-80 mg showed similar improvements from baseline in LDL-C (41.8% versus 41.4% respectively), TC, non-HDL-C, TGs, apoB and apoA1. HDL-C levels progressively increased with pitavastatin but were more variable with simvastatin; however, both groups showed a greater than 14% increase compared with the phase 1 baseline. NCEP and EAS targets were sustained over the long term, with a similarly high proportion of patients on pitavastatin 4 mg and simvastatin 40-80 mg achieving both targets after 12 and 56 weeks. In all cases, titrating the comparator dose to 80 mg did not provide a significant efficacy advantage over pitavastatin 4 mg.
A comparison of pitavastatin with atorvastatin in high-risk Japanese patients with hypercholesterolemia and metabolic syndrome (N = 53) showed that, after 12 weeks, LDL-C was reduced significantly more with pitavastatin 2 mg than with atorvastatin 10 mg (45.8% versus 39.1%; p = 0.0495) [Yokote et al. 2008]. In this subgroup analysis of patients from the phase IV CHIBA study, there were no significant differences between pitavastatin and atorvastatin on TG or HDL-C levels, but pitavastatin significantly reduced TGs by 25.2% (p < 0.001) and increased HDL-C by 6.7% (p = 0.019).
Overall, pitavastatin appears to be at least as effective as simvastatin and atorvastatin for reducing atherogenic lipids and increasing anti-atherogenic HDL-C in patients with hypercholesterolemia and high CV risk.
Lipid-lowering efficacy of pitavastatin in patients with hypercholesterolemia or combined dyslipidaemia and glucose intolerance
Type II diabetes increases the risk of CHD and stroke two- to four-fold, and may reduce life expectancy by five- to ten-years [American Diabetes Association, 2003]. In addition to hyperglycaemia, modifiable risk factors for CHD in people with diabetes include high LDL-C, low HDL-C, hypertension and smoking [UK Prospective Diabetes Study Group, 1998]. A phase III study examining the safety and efficacy of pitavastatin in Caucasian patients with type II diabetes and primary hypercholesterolemia or combined dyslipidaemia is due to report in 2011. In the meantime, the phase IV, multicenter, open-label, parallel-group PIAT study examined the effects of pitavastatin 2 mg versus atorvastatin 10 mg in 207 Japanese patients with an LDL-C level at least 140 mg/dl and glucose intolerance [Sasaki et al. 2006]. In this 52-week study, increases in HDL-C (8.2% versus 2.9%; p = 0.031) and apoA1 levels (5.1 versus 0.6; p = 0.019) were significantly greater with pitavastatin than with atorvastatin. Although the reductions in LDL-C (40.1% versus 33.0%; p = 0.002), non-HDL-C (37.4 versus 31.1; p = 0.004), apoB (35.1 versus 28.2; p < 0.001), and apoE (28.1 versus 17.8; p < 0.001) were greater with atorvastatin than with pitavastatin, reductions compared with baseline were significant for both treatment groups and there were no significant differences between treatments with respect to glucose metabolism.
Lipid-lowering efficacy of pitavastatin in elderly patients with hypercholesterolemia or combined dyslipidaemia
The risk of CVD increases with age [NCEP Adult Treatment Panel III, 2002]. A phase III noninferiority study comparing pitavastatin with low-dose pravastatin (1 mg versus 10 mg; 2 mg versus 20 mg; and 4 mg versus 40 mg) in 942 elderly patients (age > 65 years) with primary hypercholesterolemia or combined dyslipidaemia showed that 12 weeks of treatment with pitavastatin was noninferior to pravastatin in reducing LDL-C at all dose comparisons (p < 0.001) and that the drug was well tolerated, irrespective of dose [Stender and Hounslow, 2009]. Moreover, improvements in LDL-C, TC, HDL-C, TGs, and apoB levels were significantly greater for pitavastatin versus pravastatin for most dose comparisons, with similar efficacy across all age groups (65-69 years versus 70-74 years versus ≥ 75 years). The proportion of patients that met the NCEP LDL-C target with pitavastatin and pravastatin were high: low doses, 83% versus 65%; medium doses, 89% versus 81%; and high doses, 91% versus 88%.
Of the volunteers that completed the 12-week study, 545 patients entered an open-label 60-week extension phase [Stender and Hounslow, 2009]. Patients that started therapy with pitavastatin 2 mg could be uptitrated to 4 mg if their LDL-C target was not met after 8 weeks. Only 17% of patients were uptitrated and less than 70% of these attained their target LDL-C level. Overall, the improvements in LDL-C, TC, TGs, non-HDL-C, apoB and apoA1 that were observed after 12 weeks were sustained after 60 week of treatment with pitavastatin 2–4 mg. As seen in the pivotal trials, an incremental increase in HDL-C with pitavastatin was observed during the extension phase (9.6% versus the initial baseline), suggesting a continued benefit for elderly patients remaining on pitavastatin. There was a numeric increase in the proportion of patients treated with pitavastatin achieving both NCEP and EAS LDL-C targets at 60 weeks versus 12 weeks, with 99% of patients attaining their NCEP target after 60 weeks. The results of this study suggest that pitavastatin is more effective than pravastatin for the reduction of LDL-C levels and improvement of lipid profile in elderly patients with hypercholesterolemia or combined dyslipidaemia and that the benefits are sustained over the long term.
Efficacy of pitavastatin in clinical practice
Although clinical trials provide useful information on the safety and efficacy of drugs in specific patient populations, the results do not always reflect those observed in the real world [Foody et al. 2010]. The Japanese long-term prospective post-marketing surveillance LIVALO Effectiveness and Safety (LIVES) study was designed to assess the efficacy and safety of pitavastatin in clinical sp practice [Kurihara et al. 2008]. Of the 20,279 patients recruited, 18,031 were analyzed for drug effectiveness. After 104 weeks, pitavastatin was associated with significant reductions in serum LDL-C (29.1%) that largely occurred within 4 weeks of treatment initiation. In patients with abnormal TG and HDL-C levels at baseline, pitavastatin reduced TGs and increased HDL-C by 22.7% and 19.9%, respectively. According to the Japan Atherosclerosis Society Guidelines for Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases [Teramoto et al. 2007], 88.2% of the primary prevention low-risk patients attained their LDL-C target compared with 82.7% of intermediate-risk patients, 66.5% of high-risk patients and 50.3% of secondary prevention patients. These rates are consistent with those from the phase III extension study in Caucasian patients with hypercholesterolemia or combined dyslipidaemia, in which 74.0% and 73.5% of patients achieved their NCEP and EAS LDL-C targets respectively at week 52 [Ose et al. 2009].
