Abstract
The common pathological mechanisms among the spectrum of neurodegenerative diseases are supposed to be shared. Multiple lines of evidence, from molecular and cellular to epidemiological, have implicated nicotinic transmission in the pathology of the two most common neurodegenerative disorders, namely Alzheimer's disease (AD) and Parkinson's disease (PD). In this review article we present evidence of nicotinic acetylcholine receptor (nAChR)-mediated protection against neurotoxicity induced by β amyloid (Aβ), glutamate, rotenone, and 6-hydroxydopamine (6-OHDA) and the signal transduction involved in this mechanism. Our studies have clarified that survival signal transduction, the α7 nAChR/Src family/PI3K/AKT pathway and subsequent upregulation of Bcl-2 and Bcl-x, would lead to neuroprotection. In addition to the PI3K/AKT pathway, two other survival pathways, JAK2/STAT3 and MEK/ERK, are proposed by other groups. In rotenone- and 6-OHDA-induced PD models, nAChR-mediated neuroprotection was also observed, and the effect was blocked not only by α7 but also by α4β2 nAChR antagonists. We also document that nAChR stimulation blocks glutamate neurotoxicity in spinal cord motor neurons. These findings suggest that nAChR-mediated neuroprotection is achieved through subtypes of nAChRs and common signal cascades. An early diagnosis and protective therapy with nAChR stimulation could be effective in delaying the progression of neurodegenerative diseases such as AD, PD and amyotrophic lateral sclerosis.
Keywords: Alzheimer's disease, amyotrophic lateral sclerosis, β amyloid, glutamate, nAChR, nicotine, Parkinson's disease
Introduction
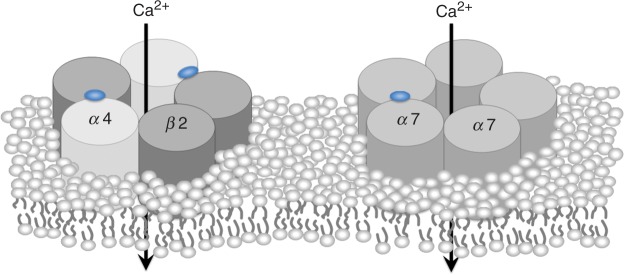
To date, available therapeutic agents against Alzheimer's disease (AD) are acetylcholinesterase inhibitors (AChEI), while the most reproducible epidemiologically relevant factor against Parkinson's disease (PD) is cigarette smoking habits [Quik, 2004; Dorn, 1959]. Both clearly show that placing the importance on modulation of acetylcholine systems must be the key therapeutic targets of these most common devastating neurodegenerative diseases, whose socio-economic impacts have been striking. Acetylcholine (ACh) is one of the major neurotransmitters in the central nervous system (CNS). ACh receptors are classified into two groups; nicotinic ACh receptors (nAChRs) and muscarinic ACh receptors (mAChRs). In the brain, nAChRs show additional complexity, as there are multiple receptor subtypes with differing properties and functions [Lindstrom et al. 1995; Clarke et al. 1985]. At least nine α subunits (α2-α7, α9, and α10 in mammals; α8 in chicks) and three β subunits (β2-β4) have been identified in the brain. Both α and β subunits are required to form functional heteropentametric receptors, with the exception of α7-α10 subunits, which apparently form functional homopentameric receptors. In the brain, α7 homometric and α4β2 heterometric nAChRs are the major two subtypes. Both α4β2 and α7 subtypes have been implicated in the mechanism of neuroprotection provided by nicotine (Figure 1) [Kihara et al. 2001, 1998]. Implication of heterometric nAChR-containing α6 subunits, α6β2*, is also emphasized in dopaminergic systems in the CNS (* indicates possible additional subunits) [Bordia et al. 2007; Bohr et al. 2005; Quik, 2004; Champtiaux et al. 2002].
Figure 1.
Composition of two major nicotinic acetylcholine receptors, α4β2 and α7, in the central nervous system.
There is evidence that neuronal nAChRs are involved in synaptic plasticity as well as in neuronal survival and neuroprotection. Moreover, presynaptic nAChRs can modulate the release of many neurotransmitters, including dopamine, noradrenaline, serotonin, ACh, γ-aminobutyric acid (GABA), and glutamate. These neurotransmitter systems play an important role in cognitive and noncognitive functions such as learning, memory, attention, locomotion, motivation, reward, reinforcement, and anxiety. Thus, nAChRs are considered promising therapeutic targets for new treatments of neurodegenerative disorders. It is also known that α4 and β2 nAChR genes, CHRNA4 and CHRNB2, are causative genes of autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) [De Fusco et al. 2000; Steinlein et al. 1995]. The benign nature of this form of epilepsy is explained by a compensatory mechanism of the nAChRs. Analyzing the polymorphism of the nAChRs genes in AD patients and controls, we concluded that genetic polymorphisms of the neuronal nAChR genes might be related to the pathogenesis of sporadic AD [Kawamata and Shimohama, 2002].
To date, mainly three possible mechanisms of nAChRs-mediated neuroprotection have been proposed, as follows [Buckingham et al. 2009]:
Phosphatidylinositol 3-kinase/v-akt murine thymoma viral oncogene homolog 1 (PI3K/AKT) pathway.
Janus kinase-2/signal transducer and activator of transcription-3 (JAK2/STAT3) pathway.
MEK (MAPK/ERK kinase; Mitogen-activated protein kinase/Extracellular signal regulated kinase kinase)/Extracellular signal regulated kinase (MEK/ERK) pathway.
In this review we focus mainly on the PI3K/AKT pathway initially proposed by our collaborators and present evidence for nAChR-mediated protection against β amyloid (Aβ)- and glutamate-induced neurotoxicity and in rotenone- and 6-hydroxydopamine (6-OHDA)-induced PD animal models, based mainly on our studies.
Alzheimer's disease
AD and nicotinic transmission
AD pathology is characterized by the presence of two hallmarks, senile plaques (SPs) and neurofibrillary tangles (NFTs), and by extensive neuronal loss [Giannakopoulos et al. 1996]. Aβ is a major element of SP and one of the candidates for the cause of the neurodegeneration found in AD. It has been shown that the accumulation of Aβ precedes other pathological changes and causes neurodegeneration or neuronal death in vivo [Yankner et al. 1990]. Several mutations of the Aβ precursor protein (APP) are found in familial AD, and these mutations are involved in amyloidogenesis [Citron et al. 1992]. Also, familial AD mutations of presenilin 1 (PS-1) enhance the generation of Aβ 1-42 [Tomita et al. 1997]. It is also known that Aβ binds very strongly to α7 nAChR [Wang et al. 2000] and upregulation of α7 nAChR is observed in transgenic mice co-expressing mutant human presenilin 1 and APP [Dineley et al. 2002].
