Abstract
Only a few randomized controlled trials investigating antiosteoporotic agents with fracture endpoints have included participants over the age of 80 years. The pivotal trial with alendronic acid had an upper age range of 80 years, although a separate trial that showed a significant reduction in nonvertebral fractures included some participants up to age of 84 years. Risedronate and zoledronic acid are the only bisphosphonates to show a significant reduction in new vertebral, hip and nonvertebral fractures during a 3-year period in those over 80 years of age. In addition, zoledronic acid was associated with a reduction in the rate of new clinical fractures and improved survival in elderly subjects after hip fracture. More recently, denosumab was found to significantly reduce the risk of new radiographic vertebral, hip and nonvertebral fractures in women up to the age of 89 years with osteoporosis. Strontium ranelate and teriparatide have shown fracture reductions in populations that have included subjects over the age of 80 years. There has been evidence to show that a combination of calcium and vitamin D reduces nonvertebral fracture in older populations. The role of vitamin D alone is less clear, although there is the suggestion that it may be effective at higher doses. The burden of osteoporosis is unquestionably rising within our ageing population. More emphasis is therefore required on researching the benefits of these pharmacological agents in very elderly people.
Keywords: bisphosphonates, calcium, denosumab, elderly, fracture, fracture prevention, osteoporosis, strontium ranelate, teriparatide, vitamin D
Introduction
Osteoporosis is a common skeletal disorder that is characterized by compromised bone strength predisposing to an increased risk of fracture. Bone strength is reflected by both bone mass and bone quality [Raisz, 2005]. After the age of 50 years the prevalence of osteoporosis and incidence of osteoporotic fractures rise substantially with age. The number of people over the age of 60 years is projected to more than triple globally in the next half century, from 593 million to 1.97 billion. The share of older people in the population is set to increase from 10% to 22%. It is estimated that 30% of all fragility fractures in women occur over the age of 80 years and this figure increases to 60% when hip fracture is considered separately [Population Division of the Department of Economic and Social Affairs of the Nations Secretariat, 2002].
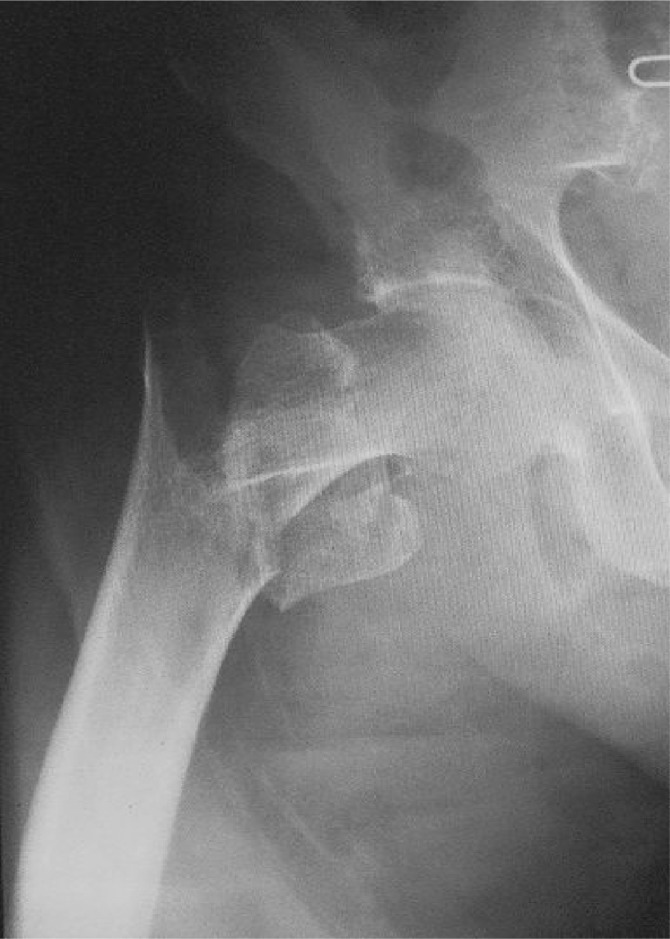
The three most common osteoporotic fractures occur at the vertebrae, forearm and hip; although fragility fractures at other sites, including the upper arm, pelvis, ribs and clavicle, also contribute to the overall morbidity of osteoporosis. Hip fractures are associated with the highest levels of morbidity and mortality, and are considered to be the most economically important osteoporotic fractures (Figure 1). Following a hip fracture, approximately 20% of patients do not survive the next year and 50% do not regain their previous level of independence [Chrischilles et al. 1991]. Vertebral fractures are also associated with adverse outcomes, including height loss, back pain, kyphosis, functional impairment and hospitalization [Greendale et al. 2000; Ismail et al. 1999]. The prevalence of vertebral deformities increases from 5-10% in women in their early fifties to 45-55% in those aged between 80 and 90 years [O'Neill et al. 1996]. Individuals with vertebral fractures are at an increased risk of further vertebral fractures as well as hip fractures [Black et al. 1999].