A subanalysis of LIVES data focussing on the effects of pitavastatin on HDL-C levels showed that HDL-C was elevated by 5.9% in all patients and by 24.6% in those with low (<40mg/dl) HDL-C levels at baseline (p < 0.0001). A time-course analysis showed that the elevation in HDL-C in the low HDL-C group was enhanced by 14.0% and 24.9% at 12 weeks and 104 weeks respectively. In contrast, previous studies have shown that other statins have inconsistent effects on HDL-C levels, with elevations ranging from 0% to 12% [Sviridov et al. 2007]. According to the LIVES subanalysis, pitavastatin produced a significant increase in HDL-C levels in patients switching from other statins, suggesting that patients with an unacceptably low level of HDL-C on other statins might benefit from switching to pitavastatin [Teramoto et al. 2009].
Effects of pitavastatin on estimated glomerular filtration rate in patients with chronic kidney disease
Chronic kidney disease (CKD) is associated with an elevated risk of CVD and an increased risk of progression to end stage renal disease [Go et al. 2004]. In a subanalysis of LIVES data, 958 patients with hypercholesterolemia and baseline estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 m2 were analyzed to evaluate the effects of pitavastatin treatment on eGFR [Kimura et al. 2010]. A significant increase in eGFR (5.4 ml/min/1.73 m2) was observed after 104 weeks of pitavastatin treatment (p < 0.001; one-sample t-test). Moreover, multivariate analysis showed that the degree of change was related to the presence/absence of proteinuria and the initial level of HDL-C. These results suggest that pitavastatin might be a useful treatment option for people with hypercholesterolemia and CKD. However, head-to-head studies are needed to test pitavastatin's efficacy versus other available statins in this patient group.
Safety and tolerability of pitavastatin in patients with hypercholesterolemia or combined dyslipidaemia
Safety in phase III/IV clinical trials
Phase III and IV clinical trials demonstrate that pitavastatin is well tolerated in a wide range of patients with hypercholesterolemia or combined dyslipidaemia (Table 2), with similar safety profiles to comparable doses of atorvastatin, simvastatin and low-dose pravastatin [Hounslow et al. 2010; Ose et al. 2010, 2009; Watson, 2010; Budinski et al. 2009; Stender and Hounslow, 2009; Kawashiri et al. 2008; Yokote and Saito 2008]. The most commonly reported system order class disorders in every patient population at every dose were infections and infestations, gastrointestinal disorders and musculoskeletal and connective tissue disorders. The incidence of myopathy, myositis or rhabdomyolysis was low for every patient population. During the 52-week open-label extension study in patients with hypercholesterolemia and mixed dyslipidaemia, only 4.1% of patients receiving the highest recommended dose of pitavastatin (4 mg) withdrew because of treatment emergent adverse events (TEAEs) (Table 2) [Ose et al. 2010]. The investigators did not consider that any of the serious adverse events reported during this study were related to pitavastatin. No clinically significant abnormalities were associated with pitavastatin in routine laboratory variables, urinalysis, vital signs or 12-lead ECG. Increased creatine phosphokinase (2.74%), nasopharyngitis (5.4%) and myalgia (4.1%) were the most common TEAEs.
Table 2.
Pleiotropic effects of pitavastatin [modified from Saito, 2009].
| Pleiotropic effects | |
|---|---|
| Endothelial function | eNOS mRNA expression↑ ET-I mRNA expression↓ [Morikawa et al. 2002] |
| Monocyte activation/endothelium adhesion/migration | Monocyte adhesion on endothelium↓ [Hiraoka et al. 2004], MCP-I mRNA expression↓ IL-8 production/mRNA expression↓ [Kibayashi et al. 2005; Morikawa et al. 2002] NF-κB transactivation↓ [Inoue et al. 2002] ICAM-I mRNA expression↓ [Sagara et al. 2007] |
| Foam cell formation/cholesterol accumulation | SMC proliferation↓ [Nakano and Egashira 2009; Kohno et al. 2002] SMC migration↓ [Kohno et al. 2002] CD36 mRNA/protein expression↓ [Han et al. 2004] cholesterol accumulation in macrophage↓ apoB48R expression↓ [Kawakami et al. 2005] |
| Plaque stabilisation | Accumulation of macrophages↓ collagen↑MMPs↓ [Suzuki et al. 2003] |
| Thrombosis formation | TF mRNA/protein expression↓ PAI-I mRNA expression/antigen secretion/activity↓ t-PA mRNA expression/antigen secretion↑ |
| TM mRNA expression/cellular antigen/transcription rate↑[Markle et al. 2003; Morikawa et al. 2002] | |
| Inflammatory markers | CRP↓ [Motomura et al. 2009; Kibayashi et al. 2005; Hiraoka and Yoshida, 2003] PTX3↓ [Ohbayashi et al. 2009; Morikawa et al. 2004] |
CRP, C-reactive protein; eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; ICAM-1, intercellular adhesion molecule-1; IL-8, interleukin-8; MCP-1, monocyte chemoattractant protein-1; MMP, matrix metalloproteinases; NFκβ nuclear factor kappa beta; PAI-1, plasminogen activator inhibitor-1; PTX 3, pentraxin 3; SMC, smooth muscle cell; TF, tissue factor; TM, thrombomodulin; t-PA, tissue plasminogen activator.
Safety in clinical practice
To assess the long-term safety and tolerability of pitavastatin in clinical practice, the postmarketing LIVES surveillance study analysed safety records from 19,925 Japanese patients with hypercholesterolemia [Kurihara et al. 2008]. During a 104-week follow up, 10.4% of pitavastatin-treated patients experienced adverse events, of which, approximately 84% were mild and only around 1% were severe. Increases in blood creatine phosphokinase (2.74%), alanine amino-transferase (1.79%), myalgia (1.08%), aspartate aminotransferase and gamma-glutamyltransferase (1.00%) were the most common adverse events and only 7.4% of patients discontinued pitavastatin because of adverse events. Regression analysis demonstrated that age was not a significant factor for the incidence of any adverse event.