The cerebral cortex contains a dense plexus of cholinergic axon terminals that arise from the cells of the basal forebrain including the nucleus basalis of Meynert [Mesulam et al. 1983; Bigl et al. 1982]. Degeneration of this cholinergic projection is recognized as one of the most prominent pathological changes in the AD brain [Rosser et al. 1982; Whitehouse et al. 1981]. In AD, the cholinergic system is affected, and a reduction in the number of nAChRs has been reported [Whitehouse and Kalaria, 1995; Shimohama et al. 1986]. This, in conjunction with the memory-enhancing activity of nicotine and selective nAChR agonists such as the α7 nAChR agonist, 3-(2,4)-dimethoxybenzylidene anabaseine (DMXB) [Meyer et al. 1997], suggests a significant role for nAChRs in learning and memory. Therefore, it is generally recognized that downregulation of nAChRs is involved in the intellectual dysfunction in AD. Our studies showed that nAChR stimulation protected neurons from Aβ- and glutamate-induced neurotoxicity. This allowed us to hypothesize that nAChRs are involved in a neuroprotective cascade [Kihara et al. 2001, 1998, 1997; Kaneko et al. 1997; Shimohama et al. 1996; Akaike et al. 1994] as described in following sections.
In 2009, Dziewczapolski and colleagues reported interesting but controversial findings about the interaction between Aβ and α7 nAChR. They used a transgenic mouse model of AD, PDAPP, overexpressing a mutated form of the APP (both ‘Swedish’ and ‘Indiana’ mutations) and lacking the α7 nAChR gene. They reported that despite the presence of high amounts of APP and amyloid deposits, deleting the α7-nicotinic subunit in the mouse model of AD rescues the mice from expressing the phenotype of the dysfunction in synaptic integrity and learning and memory behavior in aged stage (13-22 months) [Dziewczapolski et al. 2009]. One of the possible explanations is that pathological intracellular translocation of Aβ through α7 nAChR with high affinity binding is hampered by deleting the α7 nAChR itself. Recently, Hernandez and colleagues reported that loss of α7 nAChR enhances Aβ oligomer accumulation, exacerbating early-stage cognitive decline in transgenic mouse model of AD, the Tg2576, overexpressing a mutated form of APP (‘Swedish’ mutation) [Hernandez et al. 2010]. Contribution of α7 nAChR to AD pathology may be different depending on the stage of neurodegeneration.
Protection against Aβ toxicity
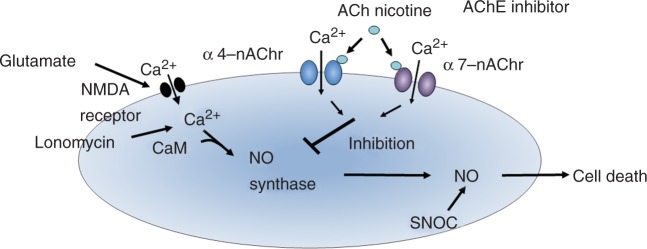
A 48-hour exposure to 20 μM neurotoxic Aβ 25-35 caused a significant reduction in the neuronal cells of rat fetal primary culture. Simultaneous incubation of the cultures with nicotine and Aβ significantly reduced the Aβ-induced cytotoxicity. The protective effect of nicotine was reduced by both dihydro-β-erythroidine (DHβE), an α4β2 nAChR antagonist, and α-BTX, an α7 nAChR antagonist. The effect of a selective α4β2 nAChR agonist, cytisine, and a selective α7 nAChR agonist, DMXB [Hunter et al. 1994], on Aβ cytotoxicity was examined. Aβ cytotoxicitywas significantly reduced when 10 μM cytisine or 1 μM DMXB was co-administered. These findings suggest that both α4β2 and α7 nAChR stimulation are protective against Aβ cytotoxicity [Kihara et al. 2001, 1998, 1997]. In addition, MK-801, an N-methyl-D-aspartate (NMDA) receptor antagonist, inhibited Aβ cytotoxicity when administrated simultaneously with Aβ, suggesting that Aβ cytotoxicity is mediated via the NMDA receptor, or via glutamate in cultured cortical neurons (Figure 2).
Figure 2.
Proposed hypothesis for the mechanism of nicotinic acetylcholine receptor (nAChR)-mediated survival signal transduction against glutamate-induced necrosis. AChE, acetylcholinesterase; NMDA, N-methyl-D-aspartate; NO, nitric oxide; SNOC, NO-generating agent.
Protection against glutamate cytotoxicity
Glutamate cytotoxicity is one of the most suspected causative pathways in neurodegenerative processes such as AD, PD, and amyotrophic lateral sclerosis (ALS). It is also assumed that glutamate plays an important role in the neurodegeneration observed in hypoxic-ischemic brain injury [Meldrum and Garthwaite, 1990; Choi, 1988]. Several investigators have also suggested that cortical neurodegeneration in AD is attributable to glutamate [Mattson, 1988; Maragos et al. 1986]. Moreover, brief glutamate exposure induces delayed cell death in cultured neurons from certain brain regions such as the cerebral cortex and hippocampus. In these brain regions, the NMDA glutamate receptor subtype plays a crucial role in glutamate neurotoxicity. Several studies have indicated the existence of nitric oxide (NO) synthase in the CNS, including the cerebral cortex. NMDA receptor stimulation induces Ca2+ influx into cells through ligandgated ion channels, thereby triggering NO formation. NO is also thought to diffuse to the adjacent cells, resulting in the appropriate physiological response and/or glutamate-related cell death [Dawson et al. 1991; Choi et al. 1989; Hartley and Choi, 1989]. Although there has been only limited information concerning the cholinergic interaction with glutamate neurotoxicity, Mattson and Olney and colleagues have demonstrated that stimulation of the muscarinic AChR potentiates neurodegeneration [Olney et al. 1991; Mattson, 1989].
We examined the effects of nicotine on glutamate-induced neurotoxicity using primary cultures of rat cortical neurons. Cell viability was decreased by treatment with 1 mM glutamate for 10 minutes followed by incubation in glutamate-free medium for 1 hour. Incubating the cultures with 10 μM nicotine for 24 hours prior to glutamate exposure significantly reduced glutamate cytotoxicity. To investigate whether nicotine-induced neuroprotection is due to a specific effect mediated by nAChRs, the effects of cholinergic antagonists were examined. An addition of DHβE or α-BTX to the medium containing nicotine reduced the protective effect of nicotine. We also examined the protection of nicotine against the effects of ionomycin, a calcium ionophore, and SNOC, a NO-generating agent. Incubating the cultures for 10 minutes in either 3 μM ionomycin- or 300 μM SNOC-containing medium markedly reduced cell viability. A 24-hour pretreatment with nicotine significantly attenuated the ionomycin cytotoxicity, but did not affect the SNOC cytotoxicity (Figure 2) [Shimohama et al. 1998, 1996; Kaneko et al. 1997; Akaike et al. 1994].
Protection against Aβ-enhanced glutamate toxicity
Whilst it is thought that PS-1 mutations enhance the generation of Aβ 1-42, it is controversial whether Aβ is directly toxic to neurons. We found that Aβ 25-35-induced neurotoxicity was inhibited by MK801. It can therefore be hypothesized that Aβ might modulate or enhance glutamate-induced cytotoxicity. Indeed, Aβ causes a reduction in glutamate uptake in cultured astrocytes [Harris et al. 1996], indicating that, to some extent, Aβ-induced cytotoxicity might be mediated via glutamate cytotoxicity.
