Abstract
It is increasingly accepted that the effects of gastro-oesophageal reflux are not limited to the gastrointestinal tract. The adjacent respiratory structures are also at risk from material ejected from the proximal oesophagus as a result of the failure of anatomical and physiological barriers. There is evidence of the influence of reflux on several respiratory and otorhinological conditions and although in many cases the precise mechanism has yet to be elucidated, the association alone opens potential novel avenues of therapy to clinicians struggling to treat patients with apparently intractable respiratory complaints. This review provides a description of the airway reflux syndrome, its effects on the lung and current and future therapeutic options.
Keywords: airways reflux, cough, respiratory disease, therapy
Introduction
The relationship between gastro-oesophageal reflux (GOR) and respiratory disease is controversial. There is no doubt that the lungs can suffer damage as a direct result of aspiration of gastric contents causing a chemical injury, but aspiration pneumonitis is merely the most obvious clinical manifestation of a more complex and diverse process. GOR can be the primary cause of respiratory symptoms such as chronic cough and can also complicate established respiratory disease.
The terminology employed to describe GOR affecting the respiratory tract is varied and contributes to the lack of clarity in diagnosis. Laryngopharyngeal reflux (LPR) is recognised as a distinct clinical entity caused by reflux of gastric contents to the laryngopharynx [Pontes and Tiago, 2006]. A similar condition with fluoroscopic oesophageal abnormalities has been termed esophagopharyngeal reflux [Belafsky et al. 2008] and the cumbersome alternative terms supraoesophageal and extraoesophageal reflux also encompass the effects of gastric content entering the remainder of the upper respiratory tract [Wiener et al. 2009; Koufman et al. 2002]. We prefer the simpler term ‘airway reflux’ to describe the broader phenomenon.
Evolution of humans into upright, bipedal organisms has led to a unique predisposition to GOR and consequent aspiration. Recent advances in characterisation of the pathophysiology, including both the composition of the refluxate, which can be gaseous and nonacid, and the mechanisms of damage to the respiratory epithelium have led to insights into the development of clinical manifestations in the upper and lower respiratory tracts. We present an overview of the syndrome which, when recognised, makes a wide variety of therapeutic options available to the clinician.
Epidemiology
The association of GOR with various respiratory conditions is well described in epidemiological studies. The diagnostic criteria used are diverse, ranging from acid reflux symptoms coexisting with asthma and other respiratory symptoms as in the Nord-Trøndelag Health Survey [Nordenstedt et al. 2006] to the presence of erosive oesophagitis or strictures as investigated by el-Serag and Sonnenberg [El-Serag and Sonnenberg, 1997] in a retrospective review of over 100,000 cases. The latter study found positive associations with sinusitis, aphonia, laryngitis and laryngeal stenosis in the upper respiratory tract as well as lower tract features such as chronic bronchitis, asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, bronchiectasis, pulmonary collapse and pneumonia. Smaller studies have shown that the presence of a hiatus hernia or reflux oesophagitis is associated with an increased likelihood of respiratory related hospitalisation [Ruhl et al. 2001] and the RHINE study group demonstrated that nocturnal GOR is independently associated with the onset of asthma and respiratory symptoms [Gunnbjörnsdóttir et al. 2004]. Data from a cross-sectional questionnaire survey of 4000 patients in Yorkshire showed a relationship between chronic cough, regurgitation and irritable bowel syndrome [Ford et al. 2006].
Whether the relationship is causal or coincidental is difficult to determine from retrospective studies. Analysis from the prospective ProGERD study [Jaspersen et al. 2003] found a high prevalence (32.8%) of extraoesophageal symptoms developing in patients presenting with heartburn which was significantly higher in patients with erosive oesophagitis, particularly in those with more severe endoscopic grading of oesophageal disease implying an exposure—response relationship.
The use of acid-related GOR disease and symptomatology as a marker may in fact underestimate the true prevalence of airway reflux but the diversity of clinical manifestations and lack of a reliable diagnostic test precludes direct study.
Pathophysiology
Reflux reaches the airway as a result of an inherent susceptibility to the condition in humans combined with the failure of physiological defence mechanisms. Several components of gastric contents then have the potential to stimulate sensory receptors and trigger respiratory symptoms.
Evolutionary disadvantages
Two factors in human evolution, which are significant in terms of survival advantage, mean we are uniquely predisposed to airway reflux. Firstly, the relatively rapid transition to bipedal locomotion was not matched by compensatory changes in the gastrointestinal tract. In other mammals, the oesophagus is near horizontal, with food boluses dropping into the vertically hanging stomach as they traverse the diaphragm. In humans, the upper gastrointestinal tract is straightened out, removing the anatomical barrier preventing reflux when the lower oesophageal sphincter (LOS) opens. The crura of the diaphragm partially compensate by creating a bend in the lower oesophagus, but using a mobile structure to accomplish this has drawbacks; reflux on phonation is common, particularly with laughing as the intra-abdominal pressure oscillates. An even more significant evolutionary advantage is the development of speech and language, but this has also increased the risk of aspiration because of the changes in the laryngeal sphincter. The deepening of the larynx and separation of the soft palate from the epiglottis and arytenoids leaving the oropharynx permanently expanded is particularly ineffective at preventing transit of material, either from the oral cavity or regurgitated from the stomach [Laitman and Reidenberg, 1997].
Physiological defences
Despite the evolutionary predisposition, reflux is not universal. There remain barriers which prevent the free transit of gastric material to the airway, namely the lower and upper oesophageal sphincters. Abnormalities of these increase the likelihood of symptomatic GOR or airway reflux, for example patients with a hiatus hernia are inherently more susceptible to GOR because of the malposition of the LOS in the thoracic cavity with the consequent loss of the diaphragmatic component of sphincter tone. LOS hypotonia and an increased frequency of transient LOS relaxation (TLOSR) episodes are the principle mechanisms thought to contribute to GOR, with studies suggesting that the former is the more significant factor for reflux to the proximal oesophagus [Grossi et al. 2001]. There is also evidence which points to TLOSR as a likely culprit, particularly in airway reflux. The episodes are detectable in normal controls, with early investigations in asymptomatic volunteers demonstrating up to 24 episodes related to TLOSRs over a 12 h period [Dent et al. 1980]. Increased TLOSR frequency was thought to be contributory to the development of GOR symptoms, however more recent work suggests that there is no difference in the frequency between patients and controls [Trudgill and Riley, 2001]. In the latter study, there was also no difference in the number of reflux episodes detected using intraluminal pressure monitoring but episodes in patients with GOR were more frequently associated with a drop in pH, suggesting that acidity of the reflux is key to the development of the classical GOR symptom of dyspepsia. Clearance of acid from the oesophagus and a return to baseline pH is considered a useful marker of oesophageal function [Helm, 1986]. Postma and colleagues used dual probe pH monitoring to show that oesophageal pH returns to normal more quickly in patients with LPR alone than in those with GOR [Postma et al. 2001]. The authors concluded that patients with LPR alone had superior oesophageal function; however they also found that the LPR group had a longer clearance time than normal controls. The degree of acidity does not seem to affect the opening of the upper oesophageal sphincter (UOS) which is normally associated with TLOSR episodes and changes in oesophageal intraluminal pressure [Pandolfino et al. 2007]. Investigation of patients with chronic cough showed a third of patients had normal pH studies despite abnormal oesophageal manometry [Kastelik et al. 2003]. Taken together, these data support two distinct pathophysiological phenotypes. Acidic reflux causing classical GOR with heartburn, related to LOS hypotonia or anatomical abnormalities, and nonacid, gaseous reflux related to TLOSR episodes and leading to airway reflux.