Figure 1.
Radiograph showing an intertrochanteric hip fracture.
Even though treatments for osteoporosis are now available, only a small proportion of older women with osteoporosis, particularly those above the age of 80 years, receive treatment [Freedman et al. 2000; Torgerson and Dolan, 1998]. One possible reason is that clinicians may presume that it is too late to alter the course of the disease in more elderly age groups. It is important therefore to consider the evidence available in the literature relating to osteoporosis treatment and fracture prevention in older populations aged 80 years and over. Thus, for the purposes of this review, we define ‘very elderly’ as people aged 80 years and above. It is also worth noting that most of the evidence available in this older age group for osteoporosis treatments is in women. This is a comprehensive literature search rather than a systematic review. We have included, where appropriate, post hoc analyses of pivotal studies, although it is important to recognize the limitations of such data.
Antiresorptive agents
Most of the evidence, in the form of randomized controlled trials (RCTs), for antiosteoporotic agents in reducing fragility fractures exists in women up to the age of 80 years. A previous meta-analysis of 13 studies of antiresorptives in women showed that there was a greater reduction in the risk vertebral fracture with increasing age [Maraldo et al. 2010]. For antiresorptives other than bisphosphonates, no fracture outcome trials that included subjects over the age of 80 years were found for the selective oestrogen receptor modulator raloxifene or for calcitonin. For the bisphosphonates, no fracture outcome trials were found for etidronate or ibandronate that included subjects over the age of 80 years. The pivotal alendronate study (FIT) did not include patients above the age of 80 years [Black et al. 2000]. In a separate study using alendronate, in which bone mineral density was the primary endpoint, a reduction in nonvertebral fractures was seen (n = 1908, relative risk [RR] = 0.53; confidence interval [CI] 0.30-0.90). Women up to the age of 84 years were included, but the mean age of the population was only 62 years [Pols et al. 1999].
The pivotal risedronate studies include, from three RCTs, almost 1400 individuals aged over 80 years with confirmed osteoporosis: these are the Hip Intervention Program (HIP) trial, the Vertebral Efficacy with Risedronate Therapy — Multinational (VERT-MN) trial, and the VERT-North America (NA) trial [McClung et al. 2001; Reginster et al. 2000; Harris et al. 1999]. Pooled analysis from these three RCTs showed that, in those aged 80 years and over, the risk of new vertebral fractures in the risedronate group was 81% lower (n = 1392, RR = 0.19, 95% CI: 0.60-0.90) at 1 year, and the antifracture efficacy was confirmed after 3 years (RR = 0.56, CI 0.19-0.61). The number needed to treat (NNT) to prevent one new vertebral fracture at 1 year was 12. In this pooled analysis, no significant reduction was seen in osteoporosis-related nonvertebral fractures [Boonen et al. 2004].
