Abstract
Objectives:
In this paper we aim to: (1) identify and review midlife risk factors that may contribute to the development of dementia and that may be amenable to intervention; (2) review advances made in our understanding of the most common cause of dementia, Alzheimer's disease (AD), where current pharmacological studies have aimed to modify the disease course; and (3) explore other interventions that may slow cognitive decline in those with AD.
Methods:
A review of the literature was conducted to look for interventions that may modify the risk of incident dementia or that may modify symptom progression in those with diagnosed dementia.
Results:
(1) Midlife risks identified as amenable to intervention include blood pressure, diabetes, elevated cholesterol, poor psychosocial and lifestyle factors. (2) The leading drugs in development can be grouped by their principal target: anti-amyloid, anti-tau and mitochondrial stability. However to date, there have been no successes in late stage Phase III trials of putative disease-modifying drugs for AD. (3) Once the diagnosis of dementia has been made there is little that can slow the rate of decline. Possible exceptions include the use of exercise and antihypertensive medication with some nootropic medication showing promise in small trials.
Conclusion:
(1) It is clear that there are several risk factors in midlife that may lead to a greater likelihood of developing dementia. However, there is no simple intervention to modify these risks. It seems sensible to conclude from the data that avoiding high blood pressure, controlling cholesterol and diabetes as well as maintaining a healthy diet and lifestyle may lower the risk of developing dementia. (2) The need for better outcome measures in clinical trials is evident and may, in part, explain the numerous failures in late-stage clinical trials of disease-modifying drugs. Improved diagnostic test batteries to reduce population heterogeneity in early intervention studies will be required for robust clinical trials in the future. (3) Current research indicates that there is little that can delay decline; however, future trials may wish to focus on nootropics.
Keywords: ageing, Alzheimer's disease, delay, dementia, cognitive decline, prevent, risk factors
Introduction
Currently 24 million people worldwide suffer from dementia [Ferri et al. 2005]. This figure is expected to more than treble to 81 million by 2040. In the UK alone, there are currently over 800,000 cases of dementia and this is predicted to grow to more than 1.4 million by 2040 [Luengo-Fernandez et al. 2010; Comas-Herrera et al. 2007]. The associated cost to the UK economy of dementia presently stands at £23 billion per year [Luengo-Fernandez et al. 2010], with this estimated to double to over £50 billion per year by 2040 [Comas-Herrera et al. 2007]. In addition, its effects are devastating and far-reaching for patients, carers, families and society alike. The globally ageing society and the increased risk of dementia at older age means that the impact of dementia both in terms of financial and personal cost will be felt worldwide [Lobo et al. 2000].
Alzheimer's disease (AD) is the most common cause of dementia and accounts for more than half of all dementia cases. AD is a progressive neurodegenerative disease which gradually deprives individuals of their memory, perception, judgment, abstraction and language skills. This cognitive decline mediates both functional and behavioural deterioration. The incidence and prevalence of AD rise exponentially with age, as every 5 years after the age of 65 the probability of developing the disease doubles [Fratiglioni et al. 2000a]. It is estimated that 2-3% of people aged 65 and over suffer from AD whilst 25-50% of people aged 85 have symptoms of AD and a greater number have histopathological hallmarks of the disease without the characteristic symptoms [Luengo-Fernandez et al. 2010]. The second most frequent cause of dementia is thought to be one of vascular origin [Lobo et al. 2000] although it is likely that the overlap between these two dementia types is large with the AD pathology occurring alongside vascular damage such as stroke [Barker et al. 2002; Jellinger, 2002; Gold, 1998; Sparks et al. 1995]. This is further supported by the literature which indicates that the risk factors for both tend to be similar [Kivipelto et al. 2006; Whitmer et al. 2005a].
Identifying those who are at risk for developing dementia may provide an opportunity for intervention. Delaying the onset of AD by just 5 years would reduce the prevalent cases by 50% [Brookmeyer et al. 1998] and have huge public health impact. This article aims first to review risk factors identified in midlife that may lead to an increased chance of developing dementia in later life and interventions that could reduce these risks and delay the onset of dementia. Second, it will then review pharmacological interventions that will specifically affect the AD process and, therefore, prevent further cognitive, behavioural and functional decline and third it will review nonpharmacological interventions, the pharmacological treatment of risk factors (specifically cardiovascular risks), and the use of other drugs or products not licensed specifically for use in dementia which may delay or ameliorate dementia.
Modifying disease course: 1
There are several risk factors that have been identified in midlife that may lead to an increased risk of dementia. Midlife is generally described as being the period from 40 to 60 years of age. Midlife risks identified to be amenable to intervention include blood pressure [Singh-Manoux and Marmot, 2005; Qiu and Winblad, 2005], diabetes [Allen et al. 2004], elevated cholesterol [Reiman et al. 2010] and poor psychosocial and lifestyle factors which will be explored in turn. Knowledge of these risk factors is relevant to the primary prevention of dementia.
Blood pressure
Evidence indicates that both low diastolic blood pressure (DBP) and high systolic blood pressure (SBP) are risk factors for developing dementia [Qiu et al. 2003]. Several longitudinal studies including a large prospective, population-based study have indicated that raised SBP in midlife can increase the risk of AD in later life [Kivipelto et al. 2001]. This study also suggests that DBP in midlife had no significant effect on the risk of AD. It appears that findings from these studies indicate that both high SBP and low DBP affect the risk of developing dementia; however, this relationship appears complicated. A recent Cochrane review [McGuinness et al. 2009b] examined evidence from four large trials. This review concluded that lowering blood pressure in late-life did not prevent the development of dementia or cognitive impairment in hypertensive patients.
A review carried out by Qiu and Winblad investigated whether treatment of blood pressure with antihypertensive medications in midlife leads to better cognitive function in later life [Qiu and Winblad, 2005]. They concluded that there is ‘moderately strong evidence’ to indicate that the treatment of hypertension in midlife has a positive impact on cognition in later life. Furthermore, evidence suggests that high blood pressure in midlife initiates a cumulating effect of increased severity associated with atherosclerosis and thereafter greater vascular comorbidities in later life. This is supported by a meta-analysis undertaken by Feigen and colleagues which analysed four large blood pressure trials [Feigen et al. 2005]. Although this meta-analysis did not reach significance it was concluded that administering antihypertensive medication may offer a protective effect against cognitive decline.
