Abstract
While lithium is generally regarded as the first-line agent for patients with bipolar disorder, it does not work for everyone, which raises the question: can we predict who will be most likely to respond? In this paper, we review the most compelling clinical, biologic, and genetic predictors of lithium response in bipolar disorder. Among clinical factors, the strongest predictors of good response are fewer hospitalizations preceding treatment, an episodic course characterized by an illness pattern of mania followed by depression, and a later age at onset of bipolar disorder. While several biologic predictors have been studied, the results are preliminary and require replication with studies of larger patient samples over longer observation periods. Neuroimaging is a particularly promising method given that it might concurrently illuminate pathophysiologic underpinnings of bipolar disorder, the mechanism of action of lithium, and potential predictors of lithium response. The first genome-wide association study of lithium response was recently completed. No definitive results emerged, perhaps because the study was underpowered. With major new initiatives in progress aiming to identify genes and genetic variations associated with lithium response, there is much reason to be hopeful that clinically useful information might be generated within the next several years. This could ultimately translate into tests that could guide the choice of mood-stabilizing medication for patients. In addition, it might facilitate pharmacologic research aimed at developing newer, more effective medications that might act more quickly and yield fewer side effects.
Keywords: bipolar disorder, genetics, lithium, neuroimaging, predicting response
Bipolar disorder
Bipolar disorder (BP), formerly called manic-depressive illness, is a mood disorder characterized by two poles of mood, mania and depression. With a lifetime prevalence of 1.3-3.1% of the population [Merikangas et al. 2007], BP places those affected at risk for episodes at the two poles, but also for hypo-manic and mixed mood episodes. There are two types of BP, BP I and BP II, based on current diagnostic criteria as described in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV). Mania serves as the clinical feature that distinguishes not only BP from major depression (sometimes called unipolar depression), but also BP I from BP II. That is, the presence of at least one lifetime episode of mania warrants a diagnosis of BP I. In BP II, patients have hypomania, a milder form of mania. While patients typically experience depressive episodes in both forms of BP, a lifetime history of clinical depression is not required for the diagnosis of BP I. The goal of this section is to review the clinical features of BP.
Mania is characterized by elated to irritable mood, increased activity, inflated sense of self worth, racing thoughts, diminished sleep requirement, distractibility, and pressured speech. Psychotic symptoms, such as grandiose delusions or auditory hallucinations, are not uncommon in BP, particularly in the manic phase [Potash et al. 2001]. Another sign of mania is impaired judgment, which may lead to uncharacteristic behaviors such as spending sprees or promiscuous sexual activity. By contrast, hypomanic episodes are milder versions of manic ones, with no psychotic symptoms, and less impairment, or sometimes none at all. Further, mood symptoms must persist for at least 4 days in hypomania, as opposed to a full week in mania.
During the depressive phase of BP, patients typically develop a low, sad, or apathetic mood, physical exhaustion, changes in appetite, impaired concentration, decreased hedonic capacity, and insomnia. Another cardinal symptom of clinical depression is the presence of a diminished sense of self worth or feelings of guilt. Hopelessness frequently ensues, which may lead to thoughts of death or suicide. In a depressive episode, symptoms are present for at least 2 weeks and lead to significant impairment in functioning.
Mixed states arise when manic and depressive symptoms are present at the same time. To meet current diagnostic criteria for a mixed episode, patients must demonstrate full criteria for mania and major depression for at least 1 week. While few patients meet this very high diagnostic threshold, many experts believe that clinically important mixed states not meeting these criteria occur fairly commonly.
BP is associated with many costs, the most alarming of these being the drastically elevated suicide rate seen in the illness, with the risk being around 20-fold greater in those with BP compared with the general population [Osby et al. 2001]. Further, the problems with cognition, motivation, and impulse control often lead to significant impairment in the workplace, as well as at home and in interpersonal relationships. According to the World Health Organization, BP is the sixth leading cause of disability among all diseases worldwide [Lopez and Murray, 1998].
Given the significant morbidity and mortality associated with BP, timely diagnosis and initiation of appropriate medication therapy is highly desirable. Although no cure is currently available, BP can be managed effectively using a variety of pharmacologic and psychotherapeutic treatments. The mainstay of pharmacologic treatment for BP is the class of medications known as mood stabilizers. These are agents that have been shown to be beneficial in the treatment of acute episodes and in the prevention of recurrence of bipolar illness. There are four mood stabilizers for which substantial evidence of efficacy exists: lithium, valproate, carbamazepine, and lamotrigine, with lithium being the one for which the body of supporting data is most substantial. Neuroleptic agents, such as olanzapine, also have mood-stabilizing qualities. In addition to its use as a mood stabilizer, lithium has been shown to be an effective augmenting agent in the treatment of unipolar major depression [Crossley and Bauer, 2007].
Lithium is generally regarded as the first-line agent for the management of BP, although it does not work for everyone. For instance, Geddes and colleagues performed a meta-analysis of five randomized controlled trials comparing prophylactic lithium therapy with placebo in BP and found that lithium is more effective than placebo in preventing recurrence of illness, with 60% in the lithium group remaining well over 1–2 years compared with 40% in the placebo group [Geddes et al. 2004]. A meta-analysis of six studies of lithium in the treatment of acute mania found that 47% of patients responded compared with 32% of controls [Yildiz et al. 2011].
The results cited above raise the question: can we predict who will be most likely to respond? This type of question is one that is being asked throughout psychiatry and other clinical fields in the context of the quest for personalized medicine. One can ask the question with a focus on clinical and demographic factors, though in recent years, such research has been centered on patient-specific molecular and genetic factors that might predict which treatment(s) are expected to provide the best clinical outcomes for a given patient with a particular illness [de Leon, 2009]. After a brief review of the history of lithium as a treatment for BP, we will review the most compelling clinical, biologic, and genetic predictors of lithium response in BP with an overarching aim of advancing personalized medicine within the field of psychiatry. Table 1 provides an overview of the results of our review of these potential predictors.
Table 1.
Level of support for the clinical, biologic, and genetic predictors of lithium response in bipolar disorder.
| Variables | Level of support |
|---|---|
| Clinical variables | |
| Course of illness | +++ |
| Family history of BP | ++ |
| Family history of Li response | ++ |
| Age of BP onset | +++ |
| Number of BP hospitalizations | +++ |
| Classic BP symptomatology | + |
| Biologic variables | |
| Normal EEG | + |
| ERPs | + |
| Brain imaging | + |
| Genetic variables | |
| Single-gene association studies | + |
| Genome-wide association study | ++ |
BP, bipolar disorder; EEG, electroencephalography; ERP, event-related potential; Li, lithium; +, weak evidence; ++, moderate evidence; +++, strong evidence.
The history of lithium use in bipolar disorder
Lithium has been used for the treatment of medical and psychiatric conditions such as gout, seizures, mania, and ‘general nervousness’ since the mid 1800s. However, its modern introduction into psychiatry began in 1949, when Australian psychiatrist John Cade successfully used the drug in the treatment of 10 patients with mania. Several of these patients were able to be discharged from a chronic mental hospital despite years of psychiatric instability [Cade, 1949].
These findings spurred research resulting in a plethora of papers about various biologic aspects of lithium [Schou, 1999]. In 1954, Schou and colleagues performed the first randomized controlled trial of lithium in which he showed the treatment was effective and an alternative to electroconvulsive therapy (ECT) in the treatment of mania [Schou et al. 1954]. In the ensuing decade, a number of publications supported Schou and colleagues' findings that lithium is a successful antimanic agent.