Reflux composition
Gaseous reflux has been well described with studies using multichannel impedance monitoring showing over a third of normal controls having reflux with a detectable gaseous component. Unsurprisingly, the gaseous material was more likely to reach the proximal oesophagus than the liquid component, suggesting that it is the more likely mechanism of airway reflux [Balaji et al. 2003]. This raises the question of whether the damaging agent in reflux can be acid alone since the pH of gaseous reflux can be mildly acidic [Kawamura et al. 2004]. Studies in the oesophagus have shown that the mucosal effects are similar on perfusion with acidic and nonacid solutions [Farré et al. 2010] and that bile exposure is commonly present in patients with severe mucosal damage and is associated with worse oesophageal dysfunction [Oh et al. 2006]. Experimental exposure of laryngeal epithelial cells to pepsin has shown uptake and cell damage at pH 7.4 [Johnston et al. 2009], illustrating that damage to airway epithelium can indeed occur as a response to nonacid reflux. The question of how airway damage can occur despite a lack of apparent oesophageal symptoms can be explained by the increased susceptibility of the airway to damage compared with the robust oesophageal mucosa. Experimental evidence shows that carbonic anhydrase III activity is increased in the oesophagus in patients with GOR, theoretically providing a defence mechanism against acid injury, but this activity is reduced in the laryngeal tissue of patients with LPR [Axford et al. 2001]. Other mechanisms such as depletion of E-cadherin cell adhesion molecules may also contribute [Johnston et al. 2003].
Cough reflex hypersensitivity
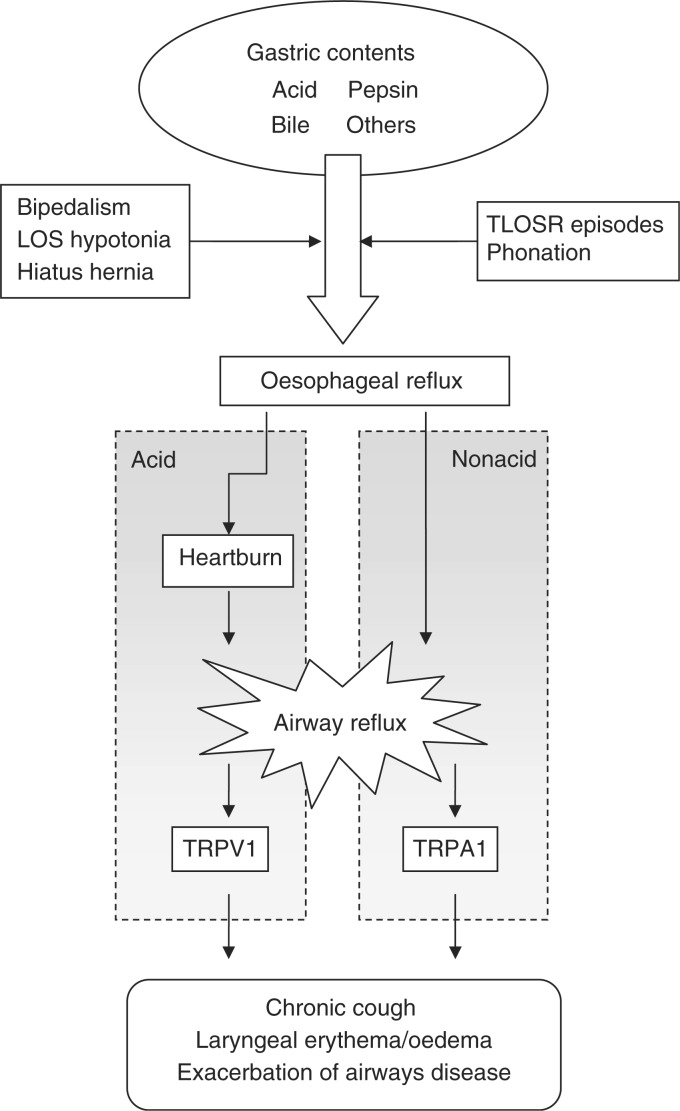
The process by which reflux causes respiratory symptoms, and in particular chronic cough is likely to be a combination of a heightened sensitivity to cough stimuli and the recurrent trigger of reflux episodes. Coughing is a physiological defence mechanism which is thought to be neurogenically mediated via transient receptor potential (TRP) ion channels on airway sensory neurones (Figure 1). The vanilloid sensitive (TRPV1) receptor is present in the airway [Watanabe et al. 2005] and is stimulated by a group of compounds which includes capsaicin, a substance derived from chilli peppers used as a tussive agent in cough challenge testing [Midgren et al. 1992]. It is also activated by acid [Bevan and Gepetti, 1994], providing a link both to the use of citric acid as a cough stimulant and to acidic reflux events. The molecularly similar TRPA1 (ankyrin) receptor is coexpressed with TRPV1 [Story et al. 2003] and is stimulated by several other substances including foodstuffs [Jordt et al. 2004] which have potential significance in nonacid reflux. Both receptors have been shown to contribute to neurogenic inflammation in animal studies [Wei et al. 2010; Trevisani et al. 2004a). Expression of TRPV1 is increased on afferent neurones in the respiratory tract in patients with chronic cough [Mitchell et al. 2005] and also in patients with oesophagitis [Matthews et al. 2004].
Figure 1.
Proposed mechanism of reflux to the airway causing respiratory symptoms. LOS, lower oesophageal sphincter; TLOSR, transient lower oesophageal sphincter relaxation; TRPA1, transient receptor potential (ankyrin receptor); TRPV1, transient receptor potential (vanilloid sensitive).
Capsaicin cough sensitivity increases in response to upper respiratory tract infection [O'Connell et al. 1996] and has also been demonstrated in patients with asthma, COPD [Doherty et al. 2000a] and fibrosing alveolitis [Doherty et al. 2000b]. TRPV1 anatagonists have been shown to reduce cough frequency experimentally [Trevisani et al. 2004b] and have the potential to become useful therapeutic agents in clinical practice.