However, caution must be exercised as results may also be confounded by studies using insufficient power to detect modest treatment effects, missing data, age at which baseline levels of blood pressure were taken and inclusion of patients with cognitive impairment at entry. Trials were also often designed with cardiovascular endpoints so were inherently limited to comment on cognitive outcomes. Results may also be due to antihypertensive agents reducing the risk of developing dementia through another pathway unconnected to the lowering of blood pressure. However, if the effect of lowering blood pressure on the incidence of dementia is confirmed it would be important not only in terms of our understanding of the aetiology of dementia but also in promoting blood pressure lowering strategies for broader public health.
Diabetes mellitus
Diabetes mellitus is a complex metabolic disorder that shares other risk factors, including hypertension and vascular disease, associated with cognitive decline. Several large longitudinal population-based studies have shown that the rate of cognitive decline is accelerated in elderly people with type II diabetes [Allen et al. 2004].
A systematic review which examined 14 studies looking at incident dementia in those with diabetes mellitus concluded that the incidence of ‘any dementia’ was higher in individuals with diabetes than those without the condition [Biessels et al. 2006]. However, the reasons for this were unclear with little detailed information recorded on the effects of glycaemia management, vascular, hypertension and stroke incidences. They also concluded that vascular disease, alterations in glucose, insulin, and amyloid metabolism underlie the pathology of dementia, but it is still unclear as to which of these processes are clinically relevant.
Three studies in this systematic review [Whitmer et al. 2005a; Schnaider Beeri et al. 2004; Yamada et al. 2003] looked at midlife diabetes and followed these patients up 25-35 years later [Whitmer et al. 2005b; Schnaider Beeri et al. 2004; Yamada et al. 2003]. Findings indicated that the incidence of dementia was higher in those with diabetes. However, there were generally long intervals between measurement of diabetes and dementia assessment which may cast doubt on these findings. Dropout rates in these studies were also large with death before follow up the most common cause of dropout and (as expected) was higher in the diabetic group. A further paper from this systematic review [Luchsinger et al. 2005] investigated the interaction between hypertension and diabetes. Interestingly, the risk of AD was greater in participants with diabetes but without hypertension suggesting that hypertension may mediate the effect of diabetes on cognition. However, a recent study [Xu et al. 2010] concluded that borderline diabetes may interact with severe systolic hypertension to multiply the risk of AD. It is clear that clarity is needed and future trials may want to focus on this relationship.
Xu and colleagues assessed a community-based cohort of 1,173 participants without diabetes or AD for a period of 9 years [Xu et al. 2007]. Borderline diabetes was associated with increased risk of AD and this risk effect was independent to the development of diabetes in the future. Furthermore, in a subsequent study they looked at 13,693 twin individuals aged >65 years and concluded that diabetes increased the risk of AD and vascular dementia [Xu et al. 2009]. They found that the risk effect was stronger when diabetes occurred at mid-life rather than in late life suggesting that the associated between diabetes and dementia may develop across a lifespan. They also reported that diabetes and prediabetes substantially accelerated the progression from mild cognitive impairment (MCI) to dementia, they concluded that diabetes preceded dementia occurrence by more than 3 years in people with MCI [Xu et al. 2010]. They also suggested that diabetes may actually speed up the process of memory decline by bypassing MCI or shortening the MCI phase. Further studies may choose to focus on early life and adulthood environmental factors as well as genetic features that may be involved in an association between diabetes and dementia.
The evidence linking diabetes with dementia highlights the need to detect and control both prediabetic states and diabetes in order to prevent or postpone dementia. However, there are few epidemiological studies focused on risk factors or interventions. Additionally a Cochrane review conducted in 2009 concluded that there was no reliable evidence to suggest that type or intensity of diabetic treatment either to prevent or manage the risk of cognitive impairment was effective in type II diabetes [Grimley Evans and Areosa Sastre, 2009]. No trials were thought to be appropriate for inclusion in this meta-analysis as studies did not include data on cognitive function. In order to develop treatments to be used in midlife the mechanisms that drive the association between diabetes and cognitive change must be identified. Studies must include assessment of cognition as well as assessment of diabetes, metabolic factors, blood pressure and vascular disease.
Cholesterol
Experimental studies have suggested that cholesterol may encourage the aggregation of amyloid-β (Aβ) and amyloid plaques in the brain, which are important features in the pathology of AD [Reiman et al. 2010]. Evidence from epidemiological studies also points to a strong relationship between elevated low-density lipoprotein cholesterol (LDL-C) levels and cerebrovascular disease risk. These studies also suggest that elevated serum total cholesterol during midlife, but not during late life, increase the risk of AD (Reiman et al. 2010). Recent findings from imaging studies have suggested that higher cholesterol levels and other cardiovascular risk factors accelerate brain changes associated with normal ageing, and when combined with possession of an APOE e4 allele there is an even greater risk of developing AD [Reiman et al. 2010].
Studies that have looked at cholesterol-lowering treatment and its impact on cognitive impairment have demonstrated mixed results. Some studies have indicated that cholesterol-lowering treatment reduces the risk of cognitive decline and dementia [Haag et al. 2009; Cramer et al. 2008]; others have failed to identify such benefits [Szwast et al. 2007; Zandi et al. 2005]. However, the differing methods used in the assessment of cognitive function in these studies may have resulted in these findings. Inconsistent findings may also be attributed to cholesterol-lowering medications being treated similarly when evidence suggests that only statins are associated with lowering the risk of AD [Reiman et al. 2010]. Statins may exercise a protective effect by increasing α-secretase activity and thereby decreasing the more toxic long Aβ levels and also cutting the risk of cardiovascular disease [Reiman et al. 2010].
Observational and prospective cohort studies have also not found significant effects. Two large, randomized, controlled statin trials, the Heart Protection Study [Collins et al. 2002; Heart Protection Study Collaborative Group, 2002] and the Prospective Study of Pravastatin in Elderly at Risk [Shepherd et al. 2002], assessed cognition only as part of secondary subanalyses, and both concluded that there was no significant effect of statin use on cognition. These trials were also included in a recent Cochrane review [McGuinness et al. 2009a] which concluded that statins could not be recommended for the prevention of AD or dementia. It is important to remember that cognitive measures were limited in these studies. This coupled with short follow-up times makes it difficult to draw conclusions on the long-term benefits of statins on cognitive decline. Further longitudinal studies on midlife effects of statins on cognition would be highly beneficial and are currently ongoing [Golomb et al. 2004].