The HIP study investigated the efficacy of risedronate on the risk of hip fracture. There were two groups of women recruited. In the group aged between 70 and 79 years with osteoporosis there was a significant 40% reduced risk of hip fracture in the treatment group (n = 5445, RR = 0.60, CI: 0.40-0.90). In the other group of women aged 80 years or over who were selected primarily on the basis of nonskeletal risk factors, no significant reduction in hip fractures was seen (n = 3886, RR = 0.8, CI: 0.60-1.20). In the combined intention-to-treat population (age 70-100 years) a significant 30% hip fracture risk reduction was seen (n = 9331, RR = 0.70, CI: 0.60-0.90) [McClung et al. 2001].
A post hoc analysis of the HIP and VERT studies showed that, compared with placebo, risedronate reduced the risk of hip fracture by 46% (RR = 0.54, CI: 0.32-0.91) in women aged 70-100 years with established osteoporosis [Masud et al. 2009].
Zoledronic acid is a potent bisphosphonate that can be administered intravenously once yearly. The pivotal Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Trial (HORIZON-PFT) showed that a once-yearly infusion of 5 mg intravenous zoledronic acid during a 3-year period significantly reduced the risk of vertebral and hip fractures by 70% (n = 5675, RR = 0.30, CI: 0.24-0.38) and 41% (RR = 0.59, CI: 0.42-0.83), respectively. Thirty-nine per cent of the patients recruited in this study were aged 75 years or over (age range 65-89 years). The incidence of nonvertebral fractures was similarly significantly reduced in the zoledronic acid group by 25% (RR= 0.75, CI: 0.64-0.87) [Black et al. 2007].
A subsequent trial (HORIZON-Recurrent Fracture Trial) looked at the efficacy and safety of zoledronic acid administered once-yearly for the prevention of new clinical fractures in women and men who had undergone recent surgical repair of a hip fracture. More than half of those recruited were over the age of 75 years (age range 50-85 years). This study showed a reduction of 35% (n = 2127, RR = 0.65, CI: 0.50-0.84) in the risk of any new clinical fracture and a 28% (RR= 0.72, CI: 0.56-0.93) risk reduction in death from any cause in the zoledronic acid group [Lyles et al. 2007].
Denosumab
Denosumab is a recently launched human monoclonal antibody that targets and binds with high affinity and specificity to the receptor activator of nuclear factor-kappa B ligand (RANKL), preventing activation of the RANK receptor, which is found on osteoclast precursors and osteoclasts. This leads to a decrease in bone resorption and an increase in bone density. The Fracture Reduction Evaluation of Denosumab in Osteoporosis every 6 months (FREEDOM) Trial investigated the efficacy of denosumab on the incidence of new vertebral, nonvertebral and hip fractures. Postmenopausal women aged 60-90 years with osteoporosis were recruited, of whom almost a third were over the age of 75 years in the treatment group. The study showed that denosumab 60 mg, administered subcutaneously every 6 months for 36 months, significantly reduced the risk of new radiographic vertebral, hip and nonvertebral fractures in women with osteoporosis by 68% (n = 7808, RR = 0.32, CI: 0.26-0.41), 40% (RR = 0.60, CI: 0.37-0.97) and 20% (RR= 0.80, CI: 067-0.95), respectively. The reduction in the risk of vertebral fracture was similar in the first and subsequent years and for both clinically diagnosed and multiple vertebral fractures [Cummings et al. 2009].
Anabolic agents
Parathyroid hormone (PTH) or its analogues, given by subcutaneous injection once daily, are anabolic agents that directly stimulate osteoblastic bone formation, resulting in substantial increases in trabecular bone density and connectivity in women with postmenopausal osteoporosis. This mechanism of action is very different from that of the antiresorptive agents mentioned previously, which reduce bone resorption.