It was not until 1967 that Baastrup and Schou systematically demonstrated that lithium confers protection against the recurrence of illness using a nonblinded mirror design in 88 women [Baastrup and Schou, 1967]. In this study, the authors demonstrated that lithium not only reduced the frequency of relapses, but also shortened the length of relapses that occurred on the medication. Baastrup and colleagues followed up with a double-blind discontinuation study of the prophylactic qualities of lithium [Baastrup et al. 1970]. Among the 45 patients treated with lithium, none had recurrences of their mood illness, while 21 of 39 patients treated with placebo experienced a relapse. Subsequently, the efficacy of lithium as a long-term prophylactic agent for the treatment of BP was corroborated by a series of double-blind trials conducted in the 1970s and 1980s [Grof and MullerOerlinghausen, 2009].
Despite its well established antimanic and prophylactic qualities, lithium became the focus of close scrutiny in the 1990s. For example, there has been concern that the prophylactic efficacy of lithium dissipates over time. In their review, Grof and Muller-Oerlinghausen offered a series of compelling arguments in favor of the mood-stabilizing properties of lithium [Grof and Muller-Oerlinghausen, 2009]. The authors asserted that the efficacy of lithium was rigorously proven using a variety of study designs including randomized, double-blind; double-blind discontinuation; and double-blind crossover trials. Further, they argued, these older studies were not influenced by the pharmaceutical industry, a powerful force, more recently, in promoting newer mood-stabilizing medications.
Predictors of lithium response: Clinical epidemiology
Since the late 1960s, a vast number of studies have investigated various clinical factors that might predict lithium response in patients with BP. In their 2005 review paper, Kleindienst and colleagues identified nearly 2000 articles published from 1966 to 2003 on this topic [Kleindienst et al. 2005]. In Table 2 we have summarized some of the most important of these papers. Although dozens of clinical features have been examined as potential predictors, the results of these analyses have not always produced consistent results. Among these factors, three have been shown most consistently to predict a positive response: fewer hospitalizations preceding treatment; an episodic course characterized by an illness pattern of mania, followed by depression, and then euthymia; and a later age at onset of BP. In Figure 1 we show a re-analysis of the Kleindienst meta-analysis of the strongest clinical predictors, including several more recent papers on age at onset not included in their study.
Table 2.
Selected studies of clinical predictors of lithium response.
| Authors | Year | N | Phase and duration of treatment | Li level | Design | Clinical variable(s) | Outcome evaluation | Findings |
|---|---|---|---|---|---|---|---|---|
| Dunner and Fieve | 1974 | 55 | Prophylaxis ≥6 months | 0.7–1.2 mEq/liter | Case–control | Age of illness onset | Recurrence of mood episodes; poor response defined as patient hospitalized, required tx, or sx present for ≥2 weeks | Age of illness onset not related to Li response (p value NA) |
| McKnew et al. | 1981 | 6 | Acute tx, 16–18 weeks in duration | 0.8–1.2 mEq/liter | Double-blind, crossover | Family history of Li responsiveness | Improvement in CPRS, CARS, Conner's TRS, and global rating of child's status | 2 children with BP, mixed, who had Li responsive relatives were deemed responsive to Li (p <0.05 for first child's change in global rating; p-value NA for second child.) |
| Sarantidis and Waters | 1981 | 46 | Prophylaxis ≥2 years | NA | Case–control | Age of illness onset | Reduction in time spent in hospital under Li tx; excellent response defined as no hospitalizations during Li tx | Age of illness onset not related to Li response (p value NA) |
| Yang | 1985 | 101 | Prophylaxis >2 years | Mean level 0.73 mEq/liter | Case–control | Age of illness onset | Reduction of episode frequency and/or admissions; patients with good response had no episodes under Li tx | Age of illness onset not related to Li response (p value NA) |
| Maj et al. | 1986 | 69 | Prophylaxis, 2 years in duration | 0.30–0.50 mEq/liter (sub-group analysis) | Cohort study | Age of illness onset | Recurrence of mood episodes on Li; patients who responded to Li had no relapses of mood disorder under Li tx | Age of illness onset not related to Li response (p value NA) |
| Okuma | 1993 | 215 | Prophylaxis ≥2 years | NA | Case–control | Age of illness onset | Time ill under Li tx; markedly effective response was defined as complete suppression of mood episodes | Age of illness onset not related to Li response (p value NA) |
| Grof et al. | 1994 | 1024 | Prophylaxis, 3–20 years in duration | ≥0.7 mEq/liter | Cohort study | Family history of BP | Recurrence under Li; patients who responded to Li had no further mood episodes during Li tx | BP more common in first-degree relatives of patients who responded to Li compared with those who did not respond, (p = 0.005) |
| Kusalic and Engelsmann | 1998 | 29 | Prophylaxis, 2 years in duration | 0.8–1.3 mEq/liter | Prospective | Quality of BP symptomatology | Recurrence of mood episodes and presence of interepisodic sx during Li tx | Classic sx of mania present in 85% of patients who responded to Li compared with those who did not respond (p < 0.028] |
| Yazici et al. | 1999 | 141 | Prophylaxis ≥3 years | NA | Case–control | Age of illness onset | AMI during Li tx: good response group had AMI < 0.2 | Greater age of disease onset associated with better Li response (p = 0.02) |
| Coryell et al. | 2000 | 186 | Prophylaxis ≥26 weeks | NA | Cohort study | Age of illness onset | Change in morbidity: low morbidity on Li considered marker for Li response | Greater age of disease onset associated with better Li response (p = 0.0023] |
| Kato et al. | 2000 | 32 | Prophylaxis >1 year | 0.3–1.0 mEq/liter | Case–control | Age of illness onset | Recurrence under Li; patients who responded had no mood episodes under Li tx | Age of illness onset not related to Li response (p value NA) |
| Schuroff et al. | 2000 | 97 | Prophylaxis ≥1 year | NA | Not described | Age of illness onset | Recurrence under Li; response defined as relapse once Li stopped after ≥1 year of tx or no relapse of illness after 3 years of tx | Later disease onset (>40 years) associated with better Li response compared with younger disease onset (<18 years) (p = 0.04) |
| Tondo et al. | 2001 | 360 | Prophylaxis ≥1 year | Mean level 0.615 mEq/liter | Cohort study | Age of illness onset | Response defined as less time ill during Li tx | Greater age of disease onset associated with better Li response (p = 0.005] |
| Grof et al. | 2002 | 170 | Prophylaxis ≥1 year | NA | Case–control | Family history of Li responsiveness | Alda tx response scale; score ≥7 indicates full tx response | 67% of BP relatives of patients who responded to Li responded themselves compared with 35% in the control group (p = 0.014) |
| Grof | 2003 | 868 | Prophylaxis ≥3 years | NA | Cross-sectional assessment of first-degree relatives | Family history of BP | Alda tx response scale; score ≥7 indicates full tx response | Patients who responded to Li had 13 first-degree relatives with BP compared with 4 and 6 relatives with BP of patients who responded to lamotrigine and olanzapine respectively (p value NA, but difference statistically significant] |