Psychosocial and lifestyle factors
Evidence suggests both psychosocial and lifestyle factors may have an influence on the risk of developing dementia. Psychosocial factors include a lack of social activity or social support networks [Hakansson et al. 2009; Fratiglioni et al. 2004], living alone [Fratiglioni et al. 2000b], crystallized intelligence [Ritchie et al. 2010; Hall et al. 2009] and depressive episodes [Ritchie et al. 2010; Jorm, 2000; Alexopoulos et al. 1993]. Depression can be associated with significant cognitive deficits and can be comorbid with dementia. Meta-analyses [Ownby et al. 2006; Jorm, 2001] concluded that a history of depression may confer an increased risk for later developing AD and further concluded that depression may act as an independent risk factor for the disease. Three recent articles in the journal Neurology also point to a link between depression and dementia [Saczynski et al. 2010; Dotson et al. 2010; Wilson et al. 2010]. However, causality is far from clear and it may be that depression represents an early response to undiagnosed cognitive decline, or that both depression and dementia are related to higher cardiovascular risk. There are very few longitudinal studies that have been carried out in midlife to determine whether effective treatment of depression can reduce the risk of dementia in later life.
Lifestyle factors in midlife that have been suggested to decrease the risk of dementia include limited alcohol consumption [Anstey et al. 2009], smoking cessation [Peters et al. 2008] and following a ‘Mediterranean-type diet’ [Scarmeas et al. 2009; Sofi et al. 2008]. Antioxidant or vitamin intake may also be protective. Dietary rather than supplemental intake may be most beneficial, for example dietary vitamin E rather than a-tocopherol alone as found in supplements has been associated with a greater reduced risk of incident AD [Devore et al. 2010; Dai et al. 2006; Morris et al. 2005; Larrieu et al. 2004; Barberger-Gateau et al. 2002].
Levels of physical activity in midlife have also been linked to the development of AD in later life [Hamer and Chida, 2009; Lautenschlager et al. 2008; Rovio et al. 2005]. A large longitudinal study looked at whether leisure time physical activity at least twice a week delayed cognitive decline and eventual development of dementia [Rovio et al. 2005]. The study looked at 1449 participants over 20 years with results suggesting that regular physical activity may decrease the risk of dementia and specifically AD in later life. This finding is supported by a large study of twins where levels of midlife physical activity were recorded. Twins with greater levels of physical activity had lower levels of cognitive decline when compared with their cotwin [Andel et al. 2008].
Furthermore, there is strong evidence to suggest that obesity in midlife is associated with an increased risk of dementia and AD independent of comorbid conditions [Whitmer et al. 2008, 2005b; Kivipelto et al. 2005; Rosengren et al. 2005; Kalmijn et al. 2000]. In a recent longitudinal study a higher midlife body mass index (BMI) score preceded lower general cognitive ability and steeper cognitive decline in both men and women. Interestingly, the association between midlife BMI and general cognitive ability remained even when those diagnosed with AD were excluded from analysis. It seems logical that a public health intervention may wish to focus on reducing obesity by increasing physical activity and improving diet in midlife which may result in better cognitive outcomes [Bendlin et al. 2010].
A recent paper aimed to provide a hierarchy of priorities for public health interventions [Ritchie et al. 2010]. The findings suggest that the ‘removal’ of diabetes and depression, increasing crystallized intelligence and improving diet would provide the biggest impact for the reduction of dementia incidence. However, caution must be applied to research into the impact of lifestyle factors on later incident dementia as diet, physical activity, smoking, alcohol consumption, education, occupational attainment and leisure activities are highly likely to interact.
It is clear that there are several risk factors which are present and potentially modifiable in middle life that may lead to a greater likelihood of developing dementia in later life. However, there is no simple intervention to modify these risks. It seems sensible to conclude that avoiding high blood pressure, controlling cholesterol and diabetes as well as maintaining a healthy diet and lifestyle may lower the risk of developing dementia. It is essential that future studies collect data on cognitive function at baseline and follow up and that studies record details of other comorbidities. It is only then that risks can be defined with more certainty and large-scale public health interventions can be trialled.
Modifying disease course: 2
In theory, once AD has developed, pharmacological interventions can be given that will specifically affect the disease process and therefore prevent further synaptotoxicity, neurotoxicity and neurodegeneration. It is outside the scope of this article to provide a comprehensive review of our knowledge regarding Alzheimer's pathology. However, it is necessary to summarize the advances made in our understanding of AD where they are relevant to current pharmacological studies aiming to modify the course of disease.
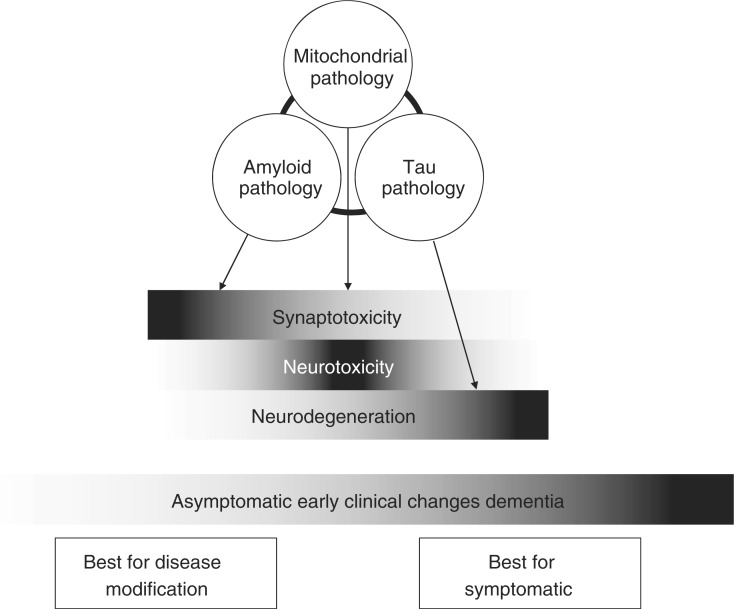
There are three top-level disease processes that are the favoured targets for pharmacological intervention, debate rages as to what processes are upstream of the others or more important in terms of the genesis of the disease and accordingly better candidates for pharmacological targeting. This debate is probably both irrelevant and an inaccurate conceptualization as it infers a linear course, when, in fact, we are in a complex disease system; a ‘perfect storm’ of factors probably needs to arise from all three to mediate a clinically manifest disease state. These three factors are mitochondrial dysfunction, the genesis and oligomerization of Aβ from amyloid precursor protein (APP) and the hyperphosphorylation of Tau with an impact on the intraneuronal cytoskeletal integrity of tubulin. These three disease processes lead to three neuropathological consequences that lead to symptoms (Figure 1). These consequences are synaptotoxicity, neurotoxicity and neurodegeneration. Each of these three consequences will have an impact on the genesis of symptoms depending upon the location of the pathology and the extent of damage. It can also be conceptualized that each of the three processes have differing potentials for salvation, with synaptotoxicity being highly dynamic and amenable to intervention with hope of full recovery and neurodegeneration being the final chronic effect of the other two processes with little if any opportunity for recovery.
