Abstract
Lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH) affect many older men and can have a significant impact on health-related quality of life. BPH is a progressive condition that may lead to complications including acute urinary retention. There exists an unmet need for a safe and effective, office-based, catheter-free therapy for BPH patients. NX-1207 is a promising first-in-class drug currently in phase III trials for the treatment of BPH. This review provides an overview of the NX-1207 trial program and considers its potential application for patients with symptoms related to BPH. NX-1207 is administered as an office-based procedure by transrectal intraprostatic injection under ultrasound guidance. NX-1207 has selective pro-apoptotic properties, which induce focal cell loss in prostate tissue, leading to prostate volume reduction with both short- and long-term symptomatic improvement. In four US clinical trials to date, NX-1207 has shown evidence of symptomatic improvement substantially better than currently approved BPH medications with no significant safety issues. Larger phase III trials are ongoing to confirm further the efficacy, safety, and tolerability for this minimally invasive, anesthetic-free, clinic-based treatment for BPH.
Keywords: benign prostatic hypertrophy, efficacy, lower urinary tract symptoms, NX-1207, safety
Introduction
Benign prostatic hyperplasia (BPH) is a common condition of middle-aged and elderly men, and is caused by the progressive age-related hyperplasia of prostate glandular and stromal tissues in the prostate’s periurethral transition zone [Wei et al. 2007; Berry et al. 1984]. BPH is the fourth most commonly diagnosed condition of men 50 years of age or older [Issa et al. 2006]. The chief complaint of the patient with clinical BPH is usually bothersome lower urinary tract symptoms (LUTS) typified by nocturia, frequent urination, urgency, weak stream, and/or incomplete emptying of the bladder. These symptoms are generally believed to be associated with bladder outlet and urethral compression from the enlarged prostate gland, but the relationship between BPH and LUTS is complex and unresolved. Not all men with histological evidence of BPH will develop LUTS; conversely, not all men with LUTS have BPH. Histological evidence of BPH is present at autopsy in 50% of men aged 51–60 years, increasing to 90% for men older than 80 years of age. Clinical BPH (moderate-to-severe LUTS) has an estimated prevalence of about 26% of men between the ages of 40 and 49 years and 46% in men older than 70 years [Chute et al. 1993]. BPH is a progressive disease with symptoms typically worsening over time [Fitzpatrick, 2006].Without treatment, BPH can lead to significant complications, including acute urinary retention, urinary tract infection (UTI), and, more infrequently, bladder calculi and renal deterioration.
Diagnosis
The initial evaluation of a patient presenting with LUTS includes a medical history to identify possible other causes of voiding dysfunction or comorbidities. Prostate cancer, bladder cancer, carcinoma in situ of the bladder, UTIs, urethral strictures, distal urethral stones, and bladder stones are among the conditions that can produce LUTS in older men. Severe BPH is associated with refractory retention, persistent gross hematuria, bladder stones, recurrent UTIs, and renal insufficiency. A digital rectal examination should be performed and where appropriate prostate specific antigen (PSA) testing offered. A urine dipstick should be performed to screen for hematuria and UTI [National Clinical Guideline Centre, 2010; American Urological Association, 2003].
Symptom severity is assessed by a validated symptom score such as the American Urological Association Symptom Index (AUASI) or the International Prostate Symptom Score (IPSS). The AUASI and IPSS use identically worded questions to assess symptom severity on a scale of 0–35 points, based on the individual scores (0–5) assigned by the patient to seven questions about incomplete bladder emptying, frequency and urgency of urination, hesitation and pushing and straining in urination, weak urine stream, and nocturia. The IPSS questionnaire also includes an additional disease-specific quality-of-life question scored on a scale of 0–6 points. Symptom severity is typically classified as mild (0–7), moderate (8–19), or severe (20–35). Other diagnostic testing for patients with moderate or severe symptoms may include, for example, peak urinary flow rate (Qmax) as measured by uroflowmetry, postvoid residual volume as measured by ultrasound bladder scan, and prostate volume as measured by transrectal ultrasound (TRUS) [Wei et al. 2007; European Association of Urology, 2004; American Urological Association, 2003].
Treatment
Current treatment guidelines [Roehrborn, 2008; European Association of Urology 2004; American Urological Association, 2003] recommend various treatment options depending upon symptom severity and bother to the patient, ranging from watchful waiting to medical therapy with an alpha-adrenergic blocker (αB) or 5 alpha-reductase inhibitor (5ARI) or a combination of αB and 5ARI, to surgical intervention through minimally invasive surgical therapy (MIST), laser ablation, transurethral resection of the prostate (TURP) or open surgery [European Association of Urology, 2004; American Urological Association, 2003; Verhamme et al. 2003]. Other medications not yet approved for BPH and in trials include phosphodiesterase-5 inhibitors and botulinum toxin.