| Passmore et al. | 2003 | 164 | Prophylaxis, duration NA | NA | Family study | Pretx episodic illness course Family history of BP | Alda tx response scale; response defined as full stability during tx period with no episodes of abnormal mood | Episodic course observed in 86% of patients who showed full response compared with 14% who showed full response with minor, residual pretx sx (p < 0.001); among patients who responded to Li, 16.6% of first-degree relatives had BP compared with 2.5% of such relatives of patients who responded to lamotrigine (p = 0.05] |
| Tondo et al. | 2003 | 1856 | Prophylaxis ≥4 months | NA | Review paper with metaanalysis | History of rapid-cycling BP | Rates of tx failure based on recurrence and nonimprovement under tx | Li was less effective for rapid cycling BP, but so were the anticonvulsants. No clear difference for any specific medication emerged. |
| Kleindienst et al. | 2005 | 1138 677 400 1151 904 | Prophylaxis | NA | Review paper with metaanalysis | Age of illness onset; no. of previous hospitalizations; CC illness course; DMI illness course; MDI illness course | Variable, as per original publications | High age of onset correlated with Li response: (r = 0.11, p = 0.003); high number of previous hospitalizations correlated with poor Li response (r = −0.35, p <0.001); CC course negatively correlated with Li response (r = −0.21, p<0.001); DMI course negatively correlated with Li response (r = −0.12, p = 0.02); MDI course positively correlated with Li response (r = 0.20, p <0.001) |
| Duffy et al | 2007 | 15 | Prophylaxis ≥1 year | ≥0.7 mEq/liter | Case–control | Family history of Li responsiveness | Alda tx response scale; score ≥7 indicates full tx response | All patients who responded to Li (n = 9) had Li-responsive parents (n = 10) (p = 0.001) |
| Garnham et al. | 2007 | 120 | Prophylaxis ≥6 months | NA | Chart review | Age of illness onset Pretx episodic illness course | Alda tx response scale; score ≥7 indicates full tx response | Younger age of disease onset associated with better Li response (p = 0.03); episodic course observed in 44% of patients who had full response to Li compared with 15% in those with nonepisodic course (p = 0.004) |
| Rybakowski et al. | 2007 | 111 | Prophylaxis ≥5 years | NA | Case–control | Age of illness onset | Reduction in episode index; excellent response in patients with no mood episodes under tx | Age of illness onset not related to Li response (p value NA) |
| Berghofer et al. | 2008 | 242 | Prophylaxis, average duration of tx 10 ± 6.4 years | ≥0.5 mEq/liter | Cohort study | Quality of BP symptomatology | Total MI, depressive MI, and manic MI | Of the 242 patients studied, 142 had more typical sxs of BP than atypical sxs. There was no significant difference between the total MI (p = 0.17), depressive MI (p = 0.198), and manic MI (p = 0.47) based on the quality of BP symptomatology |
| Masui et al. | 2008 | 161 | Prophylaxis ≥1 year | 0.4–1.2 mEq/liter | Case–control | Age of illness onset | Recurrence under Li; patients who responded had no mood episodes under Li tx | Greater age of disease onset associated with better Li response (p<0.01) |
| Backlund et al. | 2009 | 100 | Prophylaxis, average duration of tx 9.7 ± 5.8 years | 0.5–0.9 mEq/liter | Retrospective, life chart method | Quality of BP symptomatology | Four measures: difference in no. mood episodes/year before/after start of Li; difference in annual burden of illness before/after start; total remission for >3 years after start; burden of illness after start | Absence of mixed episodes prior to Li (RR 3.5) and absence of rapid cycling prior to Li (RR 7.3) predicted good response defined as absence of mood episodes during first 3 years of Li initiation; absence of mixed episodes prior to Li (RR 2.8) and absence of rapid cycling prior to Li (RR 9.6) predicted good Li response defined as low disease burden after start of Li tx |
AMI, Affective Morbidity Index; BP, bipolar disorder; CARS, Children's Affective Rating Scale; CC, continuous cycling; CPRS, Children's Psychiatric Rating Scale; DMI, depression-mania-free interval; Li, lithium; MI, morbidity index; MDI, mania-depression-free interval; NA, not available or not applicable; no., number; pretx, pretreatment; RR, relative risk; sx, symptoms; TRS, Teacher's Rating Scale; tx, treatment.
Figure 1.
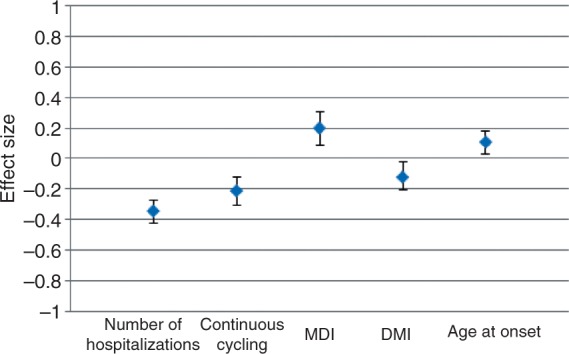
Correlation between clinical predictors and response to lithium treatment. Shown is the meta-analytic effect size (r) for the clinical predictors by descending order of strength. A positive r value indicates a positive correlation between the clinical predictor and a positive response to lithium. A negative r value indicates a negative correlation between the clinical predictor and a positive response to lithium. Meta-analysis performed under a random effects model as described by Kleindienst and colleagues [Kleindienst et al. 2005]. The result for age at onset has been updated to include three additional papers examining this relationship. DMI, depression-mania-free interval; MDI, mania-depression-free interval.
The clinical course of illness prior to initiation of lithium has been shown to be an important predictor of response. For instance, an episodic course of BP has generally been positively correlated with response. Garnham and colleagues performed a retrospective chart review of 120 patients with BP who were treated in an outpatient setting [Garnham et al. 2007]. Among the 78 lithium treatment trials considered, a full response to lithium was observed 30% of the time, whereas the rate of partial improvement was 58%. Of the patients who achieved a full response to lithium, 44% were found to have an episodic course of illness prior to initiating treatment compared with 15% in those with a nonepisodic course. In a retrospective study of 14 patients who had full stability in their mood while taking lithium, it was noted that nearly 90% of these patients had an illness course characterized by full remission between mood episodes [Passmore et al. 2003].
Furthermore, the pattern of mood episodes has also been shown to predict lithium response. Specifically, a pattern of mania (M), depression (D), and euthymic interval (I) has been shown to positively predict lithium response. In their review paper, Kleindienst and colleagues offered an impressive analysis of 42 potential clinical predictors of lithium response, and included in their analysis was a comparison of the MDI and DMI patterns of BP [Kleindienst et al. 2005]. The authors concluded based on aggregated data that the MDI pattern is a positive predictor of response, while the DMI pattern was negatively correlated to lithium response.
A family history of BP has been shown to be another favorable predictor of response to lithium, based on studies extending back to the 1970s [Grof et al. 1994]. In his review paper, Grof and colleagues cite a study in which family history was obtained on 756 first-degree relatives of 112 patients who demonstrated an unequivocal response to lithium, lamotrigine, or olanzapine [Grof et al. 1994]. In the patients who responded to lithium, there was a higher rate of BP in their relatives compared with the relatives of patients who responded to lamotrigine and olanzapine. Passmore and colleagues found a higher prevalence of BP in 103 first-degree relatives of 14 cases with an unequivocal lithium response compared with those who responded to lamotrigine [Passmore et al. 2003]. Another study examined 903 first-degree relatives and spouses of 121 patients with BP, major depression, or schizoaffective disorder [Grof et al. 1994]. The results of the study likewise demonstrate a greater frequency of BP in the family members of patients who responded to lithium.