Figure 1.
Schematic interaction between disease process, biological impact, clinical manifestation and opportunity for pharmacological intervention.
Before we consider the drugs currently in development for disease modification there are two further linked hypotheses which need articulating. First, it is emerging as very likely that each disease process is initiated as a restorative physiological response to another intracerebral insult and, second, that the current human organism has evolved to a point where long life into a postreproductive stage was not anticipated by the evolution of these physiological responses. That is to say that the aggregation of stresses that accumulate as we age are dealt with by responses that keep the organism alive to middle life but are maladaptive when present over longer durations. The net result of these two hypotheses is that treatments aimed at decreasing an identified pathology may run the risk of inadvertently impacting upon a physiological process. By way of supporting evidence for this, it is now generally considered that monomeric Aβ is a powerful antioxidant [Zou et al. 2002] and involved in central trace metal regulation [Maynard et al. 2005]. If the genesis or presence of monomeric Aβ was affected by a disease-modifying therapy, then the resultant reduction in the physiological properties of Aβ may lead to net detriment to neuronal function. Moreover, if we assume that oligomers only have a pathological impact on the neurone and synapse, then anti-aggregation strategies may be useful if they prevent monomers from oligomerizing, but be harmful if they prevent toxic oligomers further aggregating into plaque.
The conclusion to this preamble is that we must show remarkable caution in the targeting of our therapies and be using them judiciously at the earliest stage of disease as possible.
The identification of premanifest AD remains elusive however. There has been a recent refocusing of attention on the accurate characterization of premanifest dementia using clinical signatures (e.g. executive and episodic memory dysfunction), biomarkers and neuroimaging. The main therapeutic reasons for the accurate identification of the premanifest population are, first, to identify a population who may benefit from secondary prevention through risk modification strategies and, second, to create the criteria for the creation of a homogenous population for disease-modifying trials when disease is much less advanced (and possibly intractable) than observed in patients with clinical dementia.
To date, there have been no successes in late-stage phase III trials of putative disease-modifying drugs for AD. There are numerous methodological and operational reasons why this may be the case including the heterogeneity of the sample population in terms of concomitant medication and comorbidities, poor psychometric properties of outcome scales and the presence of severe (hypothetically unmodifiable) disease in even mild dementia. The first problem can be addressed through better trial execution; the latter two await the conclusions from ongoing outcomes and diagnostic test accuracy research [Mason et al. 2010] respectively.
The leading drugs in development can be grouped by their principal target and then sub-grouped by the part of the process they putatively interdict. The groups are anti-amyloid, anti-tau and mitochondrial stabilizers.
Anti-amyloid
Given the prominence in scientific discussions for the principal role of Aβ in the development of AD pathology in the late 20th Century, it is not surprising that the most advanced disease-modifying strategies affect this protein's processing from APP to plaque.
The drugs either in testing or recently completed phase III work either inhibit the secretase enzymes that cleave APP to create Aβ42 [e.g. Green et al. 2009], prevent oligomerization [e.g. Lannfelt et al. 2008] or raise antibody responses to various oligomerization states of Aβ amyloid [e.g. Salloway et al. 2009]. To date no phase III trial in patients with AD has achieved its primary endpoints.
Tau regulation
There is less developmental work in this area and there have to date been very few convincing phase II studies testing drugs that effect tau hyperphosphorylation. The main target of pharmacological intervention has been inhibition of protein kinases considered responsible for the hyperphosphorylation of tau [Imahori and Uchida, 1997]. Clinical testing of kinase inhibitors (for example, lithium and sodium valproate) have however proven disappointing [e.g. Hampel et al. 2009] with regards to lithium and the results from the US-based sodium valproate (VALID) study which recently completed are eagerly awaited. Another approach is to inhibit the aggregation of tau [Bulic et al. 2010]. As yet neither of these approaches has been subject to phase III trials.
Mitochondrial stability
This is a target that has received a lot of attention recently, not least due to the development work on dimebon that enjoyed early success [Doody et al. 2008] but more recent disappointment [Pfizer and Medivation, 2010] and the emergence of the TOMM40 gene as a predictor of disease onset in AD [Roses et al. 2009]. In some regards, mitochondrial dysfunction can be viewed as both an antecedent and consequence to amyloid and tau pathology, perhaps making this the pivotal target for drug development [Moreira et al. 2010].
In summary, a clearer understanding of the disease process in AD has raised hope and expectation of a major therapeutic breakthrough. However, the heterogeneity of patients with clinical dementia entering trials with advanced disease coupled with the poor psychometric properties of clinical outcome measures have conspired to, in part, explain the numerous failures in late-stage clinical trials of disease-modifying drugs. These failures have forced the pharmaceutical industry and academics to pause for reflection. In this pause, better outcome measures will be developed and better diagnostic test batteries will be constructed that will be used in the methodologically more robust and thoughtful clinical trials of the future.
Modifying disease course: 3
In addition to the pharmacological interventions designed specifically to ameliorate the symptom progression in AD various other interventions have been suggested once a diagnosis of dementia or cognitive decline has been reached. These can be divided into three groups. Nonpharmacological interventions, the pharmacological treatment of risk factors (specifically cardiovascular risks), and the use of other drugs or products not licensed specifically for use in dementia, for example nootropics.