Two PTH-related analogues are currently in use: teriparatide (PTH 1,34) and recombinant human PTH 1,84. For teriparatide, the pivotal trial was the Fracture Prevention Trial (FPT), which showed that after daily subcutaneous injection of teriparatide 20 μg for a median duration of 21 months, new vertebral fractures were reduced by 65% (n = 892, RR = 0.35, CI: 0.22-0.55) and new nonvertebral fragility fractures were reduced by 53% (n = 1085, RR = 0.47, CI: 0.25-0.88), compared with placebo [Neer et al. 2001]. The full age range, however, was not stated in the paper. In a subsequent published post hoc analysis from the FPT, the age range of the intention-to-treat postmenopausal population studied was reported as 42-86 years [Boonen et al. 2006]. In this analysis, subgroups were defined according to patient age <75 years (n = 841) and 75 years or older (n = 244). There were no significant treatment-by-age interactions for vertebral or nonvertebral fragility fractures, indicating that the clinical effects of teriparatide were consistent in the older and younger women [Boonen et al. 2006].
PTH 1,84 was shown to reduce the relative risk of new and worsened vertebral fracture in postmenopausal women with osteoporosis. The degree of relative risk reduction varied according to the assumed fracture risk in those that did not complete the study. The authors reported that on subgroup analysis by age, results achieved for each group were comparable with the overall study findings. The latter included a subgroup of patients aged over 75 years [Greenspan et al. 2007].
Dual-action bone agents
Strontium ranelate is an antiosteoporotic agent thought to have a dual action (antiresorptive and bone forming). The phase III programme included the SOTI study (Spinal Osteoporosis Therapeutic Intervention, n = 1649, mean age 70 years, range 50-96) and the TROPOS study (Treatment Of Postmenopausal Osteoporosis, n = 5091, mean age 77 years, range 70-100) [Reginster et al. 2005; Meunier et al. 2004].
The SOTI study showed a significant 49% reduction in new vertebral fractures at 1 year in the treatment group (RR = 0.51, CI: 0.36-0.74) and a 41% reduction over 3 years (RR = 0.59, CI: 0.48-0.73) [Meunier et al. 2004].
The TROPOS study showed that in the intention-to-treat population there was a significant 16% reduction in all nonvertebral fractures over 3 years with strontium ranelate (RR = 0.84, CI: 0.70-0.99). The risk of major nonvertebral fractures (hip, wrist, pelvis, sacrum, ribs, sternum, clavicle or humerus) was reduced by 19% (RR = 0.81, CI: 0.66-0.98). There was a trend towards a reduction in hip fracture in the intention-to-treat population of 15%, but this did not reach statistical significance as the study was not powered for this parameter. An analysis on a high-risk group suggested by the European regulatory authorities [women aged 74 years and over plus a femoral neck bone mineral density T score of less than −3 (equivalent to the US National Health and Nutrition Examination Survey reference −2.4)] showed that strontium ranelate reduced hip fracture risk by 36% (RR = 0.64, CI: 0.41-0.99) [Reginster et al. 2005].
In a preplanned pooled analysis of SOTI plus TROPOS in women aged 80 years or more (n = 1488), strontium ranelate reduced the risk of vertebral fracture in the first year by 59% (RR = 0.41, CI: 0.22-0.75) and nonvertebral fractures by 41% (RR = 0.59, CI: 0.37-0.95). At 3 years, the risk reductions were 32% (RR = 0.68, CI: 0.50-0.92) and 31% (RR = 0.69, CI: 0.52-0.92), respectively [Seeman et al. 2006]. In a follow-up pooled analysis at 5 years in this age group, the relative risk reductions were maintained: 31% for vertebral fractures (RR = 0.69, CI: 0.52-0.92) and 27% for nonvertebral fractures (RR = 0.73, CI: 0.57-0.95) [Seeman et al. 2010].
Calcium and vitamin D
Intestinal absorption of calcium occurs first by a passive mechanism and second by a vitamin D-mediated active transport mechanism. The efficiency of both of these mechanisms decreases with age [Heaney et al. 1982]. Furthermore, vitamin D insufficiency is more likely with advancing age because of several factors, including a declining capability of ageing skin to synthesize vitamin D, lower exposure to the sun in frailer, older, housebound or institutionalized people, and a decreased dietary vitamin D intake [Lips, 2001; Pattanaungkul et al. 2000; Hollick et al. 1989; Heaney et al. 1982]. A tendency towards low vitamin D levels leads to secondary hyperparathyroidism in order to maintain normocalcaemia. In doing so, bone resorption and bone loss occurs.