In our previous study [Kihara et al. 2000], incubation of the cortical neurons with both Aβ 1-40 (1 nM) and Aβ 1-42 (100 pM) for 7 days did not induce cell death: these are the concentrations of Aβ in the cerebrospinal fluid (CSF) of AD patients [Jensen et al. 1999]. Although 20 μM glutamate alone did not significantly induce cell death, exposure to 20 μM glutamate for 24 hours caused a significant reduction in the neuronal cells in the Aβ-treated group, showing that Aβ itself is not toxic at low concentrations, but makes neurons vulnerable to glutamate. Conversely, co-incubation of the cultures with nicotine (50 μM for 7 days) and Aβ significantly reduced Aβ-enhanced glutamate cytotoxicity [Kaneko et al. 1997; Shimohama et al. 1996; Akaike et al. 1994].
Involvement of the phosphatidylinositol 3-kinase pathway in neuroprotection
To investigate the mechanism of the protective effect of nicotine, we focused on the phosphatidylinositol 3-kinase (PI3K) pathway because PI3K has been shown to protect cells from apoptosis [del Peso et al. 1997]. Long exposure to low concentrations of glutamate (50 μM for 24 hours) induced cytotoxicity. Incubating the cultures with nicotine (10 μM for 24 hours) prior to glutamate exposure significantly suppressed glutamate cytotoxicity. Simultaneous application of LY294002, a PI3K inhibitor, with nicotine cancelled the protective effect of nicotine. α-BTX, an α7 nAChR antagonist, blocked the protection provided by nicotine and by DMXB, an α7 selective nAChR agonist. Furthermore, this DMXB-induced protection was also reduced by LY294002. Although α4β2 nAChR stimulation also had a protective effect on Aβ- and glutamate-induced cytotoxicity, this effect was not inhibited by LY294002, suggesting PI3K system is not directly involved in α4β2 nAChR-mediated neuroprotection. PD98059, a mitogen-activated protein (MAP) kinase kinase (MEK) inhibitor, did not reduce the protective effect of nicotine, also suggesting that the MEK/ERK pathway is not directly involved in the protective effect of nicotine (Figure 3). A nonreceptor tyrosine kinase inhibitor, PP2, did reduce the protective effect of nicotine, suggesting that Src is involved in the mechanism of the protective effect. Cycloheximide also inhibited the protection, implying that some protein synthesis is necessary for this effect.
Figure 3.
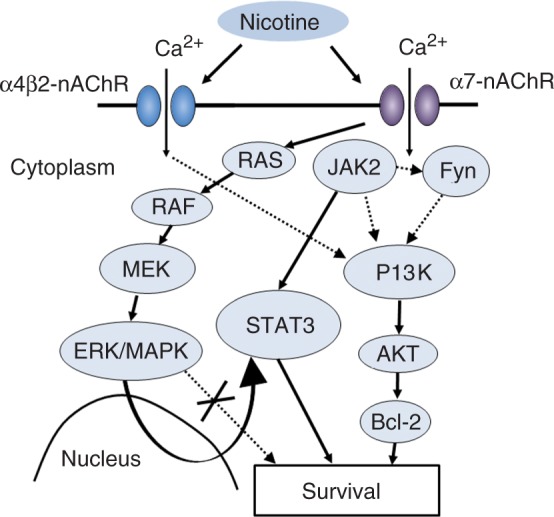
Proposed three pathways for the mechanism of nicotinic acetylcholine receptor (nAChR)-mediated survival signal transduction. 1: PI3K/AKT pathway; 2: JAK2/STAT3 pathway; 3: RAS/RAF/MEK/ERK/MAPK pathway.
AKT is a serine/threonine protein kinase and a putative effector of PI3K. To investigate the activation of AKT by nicotine through PI3K, we examined the level of phosphorylated AKT using an antiphosphospecific AKT antibody. The phosphorylated form of AKT appeared just after the application of nicotine. Nicotine-induced AKT phosphorylation was blocked by simultaneous application of LY294002, but not of PD98059, indicating that PI3K, but not MAPK is involved. The AKT phosphorylation is blocked by α-BTX, but not by DHβE, implying that nicotine-induced AKT phosphorylation is mediated by α7 but not by α4β2 nAChRs. PP2 also blocked AKT phosphorylation, which suggests the involvement of tyrosine kinase. The level of total AKT protein which was detected with anti-AKT antibody remained unchanged.
Bcl-2 and Bcl-x proteins are anti-apoptotic proteins that can prevent cell death induced by a variety of toxic attacks [Zhong et al. 1993]. It has been reported that AKT activation leads to the overexpression of Bcl-2 [Matsuzaki et al. 1999]. Because nicotine can activate AKT via PI3K, we examined the protein levels of Bcl-2 and Bcl-x. We found that treatment with nicotine for 24 hours increased the levels of Bcl-2 and Bcl-x, and this was inhibited by LY294002, which indicates the involvement of the PI3K pathway in nicotine-induced Bcl-2 and Bcl-x upregulation (Figure 3). These results suggest that nAChR stimulation protects neurons from glutamate-induced cytotoxicity by activating PI3K, which in turn activates AKT and upregulates Bcl-2 and Bcl-x.
Galantamine modulates nAChR and blocks Aβ-enhanced glutamate toxicity
Galantamine is an AChEI that is currently used for the treatment of AD. In addition to the inhibition of AChE, galantamine binds to nAChRs and allosterically potentiates their synaptic transmission. Consequently, galantamine is called an allosteric potentiating ligand (APL) of nAChRs. This APL effect is present on both α7 and α4β2 nAChRs [Maelicke et al. 2001]. Thus, galantamine could stimulate cholinergic transmission in two ways: (1) by inhibiting AChE and increasing AChs; and (2) by potentiating cholinergic transmission through the APL effect. We demonstrated that galantamine protected cortical neurons against Aβ-enhanced glutamate toxicity by, at least partially, the α7nAChR/PI3K/AKT pathway [Kihara et al. 2004].
Donepezil promotes internalization of NMDA receptors by stimulating α7 nAChRs and protects against glutamate cytotoxicity
Donepezil is one of the most widely prescribed AChEIs for the treatment of AD and related dementias. Our group recently reported that in addition to upregulating the PI3K/AKT pathway, there is another mechanism underlying neuroprotection by donepezil: decreased glutamate toxicity through downregulation of NMDA receptors, following stimulation of α7 nAChRs in primary rat neuron cultures [Shen et al. 2010].
Parkinson's disease
PD and nicotinic transmission
PD is the second most common progressive neurodegenerative disorder next to AD.