Safety in people with renal impairment
It is well known that renal impairment is a risk factor for adverse events in patients receiving statin therapy. Treatment guidelines therefore recommend that clinicians should closely monitor all patients with moderate or severe renal disease who are treated with statins and suggest avoiding higher doses of statins when possible [Graham et al. 2007; Pitavastatin Prescribing Information, 2007; Grundy et al. 2004; NCEP Adult Treatment Panel III, 2002]. Consistent with these guidelines, an analysis of LIVES data from 720 patients treated with pitavastatin showed that higher rates of adverse events were reported in patients with renal disease than in those without (13.6% versus 10.3%; p = 0.04) [Kurihara et al. 2008]. Most patients with a history of renal disease had moderate (43%) or severe (26%) renal impairment as judged by eGFR, and the majority of adverse events occurred in the first year of treatment.
Effects of pitavastatin on glucose metabolism
Several studies have reported adverse effects on glucose metabolism following statin treatment [Ridker et al. 2008; Sasaki et al. 2006; Colhoun et al. 2004; Sever et al. 2003]. However, the effects of pitavastatin on glucose metabolism, although not extensively tested, appear neutral [Yokote and Saito, 2009; Tamakawa et al. 2008; Kawai et al. 2005]. In one study, 12 weeks of treatment with pitavastatin 1–2 mg had no significant effect on fasting plasma glucose levels (8.20 ± 2.71 to 8.27 ± 2.10 mmol/l) or HbA1c levels (7.25 ± 1.60 to 7.27 ± 1.47%) in 79 statin treatment-naive patients with hypercholesterolemia and type II diabetes [Kawai et al. 2005]. Changes in AST, ALT, γ-GTP, and CK levels were also not significant. Similarly, a retrospective analysis of glycemic control in 279 patients with hypercholesterolemia and type II diabetes receiving atorvastatin 10 mg, pitavastatin 2 mg, or pravastatin 10 mg for 3 months showed that glycaemic control remained the same in the pitavastatin and pravastatin groups but deteriorated in the atorvastatin group [Tamakawa et al. 2008]. In this study, nonfasting blood glucose levels increased from 147 ± 51 mg/dl (mean ± SD) to 176 ± 69 mg/dl with atorvastatin (p < 0.001), but decreased from 155 ± 53 to 154 ± 51 mg/dl with pitavastatin and from 136 ± 31 to 134 ± 32 mg/dl with pravastatin (p = NS for both). Respective changes in HbA1C were 7.0 ± 1.1% to 7.4 ± 1.2% (p < 0.001), 7.3 ± 1.0% to 7.2 ± 1.0% (p = NS), and 6.9 ± 0.9% to 6.9 ± 1.0% (p = NS). Similar results were obtained by the glycemic control subanalysis of the 12-week CHIBA study [Yokote and Saito, 2009]. Here, serum glycoalbumin increased by 0.67 ± 1.31% versus baseline in Japanese patients with hypercholesterolemia and type II diabetes treated with atorvastatin 10 mg (p = 0.026), whereas there was no significant change in those treated with pitavastatin 2 mg. HbA1c levels tended to increase (p = 0.098) with atorvastatin, but not with pitavastatin, and although there were no significant changes in fasting plasma glucose, insulin or the homeostasis model assessment ratio with pitavastatin or atorvastatin, all these parameters tended to decrease with pitavastatin. The ongoing Japan Prevention Trial of Diabetes by Pitavastatin in Patients with Impaired Glucose Tolerance (J-PREDICT) study has been designed to evaluate the effects of pitavastatin on the prevention of diabetes in people with impaired glucose tolerance (http://clinicaltrials.gov/ct2/show/NCT00301392). The study is due to report in 2013.
Possible pleiotropic effects beyond lipid-lowering: Plaque stabilization and regression
It is widely accepted that the incidence of secondary CV events can be significantly reduced by intensive statin therapy in patients with acute coronary syndrome (ACS). Although studies have yet to show that pitavastatin reduces CV risk, the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome (JAPAN-ACS) study showed that pitavastatin induces plaque regression in patients with ACS [Hiro et al. 2009]. In this multicenter, prospective, randomized, open-label, parallel-group study, the effects of 8-12 months of treatment with pitavastatin 4 mg/day were noninferior to those of atorvastatin 20 mg/day on coronary plaque regression in nonculprit lesions of the culprit vessel treated by percutaneous coronary intervention (−16.9 ± 13.9% versus −18.1 ± 14.2%; p = 0.5). Unlike other intravascular ultrasound studies in which the rate of atherosclerotic progression was inversely proportional to the level of LDL-C [Crouse et al. 2007; Nissen et al. 2006, 2004] multivariate analyses of the JAPAN-ACS data showed that plaque volume regression did not correlate with LDL-C levels in patients without diabetes. These data suggest more intensive LDL-C reduction is required to achieve a greater degree of plaque volume regression in patients with ACS and diabetes, and suggests the existence of both LDL-C-dependent and LDL-C-independent mechanisms for statin-induced plaque regression in Japanese patients with ACS.
Consistent with the results from this study, Suzuki and colleagues [Suzuki et al. 2003] showed that 16 weeks of pitavastatin treatment (0.5 mg/kg) in WHHL rabbits reduced plasma lipid levels and decreased the area of the aortic lesion by 38.6%. In this study, pitavastatin reduced the macrophage-positive area in the aortic plaque by 39.4%, increased the areas occupied by collagen and alpha-smooth muscle actin (SMA) by 66.4% and 91.7%, respectively, increased the average thickness of alpha-SMA in the plaque by 96.7% and reduced the vulnerability index by 76.0%. Other changes included reductions in the positive areas of monocyte chemoattractant protein-1 (39.1%), and in matrix metalloproteinase-3 (40.6%) and matrix metalloproteinase-9 (52.3%).
In addition to the pleiotropic effects demonstrated in rabbits, a number of in vitro studies have shown that pitavastatin inhibits CRP-induced interleukin-8 production by endothelial cells and reduces monocyte adhesion to endothelial cells [Kibayashi et al. 2005; Hiraoka et al. 2004]. Other effects include inhibition of plasminogen activator inihibitor-1 and tissue factor-mediated thrombosis formation [Markle et al. 2003], increased tissue plasminogen activator and thrombomodulin mRNA expression [Inoue et al. 2007; Morikawa et al. 2002], and greater inhibition of vascular smooth muscle cell (SMC) proliferation than atorvastatin, simvastatin, fluvastatin, rosuvastatin, or pravastatin [Nakano and Egashira, 2009]. Together, these pleiotropic effects have the potential to improve the composition of vulnerable plaques and improve plaque stability, improve endothelial function, reduce monocyte activation, adhesion and migration, inhibit foam cell formation and cholesterol accumulation, inhibit thrombosis formation, and reduce inflammation [Saito, 2009].