Respiratory disease and airway reflux
The association of clinical respiratory symptoms and reflux has been investigated from several perspectives and as a consequence is marred with confusing terminology. The gastroenterological view is of extraoesophageal manifestations appended to the spectrum of GOR disease, while the respiratory-biased reflux cough and reflux asthma syndromes focus on the effects rather than the cause of airway reflux [Vakil et al. 2006]. Laryngopharyngeal reflux sits both anatomically and physiologically between the two. A closer inspection reveals a common symptomatology between all three groups, with complaints such as globus, throat clearing and soreness, dysphonia, an unpleasant or metallic taste in the throat and often a chronic intractable cough featuring prominently [Everett and Morice, 2007; Pontes and Tiago, 2006]. More typical symptoms of breathlessness and wheeze and the corresponding heartburn and dysphagia can be absent, with estimates ranging from 30% to 75% of the time [Everett and Morice, 2007; Jaspersen et al. 2006; Irwin et al. 1990] and are thus less useful in diagnosis.
Chronic cough
The most ubiquitous symptom of airway reflux is chronic cough, often occurring in the absence of heartburn [Jaspersen et al. 2006]. The precise mechanism is again a matter of debate. Impairment of laryngopharyngeal mechanosensitivity has been demonstrated [Phua et al. 2005] and there is also evidence that weakly acidic reflux can be significant [Pauwels et al. 2009]. In our own survey, regurgitation rather than heartburn was most strongly associated with cough [Ford et al. 2006]. Although patients with chronic cough have not been shown to have an increased frequency of reflux episodes, there is evidence that nonacid reflux events reach the pharynx more often in patients when there is a positive symptom association probability (SAP) between cough and oesophageal reflux episodes [Patterson et al. 2009]. A validated questionnaire (available at www.issc.info) has been developed to capture the symptom complex associated with airway reflux induced cough [Morice et al. 2011].
Asthma
Asthma is a heterogeneous entity with few adult patients conforming to the typical childhood atopic phenotype. In cough-variant asthma, isolated chronic cough may be the only presenting feature, with the final diagnosis being dependent on a response to antiasthma therapy [Dicpinigaitis, 2006]. Difficult asthma is often thought to be principally related to poor adherence to treatment. However, GOR and microaspiration may contribute to airway inflammation and exacerbation in allergic asthma [Spaulding et al. 1982; Mays, 1976] and patients with GOR may develop eosinophilic airway hypersensitivity and an asthma-like syndrome characterised by unpredictable paroxysms of coughing, wheezing and breathlessness [Kiljander and Laitinen, 2004] which suggests a degree of overlap between typical or cough variant asthma and reflux-induced chronic cough. Up to 80% of patients with asthma have been found to have abnormal acid reflux on 24 h pH monitoring [Sontag et al. 1990] and tracheal aspiration has also been demonstrated [Jack et al. 1995]. Wu et al. [2002] showed an increase in capsaicin challenge cough sensitivity in response to acid perfusion of the oesophagus in patients with mild asthma and chronic cough.
Recent work has revealed a potential link between airway hypersensitivity and GOR with increased airway tachykinin levels found in induced sputum samples from patients with asthma and chronic cough [Patterson et al. 2007]. Tachykinin-mediated neurogenic inflammation has been suggested as a mechanism in the development of a response to inhaled irritants and is potentially important in patients with asthma [Barnes, 2001]. Paradoxically, treatments for asthma may also worsen reflux, with theophyllines [Berquist et al. 1981] and beta-2 adrenoreceptor agonists [Lacy et al. 2008] having the potential to reduce the LOS pressure.
Chronic obstructive pulmonary disease
Patients with established lung disease are likely to be more susceptible to the effects of airway reflux. Patients with COPD have a high prevalence of reflux into the proximal oesophagus, perhaps contributed to by the changes in thoracic anatomy seen in the later stages of the condition. Interestingly, only a minority of patients studied reported typical heartburn [Kempainen et al. 2007; Casanova et al. 2004]. The combination of LPR symptoms and laryngoscopic findings with COPD has also been investigated with the dual diagnosis made in 44% of patients studied [Eryuksel et al. 2009]. Many patients with COPD are recurrently admitted to secondary care in exacerbation with no clinical evidence of acute infection. If a proportion of these are due to airway reflux, a new and potentially important avenue of treatment becomes available for these highly resource consuming patients.
Cystic fibrosis
An increased prevalence of symptomatic GOR is well documented in patients with cystic fibrosis [Blondeau et al. 2008a]. Recent work shows this may persist despite acid suppression therapy and can be associated with worsening of respiratory symptoms [Sabati et al. 2010]. Objective measurements have demonstrated abnormal oesophageal function with reduced LOS pressures and positive 24 h pH studies [Ledson et al. 1998].
Interstitial lung disease
Numerous studies have shown an association between GOR and interstitial lung disease (ILD) based on an association with hiatus hernia [Mays et al. 1976] and demonstrable reflux on pH monitoring [Salvioli et al. 2006]. Chronic microaspiration has been suggested as a mechanism, although the specific pathophysiology, particularly in relation to the newer definitions of ILD, has yet to be determined [Lee et al. 2010]. ILD, and in particular usual interstitial pneumonia, is generally a progressive disease with few effective treatments available. Confirmation of an airway reflux aetiology would again provide new options.
Other respiratory disease
Links have been made between the presence of airway reflux and several other respiratory diseases, however whether this simply a chance finding or a causal relationship requires further study. Obstructive sleep apnoea has been associated with chronic cough, with changes in nocturnal oesophagaeal function demonstrated. Interestingly these appear to be protective against reflux [Kuribayashi et al. 2010] despite obesity being a major risk factor for both obstructive sleep apnoea and GOR [Jacobson et al. 2006]. Both acid reflux and nonacid reflux are prevalent in lung transplant recipients [Blondeau et al. 2008b] and early development of the bronchiolitis obliterans syndrome causing chronic rejection is associated with the presence of bile acids in bronchoalveolar lavage fluid [D'Ovidio et al. 2006].
Otorhinological manifestations
The diagnosis of laryngopharyngeal reflux (LPR), the most frequently used descriptor, is essentially a clinical one based on typical symptoms of globus pharyngeus, dysphonia, excessive throat clearing and soreness [Pontes and Tiago, 2006]. In addition, endoscopic visualisation and scoring of the laryngeal appearance [Belafsky et al. 2001] is used to exclude malignancy and assess for abnormalities. Features of vocal cord oedema with mucosal abnormalities of the cords and intra-arytenoid notch were found to reliably distinguish symptomatic cases from controls [Jonaitis et al. 2006]. It is comparatively widely recognised by otolaryngologists and commonly treated with proton pump inhibitors (PPIs) [Karkos et al. 2007].
Investigation
To date, there is no gold standard test to confirm the presence of airway reflux or to clearly differentiate it from other causes of respiratory symptoms. Eliciting the characteristic clinical features, with an empirical trial of antireflux medication is the mainstay of the current diagnostic process. A successful trial of high-dose proton pump inhibitor was considered diagnostic and is recommended in guidelines for chronic cough [Morice et al. 2007] and LPR [Koufman et al. 2002].