Nonpharmacological interventions
Nonpharmacological interventions include the use of cognitive and physical training. Cognitive interventions either aim to improve cognitive function directly or to facilitate the learning of strategies to help compensate for the effects of the disease. Interventions may take the form of reminiscence therapy or the use of errorless learning techniques and be compared with no interaction or an interaction without specific cognitive training. A meta-analysis in this area found several studies and reported a suggestion of benefit, but highlighted several limitations. When the few high-quality studies were examined separately there was an even lower overall effect size [Sitzer et al. 2006] and the overall evidence in favour of cognitive training is limited. Physical training on the other hand does seem to have a positive outcome. A meta-analysis including trials where participants were suffering from dementia, cognitive impairment or had an MMSE <26 found a combined effect size of 0.57 (95% confidence intervals 0.38-0.75), in favour of exercise [Heyn et al. 2004].
Exercise may increase cerebral blood flow and decrease blood pressure and cardiovascular risk. Since cardiovascular risk factors have been shown to increase the risk of developing AD [Helzner et al. 2009] decreasing that risk may help to ameliorate its progression, possibly even after diagnosis. Although in at least one study multiple cardiovascular interventions were attempted and had no impact on disability, cognitive or behavioural outcome [Richard et al. 2009], for individual risk factors the evidence differs.
Pharmacological treatment of risk factors
Studies have found a higher rate of cognitive decline associated with hypertension and a lower rate with antihypertensive medication. In the Cache County study participants who developed incident AD were followed after diagnosis and blood pressures ≥160 mmHg at the visit where diagnosis was made were associated with greater rates of subsequent cognitive decline. Conversely, antihypertensive use was associated with lower rates of decline [Mielke et al. 2007]. Antihypertensive use was associated with lower rates of cognitive decline in a further observational study and angiotensin-converting enzyme (ACE) inhibitors, particularly brain-penetrating ACE inhibitors with lower rates of decline in mild cognitive impairment and mild-to-moderate AD, respectively [Duron et al. 2009; Rozzini et al. 2006; Ohrui et al. 2004]. It seems therefore that antihypertensive use may be important in ameliorating cognitive decline although it is not clear whether antihypertensive type is important [Shah et al. 2009], and not all studies support this [Bellow et al. 2004]. Double-blind, placebo-controlled trials are now needed.
Cholesterol has also been linked to an increased risk of dementia and in this area a robust doubleblind, placebo-controlled trial of atorvastatin has just finished. The ‘Lipitor's Effect in AD’ (LEADe) trial included 640 participants with MMSE scores between 13 and 25 and found no effect of this statin on cognitive functioning [Feldman et al. 2010]. This effectively answers the questions raised by earlier work and small trials such as one published in 2006 [Sparkes et al. 2006] which had suggested a possible benefit from a statin in mild-to-moderate AD albeit with a slightly broad range of cognitive function scores (MMSE 12-28) and only 63 participants completing their first quarterly visit [Sparkes et al. 2006].
Use of other drugs or products not licensed specifically for dementia
Further interventions that have been explored include the use of vitamins B and E or other nootropics, defined as drugs, supplements or functional foods that are purported to improve mental functions. Unfortunately, there is little evidence for nootropics or supplementation. Using B vitamins, two double-blind randomized controlled trials found no effect on cognitive function [Sun et al. 2007] or mild-to-moderate AD [Aisen et al. 2008]. Furthermore, no effect of vitamin E (specifically alpha-tocopherol) was seen in MCI [Peterson et al. 2005]. Although there are some nootropics that have shown promise in small trials such as ginseng [Lee et al. 2008] and saffron or Crocus sativus [Akhondzadeh et al. 2010]; however, these are very small initial trials, in the case of ginseng not blinded and all require replication in a larger more robust context.
In summary, once the diagnosis of cognitive impairment or dementia has been made there would seem to be very little that can slow the rate of decline. Possible exceptions include the use of exercise and antihypertensive medication although this may depend on antihypertensive class. Other potential interventions include some nootropics but these require much more rigorous testing.
Conclusions
Dementia is a progressive, inevitably fatal neurodegenerative disease with wide-ranging effects on the individual affected, their families and carers as well as wider society. The ability to identify and modify risk factors for dementia would have far-reaching implications for each of these groups. If it were possible to intervene at the premanifest stage of disease with, for example, an effective lifestyle intervention it would intuitively be clinically optimal; however, evidence for the effectiveness of such intervention programmes is currently limited. Medications that are being developed to modify the path of dementia are limited by problems with trial execution and inconsistent and inaccurate definition of the diseased population before dementia develops. Moreover, once cognitive impairment has developed there is little evidence to demonstrate that other interventions are able to slow down the rate of decline, with the possible exception of exercise and some antihypertensive agents. These conclusions must though be taken with some caution as all proposed disease-modifying strategies applied at either a population or clinical level must be subject to large and long duration trials where cognition or the development of dementia are the principal outcomes before one can draw firm, unambiguous conclusions.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CWR has been involved as a Chief Investigator for commercially sponsored trials of drugs in development for Alzheimer's disease and has been provided with consultancy fees from several such companies. CWR is also a shareholder with Prana Biotechnology who is developing PBT2 which is a drug in development to treat Alzheimer's disease and cited in this manuscript.