There is also some evidence that patients with a family history of response to lithium are more likely to respond as well. The first study to report on familial correlation of lithium response involved just six children of parents who responded to lithium. The two children in the study who had BP both had clear responses to lithium [McKnew et al. 1981]. In the only sizable study of familiality of lithium response, 24 BP relatives of patients who responded to lithium were assessed, along with 40 lithium-treated patients from an outpatient clinic. The prevalence of unequivocal response among the relatives was 67%, as compared with a response rate of 35% in the comparison clinic group [Grof et al. 2002]. More recently, Duffy, and colleagues examined 15 adolescents who met criteria for bipolar spectrum disorder and had a parent with BP. Nine of the adolescents were observed to have a positive response to lithium treatment; this observation was associated with lithium responsiveness in the parents [Duffy et al. 2007].
Historically, the presence of classic symptomatology of BP, such as euphoric mania, has been considered to prognosticate a good response to prophylactic lithium. In a prospective trial of 29 patients over the course of 2 years, classic manias consisting of euphoria and flight of ideas were associated with lithium response [Kusalic and Engelsmann, 1998]. Furthermore, patients with atypical features of BP, such as rapid cycling and mixed states, have been thought to poorly respond to lithium treatment. In support of this viewpoint are the findings of a retrospective analysis of 100 patients with BP that employed the semi-structured life-charting protocol [Backlund et al. 2009]. The authors demonstrated that the absence of rapid cycling and mixed episodes prior to the initiation of treatment predict a good response to lithium.
However, some more recent work has questioned whether atypical features of BP truly predict poorer outcomes with lithium or poorer outcomes than for other medications. One study followed 242 patients prospectively for a mean period of 10 years [Berghofer et al. 2008]. The authors surveyed the patients for atypical features of BP such as mood-incongruent psychosis and the presence of residual, subsyndromal symptoms between mood episodes. Among the study participants, 142 demonstrated more typical features of BP than atypical, while 100 displayed more atypical features than typical or had an equal number of typical and atypical features. Despite these differences in clinical presentation, both groups had similar responses to prophylactic lithium treatment as measured by the mean morbidity index. Another study performed a meta-analysis of 16 studies and 1856 patients demonstrated that those with rapid-cycling BP were less responsive to lithium, but also to anticonvulsant mood stabilizers, compared with patients with nonrapid-cycling illness [Tondo et al. 2003]. Importantly, this analysis found no clear evidence to suggest that the anticonvulsants confer better treatment outcomes than lithium in patients with rapid-cycling BP.
The relative frequency of different mood episodes prior to the initiation of treatment with a mood stabilizer has also been investigated. It has been argued that a predominance of depressive episodes over manic episodes prior to the initiation of lithium is associated with better lithium responsiveness. Evidence for this viewpoint includes the findings from a retrospective study of 141 patients with BP: a greater ratio of pre-treatment manic to depressive episodes predicted a worse response to lithium [Yazici et al. 1999]. However, Kleindienst and colleagues aggregated data from two studies consisting of 147 patients and concluded that neither the number of previous manic episodes nor depressive episodes was associated with lithium response [Kleindienst et al. 2005].
Predictors of lithium response: Biologic studies
Numerous biologic variables, ranging from cerebrospinal fluid levels of central nervous system metabolites to changes in the potential difference across rectal mucosa, have been studied as potential biologic predictors of lithium response. While the hope has been that a simple laboratory test might predict lithium response, decades of research on this topic have yet to identify a biologic measure of unequivocal clinical utility. However, the identification of biologic predictors of lithium response remains an important step in the pursuit of personalized medicine for the treatment of BP. The goal of this section is to review promising biologic markers of response based on key neurophysiologic and neuroimaging findings.
In the realm of neurophysiology, electroencephalography (EEG) is attractive because it is a non-invasive test of brain function. It seems reasonable to hypothesize that lithium response might be associated with EEG findings given that several other mood stabilizers are antiepileptic agents. However, only a few studies have examined the relationship between EEG and lithium response. In one such study, the EEG recordings of 27 patients taking lithium for BP were examined [Ikeda et al. 2002]. None of the five patients who responded to lithium were found to have abnormal EEGs, while five of the 22 patients who did not respond to lithium showed an abnormality. Reeves and colleagues examined relative EEG differences in 20 patients who responded to lithium and 20 patients who responded to valproate who presented for treatment during a manic episode [Reeves et al. 2001]. Seventy percent of the patients who responded to valproate were found to have EEG abnormalities, while only 30% of those who responded to lithium had abnormal EEGs. Thus, the authors concluded that patients with EEG abnormalities were more likely to respond to valproate than lithium. In another series of papers, patients who did not respond to treatment were found to have increased generalized theta activity at baseline [Small et al. 1999, 1998]. However, the group who did not respond to treatment consisted of patients who received five different psychotropic regimens, such as lithium monotherapy or combination therapy with lithium and haloperidol. Thus, this EEG finding was not specific to lithium treatment.
Event-related potentials (ERPs) have also been examined as a possible predictor of lithium response. ERPs, which are measured by EEG, assess changes in brain activity following the application of sensory stimuli. Among the initial evidence for this predictor was a pilot study that demonstrated greater intensity dependence of the N1/P2 component of auditory evoked potentials in patients who responded to prophylactic lithium therapy [Hegerl et al. 1987]. This group subsequently confirmed these results in a replication study of 34 patients with affective illness [Hegerl et al. 1992]. In another study, stronger loudness dependency of auditory-evoked potentials (LDAEPs) of the primary auditory cortex was associated with a positive response to prophylactic lithium in a study of 30 patients with major depression and BP [Juckel et al. 2004]. Further, the authors studied lithium response among patients with BP and similarly found greater LDAEPs of the primary auditory cortex in patients who responded compared with those who did not respond. These studies suggest that ERPs may serve as a clinically useful predictor of lithium response, although several caveats make it hard to draw firm conclusions: the studies were small; they measured different aspects of ERPs; and they included patients with both unipolar depression and bipolar disorder.
Neuroimaging is another promising tool for in vivo investigation of lithium response in BP. In addition to its noninvasive methodology, brain imaging confers other advantages. For example, certain imaging techniques, such as magnetic resonance spectroscopy (MRS), can be repeated serially because the scans do not subject the patient to ionizing radiation. Further, neuroimaging permits the study of brain mechanisms underlying mood disorders, as well as the therapeutic action of lithium. Thus, it is anticipated that brain imaging will facilitate the identification and investigation of rational biologic predictors of lithium response. Table 3 summarizes some of the most promising results to date in this area.
Table 3.