It is characterized by relatively selective degeneration of dopaminergic neurons in the substantia nigra and loss of dopamine in the striatum resulting in resting tremor, rigidity, bradykinesia, and postural instability [Obeso et al. 2010; Shimohama et al. 2003]. Although the pathogenesis of PD is still unclear, it is thought that the interaction of gene and the environment plays roles in causing the multifactorial disease. Rural residency, pesticides, and intrinsic toxic agents were reported as environmental risk factors for sporadic PD. Recent studies revealed several mutations in familial PD genes such as α-synuclein, parkin, PINK1, LRRK2, DJ-1, UCHL1, and ATP13A2 [Hardy, 2010]. Epidemiological studies suggest that the use of pesticides increases the risk of PD, possibly via reduced activity of complex I in the mitochondrial respiratory chain in the substantia nigra [Mizuno et al. 1998; Mann et al. 1992; Parker et al. 1989]. The H2O2 pro-oxidant, 6-OHDA, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a mitochondrial complex I inhibitor, have been widely used to produce toxin models of sporadic PD. Chronic exposure to rotenone, a nature-derived pesticide, could be an appropriate animal PD model because rotenone-treated animals show slowly progressive dopamine neuronal loss, and Lewy body-like particles, which are primarily aggregations of α-synuclein [Inden et al. 2007; Mizuno et al. 1998].
Grady and colleagues examined mouse brain using in situ hybridization to characterize the mRNA expression pattern. The ventral tegmental area (VTA) and substantia nigra express high concentrations of α4 and α6 and β2, and β3 mRNAs, intermediate levels of α5 mRNA, and low levels of the α3 and α7 mRNAs. No signal for α2 and β4 mRNA was detected [Grady et al. 2007; Le Novère et al. 1996]. They reviewed the subtypes of nAChRs on dopaminergic terminals of mouse striatum reporting five nAChR subtypes that expressed on dopaminergic nerve terminals, three of which are α6-containing subunits, namely α4α6β2β3, α6β2β3, and α6β2. The remaining two subtypes, α4β2 and α4α5β2, are more numerous than the α6-containg subtypes. The α6-containing nAChRs, which do not contribute to dopamine release induced by nicotine, are mainly located on dopaminergic neuronal terminals and probably mediating the endogenous cholinergic modulation of dopamine release at the terminal level. In contrast, α4β2 nAChR represent the majority of functional heteromeric nAChRs on dopaminergic neuronal soma. α7 nAChRs are present on dopaminergic neuronal soma, contributing to nicotine reinforcement [Champtiaux et al. 2003].
There are several studies analyzing the decline of specific nAChRs in PD patients. Court and colleagues reported the decline of α3 subunits and no change of α7 subunits [Court et al. 2000]. Gotti and colleagues as well as Court and colleagues reported a decreased level of α4 subunits, in contrast to Guan and colleagues who did not report this [Guan et al. 2002; Court et al. 2000; Gotti et al. 1997]. Bordia and colleagues reported the decline of α6 subunits in caudate and putamen. They further specified that the most vulnerable subtype in striatum of MPTP-treated mice and monkeys is α6α4β2β3 rather than α6β2β3 and further identified the specific loss of α6α4β2β3 subtype in PD brains [Bordia et al. 2007].
These results seem to indicate that the decline of nigrostriatal specific α6* subtypes is highly specific and relevant to the PD pathogenesis but not is α7 subtype. Functional studies on α6* nAChRs should be undertaken to confirm its pathological importance in PD.
Current drug therapy against PD is limited to supplementing dopamine or enhancing dopaminergic effect. Some may have neuroprotective effects, but their effects remain controversial [Iravani et al. 2006; Du et al. 2005; Quik, 2004]. It has also been reported that smokers have a lower risk for PD [De Reuck et al. 2005; Wirdefeldt et al. 2005], and nAChRs were decreased in the brains of PD patients [Fujita et al. 2006] and PD model animals [Quik et al. 2006]. Nicotine may upregulate dopamine release at the striatum from nigral dopaminergic neurons [Morens et al. 1995], followed by stimulation of α4β2 nAChRs [Champtiaux et al. 2003]. Furthermore, nicotine could protect mitochondria and have a protective effect from oxidative stress [Xie et al. 2005; Cormier et al. 2003]. In studies made in vivo, stimulation of nAChRs resulted in neuroprotection in PD model animals [Parain et al. 2003]. Although several clinical trials to evaluate the possible therapeutic effect of nicotine in PD patients have been conducted, it is still controversial about whether it has therapeutic effects in PD. The relatively high-dose administration of transdermal nicotine might have therapeutic effect in PD patients [Villafane et al. 2007].
Neuroprotection in rotenone-induced PD models
We investigated the neuroprotective effect of nicotine against nigral dopamine neuronal death induced by rotenone using a chronic rotenonetreated PD mouse model, and analyzed molecular mechanisms of the protection in dissociated cultures of the fetal rat ventral mesencephalon. Rotenone works as a mitochondrial complex I inhibitor. Acute lethal doses of rotenone eliminate the mitochondrial respiratory system of the cell, resulting in an anoxic status that immediately causes cell death. At sublethal doses it causes partial inhibition of mitochondrial complex I, and in this situation mitochondrial dysfunction leads to increased oxidative stress, decreased ATP production, increased aggregation of unfolded proteins, and then activated apoptotic pathway(s) that result in cell death [Betarbet et al. 2000], resembling dopamine neurodegeneration in PD.
Our mouse model (oral rotenone 30 mg/kg for 28 days) showed motor deficits, dopaminergic cell death in the substantia nigra, and nerve terminal/axonal loss in the striatum. These findings are relevant to some previous reports about rotenone PD models [Ravenstijn et al. 2008; Schmidt and Alam, 2006]. Simultaneous subcutaneous administration of nicotine (0.21 mg/kg/day) rescued both motor deficits and dopamine neuronal cell loss in the substantia nigra of rotenone-treated mice. Using primary dopamine neuronal culture, we analyzed the molecular mechanisms of dopamine neuroprotective effect of nicotine against rotenone-induced toxicity. We found that dopamine neuroprotective effects of nicotine were inhibited by 1 μM DHβE, 100 nM α-BTX, and/or PI3K/AKT/protein kinase B (PKB) inhibitors, demonstrating that rotenone toxicity on dopamine neurons are inhibited via activation of α4β2 or α7 nAChRs/PI3K/AKT/PKB pathways [Takeuchi et al. 2009]. Neuronal α4β2 nAChR stimulation causes dopamine release [Champtiaux et al. 2003], and our data showed the neuroprotective effect also occurred via α4β2 nAChRs, so the mechanism of neuroprotection could vary according to different receptor subtypes.
Synergistic effect of galantamine in the 6-OHDA-induced hemiparkinsonian rat model
Using the rat 6-OHDA-induced hemiparkinsonian model, the neuroprotective effects of galantamine and nicotine were evaluated. We injected 32 nmol 6-OHDA, with or without 4-120 nmol galantamine and/or 120 nmol nicotine, into the unilateral substantia nigra of rats. Although methamphetamine-stimulated rotational behavior and dopaminergic neuronal loss induced by 6-OHDA were not inhibited by galantamine alone, they were moderately inhibited by nicotine alone. In addition, 6-OHDA-induced neuronal loss and rotational behavior were synergistically inhibited by co-injection of galantamine and nicotine. These protective effects were abolished by mecamylamine, a nAChR antagonist. α7 nAChR was expressed on both tyrosine hydroxylase (TH)-immunopositive and TH-immunonegative neurons in the rat SNpc. A combination of galantamine and nicotine greatly suppressed 6-OHDA-induced reduction of TH-immunopositive/α7 nAChR-immunopositive neurons. These results suggest that galantamine synergistically enhances the neuroprotective effect of nicotine against 6-OHDA-induced dopaminergic neuronal loss through an allosteric modulation of α7nAChR activation [Yanagida et al. 2008].