Considering the anti-inflammatory effects of pitavastatin in vitro, it is not surprising that pitavastatin is associated with decreased pentraxin 3 (PTX3) levels [Ohbayashi et al. 2009; Morikawa et al. 2004]. Since PTX3 is rapidly produced by vascular endothelial cells, SMCs, macrophages and neutrophils in response to inflammation, it is a useful marker for inflammation caused by conditions such as acute MI and unstable angina. Recently, Ohbayashi and colleagues [Ohbayashi et al. 2009] showed that pitavastatin reduces PTX3 expression in patients with higher baseline PTX3 levels and asymptomatic hypercholesterolemia and identified a relationship between PTX3 levels and several conventional clinical markers, including CRP and carotid artery intima-media thickness. This study is the first to demonstrate the preventative effects of pitavastatin against asymptomatic atherosclerosis in patients with hypercholesterolemia and suggests a possible new therapeutic strategy for limiting atherogenic inflammation prior to the development of atherosclerotic lesions.
Further evidence for the anti-inflammatory effects of pitavastatin derive from the Kansai Investigation of Statin for Hyperlipidemic Intervention in Metabolism and Endocrinology (KISHIMEN) study [Koshiyama et al. 2008]. Here, 178 Japanese patients with hypercholesterolemia — including 103 (58%) with type 2 diabetes — received pitavastatin 1–2 mg/day for 12 months. In addition to improving lipid profiles, pitavastatin significantly reduced serum CRP levels by 34.8% versus baseline (p < 0.01). Although there have been conflicting results on the efficacy of other statins on CRP-lowering in patients with diabetes [Economides et al. 2004; Sommeijer et al. 2004; Tan et al. 2002], the KISHIMEN study [Koshiyama et al. 2008] and a more recent study by Motomura and colleagues [Motomura et al. 2009] showed that pitavastatin has a significant CRP-lowering effect in people with type II diabetes that is not related to reductions in lipid profile. Although the effects of pitavastatinmediated CRP lowering on CV morbidity and/or mortality have not yet been studied in clinical trials, a number of studies have shown that CRP reduction using other statins is associated with significant reductions in CV endpoints [Ridker et al. 2009, 2005; de Lemos et al. 2004]. In the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study, for example, rosuvastatin 20 mg/day reduced vascular events by 62% in healthy patients (N = 17,809; baseline LDL-C < 130 mg/dl (3.4 mmol/l) and high-sensitivity CRP ≥ 2.0 mg/l) that attained a CRP lower than 2 mg/l after a median 1.9 years of treatment (event rate 0.42 per 100 person years; hazard ratio 0.38, 95% CI 0.26-0.56, p < 0.0001) [Ridker et al. 2009].
Overall, studies have demonstrated that pitavastatin has a number of pleiotropic effects beyond lipid lowering that are likely to reduce residual CV risk in patients with dyslipidaemia and contribute to plaque stabilization/regression in patients with ACS. Although additional studies are required to assess the effects of pitavastatin on CV endpoints, current data suggest that pitavastatin is likely to play an important role in the future management of dyslipidaemia.
Conclusions
Despite the widespread availability of effective statins, an unacceptably large proportion of patients (47-84%, worldwide) fail to attain their target LDL-C level in clinical practice [Kotseva et al. 2009; Waters et al. 2009]. Reasons for this include undertreatment and poor adherence/persistence with therapy, especially in the large group of patients with dyslipidaemia requiring multiple medications [Shalev et al. 2009; Meade, 2007]. It therefore follows that a high-efficacy, low-dose statin with a good safety profile and a low propensity for drug-drug interactions has the potential to improve LDL-C target attainment rates by minimizing the need for uptitration and reducing the risk of nonpersistence because of adverse events. Since residual CV risk remains high (around two thirds) even in patients that attain their LDL-C target [Baigent et al. 2005], the most effective statins are those that modify lipid parameters, such as HDL-C, TC, TGs, and apoB: apoA1 ratios in addition to LDL-C, and have pleiotropic effects beyond lipid lowering.
Numerous studies have demonstrated that low doses of pitavastatin (1 mg, 2 mg and 4 mg) improve lipid profiles (Figures 1–3) and increase NCEP/EAS LDL-C target attainment rates with a similar efficacy to standard doses of atorvastatin, simvastatin, and pravastatin in patients with hypercholesterolemia or combined dyslipidaemia [Hounslow et al. 2010; Ose et al. 2010, 2009; Watson, 2010; Budinski et al. 2009; Stender and Hounslow, 2009; Kurihara et al. 2008; Yokote and Saito, 2008]. Furthermore, pitavastatin is generally associated with a greater HDL-C elevating efficacy than atorvastatin, simvastatin or pravastatin that is consistently increased over the long term [Hounslow et al. 2010; Ose et al. 2010, 2009; Watson, 2010; Budinski et al. 2009; Teramoto et al. 2009, 2007; Stender and Hounslow, 2009; Kurihara et al. 2008; Yokote and Saito, 2008]. In addition to pitavastatin's potent effects on lipid profiles, a number of pleiotropic benefits have been identified that may contribute to a reduction in residual CV risk in people with dyslipidaemia and could partly account for pitavastatin's ability to regress coronary plaques in patients with ACS [Egashira, 2009; Hiro et al. 2009; Motomura et al. 2009; Nakano and Ohbayashi et al. 2009; Koshiyama et al. 2008; Inoue et al. 2007; Sagara et al. 2007; Kawakami et al. 2005; Kibayashi et al. 2005; Han et al. 2004; Hiraoka et al. 2004; Morikawa et al. 2004, 2002; Hiraoka and Yoshida, 2003; Markle et al. 2003; Suzuki et al. 2003]. Importantly, the safety and tolerability profiles of pitavastatin 1–4 mg are similar to those of atorvastatin, pravastatin, and simvastatin at doses with comparable efficacy (Table 2). Thus, although future trials are required to assess the impact of pitavastatin treatment on CV morbidity and mortality, studies to date suggest that pitavastatin will play an important role in the future management of dyslipidaemia and in the overall reduction of CV risk.
Acknowledgements
Jackie Read, a medical writer, assisted with the drafting of this manuscript. However, the author is responsible for the final document.
Footnotes
Kowa Research Europe Ltd funded the studies and the preparation of this manuscript.
Leiv Ose received research grant funding from Merck, Pfizer, Schering-Plough, Roche and Boehringer Ingelheim; he is a consultant/advisor to Kowa; and he has received speakers' bureau fees from AstraZeneca and Kowa.