Neuroimaging studies of the relationship between structural changes and lithium treatment and response.
| Authors | Year | N | Design | Li treatment | Li level | Independent variable | Outcome variable | Findings |
|---|---|---|---|---|---|---|---|---|
| Moore et al. | 2000 | 10 | Prospective | 4 weeks | ∼0.8 mEq/liter | Li tx | Gray matter volume | ∼3% mean increase in total gray matter volume in 8/10 patients with BP treated with Li for 4 weeks (p value NA) |
| Yucel et al. | 2007 | 12 | Prospective | Prophylactic, 2–4 years' duration | Mean Li level at baseline 0.5 mEq/liter | Li tx | Hippocampal volume | Equal increases in mean right and left hippocampal volumes noted. Total hippocampal volume increase over time significant (p < 0.001). |
| Foland et al. | 2008 | 49 | Cross-sectional | NA | NA | Li tx | Hippocampal and amygdala volume | Larger total amygdala and hippocampal volumes noted in Li-treated patients compared with patients without Li (p = 0.023 and p = 0.008 respectively) |
| Moore et al. | 2009 | 28 | Prospective | 4 weeks | ∼0.8 mEq/liter | Li response defined as >50% reduction in HAM-D total score | Gray matter volumes of total brain, prefrontal cortex, and left subgenual cortex | Increased prefrontal cortex gray matter volume after 4 weeks of Li in patients who responded to tx (p = 0.003) |
| Usher et al | 2010 | 82 | Cross-sectional | NA | Mean level 0.72 mEq/liter in Li group | Li tx | Amygdala volume | Greater right amygdala volumes observed in patients with BP treated with Li compared with patients with BP without Li tx and healthy controls (p < 0.02) |
| Lyoo et al. | 2010 | 36 | Prospective | Mean Li tx 81.8 days | Mean level 0.65 mEq/liter in Li group | Li response, defined as reduction in depressive symptoms | Cerebral gray matter volume | Reduction in depressive symptoms corresponded to rate of gray matter volume increase in patients treated with Li (p = 0.03) |
HAM-D, Hamilton Depression Rating Scale; Li, lithium; NA, not available or not applicable; tx, treatment.
The therapeutic action of lithium is unknown, but one hypothesis is that lithium treatment may dampen overactive neural networks in BP by depleting myo-inositol (mI), a component of the phosphoinositide second messenger system. Thus, mI has been considered a target in studies examining lithium response. Moore and colleagues examined the relationship between mI, as measured by quantitative MRS, and lithium treatment response in 12 patients with depression and BP [Moore et al. 1999]. The mI levels in the right frontal lobe decreased by approximately 30% after 5–7 days of lithium treatment and remained reduced after 3–4 weeks of treatment. However, the authors concluded that reduction in mI levels did not predict therapeutic response to lithium because mI levels dropped prior to a significant improvement in the patient's clinical state. A subsequent study investigated mI levels in 11 children with BP who were treated with lithium in comparison to baseline scans from 11 gender and age-matched controls using proton MRS [Davanzo et al. 2001]. After 1 week of treatment, patients who responded to lithium were noted to have reduced anterior cingulate mI levels compared with pretreatment levels.
Neuroimaging has also permitted structural correlations of lithium administration (Table 3). Lithium treatment has been associated with increased gray matter volumes in general [Moore et al. 2000], and in particular brain regions such as the hippocampus [Foland et al. 2008; Yucel et al. 2007], and the amygdale [Usher et al. 2010; Foland et al. 2008]. Recently, several studies have examined whether such gray matter changes correlate with lithium response rather than simply treatment. One study revealed that a favorable clinical response in BP was associated with persistently increased cerebral gray matter volume over the course of 16 weeks of treatment [Lyoo et al. 2010]. A second studied 28 patients with BP longitudinally for 4 weeks and determined that patients who responded to lithium had increased prefrontal gray matter compared with those who did not respond [Moore et al. 2009].
Predictors of lithium response: Molecular genetics studies
Molecular genetics as the basis for predicting response to medication has become an area of intensive investigation, particularly because it holds enormous promise as the basis for personalized medicine. This concept has its origin in the work of Archibald Garrod, the English physicianscientist who coined the term ‘chemical individuality’. Barton Childs, of Johns Hopkins, was much influenced by Garrod. Childs, who made a major impact in human genetics, with his work on the X chromosome and its diseases, wrote a book called Genetic Medicine: A Logic of Disease (Childs, 1999), which lays out the basis for personalized medicine. Childs commented:
With the idea of common management [of disease], we've tended to see patients as similar, and we've tended to treat not the patient but the disease. I'm hoping that recognition of individuality in each person may cause physicians to pay more attention not to the disease but to the patient who has the disease, and maybe even change the treatment a little bit to take into account the uniqueness of the individual.
Throughout medicine, however, there are, to date, surprisingly few useful genetic tests for treatment of common diseases. Nonetheless, there are a few of them, most prominently in cancer. Two major examples are the use of genetic testing to guide clinical decisions about the use of trastuzumab (Herceptin) [Genentech, San Francisco, CA] in breast cancer, and KRAS in colorectal cancer [Lievre et al. 2006; Pietras et al. 1994]. No useful genetic tests exist yet that can guide the treatment of major psychiatric illnesses, and BP is no exception. However, molecular genetic studies of lithium response to date have been small and relatively few in number.
A number of small genetic association studies have analyzed the relationship of variation in biological candidate genes to lithium response. These are studies that typically have examined one or several genes that could plausibly be involved in lithium response because of some known or suspected role in the pathophysiology of BP or in the mechanism of action of the drug. A number of studies have examined lithium response in BP in this way. The studies have mostly utilized sample sizes that were only in the dozens, although a few were in the hundreds. Many investigators believe that samples in the thousands may be necessary to see the kind of subtle genetic effects likely to be involved in predicting drug response.
These studies have all reported either negative findings or positive findings that are modest and unconfirmed. Negative findings have been reported for the dopamine type 2, 3, and 4 receptors; for the serotonin type 1A, 2A, and 2C receptors; and for the GABAA-alpha-1, INPP1, and PLC-gamma 1 genes [Serretti, 2002]. A study of the tryptophan hydroxylase gene found a worse lithium response for patients with the A/A variant of a gene marker, though this difference was only marginally significant [Serretti et al. 1999]. One published study of the serotonin transporter gene found a significantly worse response in patients carrying two copies of the short allele in the promoter region polymorphism [Serretti et al. 2001]. Several studies have examined the brain-derived neurotrophic factor (BDNF) gene. Two studies reported that excellent response was found in patients who carried particular variants in BDNF [Dmitrzak-Weglarz et al. 2008; Rybakowski et al. 2005]. However, Masui and colleagues had negative findings in a Japanese sample [Masui et al. 2006b], and Michelon and colleagues' results were also negative [Michelon et al. 2006]. Another study found modest evidence implicating the BDNF receptor gene NTRK2 in lithium response, though the association was seen only for patients with a subtype of illness [Bremer et al. 2007].
XBP1, which encodes the X-box binding protein 1, is an important molecule in the endoplasmic reticulum stress response. There is a variant in the promoter region of the gene, called −116C/G, shown to influence XBP1-dependent transcription activity in lymphoblastoid cells [Kakiuchi et al. 2003]. In another study the same authors suggested that lithium may not be effective for patients with BP who carry the G/G genotype of XBP1 [Kakiuchi and Kato, 2005]. A second Japanese group showed that patients with the C allele were significantly more likely to be classed as responsive to lithium compared with those lacking a C allele [Masui et al. 2006a]. Interestingly, Kim and colleagues found that the opposite allele, of XBP1-116C/G, was associated with a better prophylactic treatment response to valproate in patients with BP. While intriguing, the numbers in these studies are quite small, and thus these results await replication in larger samples [Kim et al. 2009].