Amyotrophic lateral sclerosis: Stimulation of nAChRs protects spinal motor neurons
ALS is a fatal neurodegenerative disorder characterized by the rapidly progressive degeneration of motor neurons resulting in paralysis and, within a few years, death. To date, several causative genes of familial types have been reported including superoxide dismutase 1 (SOD1), Senataxin, TDP-43, and FUS. Although the cause of sporadic ALS is still unknown, recent proteomic and pathological studies reveal that pathological phosphorylated TDP-43 is accumulated in cytoplasm of neurons, whereas physiological TDP-43 resides mainly in the nucleus [Arai et al. 2006; Neumann et al. 2006]. The only available therapeutic alternative is glutamate-release inhibitor, riluzole, and no effective treatments have been identified. There are numerous clinical and experimental studies suggesting the role of glutamate-induced excitotoxicity in ALS pathogenesis [Rothstein, 2009]. Our previous study demonstrated that rat spinal cord cultures exposed to long-term (24-h) low-dose (10 μM) glutamate exhibit selective motor neuronal death and we proposed this paradigm as an in vitro model for ALS [Urushitani et al. 1998].
There is a study which demonstrates an early decrease in cholinergic input on motor neurons in the spinal cords of patients with ALS [Choi et al. 1989]. Therefore, we investigated the neuroprotective effect of nicotine and galantamine, an AChEI with APL properties, against spinal motor neuronal death induced by glutamate using dissociated cultures of fetal rat spinal cord.
The study demonstrated that administration of nicotine prevented glutamate-induced motor neuronal death in primary cultures of the rat spinal cord. The 10 μM nicotine-induced neuroprotection was inhibited by either 30 nM DHβE or 1 nM α-BTX, suggesting that it is mediated through both α4β2 and α7 nAChRs. Both α4β2 and α7 nAChRs were identified on rat spinal motor neurons by immunohistochemical methods. We also demonstrated that galantamine prevented glutamate-induced motor neuronal death [Nakamizo et al. 2005]. Aberration in the PI3K/AKT signaling system has been reported in both ALS patients [Wagey et al. 1998] and ALS transgenic mice [Warita et al. 2001]. Thus, it is possible that the PI3K/AKT pathway is also involved in nAChR-mediated neuroprotection against glutamate-induced spinal motor neuronal death.
Enhancement of nAChRs against neurodegenerative diseases through three pathways
PI3K/AKT pathway
Our studies showed that nAChR stimulation protected neurons from Aβ-, glutamate-, rotenone-, and 6-OHDA-induced neurotoxicity. From the experimental data, our hypothesis for the mechanism of nAChR-mediated survival signal transduction is as follows: activation of α7 nAChRs stimulates the Src family, which in turn activates PI3K. PI3K phosphorylates AKT, which causes upregulation of Bcl-2 and Bcl-x. α4β2 nAChR stimulation also causes neuroprotection cascade without direct involvement of PI3K system.
JAK2/STAT3 pathway
Other properties of nicotine proposed by other groups are anti-inflammatory potentials and modulating innate immune pathways mainly via α7 nAChR. Nicotine exerts its anti-inflammatory effect in activated immune cells, macrophages and microglia, by interacting with α7 nAChR. Activated α7 nAChR binds directory to JAK2 and triggers the JAK2/STAT3 pathway to interfere with the activation of TLR-induced NF-kB, which is responsible for pro-inflammatory cytokine transcription [Cui and Li, 2010].
MEK/ERK pathway
The importance of the MEK/ERK pathway in nicotinic neuroprotection has been also emphasized by several groups [Buckingham et al. 2009; Wang et al. 2003]. Dajas-Bailador and colleagues reported nicotine stimulation leads to PKA activation through α7 nAChR and a further Raf-1/MEK/ERK signaling pathway [Dajas-Bailador et al. 2002]. Another group showed that stepwise activation of Ras/Raf-1/MEK/ERK cascade provides for an increased cytoplasmic concentration of STAT3 due to an upregulated expression [Arredondo et al. 2006] activating the JAK2/STAT3 pathway in human oral keratonocytes.
Stimulation of nAChRs initiates these survival signal cascades in addition to their role as neurotransmitter receptors. Cholinotherapy is currently being applied with clinically symptomatic benefit in terms of AChE inhibition in AD and related disorders. It has been suggested that some of the inhibitors used in this therapy, including galantamine, which has additional nAChR modulating properties [Maelicke et al. 1995], may have disease-slowing effects. Selective α7 nAChR agonists, including DMXB [Meyer et al. 1997], S 24795 [Wang et al. 2009], R3487/MEM3454 [Rezvani et al. 2009], and ABT-107 [Bitner et al. 2010], have proven to be effective in AD animal disease models and may be regarded as therapeutic alternatives next to AChEIs. As shown in this review, recent studies suggest that nAChR stimulation could protect neurons in rotenone or 6-OHDA induced PD models as well as from Aβ and glutamate toxicity.
Conclusion
We have been studying the PI3K/AKT pathway in AD, PD, and ALS models in in vitro and in vivo systems to elucidate the mechanism of nAChR-mediated survival signal transduction as follows: activation of α7 nAChRs stimulates the Src family, which in turn activates PI3K. PI3K phosphorylates AKT, which causes upregulation of Bcl-2 and Bcl-x. Consideration the facts, together with other two pathways proposed by other groups, it is promising that protective therapy by nAChR stimulation could attenuate the progress of neurodegenerative diseases such as AD, PD, and ALS.
Footnotes
This work was supported in part by the grants from Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Smoking Research Foundation.
The authors have no conflicts of interest.