References
- ALLHAT Officers Coordinators for the ALLHAT Collaborative Research Group (2002) Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care. JAMA 288: 2998–3000 [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2003) Management of dyslipidemia in adults with diabetes. Diabetes Care 26(Suppl 1): S83–S86 [DOI] [PubMed] [Google Scholar]
- Aoki T., Nishimura H., Nakagawa S., Kojima J., Suzuki H., Tamaki T., et al. (1997) Pharmacological profile of a novel synthetic inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Arzneimittelforschung 47: 904–909 [PubMed] [Google Scholar]
- Baigent C., Keech A., Kearney P.M., Blackwell L., Buck G., Pollicino C., et al. (2005) Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366(9493): 1267–1278 [DOI] [PubMed] [Google Scholar]
- Bailey K.M., Romaine S.P., Jackson B.M., Farrin A.J., Efthymiou M., Barth J.H., et al. (2010) Hepatic metabolism and transporter gene variants enhance response to rosuvastatin in patients with acute myocardial infarction: The GEOSTAT-1 Study. Circ Cardiovasc Genet 3: 276–285 [DOI] [PubMed] [Google Scholar]
- Barter P., Gotto A.M., LaRosa J.C., Maroni J., Szarek M., Grundy S.M., et al. (2007) HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 357: 1301–1310 [DOI] [PubMed] [Google Scholar]
- Budinski D., Arneson V., Hounslow N., Gratsiansky N. (2009) Pitavastatin compared with atorvastatin in primary hypercholesterolemia or combined dyslipidemia. Clin Lipidol 4: 291–302 [Google Scholar]
- Chasman D.I., Posada D., Subrahmanyan L., Cook N.R., Stanton V.P., Jr, Ridker P.M. (2004) Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA 1291(23): 2821–2827 [DOI] [PubMed] [Google Scholar]
- Colhoun H.M., Betteridge D.J., Durrington P.N., Hitman G.A., Neil H.A., Livingstone S.J., et al. (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 364: 685–696 [DOI] [PubMed] [Google Scholar]
- Couvert P., Giral P., Dejager S., Gu J., Huby T., Chapman M.J., et al. (2008) Association between a frequent allele of the gene encoding OATP1B1 and enhanced LDL-lowering response to fluvastatin therapy. Pharmacogenomics 9(9): 1217–1227 [DOI] [PubMed] [Google Scholar]
- Crouse J.R., 3rd, Raichlen J.S., Riley W.A., Evans G.W., Palmer M.K., O'Leary D.H., et al. (2007) Effect of rosuvastatin on progression of carotid intimamedia thickness in low-risk individuals with subclinical atherosclerosis: The METEOR Trial. JAMA 297: 1344–1353 [DOI] [PubMed] [Google Scholar]
- de Lemos J.A., Blazing M.A., Wiviott S.D., Lewis E.F., Fox K.A., White H.D., et al. (2004) Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA 292: 1307–1316 [DOI] [PubMed] [Google Scholar]
- Economides P.A., Caselli A., Tiani E., Khaodhiar L., Horton E.S., Veves A. (2004) The effects of atorvastatin on endothelial function in diabetic patients and subjects at risk for type 2 diabetes. J Clin Endocrinol Metab 89: 740–747 [DOI] [PubMed] [Google Scholar]
- Foody J.M., Mendys P.M., Liu L.Z., Simpson R.J., Jr (2010) The utility of observational studies in clinical decision making: Lessons learned from statin trials. Postgrad Med 122: 222–229 [DOI] [PubMed] [Google Scholar]
- Fujino H., Yamada I., Kojima J. (1999) Studies on the metabolic fate of NK- 104, a new inhibitor of HMG-CoA reductase. Xenobio Metabol Dispos 14: 415–424 [Google Scholar]
- Fujino H., Saito T., Tsunenari Y., Kojima J., Sakaeda T. (2004) Metabolic properties of the acid and lactone forms of HMG-CoA reductase inhibitors. Xenobiotica 34: 961–971 [DOI] [PubMed] [Google Scholar]
- Fujino H., Yamada I., Shimada S., Yoneda M., Kojima J. (2003) Metabolic fate of pitavastatin, a new inhibitor of HMG-CoA reductase: Human UDP-glucuronosyltransferase enzymes involved in lactonization. Xenobiotica 33: 27–41 [DOI] [PubMed] [Google Scholar]
- Giroud D., Li J.M., Urban P., Meier B., Rutishauer W. (1992) Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol 69: 729–732 [DOI] [PubMed] [Google Scholar]
- Go A., Chertow G.M., Fan D., McCulloch C.E., Hsu C.-Y. (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- Gordon T., Castelli W.P., Hjortland M.C., Kannel W.B., Dawber T.R. (1977) High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 62: 707–714 [DOI] [PubMed] [Google Scholar]
- Graham I., Atar D., Borch-Johnsen K., Boysen G., Burell G., Cifkova R., et al. (2007) European guidelines on cardiovascular disease prevention in clinical practice: Full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice. Eur J Cardiovasc Prev Rehabil 14(Suppl 2): S1–113 [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Merz C.N., Brewer H.B., Jr, Clark L.T., Hunninghake D.B. (2004) Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110: 227–239 [DOI] [PubMed] [Google Scholar]
- Han J., Zhou X., Yokoyama T., Hajjar D.P., Gotto A.M., Jr, Nicholson A.C. (2004) Pitavastatin downregulates expression of the macrophage type B scavenger receptor, CD36. Circulation 109: 790–796 [DOI] [PubMed] [Google Scholar]
- Hasunuma T., Masahiko N., Takashi Y., Noriko A., Kuniaki F., Hajime I., et al. (2003) The drug-drug Interactions of pitavastatin (NK-104), a novel HMG-CoA reductase Inhibitor and cyclosporine. J Clin Ther Med 19: 381–389 [Google Scholar]