Recent work however suggests that this may be unreliable, even in patients with classic GOR symptoms [Aanen et al. 2006].
The detection of pepsin by immunoassay in sputum has been shown to be highly specific and sensitive for LPR (diagnosed by dual probe oesophageal pH monitoring) [Knight et al. 2005] and this noninvasive test may prove to be a useful marker of airway reflux and could be extended to other respiratory samples, such as exhaled breath condensate or bronchial lavage.
More invasive methods of investigation such as oesophageal pH monitoring and laryngoscopic examination are used in clinical practice and may have some utility, with studies suggesting that patients with LPR may be differentiated from controls using upper oesophageal acid exposure time [Merati et al. 2005], although other investigators have found no difference in symptoms in patients with and without proximal acid exposure [Cool et al. 2004]. The sensitivity and specificity of oesophageal pH monitoring and laryngoscopic examination may be lacking however and they cannot be considered diagnostic of airway reflux [Oelschlager et al. 2005; Vaezi, 2003]. The concept of using oesophageal pH alone as a marker of reflux has inherent failings because of the effects of nonacid and gaseous reflux. Combined monitoring with electrical impedance shows that nonacid reflux continues despite acid suppression [Zerbib et al. 2008; Mainie et al. 2006) and may be a mixture of liquid and gas which is associated with respiratory symptoms regardless of pH [Tutuian et al. 2008]. New techniques for directly measuring the pH of aerosolised liquids in the pharynx using a minimally invasive probe are promising. We have shown that gaseous reflux events can be detected using this system in patients with normal conventional oesophageal physiology and despite Nissen fundoplication [Molyneux et al. 2010a]. Current analysis however relies on the pharyngeal pH crossing a lower threshold as a marker of a reflux event, with normal values having been defined in an asymptomatic population [Ayazi et al. 2009]. This has similar limitations to oesophageal pH monitoring in that nonacid reflux events responsible for airway symptoms would not be detected. An alternative system based on variation in pH as a marker of reflux events is in development with initial results suggesting that different disease phenotypes may show different patterns of pH variation [Molyneux et al. 2010b].
Therapy
The established therapy for typical gastro-oesophageal reflux is less effective in airway reflux for reasons which are outlined below. Nevertheless, the complex pathophysiological process involved yields several therapeutic targets. Novel approaches are necessary but most can be accomplished with simple interventions, often using established, inexpensive medication.
Pharmacological agents
PPIs and histamine receptor antagonists are well established as the mainstay of therapy for typical GOR with heartburn symptoms or peptic ulceration. Both have been recommended [Pratter et al. 2006] and are widely used for a variety of airway reflux manifestations. There is some evidence of an effect, for instance a reduction in capsaicin cough sensitivity with omeprazole was demonstrated in patients with asthma [Ferrari et al. 2007] but a recent randomised trial showed no effect of PPIs in poorly controlled asthma [Mastronarde et al. 2009]. The authors concluded that reflux was not a significant contributor to poor asthma control, however the study proves only that acid reflux is not relevant. Several studies have failed to demonstrate an effect on LPR using PPI compared with placebo. A recent Cochrane review of PPI in chronic cough, the most common airway reflux syndrome, concluded that there was insufficient evidence of benefit from a number of small, uncontrolled trials and noted a strong placebo effect [Chang et al. 2006]. A randomised trial was recommended; we have recently completed such a project using twice daily esomeprazole which showed no benefit over placebo overall and suggests that any treatment effect is limited to patients with typical heartburn symptoms (unpublished data).
It is unsurprising that acid suppression alone is insufficient to prevent airway reflux because, as already described, nonacid and gaseous reflux may be more closely related to airway symptoms in contrast to the acid injury mechanism of typical GOR. A more rational approach is to target the failed mechanical barriers to reflux such as TLOSR episodes. TLOSRs are under vagal control [Mittal et al. 1995] and can be inhibited by cholinergic blockade with atropine. Peripheral anticholinergics are ineffective, implying a central mechanism [Fang et al. 1999], but vagal mechanoreceptors at the LOS respond to gastric distension and can be inhibited by gamma-aminobutyric acid (GABA) type B receptor agonists and metabotropic glutamate type 5 receptor (mGluR5) antagonists [Blackshaw, 2008]. Baclofen is an analogue of aminobutyric acid which is widely used for muscle spasticity in multiple sclerosis and other neuromuscular conditions. It has been shown to significantly reduce the frequency of TLOSR [Holloway, 2001] but has a problematic side effect profile. It is our normal practice to use low doses starting at 5 mg three times daily. Lesogaberan, a GABA(B) agonist currently in development, may avoid the adverse central nervous system effects [Bredenoord, 2009] and can also reduce TLOSR frequency and increase LOS pressure [Boeckxstaens et al. 2010]. It has the potential to become the preferred agent for reducing TLOSR frequency if tolerance is superior to baclofen in practice. mGluR5 antagonists have also shown promise in animal studies with 60-90% reduction in TLOSR episodes demonstrated experimentally [Jensen et al. 2005].
Prokinetic agents such as metoclopramide and domperidone as well as macrolide antibiotics such as erythromycin are used in the critical care setting in patients receiving enteral nutrition. Improving gastric motility and preventing delayed gastric emptying reduces the likelihood of reflux and aspiration [Deane et al. 2009]. A study on patients with reflux cough showed that a third of patients not responding to PPI improved with the addition of metoclopramide or cisapride [Poe and Kallay, 2003]. The latter agent has been withdrawn from the market because of a link to long QT syndrome leaving metoclopramide and domperidone as the first-choice agents. Domperidone may be the better option because it avoids the extrapyramidal side effects of metoclopramide and can be used for longer term therapy. Both are used at standard doses of 10 mg three times daily. Low-dose erythromycin (250 mg twice daily) is widely used for motility therapy and the newer macrolide azithromycin has been shown to reduce proximal reflux events, oesophageal acid exposure and bile aspiration in lung transplant recipients [Mertens et al. 2009].
As a consequence of the variable contributions of the different pathophysiological factors, there is no single agent which is effective for all patients. Because of this, we recommend a series of therapeutic trials of each agent, normally for a period of 4 weeks as long as the initial doses are well tolerated. The current lack of a definitive objective test for airway reflux means that any beneficial effect has to be assessed subjectively by the patient.
Nonpharmacological methods
The simplest method for the clinician to suggest is perhaps the most difficult for the patient to achieve; weight loss reduces the tendency to reflux and carries associated benefits in reducing the load on the respiratory system. More specific measures can be suggested, such as avoidance of large meals and carbonated drinks as gastric distension stimulates TLOSR episodes [Scheffer et al. 2002]. Caffeine and nicotine both worsen reflux [Pandolfino and Kahrilas, 2000; Boekema et al. 1999] but whether reducing caffeinated beverage intake or smoking cessation are effective as a treatment modality for airway reflux is difficult to quantify.