References
- Aisen P., Scheider L., Sano M., Diaz-Arrastia R., Dyck C., Weiner M., et al. for the Alzheimer Disease Cooperative Study Group (2008) High-dose B vitamin supplementation and cognitive decline in Alzheimer disease. JAMA 300: 1774–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhondzadeh S., Sabet M., Harirchian M., Togha M., Cheraghmakini H., Razeghi S., et al. (2010) A 22-week, multicenter, randomised, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psychopharmacology 207: 637–643 [DOI] [PubMed] [Google Scholar]
- Alexopoulos G., Meyers B., Young R., Mattis S., Kakuma T. (1993) The course of geriatric depression with ‘reversible dementia’: A controlled study. Am J Psychiatry 150: 1693–1699 [DOI] [PubMed] [Google Scholar]
- Allen K.V., Frier B.M., Strachan M.W.J. (2004) The relationship between type 2 diabetes and cognitive dysfunction: Longitudinal studies and their methodological limitations. Eur J Pharmacol 490: 169–175 [DOI] [PubMed] [Google Scholar]
- Andel R., Crowe M., Pedersen N.L., Fratiglioni L., Johansson B., Gatz M. (2008) Physical exercise at midlife and risk of dementia three decades later: A population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci 63: 62–66 [DOI] [PubMed] [Google Scholar]
- Anstey K.J., Mack H.A., Cherbuin N. (2009) Alcohol consumption as a risk factor for dementia and cognitive decline: Meta-analysis of prospective studies. Am J Geriatr Psychiatry 17: 542–555 [DOI] [PubMed] [Google Scholar]
- Barberger-Gateau P., Letenneur L., Deschamps V., Peres K., Dartigues J., Rnaud S. (2002) Fish, meat and risk of dementia: Cohort study. BMJ 325: 932–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker W., Luis C., Kashuba A., Luis M., Harwood D., Loewenstein D., et al. (2002) Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord 16: 203–212 [DOI] [PubMed] [Google Scholar]
- Bellow K., Pigeon J., Stang P., Fleishman W., Gardner R., Baker W. (2004) Hypertension and the rate of cognitive decline in patients with dementia of the Alzheimer type. Alzheimer Dis Assoc Disord 18: 208–213 [PubMed] [Google Scholar]
- Bendlin B.B., Carlsson C.M., Gleason C.E., Johnson S.C., Sodhi A., Gallagher C.L., et al. (2010) Midlife predictors of Alzheimer's disease. Maturitas 65: 131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R., Gray S., Kawas C. (1998) Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health 88: 1337–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels G.J., Staekenborg S., Brunner E., Brayne C., Scheltens P. (2006) Risk of dementia in diabetes mellitus: A systematic review. Lancet 5: 64–74 [DOI] [PubMed] [Google Scholar]
- Bulic B., Pickhardt M., Mandelkow E.M., Mandelkow E. (2010) Tau protein and tau aggregation inhibitors. Neuropharmacology, [ePub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Collins R., Peto R., Armitage J. (2002) The MRC/BHF Heart Protection Study: Preliminary results. Int J Clin Practice 56: 53–56 [PubMed] [Google Scholar]
- Comas-Herrera A., Wittenberg R., Pickard L., Knapp M. (2007) Cognitive impairment in older people: Future demand for long-term care services and the associated costs. Int J Ger Psych 22: 1037–1045 [DOI] [PubMed] [Google Scholar]
- Cramer C., Haan M.N., Galea S., Langa K.M., Kalbfleisch J.D. (2008) Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology 71: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q., Borenstein A., Wu Y., Jackson J., Larson E. (2006) Fruit and vegetable juices and Alzheimer's disease: The Kame project. Am J Med 119: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore E., Grodstein F., Rooij F., Hofman A., Stampfer M., Witteman J., et al. (2010) Dietary antioxidants and long-term risk of dementia. Arch Neurol 67: 819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody R.S., Gavrilova S.I., Sano M., Thomas R.G. (2008) Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: A randomised, double-blind, placebo-controlled study. Lancet 372: 207–215 [DOI] [PubMed] [Google Scholar]
- Dotson V.M., Beydoun M.A., Zonderman A.B. (2010) Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 75: 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron E., Rigaud A., Dubail D., Mehrabian S., La tour F., Seux M., et al. (2009) Effects of antihypertensive therapy on cognitive decline in Alzheimer's disease. Am J Hypertension 22: 1020–1024 [DOI] [PubMed] [Google Scholar]
- Feigen V., Ratnasabapathy Y., Anderson C. (2005) Does blood pressure lowering treatment prevents dementia or cognitive decline in patients with cardiovascular and cerebrovascular disease? J Neurol Sci 229-230: 151–155 [DOI] [PubMed] [Google Scholar]
- Feldman H., Doody R., Kivipelto M., Sparks D., Waters D., Jones R., et al. (2010) Randomised controlled trial of atorvastatin in mild to moderate Alzheimer disease. Neurology 74: 956–964 [DOI] [PubMed] [Google Scholar]
- Ferri C.P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M., et al. (2005) Global prevalence of dementia: A Delphi consensus study. Lancet 366: 2112-2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L., Launer L., Anderson K., Breteler M., Copeland J., Dartigues A. Lobo, et al. for the Neurologic Diseases in the Elderly Research Group (2000a) Incidence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology 54(11): S10–S15 [PubMed] [Google Scholar]
- Fratiglioni L., Paillard-Borg S., Winblad B. (2004) An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 3: 343–353 [DOI] [PubMed] [Google Scholar]
- Fratiglioni L., Wang H.X., Kjerstin B.A. Ericsson, Maytan M., Winblad B. (2000b) Influence of social network on occurrence of dementia: A community-based longitudinal study. Lancet 355: 1315–1319 [DOI] [PubMed] [Google Scholar]
- Gold G. (1998) Reevaluating the role of vascular changes in the differential diagnosis of Alzheimer's disease and vascular dementia. Eur Neurol 40: 121–129 [DOI] [PubMed] [Google Scholar]
- Golomb B.A., Criqui M.H., White H.L., Dimsdale J.F. (2004) The UCSD Statin Study: A randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials 25: 178–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R.C., Schneider L.S., Amato D.A., Beelen A.P., et al. (2009) Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. AMA 302: 2557–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley J. Evans, Areosa A. Sastre. (2009) Effect of the treatment of Type II diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev 1: CD003804 DOI: 10.1002/14651858.CD003804. [DOI] [PubMed] [Google Scholar]