- Hayashi T., Yokote K., Saito Y., Iguchi A. (2007) Pitavastatin: Efficacy and safety in intensive lipid lowering. Expert Opin Pharmacother 8: 2315–2327 [DOI] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group (2002) MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: A randomised placebo-controlled trial. Lancet 360: 7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Nitta N., Nagai M., Shimokado K., Yoshida M. (2004) MCP-1-induced enhancement of THP-1 adhesion to vascular endothelium was modulated by HMG-CoA reductase inhibitor through RhoA GTPase-, but not ERK1/2-dependent pathway. Life Sci 75: 1333–1341 [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Yoshida M. (2003) A novel HMG-CoA reductase inhibitor, pitavastatin inhibits IL-6-induced CRP in liver cells via ERK1/2-dependent but not STAT3-dependent signaling transduction. Circ J 67(Suppl 1): 271 [Google Scholar]
- Hiro T., Kimura T., Morimoto T., Miyauchi K., Nakagawa Y., Yamagishi M., et al. (2009) Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol 54: 293–302 [DOI] [PubMed] [Google Scholar]
- Hounslow N.J., Budinski D., Eriksson M. (2010) Pitavastatin 4 mg shows comparable LDL-cholesterol and superior triglyceride reduction to simvastatin 40 mg in high risk primary hypercholesterolemia or combined dyslipidaemia. EAS 2010: Abstract 890. [Google Scholar]
- Igel M., Arnold K.A., Niemi M., Hofmann U., Schwab M., Lütjohann D., et al. (2006) Impact of the SLCO1 B1 polymorphism on the pharmacokinetics and lipid-lowering efficacy of multiple-dose pravastatin. Clin Pharmacol Ther 79: 419–426 [DOI] [PubMed] [Google Scholar]
- Inagaki Y., Hunt T., Arana B., Gosho M., Morgan R. (2009) Drug-drug interaction study to assess the effects of multiple dose of pitavastatin on steady state warfarin in healthy adult volunteers. Atheroscler Suppl 10: e806. [DOI] [PubMed] [Google Scholar]
- Inoue I., Itoh F., Aoyagi S., Tazawa S., Kusama H., Akahane M., et al. (2002) Fibrate and statin synergistically increase the transcriptional activities of PPARalpha/RXRalpha and decrease the transactivation of NFkappaB. Biochem Biophys Res Commun 290(11): 131–139 [DOI] [PubMed] [Google Scholar]
- Inoue K., Sugiyama A., Reid P.C., Ito Y., Miyauchi K., Mukai S., et al. (2007) Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol 27: 161–167 [DOI] [PubMed] [Google Scholar]
- Jacobson T.A. (2006) The safety of aggressive statin therapy: How much can low-density lipoprotein cholesterol be lowered? Mayo Clin Proc 81: 1225–1231 [DOI] [PubMed] [Google Scholar]
- Kawai T., Tokui M., Funae O., Meguro S., Yamada S., Tabata M., et al. (2005) Efficacy of pitavastatin, a new HMG-CoA reductase inhibitor, on lipid and glucose metabolism in patients with type 2 diabetes. Diabetes Care 28: 2980–2981 [DOI] [PubMed] [Google Scholar]
- Kawakami A., Tani M., Chiba T., Yui K., Shinozaki S., Nakajima K., et al. (2005) Pitavastatin inhibits remnant lipoprotein-induced macrophage foam cell formation through ApoB48 receptor-dependent mechanism. Arterioscler Thromb Vasc Biol 25: 424–429 [DOI] [PubMed] [Google Scholar]
- Kawashiri M.A., Nohara A., Tada H., Mori M., Tsuchida M., Katsuda S., et al. (2008) Comparison of effects of pitavastatin and atorvastatin on plasma coenzyme Q10 in heterozygous familial hypercholesterolemia: Results from a crossover study. Clin Pharmacol Ther 83: 731–739 [DOI] [PubMed] [Google Scholar]
- Kibayashi E., Urakaze M., Kobashi C., Kishida M., Takata M., Sato A., et al. (2005) Inhibitory effect of pitavastatin (NK-104) on the C-reactive-protein-induced interleukin-8 production in human aortic endothelial cells. Clin Sci (Lond) 108: 515–521 [DOI] [PubMed] [Google Scholar]
- Kimura K., Shimano H., Yokote K., Urashima M., Teramoto T. (2010) Effects of pitavastatin (LIVALO tablet) on the estimated glomerular filtration rate (eGFR) in hypercholesterolemic patients with chronic kidney disease. J Atheroscler Thromb 30(17): 601–609 [DOI] [PubMed] [Google Scholar]
- Kohno M., Shinomiya K., Abe S., Noma T., Kondo I., Oshita A., et al. (2002) Inhibition of migration and proliferation of rat vascular smooth muscle cells by a new HMG-CoA reductase inhibitor, pitavastatin. Hypertens Res 25: 279–285 [DOI] [PubMed] [Google Scholar]
- Koshiyama H., Taniguchi A., Tanaka K., Kagimoto S., Fujioka Y., Hirata K., et al. (2008) Effects of pitavastatin on lipid profiles and high-sensitivity CRP in Japanese subjects with hypercholesterolemia: Kansai Investigation of Statin for Hyperlipidemic Intervention in Metabolism and Endocrinology (KISHIMEN) investigatars. J Atheroscler Thromb 15: 345–350 [DOI] [PubMed] [Google Scholar]
- Kotseva K., Wood D., De Backer G., De Bacquer D., Pyörälä K., Keil U. (2009) EUROASPIRE III: A survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Lancet 373: 929–940 [DOI] [PubMed] [Google Scholar]
- Kurihara Y., Douzono T., Kawakita K., Nagasaka Y. (2008) A large-scale, long-term prospective post-marketing surveillance of piavastatin (Livalo) — Livalo effectiveness and safety study (LIVES). Jpn Pharmacol Ther 36: 709–731 [Google Scholar]
- LaRosa J.C., Grundy S.M., Waters D.D., Shear C., Barter P., Fruchart J.C., et al. (2005) Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 352: 1425–1435 [DOI] [PubMed] [Google Scholar]
- Little W.C. (1990) Angiographic assessment of the culprit coronary artery lesion before acute myocardial infarction. Am J Cardiol 66: 44G–47G [DOI] [PubMed] [Google Scholar]
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group (1998) Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 339: 1349–1357 [DOI] [PubMed] [Google Scholar]