At the opposite end of the risk spectrum, surgical procedures are also an option to treat airway reflux. Laparoscopic Nissen fundoplication is well established as a safe and effective treatment for typical GOR [Broeders et al. 2010] and there is increasing evidence of its effectiveness in treating chronic cough and other respiratory symptoms [Fathi et al. 2009; Farrell et al. 2001; Allen and Anvari, 1998]. In the obese, a Rouxen-Y gastric bypass may be a more effective alternative with the combined benefits of weight loss and concurrent improvement in the mechanical barriers to reflux [Ikramuddin, 2008].
Conclusion
Airway reflux is a widespread condition with a characteristic combination of symptoms. It is clearly associated with various lung diseases and ongoing research is providing new insights into the pathological mechanisms involved. Current investigations are limited but provide supporting evidence for the diagnosis and new modalities are becoming available.
Recognition of the syndrome can open a variety of therapeutic possibilities to the clinician for patients who may have been labelled as intractable. The diagnosis should be considered in patients with chronic unexplained cough or episodic breathlessness in the presence of an unpleasant taste in the throat, excessive throat clearing and soreness, globus or dysphonia. Current pharmacotherapy utilises established, inexpensive drugs and there is potential for significant symptomatic improvements in patients with established respiratory disease. Further study is needed to develop more precise methods to identify patients with significant airway reflux and to investigate the effects of therapy.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflict of interest in preparing this manuscript.
References
- Aanen M.C., Weusten B.L., Numans M.E., de Wit N.J., Baron A., Smout A.J. (2006) Diagnostic value of the proton pump inhibitor test for gastrooesophageal reflux disease in primary care. Aliment Pharmacol Ther 24: 1377–1384 [DOI] [PubMed] [Google Scholar]
- Allen C.J., Anvari M. (1998) Gastro-oesophageal reflux related cough and its response to laparoscopic fundoplication. Thorax 53: 963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axford S.E., Sharp N., Ross P.E., Pearson J.P., Dettmar P.W., Panetti M., et al. (2001) Cell biology of laryngeal epithelial defenses in health and disease: Preliminary studies. Ann Otol Rhinol Laryngol 110: 1099–1108 [DOI] [PubMed] [Google Scholar]
- Ayazi S., Lipham J.C., Hagen J.A., Tang A.L., Zehetner J., Leers J.M., et al. (2009) A new technique for measurement of pharyngeal pH: Normal values and discriminating pH threshold. J Gastrointest Surg 13: 1422–1429 [DOI] [PubMed] [Google Scholar]
- Balaji N.S., Blom D., DeMeester T.R., Peters J.H. (2003) Redefining gastroesophageal reflux (GER). Surg Endosc 17: 1380–1385 [DOI] [PubMed] [Google Scholar]
- Barnes P.J. (2001) Neurogenic inflammation in the airways. Respir Physiol 125: 145–154 [DOI] [PubMed] [Google Scholar]
- Belafsky P.C., Postma G.N., Koufman J.A. (2001) The validity and reliability of the reflux finding score (RFS). Laryngoscope 111: 1313–1317 [DOI] [PubMed] [Google Scholar]
- Belafsky P.C., Rees C.J., Rodriguez K., Pryor J.S., Katz P.O. (2008) Esophagopharyngeal reflux. Otolaryngol Head Neck Surg 138: 57–61 [DOI] [PubMed] [Google Scholar]
- Berquist W.E., Rachelefsky G.S., Kadden M., Siegel S.C., Katz R.M., Mickey M.R., et al. (1981) Effect of theophylline on gastroesophageal reflux in normal adults. J Allergy Clin Immunol 67: 407–411 [DOI] [PubMed] [Google Scholar]
- Bevan S., Geppetti P. (1994) Protons: Small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci 17: 509–512 [DOI] [PubMed] [Google Scholar]
- Blackshaw L.A. (2008) New insights in the neural regulation of the lower oesophageal sphincter. Eur Rev Med Pharmacol Sci 12(Suppl 1): 33–39 [PubMed] [Google Scholar]
- Blondeau K., Dupont L.J., Mertens V., Verleden G., Malfroot A., Vandenplas Y., et al. (2008a) Gastro-oesophageal reflux and aspiration of gastric contents in adult patients with cystic fibrosis. Gut 57: 1049–1055 [DOI] [PubMed] [Google Scholar]
- Blondeau K., Mertens V., Vanaudenaerde B.A., Verleden G.M., Van Raemdonck D.E., Sifrim D. (2008b) Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J 31: 707–713 [DOI] [PubMed] [Google Scholar]
- Boeckxstaens G.E., Beaumont H., Mertens V., Denison H., Ruth M., Adler J., et al. (2010) Effects of lesogaberan on reflux and lower esophageal sphincter function in patients with gastroesophageal reflux disease. Gastroenterology 139: 409–417 [DOI] [PubMed] [Google Scholar]
- Boekema P.J., Samsom M., van Berge Henegouwen G.P., Smout A.J. (1999) Coffee and gastrointestinal function: Facts and fiction. A review. Scand J Gastroenterol Suppl 230: 35–39 [DOI] [PubMed] [Google Scholar]
- Bredenoord A.J. (2009) Lesogaberan, a GABA(B) agonist for the potential treatment of gastroesophageal reflux disease. IDrugs 12: 576–584 [PubMed] [Google Scholar]
- Broeders J.A., Draaisma W.A., Bredenoord A.J., Smout A.J., Broeders A., Gooszen H.G. (2010) Long-term outcome of Nissen fundoplication in non-erosive and erosive gastro-oesophageal reflux disease. Br J Surg 97: 845–852 [DOI] [PubMed] [Google Scholar]
- Casanova C., Baudet J.S., del Valle Velasco M., Martin J.M., Aguirre-Jaime A., de Torres J.P., et al. (2004) Increased gastro-oesophageal reflux disease in patients with severe COPD. Eur Respir J 23: 841–845 [DOI] [PubMed] [Google Scholar]
- Chang A.B., Lasserson T.J., Gaffney J., Connor F.L., Garske L.A. (2006) Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev 18: CD004823. [DOI] [PubMed] [Google Scholar]
- Cool M., Poelsmans J., Feenstra L., Tack J. (2004) Characteristics and clinical relevance of proximal esophageal pH monitoring. Am J Gastroenterol 99: 2317–2323 [DOI] [PubMed] [Google Scholar]
- Deane A.M., Fraser R.J., Chapman M.J. (2009) Prokinetic drugs for feed intolerance in critical illness: Current and potential therapies. Crit Care Resusc 11: 132–143 [PubMed] [Google Scholar]