- Haag M.D.M., Hofman A., Koudstaal P.J., Stricker B.H.C., Breteler M.M.B. (2009) Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry 80: 13–17, DOI: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- Hakansson K., Rovio S., Helkala E.L., Vilska A.R., Winblad B., Soininen H., et al. (2009) Association between mid-life marital status and cognitive function in later life: Population based cohort study. BMJ 339: b2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.B., Lipton R.B., Sliwinski R.B., Katz M.J., Derby C.A., Verghese J., et al. (2009) Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 73: 356–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Chida Y. (2009) Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol Med 39: 3–11 [DOI] [PubMed] [Google Scholar]
- Hampel H., Ewers M., Bürger K., Annas P., Mörtberg A., Bogstedt A., et al. (2009) Lithium trial in Alzheimer's disease: A randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry 70: 922–931 [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group (2002) MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: A randomised placebo controlled trial. Lancet 360: 7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner E., Luchsinger J., Scarmeas N., Cosentino S., Brickman A., Glymour M., et al. (2009) Contribution of vascular risk factors to the progression Alzheimer disease. Arch Neurol 66: 343-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn P., Abreu B., Ottenbacher K. (2004) The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Physical Med Rehab 85: 1694-1704 [DOI] [PubMed] [Google Scholar]
- Imahori K., Uchida T. (1997) Physiology and pathology of tau protein kinases in relation to Alzheimer's disease. J Biochem 121: 179–188 [PubMed] [Google Scholar]
- Jellinger K. (2002) Alzheimer disease and cerebrovascular pathology: An update. J Neural Trans 109: 813–836 [DOI] [PubMed] [Google Scholar]
- Jorm A.F. (2000) Is depression a risk factor for dementia or cognitive decline? A review. Gerontology 46: 219–227 [DOI] [PubMed] [Google Scholar]
- Jorm A.F. (2001) History of depression as a risk factor for dementia: An updated review. Aust NZ J Psychiatry 35: 776–781 [DOI] [PubMed] [Google Scholar]
- Kalmijn S., Foley L., White C.M., Burchfiel J.D., Curb H., Petrovitch G.W., et al. (2000) Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men: The Honolulu-Asia Aging Study. Arterios Thromb Vasc Bio 20: 2255–2260 [DOI] [PubMed] [Google Scholar]
- Kivipelto M., Helkala E.L., Laakso M.P., Hänninen T., Hallikainen M., Alhainen K. (2001) Midlife vascular risk factors and Alzheimer's disease in later life: Longitudinal, population based study. BMJ 322: b1447–b1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M., Ngandu T., Fratiglioni T., Viitanen M., Kåreholt I., Winblad B. (2005) Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62: 1556–1560 [DOI] [PubMed] [Google Scholar]
- Kivipelto M., Ngandu T., Laatikainen T., Winblad B., Soininen H., Tuomilehto J. (2006) Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population based study. Lancet Neurol 5: 735–741 [DOI] [PubMed] [Google Scholar]
- Lannfelt L., Blennow K., Zetterberg H., Batsman S., Ames D., Harrison J., et al. (2008) Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer's disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 7: 779–786 [DOI] [PubMed] [Google Scholar]
- Larrieu S., Letenneur L., Helmer C., Dartigues J.-F., Barberger-Gateau P. (2004) Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J Nutrition Health Aging 8: 150–154 [PubMed] [Google Scholar]
- Lautenschlager N.T., Cox K.L., Flicker L., Foster J.K., van Bockxmeer F.M., Xiao J., et al. (2008) Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: A randomized trial. JAMA 300: 1027–1037 [DOI] [PubMed] [Google Scholar]
- Lee S., Chu K., Sim J., Heo J., Kim M. (2008) Panax Cinseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord 22: 222–226 [DOI] [PubMed] [Google Scholar]
- Lobo A., Launer L., Fratiglioni L., Anderson K., Di Carlo A., Breteler M., et al. (2000) Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurol 54: S4–S9 [PubMed] [Google Scholar]
- Luchsinger J.A., Reitz C., Honig L.S., Tang M.X., Shea S., Mayeux R. (2005) Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 65: 545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo-Fernandez R., Leal J., Gray A. (2010) Dementia 2010: The economic burden of dementia and associated research funding in the United Kingdom. A report produced by the Health Economics Research Centre, University of Oxford for the Alzheimer's Research Trust.
- Mason S.E., McShane R., Ritchie C.W. (2010) Diagnostic tests for Alzheimer's disease: Rationale, methodology, and challenges. Int J Alzheimer's Dis, [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard C.J., Bush A.I., Masters C.L., Cappai R., Li Q.X. (2005) Metals and amyloid-beta in Alzheimer's disease. Int J Exp Pathol 86: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness B., Craig D., Bullock R., Passmore P. (2009a) Statins for the prevention of dementia. Cochrane Database Syst Rev 2: CD003160, DOI: 10.1002/14651858.CD003160.pub2. [DOI] [PubMed] [Google Scholar]
- McGuinness B., Todd S., Passmore P., Bullock R. (2009b) Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev 4: CD004034, DOI: 10.1002/14651858.CD004034.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M., Rosenburg P., Tschanz J., Cook L., Corcoran C., Hayden K., et al. (2007) Vascular factors predict rate of progression in Alzheimer disease. Neurology 69: 1850–1858 [DOI] [PubMed] [Google Scholar]
- Moreira P.I., Zhu X., Wang X., Lee H.G., Nunomurac A., Petersen R.B., et al. (2010) Mitochondria: A therapeutic target in neurodegeneration. Biochim Biophys Acta 1802: 212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M., Evans D., Tangney C., Bienias J., Wilson R., Aggarwal N., et al. (2005) Relation of tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr 81: 508–514 [DOI] [PubMed] [Google Scholar]
- Ohrui T., Tomita N., Sato-Nakagawa T., Matsui T., Maruyama M., Niwa K., et al. (2004) Effects of brain-penetrating ACE inhibitor on Alzheimer disease progression. Neurology 63: 1324–1325 [DOI] [PubMed] [Google Scholar]
- Ownby R.L., Crocco E., Acevedo A., John V., Loewenstein D. (2006) Depression a risk factor for alzheimer disease: Systematic review, meta-analysis, and metaregession analysis. Arch Gen Psychiatry 63: 530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R., Poulter R., Warner J., Beckett N., Burch L., Bulpitt C. (2008) Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatrics 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R., Thomas R., Grundman M., Bennett D., Doody R., Ferris S., et al. for the Alzheimer's disease cooperative study group (2005) N Engl J Med 352: 2379–238815829527 [Google Scholar]