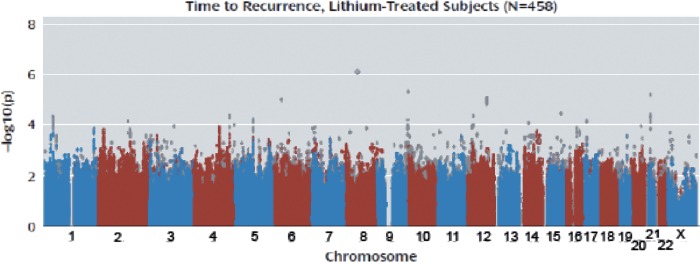
Recently, the first genome-wide association study was done to identify single nucleotide polymorphisms (SNPs) that would predict lithium response, from among hundreds of thousands of genetic variations covering most of the genes in the genome [Perlis et al. 2009]. A total of 458 patients treated with lithium from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) cohort were studied, with follow up in 359 patients with BP treated with lithium in a second cohort from the University College London. Time to recurrence of a mood episode was the outcome or phenotype variable for the first cohort, while a dichotomous poor versus intermediate or good response was the outcome in the second. No definitive results were identified in this study, perhaps because the study was underpowered (Figure 2). However, several regions of potential interest were found. One of these spanned a gene coding for the AMPA type glutamate receptor GRIA2, for which expression has been shown to be regulated by lithium treatment. Additional genes of potential interest were: SDC2 or Syndecan-2, which codes for a cell-surface proteoglycan shown to play a central role in the formation of dendritic spines in the hippocampus; SV2B, synaptic vesicle glycoprotein-2B, a protein expressed primarily in the hippocampus; and ODZ4, the human homologue of Drosophila odd Oz (odz)-4, implicated in brain patterning.
Figure 2.
Genome-wide association study of lithium response. This figure, called a Manhattan plot, shows the results of testing hundreds of thousands of genetic variations across the genome in patients taking lithium. The x axis shows the location of each on one of the 23 chromosomes. A test was performed to determine whether each variant was associated with the time to recurrence of a mood episode while on medication. The y axis shows the strength of association, with the -log10 p value of 8–9 being the range where findings are considered definitive. Each dot represents one genetic variant, and most of them are clustered in the y-axis range of 0-3, so that they are depicted as coalesced into solid bars. The strongest finding reaches just over 6, on chromosome 7. [Reproduced with permission from Perlis et al. 2009].
Future directions
One major new effort to study lithium genetics stems from the Consortium for Lithium Genetics (www.ConLiGen.org), formed in 2008 by researchers from the International Group for the Study of Lithium-Treated Patients (www.IGSLI.org) and the Unit on the Genetic Basis of Mood and Anxiety Disorders at the US National Institute of Mental Health (NIMH). Led by Thomas Schulze and Francis McMahon, this consortium has assembled the largest sample to date for genome-wide studies of lithium response in BP, currently comprising more than 1200 patients characterized for response to lithium treatment [Schulze et al. 2010]. This collaboration is basing its phenotype definition on the scale developed by Martin Alda, a leading lithium response researcher at Dalhousie University in Canada, which rates lithium response from 0 to 10, subtracting points for confounding factors such as poor compliance or need for additional medications while on lithium. Use of this scale is important because definitions of lithium response have varied widely in the literature. An initial goal of ConLiGen is to perform a genome-wide association study on this large assembled dataset.
A second major new initiative in lithium genetics is just getting under way in the USA under the leadership of John Kelsoe, a psychiatrist and leading BP geneticist at the University of California, San Diego. This project is an outgrowth of the NIMH Genetics Initiative Consortium for Bipolar Disorder, a collaboration involving up to 11 US sites. The new study, being conducted in the context of the Pharmacogenetics Trials Network of the NIH, will include many of those sites, and also international collaborators and clinical trials experts. This project, which will be carried out in coordination with ConLiGen, also aims to conduct genome-wide association studies of lithium response in BP. This study will, in addition, have a smaller divalproex sodium arm, so that it might shed light on genes that differentially predispose to response to these two major BP medications.
Finally, new studies are getting under way that would perform brain imaging of the same patients who are being characterized for lithium response, and also being genotyped for genome-wide association studies. This additional characterization might provide important information that could help clarify the mechanisms through which genetic variation leads to variation in clinical response.
Conclusions
In summary, fewer hospitalizations preceding treatment, an episodic course characterized by an illness pattern of mania followed by depression, and a later age of disease onset are important clinical predictors of a favorable response to lithium. While several biologic predictors have been studied, the results are preliminary and require replication with studies consisting of larger patient samples over longer observation periods. Neuroimaging is a particularly promising method given that it might concurrently illuminate pathophysiologic underpinnings of BP, the mechanism of action of lithium, and potential predictors of lithium response. One genome-wide association to date using lithium response as a phenotype did not yield any definitive results, though the small sample size did not provide substantial power.
With major new initiatives in progress aiming to identify genes and genetic variations associated with lithium response, there is much reason to be hopeful that clinically useful information might be generated within the next several years. This could ultimately translate into tests that could guide the choice of mood stabilizing medication for patients. In addition, it might facilitate the development of newer, more effective medications that could act more quickly and yield fewer side effects. The addition of brain imaging signatures of lithium response could provide additional valuable information that might help in several ways: by more powerfully predicting response in conjunction with genetic variations, by serving as a biomarker of response in clinical trials, and by illuminating pathophysiological pathways from gene to clinical response.
Footnotes
This research was supported by the James Wah Fund (SKT and PBM), the Johns Hopkins Brain Science Institute (PBM), Project Match (JBP), and NIH grant U01 MH092758 (JBP).
The authors have no conflicts of interest in preparing this article.