With regard to fracture risk reduction, there have been several recent meta-analyses that have looked at the role of vitamin D alone or in combination with calcium. The majority incorporated RCT data comparing each of these interventions with placebo. The updated 2005 Cochrane review included men over 65years of age and postmenopausal women. It found that vitamin D alone at daily doses of approximately 830 IU offered no significant protection against osteoporotic fracture. By contrast, pooled data for vitamin D in combination with calcium was associated with an overall significant reduction in hip fracture (46,658 participants, RR = 0.84, CI: 0.73-0.96), but not nonvertebral fracture [Avenell et al. 2009]. It was only on subsequent subgroup analysis by residential status that a significant reduction was observed in both hip and nonvertebral fracture. This analysis was based predominantly upon two French trials (n = 3853) in which the mean age of participants was above 80 years and fracture reduction was evident in the institutionalized group only [Chapuy et al. 2002, 1992].
A similar outcome was observed by Boonen and colleagues in their meta-analysis, which incorporated the same two French RCTs and data from the large-scale Women's Health Initiative trial (WHI). They looked at the effects on hip fracture in particular, and demonstrated an 18% reduction in hip fracture risk associated with coadministered vitamin D and calcium (RR = 0.82, CI: 0.71-0.94). Pooled results for vitamin D alone did not reach significance [Boonen et al. 2007]. Data from the WHI trial (n = 36,282) contributed substantially to the pooled analysis for combined calcium and vitamin D [Jackson et al. 2006]. Eighty per cent of participants in this trial were below the age of 70 years and so the findings for the pooled data must be treated with caution when considering very elderly people.
The DIPART study (vitamin D Individual Patient Analysis of Randomized Trials), by contrast with earlier meta-analyses, was designed to determine the effects of individual patient factors on the efficacy of vitamin D with or without calcium. The WHI trial was again one of the seven trials included, but in spite of this the mean age of those recruited was 69.9 years. Pooled data from all seven trials (n = 68,517) showed that vitamin D in combination with calcium was associated with a reduced risk of any fracture (RR = 0.92, CI: 0.86-0.99). However, subgroup analysis by dose of vitamin D revealed that in combination with calcium, only lower doses of vitamin D (10 μg as opposed to 20 μg) were associated with a significant reduction in risk of hip fracture (n = 45,887, RR = 0.74, CI: 0.60-0.91). Vitamin D alone (irrespective of dose), did not produce a significant risk reduction [DIPART Group, 2009].
One of the study limitations specifically highlighted by the DIPART group was a paucity of information provided on patient compliance. The meta-analysis by Bischoff-Ferrari and colleagues, which recruited participants over 65 years, addressed this issue by establishing for each of the 12 included RCTs an estimate of the received dose of vitamin D (cross-product of dose and percentage adherence). The meta-analysis, however, was limited in that compliance was only taken into account in the intervention group, not in the control group. Adose-dependent effect was noted; however, a significant reduction in hip and nonvertebral fracture risk was only apparent at doses of vitamin D above 400 IU (nonvertebral fracture, n = 33,265, RR = 0.80, CI: 0.72-0.89; hip fracture, n = 31,872, RR = 0.82, CI: 0.69-0.97). Subgroup analysis among the ‘higher dose’ trials showed that a significant risk reduction in nonvertebral fracture was maintained in the > 75 years group (n = 9879, RR = 0.83, CI: 0.74-0.92). Interestingly, an enhanced risk reduction was not observed in groups receiving calcium in addition to vitamin D. When the form of vitamin D was considered, subgroup analysis revealed that cholecalciferol resulted in a more substantial risk reduction than ergocalciferol. Nonetheless, the strength of the pooled data for ergocalciferol was limited by low numbers [Bischoff-Ferrari et al. 2009]. It is clear that more research is required to establish the most effective dosing regimen and mode of administration of vitamin D.