- Pfizer and Medivation (2010) Pfizer and Medivation announce results from two phase 3 studies in dimebon (latrepirdine) Alzheimer's disease clinical development program, http://investors.medivation.com/releasedetail.Cfm?ReleaseID=448818
- Qiu C., von Strauss E., Fastbom J., Winblad B., Fratiglioni L. (2003) Low blood pressure and risk of dementia in the Kungsholmen Project: A 6-year follow-up study. Arch Neurol 60: 223–228 [DOI] [PubMed] [Google Scholar]
- Qiu C., Winblad B. (2005) The age dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 4: 487–499 [DOI] [PubMed] [Google Scholar]
- Reiman E.M., Chen K., Langbaum J.B.S., Lee W., Reschke C., Brandy D., et al. (2010) Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer's disease and normal aging. Neuroimage 49: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E., Kuiper R., Dijkgraaf M.G., Van Gool W.A. (2009) Vascular care in patients with Alzheimer's disease with cerebrovascular lesions:a randomized clinical trial. JAm Geriat Soc 57: 797–805 [DOI] [PubMed] [Google Scholar]
- Ritchie K., Carrière I., Ritchie C.W., Berr C., Artero S., Ancelin M. (2010) Can we design prevention programs to reduce dementia incidence? A prospective study of modifiable risk factors. BMJ 5: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren A., Skoog I., Gustafson D., Wilhelmsen L. (2005) Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med 165: 321–326 [DOI] [PubMed] [Google Scholar]
- Roses A.D., Lutz M.W., Amrine-Madsen H., Saunders A.M., Crenshaw D.J., Sundseth S., et al. (2009) A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer's disease. Pharmacogenomics J 10: 375-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovio S., Kareholt I., Helkala E.L., Viitanen M., Winblad B., Tuomilehto J., et al. (2005) Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol 4: 705–711 [DOI] [PubMed] [Google Scholar]
- Rozzini L., Chilovi B., Bertoletti E., Conti M., Rio I., Trabucchi M., et al. (2006) Angiotensin converting enzyme (ACE) inhibitors modulate the rate of progression of amnestic mild cognitive impairment. Int J Geriatric Psychiatry 21: 550–555 [DOI] [PubMed] [Google Scholar]
- Saczynski J.S., Beiser A., Seshadri S., Auerbach S., Wolf P.A., Au R. (2010) Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology 5: 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S., Sperling R., Gilman S., Fox N.C., et al. (2009) A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73: 2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Luchsinger J.A., Schupf N., Brickman A.M., Cosentino S., Tang M.X., et al. (2009) Physical activity, diet, and risk of Alzheimer's disease. JAMA 302: 627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaider M. Beeri, Goldbourt U., Silverman J.M. (2004) Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology 63: 1902-1907 [DOI] [PubMed] [Google Scholar]
- Shah K., Salah Q., Johnson M., Parikh N., Schulz P., Kunik M. (2009) Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. Am J Geriatric Pharmacotherapy 7: 250–261 [DOI] [PubMed] [Google Scholar]
- Shepherd J., Blauw G.J., Murphy M.B., Bollen E.L.E.M., Buckley B.M., Cobbe S.M. (2002) Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 360: 1623-1630 [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A., Marmot M. (2005) High blood pressure was associated with cognitive function in middle-age in the Whitehall II study. J Clin Epidemiol 58: 1308–1315 [DOI] [PubMed] [Google Scholar]
- Sitzer D., Twamley E., Jeste D. (2006) Cognitive training in Alzheimer's disease: A meta-analysis of the literature. Acta Psychiatrica Scandinavia 114: 75–90 [DOI] [PubMed] [Google Scholar]
- Sofi F., Cesari F., Abbate R., Gensini G.F., Casini A. (2008) Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 337: b1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks D., Scheff S., Liu H., Landers T., Coyne C., Hunsacker J. (1995) Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci 131: 162–169 [DOI] [PubMed] [Google Scholar]
- Sparkes D., Sabbagh M., Connor D., Soares H., Lopez J., Stankovic G., et al. (2006) Statin therapy in Alzheimer's disease. Acta Neurologica Scandinavia 114 (Suppl 185): 78-86 [DOI] [PubMed] [Google Scholar]
- Sun Y., Lu C., Chien K., Chen S., Chen R. (2007) Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer's disease: A 26-week, randomised, doubleblind, placebo-controlled study in Taiwanese patients. Clin Therapeut 29: 2204–2214 [DOI] [PubMed] [Google Scholar]
- Szwast S.J., Hendrie H.C., Lane K.A., Gao S., Taylor E., Unverzagt F., et al. (2007) Association of statin use with cognitive decline in elderly African Americans. Neurology 69: 1873–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer R.A., Gunderson E.P., Barrett-Connor E., Quesenberry E.P., Jr, Yaffe K. (2005a) Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 330: 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer R.A., Sidney S., Selby J., Claiborne S. Johnston, Yaffe K. (2005b) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64: 277–281 [DOI] [PubMed] [Google Scholar]
- Whitmer R.A., Gustafson D.R., Barrett-Connor E., Haan M.N., Gunderson E.P., Yaffe K. (2008) Central obesity and increased risk of dementia more than three decades later. Neurology 71: 1057–1064 [DOI] [PubMed] [Google Scholar]
- Wilson R.S., Hoganson G.M., Rajan K.B., Barnes L.L., de Leon C.F. Martinez, Evans D.A. (2010) Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology 75: 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Caracciolo B., Wang H.X., Winblad B., Bäckman L., Qiu C., et al. (2010) Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes, [ePub ahead of print]. [DOI] [PMC free article] [PubMed]
- Xu W., Qiu C., Gatz M., Pedersen N.L., Johansson B., Fratiglioni L. (2009) Mid-and late-life diabetes in relation to the risk of dementia: A population-based twin study. Diabetes 58: 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Qiu C., Winblad B., Fratiglioni L. (2007) The effect of borderline diabetes on the risk of dementia and Alzheimer's disease. Diabetes 56: 211–216 [DOI] [PubMed] [Google Scholar]
- Yamada M., Kasagi F., Sasaki H., Masunari N., Mimori Y., Suzuki G. (2003) Association between dementia and midlife risk factors: The Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc 51:410–414 [DOI] [PubMed] [Google Scholar]
- Zandi P.P., Sparks L., Khachaturian A.S., Tschanz J., Norton M., Steinberg M., et al. (2005) Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry 62: 217–224 [DOI] [PubMed] [Google Scholar]
- Zou K., Gong J.S., Yanagisawa K., Michikawa M. (2002) A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J Neurosci 22: 4833–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]