- Dent J., Dodds W.J., Friedman R.H., Sekiguchi T., Hogan W.J., Arndorfer R.C., et al. (1980) Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest 65: 256–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicpinigaitis P.V. (2006) Chronic cough due to asthma: ACCP evidence-based clinical practice guidelines. Chest 129(1 Suppl): 75S–79S [DOI] [PubMed] [Google Scholar]
- Doherty M.J., Mister R., Pearson M.G., Calverley P.M. (2000a) Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 55: 643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M.J., Mister R., Pearson M.G., Calverley P.M. (2000b) Capsaicin induced cough in cryptogenic fibrosing alveolitis. Thorax 55: 1028–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ovidio F., Mura M., Ridsdale R., Takahashi H., Waddell T.K., Hutcheon M., et al. (2006) The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant 6: 1930–1938 [DOI] [PubMed] [Google Scholar]
- El-Serag H.B., Sonnenberg A. (1997) Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 113: 755–760 [DOI] [PubMed] [Google Scholar]
- Eryuksel E., Dogan M., Olgun S. (2009) Incidence and treatment results of laryngopharyngeal reflux in chronic obstructive pulmonary disease. Eur Arch Otorhinolaryngol 266: 1267–1271 [DOI] [PubMed] [Google Scholar]
- Everett C.F., Morice A.H. (2007) Clinical history in gastroesophageal cough. Respir Med 101: 345–348 [DOI] [PubMed] [Google Scholar]
- Fang J.C., Sarosiek I., Yamamoto Y. (1999) Cholinergic blockade inhibits gastro-oesophageal reflux and transient lower oesophageal sphincter relaxation through a central mechanism. Gut 44: 603–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré R., Fornari F., Blondeau K., Vieth M., De Vos R., Bisschops R., et al. (2010) Acid and weakly acidic solutions impair mucosal integrity of distal exposed and proximal non-exposed human oesophagus. Gut 59: 164–169 [DOI] [PubMed] [Google Scholar]
- Farrell T.M., Richardson W.S., Trus T.L., Smith C.D., Hunter J.G. (2001) Response of atypical symptoms of gastro-oesophageal reflux to antireflux surgery. Br J Surg 88: 1649–1652 [DOI] [PubMed] [Google Scholar]
- Fathi H., Moon T., Donaldson J., Jackson W., Sedman P., Morice A.H. (2009) Cough in adult cystic fibrosis: Diagnosis and response to fundoplication. Cough 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M., Benini L., Brotto E., Locatelli F., De Ioiro F., Bonella F., et al. (2007) Omeprazole reduces the response to capsaicin but not to methacholine in asthmatic patients with proximal reflux. Scand J Gastroenterol 42: 299–307 [DOI] [PubMed] [Google Scholar]
- Ford A.C., Forman D., Mosyyedi P., Morice A.H. (2006) Cough in the community: A cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 61: 975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi L., Ciccaglione A.F., Marzio L. (2001) Transient lower oesophageal sphincter relaxations play an insignificant role in gastro-oesophageal reflux to the proximal oesophagus. Neurogastroenterol Motil 13: 503–509 [DOI] [PubMed] [Google Scholar]
- Gunnbjörnsdóttir M.I., Omenaas E., Gíslason T., Norrman E., Olin A.C., Jõgi R., et al. (2004) Obesity and nocturnal gastro-oesophageal reflux are related to onset of asthma and respiratory symptoms. Eur Respir J 24: 116–121 [DOI] [PubMed] [Google Scholar]
- Helm J.F. (1986) Esophageal acid clearance. J Clin Gastroenterol 8(Suppl 1): 5–11 [DOI] [PubMed] [Google Scholar]
- Holloway R.H. (2001) Systemic pharmacomodulation of transient lower esophageal sphincter relaxations. Am J Med 111(Suppl 8A): 178S–185S [DOI] [PubMed] [Google Scholar]
- Ikramuddin S. (2008) Surgical management of gastroesophageal reflux disease in obesity. Dig Dis Sci 53: 2318–2329 [DOI] [PubMed] [Google Scholar]
- Irwin R.S., Curley F.J., French C.L. (1990) Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation and outcome of specific therapy. Am Rev Respir Dis 141: 640–647 [DOI] [PubMed] [Google Scholar]
- Jack C.I., Calverley P.M., Donnelly R.J., Tran J., Russell G., Hind C.R. (1995) Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastro-oesophageal reflux. Thorax 50: 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson B.C., Somers S.C., Fuchs C.S., Kelly C.P., Camargo C.A. (2006) Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med 354: 2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen D., Kulig M., Labenz J., Leodolter A., Lind T., Meyer-Sabelleck K., et al. (2003) Prevalence of extra-oesophageal manifestations in gastro-oesophageal reflux disease: An analysis based on the ProGERD Study. Aliment Pharmacol Ther 17: 1515–1520 [DOI] [PubMed] [Google Scholar]
- Jaspersen D., Labenz J., Willich S.N., Kulig M., Nocon M., Leodolter A., et al. (2006) Long-term clinical course of extra-oesophageal manifestations in patients with gastro-oesophageal reflux disease: A prospective follow-up analysis based on the ProGERD study. Dig Liver Dis 38: 233–238 [DOI] [PubMed] [Google Scholar]
- Jensen J., Lehmann A., Uvebrant A., Carlsson A., Jerndal G., Nilsson K., et al. (2005) Transient lower esophageal sphincter relaxations in dogs are inhibited by a metabotropic glutamate receptor 5 antagonist. Eur J Pharmacol 519: 154–157 [DOI] [PubMed] [Google Scholar]
- Johnston N., Bulmer D., Gill G.A., Panetti M., Ross P.E., Pearson J.P., et al. (2003) Cell biology of laryngeal epithelial defences in health and disease: Further studies. Ann Otol Rhinol Laryngol 112: 481–491 [DOI] [PubMed] [Google Scholar]
- Johnston N., Wells C.W., Samuels T.L., Blumin J.H. (2009) Pepsin in nonacidic refluxate can damage hypopharyngeal epithelial cells. Ann Otol Rhinol Laryngol 118: 677–685 [DOI] [PubMed] [Google Scholar]
- Jonaitis L., Pribuisiene R., Kupcinskas L., Uloza V. (2006) Laryngeal examination is superior to endoscopy in the diagnosis of the laryngopharyngeal form of gastroesophageal reflux disease. Scand J Gastroenterol 41: 131–137 [DOI] [PubMed] [Google Scholar]
- Jordt S.E., Bautista D.M., Chuang H.H., McKemy D.D., Zygmunt P.M., Högestatt E.D., et al. (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265 [DOI] [PubMed] [Google Scholar]
- Karkos P.D., Benton J., Leong S.C., Karkanevatos A., Badran K., Srinivasan V.R., et al. (2007) Trends in laryngopharyngeal reflux: A British ENT survey. Eur Arch Otorhinolaryngol 264: 513–517 [DOI] [PubMed] [Google Scholar]