References
- Akaike A., Tamura Y., Yokota T., Shimohama S., Kimura J. (1994) Nicotine-induced protection of cultured cortical neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. Brain Res 644: 181–187 [DOI] [PubMed] [Google Scholar]
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., et al. (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351: 602–611 [DOI] [PubMed] [Google Scholar]
- Arredondo J., Chernyavsky A.I., Jolkovsky D.L., Pinkerton K.E., Grando S.A. (2006) Receptor-mediated tobacco toxicity: Cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J 20: 2093–2101 [DOI] [PubMed] [Google Scholar]
- Betarbet R., Sherer T.B., MacKenzie G., Garcia-Osuna M., Panov A.V., Greenamyre J.T. (2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301–1306 [DOI] [PubMed] [Google Scholar]
- Bigl V., Woolf N.J., Butcher L.L. (1982) Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: A combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 8: 205–211 [DOI] [PubMed] [Google Scholar]
- Bitner R.S., Bunnelle W.H., Decker M.W., Drescher K.U., Kohlhaas K.L., Markosyan S., et al. (2010) In vivo pharmacological characterization of a novel selective a7 neuronal nicotinic acetylcholine receptor agonist ABT-107: Preclinical considerations in Alzheimer's disease. J Pharmacol Exp Ther 334: 875–886 [DOI] [PubMed] [Google Scholar]
- Bohr I.J., Ray M.A., McIntosh J.M., Chalon S., Guilloteau D., McKeith I.G., et al. (2005) Cholinergic nicotinic receptor involvement in movement disorders associated with Lewy body diseases. An autoradiography study using [125I]α-conotoxinMII in the striatum and thalamus. Exp Neurol 191: 292–300 [DOI] [PubMed] [Google Scholar]
- Bordia T., Grady S.R., McIntosh J.M., Quik M. (2007) Nigrostriatal damage preferentially decreases a subpopulation of α6β2* nAChRs in mouse, monkey, and Parkinson's disease striatum. Mol Pharmacol 72: 52–61 [DOI] [PubMed] [Google Scholar]
- Buckingham S.D., Jones A.K., Brown L.A., Sattelle D.B. (2009) Nicotinic acetylcholine receptor signalling: Roles in Alzheimer's disease and amyloid neuroprotection. Pharmacol Rev 61: 39–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N., Gotti C., Cordero-Erausquin M., David D., Przybylski C., Lena C., et al. (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23: 7820–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N., Han Z.Y., Bessis A., Rossi F.M., Zoli M., Marubio L., et al. (2002) Distribution and pharmacology of a 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci 22: 1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D.W. (1988) Calcium-mediated neurotoxicity: Relationship to specific channel types and role in ischemic damage. Trends Neurosci 11: 465–469 [DOI] [PubMed] [Google Scholar]
- Choi D.W., Viseskul V., Amirthanayagam M., Monyer H. (1989) Aspartate neurotoxicity on cultured cortical neurons. J Neurosci Res 23: 116–121 [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A.Y., Seubert P., et al. (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature 360: 672–674 [DOI] [PubMed] [Google Scholar]
- Clarke P.B., Schwartz R.D., Paul S.M., Pert C.B., Pert A. (1985) Nicotinic binding in rat brain: Autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-α-bungarotoxin. J Neurosci 5: 1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier A., Morin C., Zini R., Tillement J., Lagrue G. (2003) Nicotine protects rat brain mitochondria against experimental injuries. Neuropharmacology 44: 642–652 [DOI] [PubMed] [Google Scholar]
- Court J.A., Martin-Ruiz C., Graham A., Perry E. (2000) Nicotinic receptors in human brain: Topography and pathology. J Chem Neuroanat 20: 281–298 [DOI] [PubMed] [Google Scholar]
- Cui W.Y., Li M.D. (2010) Nicotinic modulation of innate immune pathways via α7 nicotinic acetylcholine receptor. J Neuroimmune Pharmacol [PMID: 20387124]. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F.A., Soliakov L., Wonnacott S. (2002) Nicotine activates the extracellular signalregulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J Neurochem 80: 520–530 [DOI] [PubMed] [Google Scholar]
- Dawson V.L., Dawson T.M., London E.D., Bredt D.S., Snyder S.H. (1991) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA 88: 6368–6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fusco M., Becchetti A., Patrignani A., Annesi G., Gambardella A., Quattrone A., et al. (2000) The nicotinic receptor β2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet 26: 275–276 [DOI] [PubMed] [Google Scholar]
- del Peso L., Gonzalez-Garcia M., Page C., Herrera R., Nunez G. (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689 [DOI] [PubMed] [Google Scholar]
- De Reuck J., De Weweire M., Van Maele G., Santens P. (2005) Comparison of age of onset and development of motor complications between smokers and non-smokers in Parkinson's disease. J Neurol Sci 231: 35–39 [DOI] [PubMed] [Google Scholar]
- Dineley K.T., Xia X., Bui D., Sweatt J.D., Zheng H. (2002) Accelerated plaque accumulation, associative learning deficits, and up-regulation of α7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J Biol Chem 277: 22768–22780 [DOI] [PubMed] [Google Scholar]
- Dorn H.F. (1959) Tobacco consumption and mortality from cancer and other diseases. Public Health Rep 74: 581–593 [PMC free article] [PubMed] [Google Scholar]
- Du F., Li R., Huang Y., Li X., Le W. (2005) Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. Eur J Neurosci 22: 2422–2430 [DOI] [PubMed] [Google Scholar]
- Dziewczapolski G., Glogowski C.M., Masliah E., Heinemann S.F. (2009) Deletion of the α7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer's disease. J Neurosci 29: 8805–8815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Ichise M., Zoghbi S., Liow J., Ghose S., Vines D., et al. (2006) Widespread decrease of nicotinic acetylcholine receptors in Parkinson's disease. Ann Neurol 59: 174–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P., Hof P.R., Kovari E., Vallet P.G., Herrmann F.R., Bouras C. (1996) Distinct patterns of neuronal loss and Alzheimer's disease lesion distribution in elderly individuals older than 90 years. J Neuropathol Exp Neurol 55: 1210–1220 [DOI] [PubMed] [Google Scholar]
- Gotti C., Fornasari D., Clementi F. (1997) Human neuronal nicotinic receptors. Prog Neurobiol 53: 199–237 [DOI] [PubMed] [Google Scholar]
- Grady S.R., Salminen O., Laverty D.C., Whiteaker P., McIntosh J.M., Collins A.C., et al. (2007) The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol 74: 1235–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z.Z., Nordberg A., Mousavi M., Rinne J.O., Hellström-Lindahl E. (2002) Selective changes in the levels of nicotinic acetylcholine receptor protein and of corresponding mRNA species in the brains of patients with Parkinson's disease. Brain Res 956: 358–366 [DOI] [PubMed] [Google Scholar]