It is worth noting that all pivotal trials of antiosteoporotic agents (antiresorptive, anabolic or dual-action agents) included supplementation with calcium and vitamin D, thereby supporting their coprescription. Recent concerns have been raised with regard to calcium supplementation and an associated reported rise in rates of myocardial infarction (MI). This has been based largely on a meta-analysis of 11 trials performed by Bolland and colleagues, which reported an almost 30% increase in relative risk (RR = 1.27, CI: 1.01-1.59) [Bolland et al. 2010]. These findings in the context of osteoporosis management must, however, be interpreted with caution, not least because the meta-analysis excluded trials that coadministered calcium and vitamin D. A linear relationship between calcium intake and MI risk was surprisingly not observed. The rise in myocardial infarction was also only seen in those patients exceeding the recommended daily intake of calcium (supplements in addition to dietary intake). By contrast with Bolland and colleagues' meta-analysis, the WHI trial group data considered specific cardiovascular endpoints but did not demonstrate an increase in MI risk with coadministered calcium and vitamin D [Hsia et al. 2007]. Nonetheless, it is clear that further trials are required to comprehensively address this issue.
In considering the treatment of osteoporosis in very elderly people, clinicians need to take into account not only the evidence for efficacy but the issue of intolerance and side effects of each therapeutic agent. These factors need to be weighed on an individual basis. For some patients who are unable to tolerate oral bisphosphonates the intravenous route may be more appropriate, as would be the subcutaneous 6-monthly route with denosumab. The other advantage of denosumab is that it can be considered in patients with low glomerular filtration rates, where bisphosphonates are inappropriate. The issue of daily self-injection of PTH preparations may be impractical for some older patients, but carers could potentially be trained to administer them.
Summary
Only a few RCTs investigating antiosteoporotic agents with fracture endpoints have included participants over the age of 80 years. With regard to bisphosphonates, only pivotal trials of risedronate and zoledronic acid showing significant fracture reductions have included participants above this age group. Zoledronic acid has also been shown to reduce the rate of new clinical fractures after repair of a low-trauma hip fracture. More recently, denosumab has been associated with a significant reduction in the risk of new radiographic vertebral, hip and nonvertebral fractures in women up to the age of 89 years with osteoporosis. Strontium ranelate and teriparatide have shown fracture reductions in populations that have included subjects over the age of 80 years. There has been evidence to show that a combination of calcium and vitamin D reduces nonvertebral fractures in older populations. The role of vitamin D alone is less clear, although there is the suggestion that it may be effective at higher doses. The burden of osteoporosis is unquestionably rising within our ageing population. More emphasis is therefore required on researching the benefits of these pharmacological agents in very elderly people. However, at present, there is sufficient evidence to treat osteoporosis in this important patient group and not to leave them untreated.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflict of interest in preparing this manuscript.