- Kastelik J.A., Redington A.E., Aziz I., Buckton G.K., Smith C.M., Dakkak M., et al. (2003) Abnormal oesophageal motility in patients with chronic cough. Thorax 58: 699–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura O., Aslam M., Rittmann T., Hofmann C., Shaker R. (2004) Physical and pH properties of gastroesophagopharyngeal refluxate: A 24-hour simultaneous ambulatory impedance and pH monitoring study. Am J Gastroenterol 99: 1000–1010 [DOI] [PubMed] [Google Scholar]
- Kempainen R.R., Savik K., Whelan T.P., Dunitz J.M., Herrington C.S., Billings J.L. (2007) High prevalence of proximal and distal gastroesophageal reflux disease in advanced COPD. Chest 131: 1666–1671 [DOI] [PubMed] [Google Scholar]
- Kiljander T.O., Laitinen J.O. (2004) The prevalence of gastroesophageal reflux disease in adult asthmatics. Chest 126: 1490–1494 [DOI] [PubMed] [Google Scholar]
- Knight J., Lively M.O., Johnston N., Dettmar P.W., Koufman J.A. (2005) Sensitive pepsin immunoassay for detection of laryngopharyngeal reflux. Larywgoscope 115: 1473–1478 [DOI] [PubMed] [Google Scholar]
- Koufman J.A., Aviv J.E., Casiano R.R., Shaw G.Y. (2002) Laryngopharyngeal reflux: Position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg 127: 32–35 [DOI] [PubMed] [Google Scholar]
- Kuribayashi S., Massey B.T., Hafeezullah M., Perera L., Hussaini S.Q., Tatro L., et al. (2010) Upper esophageal sphincter and gastroesophageal junction pressure changes act to prevent gastroesophageal and esophagopharyngeal reflux during apneic episodes in patients with obstructive sleep apnea. Chest 137: 769–776 [DOI] [PubMed] [Google Scholar]
- Lacy B.E., Mathis C., DesBiens J., Liu M.C. (2008) The effects of nebulized albuterol on esophageal function in asthmatic patients. Dig Dis Sci 53: 2627–2633 [DOI] [PubMed] [Google Scholar]
- Laitman J.T., Reidenberg J.S. (1997) The human aerodigestive tract and gastroesophageal reflux: An evolutionary perspective. Am J Med 103: 2S–8S [DOI] [PubMed] [Google Scholar]
- Ledson M., Tran J.J., Walshaw M.J. (1998) Prevalence and mechanisms of gastro-oesophageal reflux in adult cystic fibrosis patients. J R Soc Med 91: 7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Collard H.R., Raghu G., Sweet M.P., Hays S.R., Campos G.M., et al. (2010) Does chronic microaspiration cause idiopathic pulmonary fibrosis?. Am J Med 123: 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainie I., Tutuian R., Shay S., Vela M., Zhang X., Sifrim D., et al. (2006) Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: A multicentre study using combined ambulatory impedance-pH monitoring. Gut 55: 1398–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde J.G., Anthonisen N.R., Castro M., Holbrook J.T., Leone F.T., Teague W.G., et al. (2009) Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 360: 1487–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P.J., Aziz Q., Facer P., Davis J.B., Thompson D.G., Anand P. (2004) Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol 16: 897–902 [DOI] [PubMed] [Google Scholar]
- Mays E.E. (1976) Intrinsic asthma in adults. Association with gastroesophageal reflux. JAMA 236: 2626–2628 [PubMed] [Google Scholar]
- Mays E.E., Dubois J.J., Hamilton G.B. (1976) Pulmonary fibrosis associated with tracheobronchial aspiration. A study of the frequency of hiatal hernia and gastroesophageal reflux in interstitial pulmonary fibrosis of obscure etiology. Chest 69: 512–515 [DOI] [PubMed] [Google Scholar]
- Merati A.L., Lim H.J., Ulualp S.O., Toohill R.J. (2005) Meta-analysis of upper probe measurements in normal subjects and patients with laryngopharyngeal reflux. Ann Otol Rhinol Laryngol 114: 177–182 [DOI] [PubMed] [Google Scholar]
- Mertens V., Blondeau K., Pauwels A., Farre R., Vanaudenaerde B., Vos R., et al. (2009) Azithromycin reduces gastroesophageal reflux and aspiration in lung transplant recipients. Dig Dis Sci 54: 972–979 [DOI] [PubMed] [Google Scholar]
- Midgren B., Hansson L., Karlsson J.A., Simonsson B.G., Persson C.G. (1992) Capsaicin-induced cough in humans. Am Rev Respir Dis 146: 347–351 [DOI] [PubMed] [Google Scholar]
- Mitchell J.E., Campbell A.P., New N.E., Sadofsky L.R., Kastelik J.A., Mulrennan S.A., et al. (2005) Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res 31: 295–306 [DOI] [PubMed] [Google Scholar]
- Mittal R.K., Holloway R.H., Penagini R., Blackshaw L.A., Dent J. (1995) Transient lower esophageal sphincter relaxation. Gastroenterology 109: 601–610 [DOI] [PubMed] [Google Scholar]
- Molyneux I., Jackson W., Morice A.H. (2010a) Variation in pharyngeal pH in the diagnosis of respiratory reflux. Thorax 65(Suppl IV): A55, abstract. [Google Scholar]
- Molyneux I., Morice A.H., Jackson W. (2010b) Enhanced detection of airway reflux using pharyngeal pH monitoring. Am J Respir Crit Care Med 181: A5903, abstract. [Google Scholar]
- Morice A.H., Faruqi S., Wright C.E., Thompson R., Bland J.M. (2011) The cough hypersensitivity syndrome: A distinct clinical entity. Lung 189: 73–79 [DOI] [PubMed] [Google Scholar]
- Morice A.H., Fontana G.A., Belvisi M.G., Birring S.S., Chung K.F., Dicpinigaitis P.V., et al. (2007) ERS guidelines on the assessment of cough. Eur Respir J 29: 1256–1276 [DOI] [PubMed] [Google Scholar]
- Nordenstedt H., Nilsson M., Johansson S., Wallander M.A., Johnsen R., Hveem K., et al. (2006) The relation between gastro-oesophageal reflux and respiratory symptoms in a population-based study: The North-Trøndelag health survey. Chest 129: 1051–1056 [DOI] [PubMed] [Google Scholar]
- O'Connell F., Thomas V.E., Studham J.M., Pride N.B., Fuller R.W. (1996) Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 90: 279–286 [DOI] [PubMed] [Google Scholar]
- Oelschlager B.K., Chang L., Pope C.E., 2nd, Pellegrini C.A. (2005) Typical GERD symptoms and esophageal pH monitoring are not enough to diagnose pharyngeal reflux. J Surg Res 128: 55–60 [DOI] [PubMed] [Google Scholar]
- Oh D.S., Hagen J.A., Fein M., Bremner C.G., Dunst C.M., Demeester S.R., et al. (2006) The impact of reflux composition on mucosal injury and esophageal function. J Gastrointest Surg 10: 787–797 [DOI] [PubMed] [Google Scholar]