- Hardy J. (2010) Genetic analysis of pathways to Parkinson disease. Neuron 68: 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M.E., Wang Y., Pedigo N.W.J., Hensley K., Butterfield D.A., Carney J.M. (1996) Amyloid β peptide (25-35) inhibits Na+-dependent glutamate uptake in rat hippocampal astrocyte cultures. J Neurochem 67: 277–286 [DOI] [PubMed] [Google Scholar]
- Hartley D.M., Choi D.W. (1989) Delayed rescue of N-methyl-D-aspartate receptor-mediated neuronal injury in cortical culture. J Pharmacol Exp Ther 250: 752–758 [PubMed] [Google Scholar]
- Hernandez C.M., Kayed R., Zheng H., Sweatt J.D., Dineley K.T. (2010) Loss of alpha7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease. J Neurosci 30: 2442–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter B.E., de Fiebre C.M., Papke R.L., Kem W.R., Meyer E.M. (1994) A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci Lett 168: 130–134 [DOI] [PubMed] [Google Scholar]
- Inden M., Kitamura Y., Takeuchi H., Yanagida T., Takata K., Kobayashi Y., et al. (2007) Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J Neurochem 101: 1491–1504 [DOI] [PubMed] [Google Scholar]
- Iravani M., Haddon C., Cooper J., Jenner P., Schapira A. (2006) Pramipexole protects against MPTP toxicity in non-human primates. J Neurochem 96: 1315–1321 [DOI] [PubMed] [Google Scholar]
- Jensen M., Schroder J., Blomberg M., Engvall B., Pantel J., Ida N., et al. (1999) Cerebrospinal fluid Aβ42 is increased early in sporadic Alzheimer's disease and declines with disease progression. Ann Neurol 45: 504–511 [DOI] [PubMed] [Google Scholar]
- Kaneko S., Maeda T., Kume T., Kochiyama H., Akaike A., Shimohama S., et al. (1997) Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via α7-neuronal receptors and neuronal CNS receptors. Brain Res 765: 135–140 [DOI] [PubMed] [Google Scholar]
- Kawamata J., Shimohama S. (2002) Association of novel and established polymorphisms in neuronal nicotinic acetylcholine receptors with sporadic Alzheimer's disease. J Alzheimers Dis 4: 71–76 [DOI] [PubMed] [Google Scholar]
- Kihara T., Sawada H., Nakamizo T., Kanki R., Yamashita H., Maelicke A., et al. (2004) Galantamine modulates nicotinic receptor and blocks Aβ-enhanced glutamate toxicity. Biochem Biophys Res Commun 325: 976–982 [DOI] [PubMed] [Google Scholar]
- Kihara T., Shimohama S., Honda K., Shibasaki H., Akaike A. (2000) Neuroprotective effect of nicotinic agonists via PI3 kinase cascade against glutamate cytotoxicity enhanced by β amyloid. Neurology 54(Suppl. 3): A367 [Google Scholar]
- Kihara T., Shimohama S., Sawada H., Honda K., Nakamizo T., Shibasaki H., et al. (2001) α7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A β-amyloid-induced neurotoxicity. J Biol Chem 276: 13541–13546 [DOI] [PubMed] [Google Scholar]
- Kihara T., Shimohama S., Sawada H., Kimura J., Kume T., Kochiyama H., et al. (1997) Nicotinic receptor stimulation protects neurons against β-amyloid toxicity. Ann Neurol 42: 159–163 [DOI] [PubMed] [Google Scholar]
- Kihara T., Shimohama S., Urushitani M., Sawada H., Kimura J., Kume T., et al. (1998) Stimulation of α4β2 nicotinic acetylcholine receptors inhibits β-amyloid toxicity. Brain Res 792: 331–334 [DOI] [PubMed] [Google Scholar]
- Le Novère N., Zoli M., Changeux J.P. (1996) Neuronal nicotinic receptor a 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci 8: 2428–2439 [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Anand R., Peng X., Gerzanich V., Wang F., Li Y. (1995) Neuronal nicotinic receptor subtypes. Ann NY Acad Sci 757: 1001–1116 [DOI] [PubMed] [Google Scholar]
- Maelicke A., Samochocki M., Jostock R., Fehrenbacher A., Ludwig J., Albuquerque E.X., et al. (2001) Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer's disease. Biol Psychiatry 49: 279–288 [DOI] [PubMed] [Google Scholar]
- Maelicke A., Schrattenholz A., Storch A., Schroder B., Gutbrod O., Methfessel C., et al. (1995) Noncompetitive agonism at nicotinic acetylcholine receptors; functional significance for CNS signal transduction. J Recept Signal Transduct Res 15: 333–353 [DOI] [PubMed] [Google Scholar]
- Mann V., Cooper J., Krige D., Daniel S., Schapira A., Marsden C. (1992) Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson's disease. Brain 115: 333–342 [DOI] [PubMed] [Google Scholar]
- Maragos W.F., Greenamyre J.T., Penney J.B., Young A.B. (1986) Glutamate dysfunction in Alzheimer's disease: An hypothesis. Trends Neurosci 10: 65–68 [Google Scholar]
- Matsuzaki H., Tamatani M., Mitsuda N., Namikawa K., Kiyama H., Miyake S., et al. (1999) Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem 73: 2037–2046 [PubMed] [Google Scholar]
- Mattson M.P. (1988) Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res Rev 13: 179–212 [DOI] [PubMed] [Google Scholar]
- Mattson M.P. (1989) Acetylcholine potentiates glutamate-induced neurodegeneration in cultured hippocampal neurons. Brain Res 497: 402–406 [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. (1990) Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci 11: 379–387 [DOI] [PubMed] [Google Scholar]
- Mesulam M.M., Mufson E.J., Levey A.I., Wainer B.H. (1983) Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata) and hypothalamus in the rhesus monkey. J Comp Neurol 214: 170–197 [DOI] [PubMed] [Google Scholar]
- Meyer E.M., Tay E.T., Papke R.L., Meyers C., Huang G.L., de Fiebre C.M. (1997) 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat a7 receptors and improves memory-related behaviors in a mecamylaminesensitive manner. Brain Res 768: 49–56 [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Yoshino H., Ikebe S., Hattori N., Kobayashi T., Shimoda-Matsubayashi S., et al. (1998) Mitochondrial dysfunction in Parkinson's disease. Ann Neurol 44(3 Suppl. 1): S99–S109 [DOI] [PubMed] [Google Scholar]
- Morens D., Grandinetti A., Reed D., White L., Ross G. (1995) Cigarette smoking and protection from Parkinson's disease: False association or etiologic clue? Neurology 45: 1041–1051 [DOI] [PubMed] [Google Scholar]
- Nakamizo T., Kawamata J., Yamashita H., Kanki R., Kihara T., Sawada H., et al. (2005) Stimulation of nicotinic acetylcholine receptors protects motor neurons. Biochem Biophys Res Commun 330: 1285–1289 [DOI] [PubMed] [Google Scholar]
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., et al. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314: 130–133 [DOI] [PubMed] [Google Scholar]
- Obeso J.A., Rodriguez-Oroz M.C., Goetz C.G., Marin C., Kordower J.H., Rodriguez M., et al. (2010) Missing pieces in the Parkinson's disease puzzle. Nat Med 16: 653–661 [DOI] [PubMed] [Google Scholar]
- Olney J.W., Labruyere J., Wang G., Wozniak D.F., Price M.T., Sesma M.A. (1991) NMDA antagonist neurotoxicity: Mechanism and prevention. Science 254: 1515–1518 [DOI] [PubMed] [Google Scholar]