References
- Baastrup P.C., Poulsen J.C., Schou M., Thomsen K., Amdisen A. (1970) Prophylactic lithium: Double blind discontinuation in manic-depressive and recurrent-depressive disorders. Lancet 2: 326–330 [DOI] [PubMed] [Google Scholar]
- Baastrup P.C., Schou M. (1967) Lithium as a prophylactic agent. Its effect against recurrent depressions and manic-depressive psychosis. Arch Gen Psychiatry 16: 162–172 [DOI] [PubMed] [Google Scholar]
- Backlund L., Ehnvall A., Hetta J., Isacsson G., Agren H. (2009) Identifying predictors for good lithium response - a retrospective analysis of 100 patients with bipolar disorder using a life-charting method. Eur Psychiatry 24: 171–177 [DOI] [PubMed] [Google Scholar]
- Berghofer A., Alda M., Adli M., Baethge C., Bauer M., Bschor T., et al. (2008) Long-term effectiveness of lithium in bipolar disorder: A multicenter investigation of patients with typical and atypical features. J Clin Psychiatry 69: 1860–1868 [DOI] [PubMed] [Google Scholar]
- Bremer T., Diamond C., McKinney R., Shehktman T., Barrett T.B., Herold C., et al. (2007) The pharmacogenetics of lithium response depends upon clinical co-morbidity. Mol Diag Ther 11: 161–170 [DOI] [PubMed] [Google Scholar]
- Cade J.F. (1949) Lithium salts in the treatment of psychotic excitement. Med J Australia 2: 349–352 [DOI] [PubMed] [Google Scholar]
- Childs B. (1999) Genetic Medicine. Johns Hopkins Univ. Press: Baltimore [Google Scholar]
- Coryell W., Akiskal H., Leon A.C., Turvey C., Solomon D., Endicott J. (2000) Family history and symptom levels during treatment for bipolar I affective disorder. Biol Psychiatry 47: 1034–1042 [DOI] [PubMed] [Google Scholar]
- Crossley N.A., Bauer M. (2007) Acceleration and augmentation of antidepressants with lithium for depressive disorders: Two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry 68: 935–940 [DOI] [PubMed] [Google Scholar]
- Davanzo P., Thomas M.A., Yue K., Oshiro T., Belin T., Strober M., et al. (2001) Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology 24: 359–369 [DOI] [PubMed] [Google Scholar]
- de Leon J. (2009) Pharmacogenomics: The promise of personalized medicine for CNS disorders. Neuropsychopharmacology 34: 159–172 [DOI] [PubMed] [Google Scholar]
- Dmitrzak-Weglarz M., Rybakowski J.K., Suwalska A., Skibinska M., Leszczynska-Rodziewicz A., Szczepankiewicz A., et al. (2008) Association studies of the BDNF and the NTRK2 gene polymorphisms with prophylactic lithium response in bipolar patients. Pharmacogenomics 9: 1595–1603 [DOI] [PubMed] [Google Scholar]
- Duffy A., Alda M., Milin R., Grof P. (2007) A consecutive series of treated affected offspring of parents with bipolar disorder: Is response associated with the clinical profile? Can J Psychiatry 52: 369–376 [DOI] [PubMed] [Google Scholar]
- Dunner D.L., Fieve R.R. (1974) Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry 30: 229–233 [DOI] [PubMed] [Google Scholar]
- Foland L.C., Altshuler L.L., Sugar C.A., Lee A.D., Leow A.D., Townsend J., et al. (2008) Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport 19: 221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnham J., Munro A., Slaney C., Macdougall M., Passmore M., Duffy A., et al. (2007) Prophylactic treatment response in bipolar disorder: Results of a naturalistic observation study. J Affect Disord 104: 185–190 [DOI] [PubMed] [Google Scholar]
- Geddes J.R., Burgess S., Hawton K., Jamison K., Goodwin G.M. (2004) Long-term lithium therapy for bipolar disorder: Systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry 161: 217–222 [DOI] [PubMed] [Google Scholar]
- Grof P. (2003) Selecting effective long-term treatment for bipolar patients: Monotherapy and combinations. J Clin Psychiatry 64: 53–61 [PubMed] [Google Scholar]
- Grof P., Alda M., Grof E., Zvolsky P., Walsh M. (1994) Lithium response and genetics of affective disorders. J Affect Disord 32: 85–95 [DOI] [PubMed] [Google Scholar]
- Grof P., Duffy A., Cavazzoni P., Grof E., Garnham J., MacDougall M., et al. (2002) Is response to prophylactic lithium a familial trait? J Clin Psychiatry 63: 942–947 [DOI] [PubMed] [Google Scholar]
- Grof P., Muller-Oerlinghausen B. (2009) A critical appraisal of lithium's efficacy and effectiveness: The last 60 years. Bipolar Disord 11: 10–19 [DOI] [PubMed] [Google Scholar]
- Hegerl U., Ulrich G., Muller-Oerlinghausen B. (1987) Auditory evoked potentials and response to lithium prophylaxis. Pharmacopsychiatry 20: 213–216 [DOI] [PubMed] [Google Scholar]
- Hegerl U., Wulff H., Muller-Oerlinghausen B. (1992) Intensity dependence of auditory evoked potentials and clinical response to prophylactic lithium medication: A replication study. Psychiatry Res 44: 181–190 [DOI] [PubMed] [Google Scholar]
- Ikeda A., Kato N., Kato T. (2002) Possible relationship between electroencephalogram finding and lithium response in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 26: 903–907 [DOI] [PubMed] [Google Scholar]
- Juckel G., Mavrogiorgou P., Bredemeier S., Gallinat J., Frodl T., Schulz C., et al. (2004) Loudness dependence of primary auditory-cortex-evoked activity as predictor of therapeutic outcome to prophylactic lithium treatment in affective disorders - a retrospective study. Pharmacopsychiatry 37: 46–51 [DOI] [PubMed] [Google Scholar]
- Kakiuchi C., Iwamoto K., Ishiwata M., Bundo M., Kasahara T., Kusumi I., et al. (2003) Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat Genet 35: 171–175 [DOI] [PubMed] [Google Scholar]
- Kakiuchi C., Kato T. (2005) Lithium response and −116C/G polymorphism of XBP1 in Japanese patients with bipolar disorder. Int J Neuropsychopharm 8: 631–632 [DOI] [PubMed] [Google Scholar]
- Kato T., Inubushi T., Kato N. (2000) Prediction of lithium response by 31P-MRS in bipolar disorder. Int J Neuropsychopharm 3: 83–85 [DOI] [PubMed] [Google Scholar]
- Kim B., Kim C.Y., Lee M.J., Joo Y.H. (2009) Preliminary evidence on the association between XBP1-116C/G polymorphism and response to prophylactic treatment with valproate in bipolar disorders. Psychiatry Res 168: 209–212 [DOI] [PubMed] [Google Scholar]
- Kleindienst N., Engel R., Greil W. (2005) Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord 7: 404–417 [DOI] [PubMed] [Google Scholar]
- Kusalic M., Engelsmann F. (1998) Predictors of lithium treatment responsiveness in bipolar patients. A two-year prospective study. Neuropsychobiology 37: 146–149 [DOI] [PubMed] [Google Scholar]
- Lievre A., Bachet J.B., Le Corre D., Boige V., Landi B., Emile J.F., et al. (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66: 3992–3995 [DOI] [PubMed] [Google Scholar]
- Lopez A.D., Murray C.C. (1998) The global burden of disease, 1990-2020 [news]. Nat Med 4: 1241–1243 [DOI] [PubMed] [Google Scholar]
- Lyoo I.K., Dager S.R., Kim J.E., Yoon S.J., Friedman S.D., Dunner D.L., et al. (2010) Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: A longitudinal brain imaging study. Neuropsychopharmacology 35: 1743–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M., Starace F., Nolfe G., Kemali D. (1986) Minimum plasma lithium levels required for effective prophylaxis in DSM III bipolar disorder: A prospective study. Pharmacopsychiatry 19: 420–423 [DOI] [PubMed] [Google Scholar]