References
- Avenell A., Gillespie W.J., Gillespie L.D., O'Connell D. (2009) Vitamin D and vitamin D analogues for preventing fractures associated with involutional and postmenopausal osteoporosis. Cochrane Database Syst Rev 2: CD000227. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H.A., Willett W.C., Wong J.B., Stuck A.E., Staehelin H.B., Orav E.J., et al. (2009) Prevention of nonvertebral fractures with oral vitamin D and dose dependency a meta-analysis of randomized controlled trials. Arch Intern Med 169: 551–561 [DOI] [PubMed] [Google Scholar]
- Black D.M., Arden N.K., Palermo L., Pearson J., Cummings S.R. (1999) Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not new wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 14: 821–828 [DOI] [PubMed] [Google Scholar]
- Black D.M., Delmas P.D., Eastell R., Reid I.R., Boonen S., Cauley J.A., et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809–1822 [DOI] [PubMed] [Google Scholar]
- Black D.M., Thompson D.E., Bauer D.C., Ensrud K., Musliner T., Hochberg M.C., et al. (2000) Fracture risk reduction with alendronate in women with osteoporosis: The Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 85: 4118–4124 [DOI] [PubMed] [Google Scholar]
- Bolland M.J., Avenell A., Baron J.A., Grey A., MacLennan G.S., Gamble G.D., et al. (2010) Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: Meta-analysis. BMJ 314: c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonen S., Lips P., Bouillon R., Bischoff-Ferrari H.A., Vanderschueren D., Haentjens P. (2007) Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: Evidence from a comparative meta analysis of randomised controlled trials. J Clin Endocrinol Metab 92: 1415–1423 [DOI] [PubMed] [Google Scholar]
- Boonen S., Marin F., Mellstrom D., Xie L., Desiah D., Krege J.H., et al. (2006) Safety and efficacy of teriparatide in elderly women with established osteoporosis: Bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc 54: 782–789 [DOI] [PubMed] [Google Scholar]
- Boonen S., McClung M.R., Eastell R., El-Hajj G. Fuleihan, Barton I.P., Delmas P. (2004) Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: Implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc 52: 1832–1839 [DOI] [PubMed] [Google Scholar]
- Chapuy M.C., Arlot M.E., Duboeuf F., Brun J., Crouzet B., Arnaud S., et al. (1992) Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 327: 1637–1642 [DOI] [PubMed] [Google Scholar]
- Chapuy M.C., Pamphile R., Paris E., Kempf C., Schlichting M., Arnaud S., et al. (2002) Combined calcium and vitamin D3 supplementation in elderly women: Confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: The Decalyos II study. Osteoporos Int 13: 257–264 [DOI] [PubMed] [Google Scholar]
- Chrischilles E.A., Butler C.D., Davis C.S., Wallace R.B. (1991) A model of lifetime osteoporosis impact. Arch Intern Med 151: 2026–2032 [PubMed] [Google Scholar]
- Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., et al. (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361: 756–765 [DOI] [PubMed] [Google Scholar]
- DIPART (vitamin D Individual Patient Analysis of Randomized Trials) Group. (2009) Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ 339: b5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman K.B., Kaplan F.S., Bilker W.B., Strom B.L., Lowe R.A. (2000) Treatment of osteoporosis: Are physicians missing an opportunity?. J Bone Joint Surg Am 82-A: 1063–1070 [DOI] [PubMed] [Google Scholar]
- Greendale G.A., DeAmicus T.A., Bucur A., Bretsky P., Rowe J.W., Reuben D.B., et al. (2000) A prospective study of the effect of fracture on measured physical performance: Results from the MacArthur Study — MAC. J Am Geriatr Soc 48: 546–549 [DOI] [PubMed] [Google Scholar]
- Greenspan S.L., Bone H.G., Ettinger M.P., Hanley D.A., Lindsay R., Zanchetta J.R., et al. (2007) Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: A randomized trial. Ann Intern Med 146: 326–339 [DOI] [PubMed] [Google Scholar]
- Harris S.T., Watts N.B., Genant H.K., McKeever C.D., Hangartner T., Keller M., et al. (1999) Effects of risedronate treatment on vertebral and non-vertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282: 1344–1352 [DOI] [PubMed] [Google Scholar]
- Heaney R.P., Gallagher J.C., Johnston C.C., Neer R., Parfitt A.M., Whedon G.D. (1982) Calcium nutrition and bone health in the elderly. Am J Clin Nutr 36(Suppl): 986–1013 [DOI] [PubMed] [Google Scholar]