- Parain K., Hapdey C., Rousselet E., Marchand V., Dumery B., Hirsch E.C. (2003) Cigarette smoke and nicotine protect dopaminergic neurons against the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Parkinsonian toxin. Brain Res 984: 224–232 [DOI] [PubMed] [Google Scholar]
- Parker W., Jr, Boyson S., Parks J. (1989) Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol 26: 719–723 [DOI] [PubMed] [Google Scholar]
- Quik M. (2004) Smoking, nicotine and Parkinson's disease. Trends Neurosci 27: 561–568 [DOI] [PubMed] [Google Scholar]
- Quik M., Chen L., Parameswaran N., Xie X., Langston J., McCallum S. (2006) Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates. J Neurosci 26: 4681–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenstijn P.G., Merlini M., Hameetman M., Murray T.K., Ward M.A., Lewis H., et al. (2008) The exploration of rotenone as a toxin for inducing Parkinson's disease in rats, for application in BBB transport and PK-PD experiments. J Pharmacol Toxicol Methods 57: 114–130 [DOI] [PubMed] [Google Scholar]
- Rezvani A.H., Kholdebarin E., Brucato F.H., Callahan P.M., Lowe D.A., Levin E.D. (2009) Effect of R3487/MEM3454, a novel nicotinic α7 receptor partial agonist and 5-HT3 antagonist on sustained attention in rats. Prog Neuropsychopharmacol Biol Psychiatry 33: 269–275 [DOI] [PubMed] [Google Scholar]
- Rosser M.N., Svendsen C., Hunt S.P., Mounjoy C.Q., Roth M., Iversen L.L. (1982) The substantia innominata in Alzheimer's disease: A histochemical and biochemical study of cholinergic marker enzymes. Neurosci Lett 28: 217–222 [DOI] [PubMed] [Google Scholar]
- Rothstein J.D. (2009) Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol 65(Suppl. 1): S3–S9 [DOI] [PubMed] [Google Scholar]
- Schmidt W.J., Alam M. (2006) Controversies on new animal models of Parkinson's disease pro and con: The rotenone model of Parkinson's disease (PD). J Neural Transm Suppl 70: 273–276 [PubMed] [Google Scholar]
- Shen H., Kihara T., Hongo H., Wu X., Kem W.R., Shimohama S., et al. (2010) Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of a7 nicotinic receptors and internalization of NMDA receptors. Br J Pharmacol 161: 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohama S., Akaike A., Kimura J. (1996) Nicotine-induced protection against glutamate cytotoxicity. Nicotinic cholinergic receptor-mediated inhibition of nitric oxide formation. Ann NY Acad Sci 777: 356–361 [DOI] [PubMed] [Google Scholar]
- Shimohama S., Greenwald D.L., Shafron D.H., Akaike A., Maeda T., Kaneko S., et al. (1998) Nicotinic α7 receptors protect against glutamate neurotoxicity and neuronal ischemic damage. Brain Res 779: 359–363 [DOI] [PubMed] [Google Scholar]
- Shimohama S., Sawada H., Kitamura Y., Taniguchi T. (2003) Disease model: Parkinson's disease. Trends Mol Med 9: 360–365 [DOI] [PubMed] [Google Scholar]
- Shimohama S., Taniguchi T., Fujiwara M., Kameyama M. (1986) Changes in nicotinic and muscarinic cholinergic receptors in Alzheimer-type dementia. J Neurochem 46: 288–293 [DOI] [PubMed] [Google Scholar]
- Steinlein O.K., Mulley J.C., Propping P., Wallace R.H., Phillips H.A., Sutherland G.R., et al. (1995) A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 11: 201–203 [DOI] [PubMed] [Google Scholar]
- Takeuchi H., Yanagida T., Inden M., Takata K., Kitamura Y., Yamakawa K., et al. (2009) Nicotinic receptor stimulation protects nigral dopaminergic neurons in rotenone-induced Parkinson's disease models. J Neurosci Res 87: 576–585 [DOI] [PubMed] [Google Scholar]
- Tomita T., Maruyama K., Saido T.C., Kume H., Shinozaki K., Tokuhiro S., et al. (1997) The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid β protein ending at the 42nd (or 43rd) residue. Proc Natl Acad Sci USA 94: 2025–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushitani M., Shimohama S., Kihara T., Sawada H., Akaike A., Ibi M., et al. (1998) Mechanism of selective motor neuronal death after exposure of spinal cord to glutamate: Involvement of glutamate-induced nitric oxide in motor neuron toxicity and nonmotor neuron protection. Ann Neurol 44: 796–807 [DOI] [PubMed] [Google Scholar]
- Villafane G., Cesaro P., Rialland A., Baloul S., Azimi S., Bourdet C., et al. (2007) Chronic high dose transdermal nicotine in Parkinson's disease: An open trial. Eur J Neurol 12: 1313–1316 [DOI] [PubMed] [Google Scholar]
- Wagey R., Pelech S.L., Duronio V., Krieger C. (1998) Phosphatidylinositol 3-kinase: Increased activity and protein level in amyotrophic lateral sclerosis. J Neurochem 71: 716–722 [DOI] [PubMed] [Google Scholar]
- Wang H.Y., Lee D.H., D'Andrea M.R., Peterson P.A., Shank R.P., Reitz A.B. (2000) β-Amyloid(1-42) binds to α7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem 275: 5626–5632 [DOI] [PubMed] [Google Scholar]
- Wang H.Y., Li W., Benedetti N.J., Lee D.H. (2003) α7 nicotinic acetylcholine receptors mediate β-amyloid peptide-induced tau protein phosphorylation. J Biol Chem 278: 31547–31553 [DOI] [PubMed] [Google Scholar]
- Wang H.Y., Stucky A., Liu J., Shen C., Trocme-Thibierge C., Morain P. (2009) Dissociating β-amyloid from α7 nicotinic acetylcholine receptor by a novel therapeutic agent, S 24795, normalizes α7 nicotinic acetylcholine and NMDA receptor function in Alzheimer's disease brain. J Neurosci 29: 10961–10973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warita H., Manabe Y., Murakami T., Shiro Y., Nagano I., Abe K. (2001) Early decrease of survival signal-related proteins in spinal motor neurons of presymptomatic transgenic mice with a mutant SOD1 gene. Apoptosis 6: 345–352 [DOI] [PubMed] [Google Scholar]
- Whitehouse P.J., Kalaria R.N. (1995) Nicotinic receptors and neurodegenerative dementing diseases: Basic research and clinical implications. Alzheimer Dis Assoc Disord 9: 3–5 [DOI] [PubMed] [Google Scholar]
- Whitehouse P.J., Price D.L., Clark A.W., Coyle T.T., Delong M. (1981) Alzheimer's disease: Evidence for a selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol 10: 122–126 [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K., Gatz M., Pawitan Y., Pedersen N. (2005) Risk and protective factors for Parkinson's disease: A study in Swedish twins. Ann Neurol 57: 27–33 [DOI] [PubMed] [Google Scholar]
- Xie Y., Bezard E., Zhao B. (2005) Investigating the receptor-independent neuroprotective mechanisms of nicotine in mitochondria. J Biol Chem 280: 32405–32412 [DOI] [PubMed] [Google Scholar]
- Yanagida T., Takeuchi H., Kitamura Y., Takata K., Minamino H., Shibaike T., et al. (2008) Synergistic effect of galantamine on nicotine-induced neuroprotection in hemiparkinsonian rat model. Neurosci Res 62: 254–261 [DOI] [PubMed] [Google Scholar]
- Yankner B.A., Duffy L.K., Kirschner D.A. (1990) Neurotrophic and neurotoxic effects of amyloid β protein: Reversal by tachykinin neuropeptides. Science 250: 279–282 [DOI] [PubMed] [Google Scholar]
- Zhong L.T., Kane D.J., Bredesen D.E. (1993) BCL-2 blocks glutamate toxicity in neural cell lines. Mol Brain Res 19: 353–355 [DOI] [PubMed] [Google Scholar]