- Masui T., Hashimoto R., Kusumi I., Suzuki K., Tanaka T., Nakagawa S., et al. (2006a) A possible association between the −116C/G single nucleotide polymorphism of the XBP1 gene and lithium prophylaxis in bipolar disorder. Int J Neuropsychopharm 9: 83–88 [DOI] [PubMed] [Google Scholar]
- Masui T., Hashimoto R., Kusumi I., Suzuki K., Tanaka T., Nakagawa S., et al. (2006b) Lithium response and Val66Met polymorphism of the brain-derived neurotrophic factor gene in Japanese patients with bipolar disorder. Psychiatric Genet 16: 49–50 [DOI] [PubMed] [Google Scholar]
- Masui T., Hashimoto R., Kusumi I., Suzuki K., Tanaka T., Nakagawa S., et al. (2008) A possible association between missense polymorphism of the breakpoint cluster region gene and lithium prophylaxis in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 32: 204–208 [DOI] [PubMed] [Google Scholar]
- McKnew D.H., Cytryn L., Buchsbaum M.S., Hamovit J., Lamour M., Rapoport J.L., et al. (1981) Lithium in children of lithium-responding parents. Psychiatry Res 4: 171–180 [DOI] [PubMed] [Google Scholar]
- Merikangas K.R., Akiskal H.S., Angst J., Greenberg P.E., Hirschfeld R.M., Petukhova M., et al. (2007) Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64: 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelon L., Meira-Lima I., Cordeiro Q., Miguita K., Breen G., Collier D., et al. (2006) Association study of the INPP1, 5HTT, BDNF, AP-2beta and GSK-3beta GENE variants and restrospectively scored response to lithium prophylaxis in bipolar disorder. Neurosci Lett 403: 288–293 [DOI] [PubMed] [Google Scholar]
- Moore G.J., Bebchuk J.M., Parrish J.K., Faulk M.W., Arfken C.L., Strahl-Bevacqua J., et al. (1999) Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am J Psychiatry 156: 1902–1908 [DOI] [PubMed] [Google Scholar]
- Moore G.J., Bebchuk J.M., Wilds I.B., Chen G., Manji H.K. (2000) Lithium-induced increase in human brain grey matter. Lancet 356: 1241–1242 [DOI] [PubMed] [Google Scholar]
- Moore G.J., Cortese B.M., Glitz D.A., Zajac-Benitez C., Quiroz J.A., Uhde T.W., et al. (2009) A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry 70: 699–705 [DOI] [PubMed] [Google Scholar]
- Okuma T. (1993) Effects of carbamazepine and lithium on affective disorders. Neuropsychobiology 27: 138–145 [DOI] [PubMed] [Google Scholar]
- Osby U., Brandt L., Correia N., Ekbom A., Sparen P. (2001) Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 58: 844–850 [DOI] [PubMed] [Google Scholar]
- Passmore M.J., Garnham J., Duffy A., MacDougall M., Munro A., Slaney C., et al. (2003) Phenotypic spectra of bipolar disorder in responders to lithium versus lamotrigine. Bipolar Disord 5: 110–114 [DOI] [PubMed] [Google Scholar]
- Perlis R.H., Smoller J.W., Ferreira M.A., McQuillin A., Bass N., Lawrence J., et al. (2009) A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry 166: 718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras R.J., Fendly B.M., Chazin V.R., Pegram M.D., Howell S.B., Slamon D.J. (1994) Antibody to HER-2/neu receptor blocks DNA repair after cis-platin in human breast and ovarian cancer cells. Oncogene 9: 1829–1838 [PubMed] [Google Scholar]
- Potash J.B., Willour V.L., Chiu Y., Simpson S.G., MacKinnon D.F., Pearlson G.D., et al. (2001) The familial aggregation of psychotic symptoms in bipolar disorder pedigrees. Am J Psychiatry 158: 1258–1264 [DOI] [PubMed] [Google Scholar]
- Reeves R.R., Struve F.A., Patrick G. (2001) Does EEG predict response to valproate versus lithium in patients with mania? Ann Clin Psychiatry 13: 69–73 [DOI] [PubMed] [Google Scholar]
- Rybakowski J.K., Suwalska A., Skibinska M., Dmitrzak-Weglarz M., Leszczynska-Rodziewicz A., Hauser J. (2007) Response to lithium prophylaxis: Interaction between serotonin transporter and BDNF genes. Am J Med Genet B Neuropsychiatr Genet 144B: 820–823 [DOI] [PubMed] [Google Scholar]
- Rybakowski J.K., Suwalska A., Skibinska M., Szczepankiewicz A., Leszczynska-Rodziewicz A., Permoda A., et al. (2005) Prophylactic lithium response and polymorphism of the brain-derived neurotrophic factor gene. Pharmacopsychiatry 38: 166–170 [DOI] [PubMed] [Google Scholar]
- Sarantidis D., Waters B. (1981) Predictors of lithium prophylaxis effectiveness. Prog Neuropsychopharmacol 5: 507–510 [DOI] [PubMed] [Google Scholar]
- Schou M. (1999) The early European lithium studies. Aust NZJ Psychiatry 33: S39–S47 [DOI] [PubMed] [Google Scholar]
- Schou M., Juel-Nielsen N., Stromgren E., Voldby H. (1954) The treatment of manic psychoses by the administration of lithium salts. J Neurol Neurosurg Psychiatry 17: 250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze T.G., Alda M., Adli M., Akula N., Ardau R., Bui E.T., et al. (2010) The International Consortium on Lithium Genetics (ConLiGen): An initiative by the NIMH and IGSLI to study the genetic basis of response to lithium treatment. Neuropsychobiology 62: 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A. (2002) Lithium long-term treatment in mood disorders: Clinical and genetic predictors. Pharmacogenomics 3: 117–129 [DOI] [PubMed] [Google Scholar]
- Serretti A., Lilli R., Lorenzi C., Gasperini M., Smeraldi E. (1999) Tryptophan hydroxylase gene and response to lithium prophylaxis in mood disorders. J Psychiatr Res 33: 371–377 [DOI] [PubMed] [Google Scholar]
- Serretti A., Lilli R., Mandelli L., Lorenzi C., Smeraldi E. (2001) Serotonin transporter gene associated with lithium prophylaxis in mood disorders. Pharmacogenetics J 1: 71–77 [DOI] [PubMed] [Google Scholar]
- Small J.G., Milstein V., Malloy F.W., Klapper M.H., Golay S.J., Medlock C.E. (1998) Topographic EEG studies of mania. Clin Electroencephalogr 29: 59–66 [DOI] [PubMed] [Google Scholar]
- Small J.G., Milstein V., Malloy F.W., Medlock C.E., Klapper M.H. (1999) Clinical and quantitative EEG studies of mania. J Affect Disord 53: 217–224 [DOI] [PubMed] [Google Scholar]
- Tondo L., Baldessarini R.J., Floris G. (2001) Long-term clinical effectiveness of lithium maintenance treatment in types I and II bipolar disorders. Br J Psychiatry 178: S184–S190 [PubMed] [Google Scholar]
- Tondo L., Hennen J., Baldessarini R.J. (2003) Rapid-cycling bipolar disorder: Effects of long-term treatments. Acta Psychiatr Scand 108: 4–14 [DOI] [PubMed] [Google Scholar]
- Usher J., Menzel P., Schneider-Axmann T., Kemmer C., Reith W., Falkai P., Gruber O., et al. (2010) Increased right amygdala volume in lithium-treated patients with bipolar I disorder. Acta Psychiatr Scand 121: 119–124 [DOI] [PubMed] [Google Scholar]
- Yang Y.Y. (1985) Prophylactic efficacy of lithium and its effective plasma levels in Chinese bipolar patients. Acta Psychiatr Scand 71: 171–175 [DOI] [PubMed] [Google Scholar]
- Yazici O., Kora K., Ucok A., Tunali D., Turan N. (1999) Predictors of lithium prophylaxis in bipolar patients. J Affect Disord 55: 133–142 [DOI] [PubMed] [Google Scholar]
- Yildiz A., Vieta E., Leucht S., Baldessarini R.J. (2011) Efficacy of antimanic treatments: Meta-analysis of randomized, controlled trials. Neuropsychopharmacology 36: 375–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel K., McKinnon M.C., Taylor V.H., Macdonald K., Alda M., Young L.T., et al. (2007) Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: A longitudinal MRI study. Psychopharmacology 195: 357–367 [DOI] [PubMed] [Google Scholar]