- Pandolfino J.E., Ghosh S.K., Zhang Q., Han A., Kahrilas P.J. (2007) Upper sphincter function during transient lower oesophageal sphincter relaxation (tLOSR); It is mainly about microburps. Neurogastroenterol Motil 19: 203–210 [DOI] [PubMed] [Google Scholar]
- Pandolfino J.E., Kahrilas P.J. (2000) Smoking and gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol 12: 837–842 [DOI] [PubMed] [Google Scholar]
- Patterson N., Mainie I., Rafferty G., McGarvey L., Heaney L., Tutuian R., et al. (2009) Nonacid reflux episodes reaching the pharynx are important factors associated with cough. J Clin Gastroenterol 43: 414–419 [DOI] [PubMed] [Google Scholar]
- Patterson R.N., Johnston B.T., Ardill J.E., Heaney L.G., McGarvey L.P. (2007) Increased tachykinin levels in induced sputum from asthmatic and cough patients with acid reflux. Thorax 62: 491–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels A., Blondeau K., Dupont L., Sifrim D. (2009) Cough and gastroesophageal reflux: From the gastroenterologist end. Pulm Pharmacol Ther 22: 135–138 [DOI] [PubMed] [Google Scholar]
- Phua S.Y., McGarvey L.P., Ngu M.C., Ing A.J. (2005) Patients with gastro-oesophageal reflux disease and cough have impaired laryngopharyngeal mechan-osensitivity. Thorax 60: 488–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe R.H., Kallay M.C. (2003) Chronic cough and gastroesophageal reflux disease: Experience with specific therapy for diagnosis and treatment. Chest 123: 679–684 [DOI] [PubMed] [Google Scholar]
- Pontes P., Tiago R. (2006) Diagnosis and management of laryngopharyngeal reflux disease. Curr Opin Otolaryngol Head Neck Surg 14: 138–142 [DOI] [PubMed] [Google Scholar]
- Postma G.N., Tomek M.S., Belafsky P.C., Koufman J.A. (2001) Esophageal motor function in laryngopharyngeal reflux is superior to that in classic gastroesophageal reflux disease. Ann Otol Rhinol Laryngol 110: 1114–1116 [DOI] [PubMed] [Google Scholar]
- Pratter M.R., Brightling C.E., Boulet L.P., Irwin R.S. (2006) An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest 129: 222S–231S [DOI] [PubMed] [Google Scholar]
- Ruhl C.E., Sonnenberg A., Everhart J.E. (2001) Hospitalization with respiratory disease following hiatal hernia and reflux esophagitis in a prospective, population-based study. Ann Epidemiol 11: 477–483 [DOI] [PubMed] [Google Scholar]
- Sabati A.A., Kempainen R.R., Milla C.E., Ireland M., Schwarzenberg S.J., Dunitz J.M., et al. (2010) Characteristics of gastroesophageal reflux in adults with cystic fibrosis. J Cyst Fibros 9(5): 365–370, DOI: 10.1016/j.jcf.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Salvioli B., Belmonte G., Stanghellini V., Baldi E., Fasano L., Pacilli A.M., et al. (2006) Gastro-oesophageal reflux and interstitial lung disease. Dig Liver Dis 38: 879–884 [DOI] [PubMed] [Google Scholar]
- Scheffer R.C., Akkermans L.M., Bais J.E., Roelofs J.M., Smout A.J., Gooszen H.G. (2002) Elicitation of transient lower oesophageal sphincter relaxations in response to gastric distension and meal ingestion. Neurogastroenterol Motil 14: 647–655 [DOI] [PubMed] [Google Scholar]
- Sontag S.J., O'Connell S., Khandelwal S., Miller T., Nemchausky B., Schnell T.G., et al. (1990) Most asthmatics have gastroesophageal reflux with or without bronchodilator therapy. Gastroenterology 99: 613–620 [DOI] [PubMed] [Google Scholar]
- Spaulding H.S., Jr, Mansfield L.E., Stein M.R., Sellner J.C., Gremillion D.E. (1982) Further investigation of the association between gastroesophageal reflux and bronchoconstriction. J Allergy Clin Immunol 69: 516–521 [DOI] [PubMed] [Google Scholar]
- Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., et al. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829 [DOI] [PubMed] [Google Scholar]
- Trevisani M., Gazzieri D., Benvenuti F., Campi B., Dinh Q.T., Groneberg D.A., et al. (2004a) Ethanol causes inflammation in the airways by a neurogenic and TRPV1-dependent mechanism. J Pharmacol Exp Ther 309: 1167–1173 [DOI] [PubMed] [Google Scholar]
- Trevisani M., Milan A., Gatti R., Zanasi A., Harrison S., Fontana G., et al. (2004b) Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax 59: 769–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudgill N.J., Riley S.A. (2001) Transient lower esophageal sphincter relaxations are no more frequent in patients with gastroesophageal reflux disease than in asymptomatic volunteers. Am J Gastroenterol 96: 2569–2574 [DOI] [PubMed] [Google Scholar]
- Tutuian R., Vela M.F., Hill E.G., Mainie I., Agrawal A., Castell D.O. (2008) Characteristics of symptomatic reflux episodes on Acid suppressive therapy. Am J Gastroenterol 103: 1090–1096 [DOI] [PubMed] [Google Scholar]
- Vaezi M.F. (2003) Sensitivity and specificity of reflux-attributed laryngeal lesions: Experimental and clinical evidence. Am J Med 115(Suppl 3A): 97S–104S [DOI] [PubMed] [Google Scholar]
- Vakil N., van Zanten S.V., Kahrilas P., Dent J., Jones R. (2006) Global Consensus Group. The Montreal definition and classification of the gastroesophageal reflux disease: A global evidence-based consensus. Am J Gastroenterol 101: 1900–1920 [DOI] [PubMed] [Google Scholar]
- Watanabe N., Horie S., Michael G.J., Spina D., Page C.P., Priestley J.V. (2005) Immunohistochemical localization of vanilloid receptor subtype 1 (TRPV1) in the guinea pig respiratory system. Pulm Pharmacol Ther 18: 187–197 [DOI] [PubMed] [Google Scholar]
- Wei H., Koivisto A., Pertovaara A. (2010) Spinal TRPA1 ion channels contribute to cutaneous neurogenic inflammation in the rat. Neurosci Lett 479: 253–256 [DOI] [PubMed] [Google Scholar]
- Wiener G.J., Tsukashima R., Kelly C., Wolf E., Schmeltzer M., Bankert C., et al. (2009) Oropharyngeal pH monitoring for the detection of liquid and aerosolized supraesophageal gastric reflux. J Voice 23: 498–504 [DOI] [PubMed] [Google Scholar]
- Wu D.N., Yamauchi K., Kobayashi H., Tanifuji Y., Kato C., Suzuki K., et al. (2002) Effects of esophageal acid perfusion on cough responsiveness in patients with bronchial asthma. Chest 122: 505–509 [DOI] [PubMed] [Google Scholar]
- Zerbib F., Duriez A., Roman S., Capdepont M., Mion F. (2008) Determinants of gastro-oesophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut 57: 156–160 [DOI] [PubMed] [Google Scholar]