- Markle R.A., Han J., Summers B.D., Yokoyama T., Hajjar K.A., Hajjar D.P., et al. (2003) Pitavastatin alters the expression of thrombotic and fibrinolytic proteins in human vascular cells. J Cell Biochem 90: 23–32 [DOI] [PubMed] [Google Scholar]
- Mathew P., Cuddy T., Tracewell W.G., Salazar D. (2004) An open-label study on the pharmacokinetics (PK) of pitavastatin (NK-104) when administered concomitantly with fenofibrate or gemfibrozil in healthy volunteers. Clin Pharmacol Ther 75: P33 [Google Scholar]
- Meade L.T. (2007) Barriers to achieving LDL cholesterol goals. US Pharm 32: 66–71 [Google Scholar]
- Mega J.L., Morrow D.A., Brown A., Cannon C.P., Sabatine M.S. (2009) Identification of genetic variants associated with response to statin therapy. Arterioscler Thromb Vasc Biol 29: 1310–1315 [DOI] [PubMed] [Google Scholar]
- Miller M., Cannon C.P., Murphy S.A., Qin J., Ray K.K., Braunwald E. (2008) Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 51: 724–730 [DOI] [PubMed] [Google Scholar]
- Morikawa S., Takabe W., Mataki C., Kanke T., Itoh T., Wada Y., et al. (2002) The effect of statins on mRNA levels of genes related to inflammation, coagulation, and vascular constriction in HUVEC. Human umbilical vein endothelial cells. J Atheroscler Thromb 9: 178–183 [DOI] [PubMed] [Google Scholar]
- Morikawa S., Takabe W., Mataki C., Wada Y., Izumi A., Saito Y., et al. (2004) Global analysis of RNA expression profile in human vascular cells treated with statins. J Atheroscler Thromb 11: 62–72 [DOI] [PubMed] [Google Scholar]
- Morikawa S., Umetani M., Nakagawa S., Yamazaki H., Suganami H., Inoue K., et al. (2000) Relative induction of mRNA for HMG CoA reductase and LDL receptor by five different HMG-CoA reductase inhibitors in cultured human cells. J Atheroscler Thromb 7: 138–144 [DOI] [PubMed] [Google Scholar]
- Motomura T., Okamoto M., Kitamura T., Yamamoto H., Otsuki M., Asanuma N., et al. (2009) Effects of pitavastatin on serum lipids and high sensitivity C-reactive protein in type 2 diabetic patients. J Atheroscler Thromb 16: 546–552 [DOI] [PubMed] [Google Scholar]
- Mukhtar R.Y., Reid J., Reckless J.P. (2005) Pitavastatin. Int J Clin Pract 59: 239–252 [DOI] [PubMed] [Google Scholar]
- Nakano K., Egashira K. (2009) Pitavastatin has most potent pro-healing effects on endothelial cells and inhibitory effects on proliferation of vascular smooth muscle cells - a potential treatment strategy for drug-eluting stents. In The 41st Annual Scientific Meeting of the Japan Atherosclerosis Society (July 2009) general presentation No. 21.
- Nakaya N., Tateno M., Nakamura T. (2001) Pharmacokinetics of reported dose NK-104 (pitavastatin) in healthy elderly and non-elderly volunteers. J Clin Therap Med 17: 957–970 [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Circulation 106: 3143–3421 [PubMed] [Google Scholar]
- Neuvonen P.J., Niemi M., Backman J.T. (2006) Drug interactions with lipid-lowering drugs: Mechanisms and clinical relevance. Clin Pharmacol Ther 80: 565–581 [DOI] [PubMed] [Google Scholar]
- Nissen S.E., Nicholls S.J., Sipahi I., Libby P., Raichlen J.S., Ballantyne C.M., et al. (2006) Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA 295: 1556–1565 [DOI] [PubMed] [Google Scholar]
- Nissen S.E., Tuzcu E.M., Schoenhagen P., Brown B.G., Ganz P., Vogel R.A., et al. (2004) Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA 291: 1071–1080 [DOI] [PubMed] [Google Scholar]
- O'Donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P., et al. (2010) Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 376: 112–123 [DOI] [PubMed] [Google Scholar]
- Ohbayashi H., Miyazawa C., Miyamoto K., Sagara M., Yamashita T., Onda R. (2009) Pitavastatin improves plasma pentraxin 3 and arterial stiffness in atherosclerotic patients with hypercholesterolemia. J Atheroscler Thromb 16: 490–500 [DOI] [PubMed] [Google Scholar]
- Ose L., Budinski D., Hounslow N.J., Arneson V. (2009) Comparison of pitavastatin with simvastatin in primary hypercholesterolaemia or combined dyslipidaemia. Curr Med Res Opin 25: 2755–2764 [DOI] [PubMed] [Google Scholar]
- Ose L., Budinski D., Hounslow N.J., Arneson V. (2010) Long-term treatment with pitavastatin is effective and well tolerated by patients with primary hypercholesterolemia or combined dyslipidemia. Atherosclerosis 210: 202–208 [DOI] [PubMed] [Google Scholar]
- Packard C.J., Ford I., Robertson M., Shepherd J., Blauw G.J., Murphy M.B., et al. (2005) Plasma lipoproteins and apolipoproteins as predictors of cardiovascular risk and treatment benefit in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). Circulation 112: 3058–3065 [DOI] [PubMed] [Google Scholar]
- Pasterkamp G., Schoneveld A.H., van der Wal A.C., Hijnen D.J., van Wolveren W.J., Plomp S., et al. (1999) Inflammation of the atherosclerotic cap and shoulder of the plaque is a common and locally observed feature in unruptured plaques of femoral and coronary arteries. Arterioscler Thromb Vasc Biol 19: 54–58 [DOI] [PubMed] [Google Scholar]
- Pitavatstatin Prescribing Information (2007) NK-104 Pitavastatin Investigators' Brochure, Kowa Company: Tokyo [Google Scholar]
- Pitavatstatin Prescribing Information (2010) Kowa Pharmaceuticals, America. www.kowapharma.com/documents/LIVALOpitavastatinprescribinginformationV1_220100131.pdf (accessed September 2010).