- Hollick M.F., Matsuoka L.Y., Wortsman J. (1989) Age, vitamin D, and solar ultraviolet. Lancet 2: 1104–1105 [DOI] [PubMed] [Google Scholar]
- Hsia J., Heiss G., Ren H., Allison M., Dolan N.C., Greenland P., et al. (2007) Calcium/vitamin D supplementation and cardiovascular events. Circulation 115: 846–854 [DOI] [PubMed] [Google Scholar]
- Ismail A.A., Cooper C., Felsenberg D., Varlow J., Kanis J.A., Silman A.J., et al. the European Vertebral Osteoporosis Study Group (1999) Number and type of vertebral deformities: Epidemiological characteristics and relation to back pain and height loss. Osteoporos Int 9: 206–213 [DOI] [PubMed] [Google Scholar]
- Jackson R.D., LaCroix A.Z., Gass M., Wallace R.B., Robbins J., Lewis C.E., et al. (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354: 669–683 [DOI] [PubMed] [Google Scholar]
- Lips P. (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22: 477–501 [DOI] [PubMed] [Google Scholar]
- Lyles K.W., Colon-Emeric C.S., Magaziner J.S., Adachi J.D., Pieper C.F., Mautalen C., et al. (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraldo M.V., Vestergaard P., McMurdo M.E., Schwarz P. (2010) The evidence for antiresorptive osteoporosis treatment in the elderly and old. Eur Geriatr Med 5: 279–292 [Google Scholar]
- Masud T., McClung M.R., Geusens P. (2009) Reducing hip fracture risk with risedronate in elderly women with established osteoporosis. Clin Interv Aging 4: 445–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung M.R., Geusens P., Miller P.D., Zippel H., Bensen W.G., Roux C., et al. Hip Intervention Program Study Group (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344: 333–340 [DOI] [PubMed] [Google Scholar]
- Meunier P.J., Roux C., Seeman E., Ortolani S., Badurski J.E., Spector T.D., et al. (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350: 459–468 [DOI] [PubMed] [Google Scholar]
- Neer R.M., Arnaud C.D., Zanchetta J.R., Prince R., Gaich G.A., Reginster J.Y., et al. (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434–1441 [DOI] [PubMed] [Google Scholar]
- O'Neill T.W., Felsenberg D., Varlow J., Cooper C., Kanis J.A., Silman A.J. (1996) The prevalence of vertebral deformity in European men and women. The European Vertebral Osteoporosis Study. J Bone Miner Res 11: 1010–1018 [DOI] [PubMed] [Google Scholar]
- Pattanaungkul S., Riggs B.L., Yergey A.L., Vieira N.E., O'Fallon W.M., Kosla S. (2000) Relationship of intestinal calcium absorption to 1,25-dihydroxyvitamin D [1,25(OH)2D] levels in young versus elderly women: Evidence for age-related intestinal resistance to 1,25(OH)2D action. J Clin Endocrinol Metab 85: 4023–4027 [DOI] [PubMed] [Google Scholar]
- Pols H.A.P., Felsenberg D.A., Hanley D.A., Stepa'n J., Munoz-Torres N., Wilkin T.J., et al. (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: Results of the FOSIT study. Osteoporos Int 9: 461–468 [DOI] [PubMed] [Google Scholar]
- Population Division of the Department of Economic and Social Affairs of the Nations Secretariat 2002. World Population Prospects. http://esa.un.org/unpp [accessed April 2011].
- Raisz L.G. (2005) Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J Clin Invest 115: 3318–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster J.Y., Minne H.W., Sorensen O.H., Hooper M., Roux C., Brandi M.L., et al. (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11: 83–91 [DOI] [PubMed] [Google Scholar]
- Reginster J.Y., Seeman E., De Vernejoul M.C., Adami S., Compston J., Phenekos C., et al. (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90: 2816–2822 [DOI] [PubMed] [Google Scholar]
- Seeman E., Boonen S., Borgström F., Vellas B., Aquino J.P., Semler J., et al. (2010) Five years treatment with strontium ranelate reduces vertebral and nonvertebral fractures and increases the number and quality of remaining life-years in women over 80 years of age. Bone 46: 1038–1042 [DOI] [PubMed] [Google Scholar]
- Seeman E., Vellas B., Benhamou C., Aquino J.P., Semler J., Kaufman J.M., et al. (2006) Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res 21: 1113–1120 [DOI] [PubMed] [Google Scholar]
- Torgerson D.J., Dolan P. (1998) Prescribing by general practitioners after an osteoporotic fracture. Ann Rheum Dis 57: 378–379 [DOI] [PMC free article] [PubMed] [Google Scholar]