- Polisecki E., Muallem H., Maeda N., Peter I., Robertson M., McMahon A.D., et al. (2008) Genetic variation at the LDL receptor and HMG-CoA reductase gene loci, lipid levels, statin response, and cardiovascular disease incidence in PROSPER. Atherosclerosis 2008: 109-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K.K., Cannon C.P., Cairns R., Morrow D.A., Ridker P.M., Braunwald E. (2009) Prognostic utility of apoB/AI, total cholesterol/HDL, non-HDL cholesterol, or hs-CRP as predictors of clinical risk in patients receiving statin therapy after acute coronary syndromes: Results from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol 29: 424–430 [DOI] [PubMed] [Google Scholar]
- Ridker P.M., Genest J., Boekholdt S.M., Libby P., Gotto A.M., Nordestgaard B.G., et al. (2010) HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: An analysis from the JUPITER trial. Lancet 376: 333–339 [DOI] [PubMed] [Google Scholar]
- Ridker P.M., Cannon C.P., Morrow D., Rifai N., Rose L.M., McCabe C.H., et al. (2005) C-reactive protein levels and outcomes after statin therapy. N Engl J Med 352: 20–28 [DOI] [PubMed] [Google Scholar]
- Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr, Kastelein J.J., et al. (2008) Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359: 2195–2207 [DOI] [PubMed] [Google Scholar]
- Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr, Kastelein J.J., et al. (2009) Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 373: 1175–1182 [DOI] [PubMed] [Google Scholar]
- Sagara N., Kawaji T., Takano A., Inomata Y., Inatani M., Fukushima M., et al. (2007) Effect of pitavastatin on experimental choroidal neovascularization in rats. Exp Eye Res 84: 1074–1080 [DOI] [PubMed] [Google Scholar]
- Saito Y. (2009) Critical appraisal of the role of pitavastatin in treating dyslipidemias and achieving lipid goals. Vasc Health Risk Man 5: 921–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Yamada N., Teramoto T., Itakura H., Hata Y., Nakaya N., et al. (2002) Clinical efficacy of pitavastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, in patients with hyperlipidemia. Dose-finding study using the double-blind, three-group parallel comparison. Arzneimittelforschung 52: 251–255 [DOI] [PubMed] [Google Scholar]
- Sasaki J., Iwashita M., Kono S. (2006) Statins: Beneficial or adverse for glucose metabolism. J Atheroscler Thromb 13: 123–126 [DOI] [PubMed] [Google Scholar]
- Scandinavian Simvastatin Survival Study Group (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383–1389 [PubMed] [Google Scholar]
- Sever P.S., Dahlöf B., Poulter N.R., Wedel H., Beevers G., Caulfield M., et al. (2003) Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet 361: 1149–1158 [DOI] [PubMed] [Google Scholar]
- Shalev V., Chodick G., Silber H., Kokia E., Jan J., Heymann A.D. (2009) Continuation of statin treatment and all-cause mortality: A population-based cohort study. Arch Intern Med 169: 260–268 [DOI] [PubMed] [Google Scholar]
- Sharma R.K., Singh V.N., Reddy H.K. (2009) Thinking beyond low-density lipoprotein cholesterol: Strategies to further reduce cardiovascular risk. Vasc Health Risk Manag 5: 793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J., Cobbe S.M., Ford I., Isles C.G., Lorimer A.R., MacFarlane P.W., et al. (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med 333: 1301–1307 [DOI] [PubMed] [Google Scholar]
- Shitara Y., Sugiyama Y. (2006) Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther 112: 71–105 [DOI] [PubMed] [Google Scholar]
- Sommeijer D.W., MacGillavry M.R., Meijers J.C., Van Zanten A.P., Reitsma P.H., Ten Cate H. (2004) Anti-inflammatory and anticoagulant effects of pravastatin in patients with type 2 diabetes. Diabetes Care 27: 468–473 [DOI] [PubMed] [Google Scholar]
- Stender S., Hounslow N. (2009) Robust efficacy of pitavastatin and comparable safety to pravastatin. Atheroscler Suppl 10: P770 [Google Scholar]
- Suzuki M., Iwasaki H., Fujikawa Y., Kitahara M., Sakashita M., Sakoda R. (2001) Synthesis and biological evaluations of quinoline-based HMG-CoA reductase inhibitors. Bioorg Med Chem 9: 2727–2743 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kobayashi H., Sato F., Yonemitsu Y., Nakashima Y., Sueishi K. (2003) Plaque-stabilizing effect of pitavastatin in Watanabe heritable hyperlipidemic (WHHL) rabbits. J Atheroscler Thromb 10: 109–116 [DOI] [PubMed] [Google Scholar]
- Sviridov D., Nestel P., Watts G. (2007) Statins and metabolism of high density lipoprotein. Cardiovasc Hematol Agents Med Chem 5: 215–221 [DOI] [PubMed] [Google Scholar]
- Tamakawa T., Takano T., Tanaka S., Kadonosono K., Terauchi Y. (2008) Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J Atheroscler Thromb 15: 269–275 [DOI] [PubMed] [Google Scholar]
- Tan K.C., Chow W.S., Tam S.C., Ai V.H., Lam C.H., Lam K.S. (2002) Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab 87: 563–568 [DOI] [PubMed] [Google Scholar]
- Teramoto T., Sasaki J., Ueshima H., Egusa G., Kinoshita M., Shimamoto K., et al. (2007) Executive summary of Japan Atherosclerosis Society JAS guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb 14: 45–50 [DOI] [PubMed] [Google Scholar]
- Teramoto T., Shimano H., Yokote K., Urashima M. (2009) Effects of pitavastatin (LIVALO tablet) on high density lipoprotein cholesterol (HDL-C) in hypercholesterolemia. J Atheroscler Thromb 16: 654–661 [DOI] [PubMed] [Google Scholar]
- Thompson J.F., Man M., Johnson K.J., Wood L.S., Lira M.E., Lloyd D.B., et al. (2005) An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics J 5: 352–358 [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group. (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Brit Med J 317: 703–713 [PMC free article] [PubMed] [Google Scholar]
- Waters D.D., Brotons C., Chiang C.W., Ferrières J., Foody J., Jukema J.W., et al. (2009) Lipid treatment assessment project 2: A multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation 120: 28–34 [DOI] [PubMed] [Google Scholar]
- Watson K.E. (2010) Pitavastatin: The newest HMG-CoA reductase inhibitor. Rev Cardiovasc Med 11: 26–32 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2010) Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus. Available at: www.who.int/diabetes/publications/en/ (accessed May 2010).
- Yamazaki H., Fujino H., Kanazawa M. (2004) Pharmacological and pharmacokinetic features and clinical effects of pitavastatin (Livalo Tablet). [In Japanese] Folia Pharmacol Jpn 123: 349–362 [DOI] [PubMed] [Google Scholar]
- Yokote K., Bujo H., Hanaoka H., Shinomiya M., Miyashita Y., Nishikawa T., et al. (2008) Multicenter collaborative randomized parallel group comparative study of pitavastatin and atorvastatin in Japanese hypercholesterolemic patients Collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). Atheroscler 201: 345–352 [DOI] [PubMed] [Google Scholar]
- Yokote K., Saito Y. (2009) Influence of statins on glucose tolerance in patients with type 2 diabetes mellitus: Subanalysis of the collaborative study on hypercholesterolemia drug intervention and their benefits for atherosclerosis prevention (CHIBA study). J Atheroscler Thromb 16: 297–298 [DOI] [PubMed] [Google Scholar]
- Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F., et al. (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 364: 937–952 [DOI] [PubMed] [Google Scholar]