NX-1207
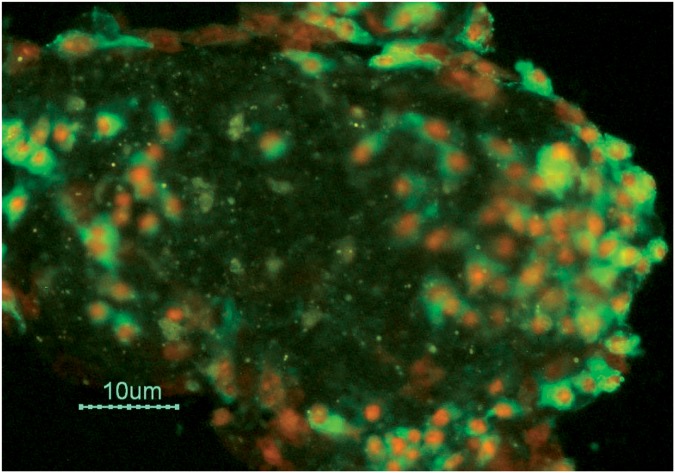
NX-1207 is an investigational drug for the treatment of BPH that is currently in phase III double-blinded, placebo-controlled, multicenter clinical trials in the USA. It is a new therapeutic protein of proprietary composition with selective pro-apoptotic properties [Nymox Pharmaceutical Corporation, data on file; Shore et al. 2008, 2007; Cowan et al. 2008, 2007; ClinicalTrials.gov]. Apoptosis is programmed cell death and is a natural mechanism in the body for cell suicide. NX-1207-treated cells in vivo and in vitro show strong positivity for standard apoptotic cell-death markers, such as caspases and Annexin V (Figure 1). The drug is sterile formulated in phosphate buffered saline (PBS) at physiologic pH (7.4) and is administered through TRUS guidance by injection directly into the periurethral transitional zone of the prostate. The transitional zone of the prostate surrounds the urethra; the enlargement of the transitional zone is believed to play a significant role in BPH and the association of symptoms with LUTS. NX-1207 has been demonstrated to induce focal cell loss in prostate tissue through apoptosis, leading to prostate tissue shrinkage with resultant short- and long-term symptomatic improvement. In the current ongoing phase III clinical trials of NX-1207 for BPH, NX-1207 is administered to patients with moderate-to-severe BPH (AUASI ≥ 15), and with prostate volumes between 30 ml and 70 ml (Table 1).
Figure 1.
Cell culture photomicrograph illustrating NX-1207-induced apoptotic cell death, strong diffuse Annexin V positivity and diffuse loss of membrane integrity. Scale bar = 10 µm. (Nymox Pharmaceutical Corporation, Hasbrouck Heights, NJ, USA).
Table 1.
Inclusion and exclusion criteria for phase III studies NX02-0017 and NX02-0018.
|
Inclusion criteria Male. Age 45 years. Informed consent (the informed consent form should meet all of the criteria of current local health regulations). No clinically significant deviation from normal in medical history, physical examination, clinical laboratory determinations and electrocardiograms. History of BPH for at least 1 year. American Urology Association Symptom Index score in the ≥15 range. Prostate volume ≥30 ml (30 g) and ≤70 ml (70 g) as determined by ultrasound carried out no more than 6 months prior. No BPH medications prior to baseline assessment, and for the duration of the trial. Urine peak flow rate <15 ml/s. |
|
Exclusion criteria Acute or chronic prostatitis or suspected prostatitis. History or evidence from physical examination, clinical laboratory tests, or electrocardiogram of any acute or chronic disease that may interfere with the study or endanger the subject. Any condition that would interfere with the subject’s ability to provide informed consent, to comply with study instructions, provide an objective assessment of his symptoms, or that might confound the interpretation of the study results. Any other sound medical, psychiatric, and/or social reason (including desire to maintain fertility) as determined by the investigator. History of any acute illness in the 15 days preceding screening. Participation in a study of any investigational drug or device within the previous 90 days. Use of any of the following concomitant medications: immunosuppressants, anticoagulants, alpha-blockers, 5 alpha-reductase inhibitors, antipsychotics, cancer chemotherapy, medication prescribed for dementia, male hormonal replacement, and medication prescribed for overactive bladder. History of any significant drug allergy. Documented urinary tract infection more than once in the past 12 months. Microscopic hematuria that has not been evaluated by a urologist and has not been attributed to BPH. PSA ≥10 ηg/ml. For subjects with PSA ≥4 ηg/ml and <10 ηg/ml a negative prostatic biopsy required within prior 12 months. Presence of a symptomatic median lobe of the prostate. Any urethral disease or condition. Prostate or bladder cancer. History of pelvic irradiation. Neurogenic bladder or lower urinary tract symptoms secondary to neurologic disease. History of central nervous system injuries within prior 6 months. History of pelvic trauma or surgery. Clinical evidence of Mullerian duct cysts or atonic, decompensated, or hypocontractile bladder. Previous surgical or invasive prostate treatments such as transurethral resection of the prostate, transurethral microwave thermotherapy, transurethral needle ablation, laser, or any other minimally invasive treatment. Lower urinary tract instrumentation of any type within previous 30 days. Postvoid residual urine volume >200 ml. History of urinary retention in the previous 12 months. Clinically significant renal or hepatic impairment. Bleeding disorder. Uncontrolled diabetes type 1 or type 2. History of drug, alcohol, or other substance abuse within previous 6 months. |
BPH, benign prostatic hyperplasia, PSA, prostate-specific antigen.
Mode of administration
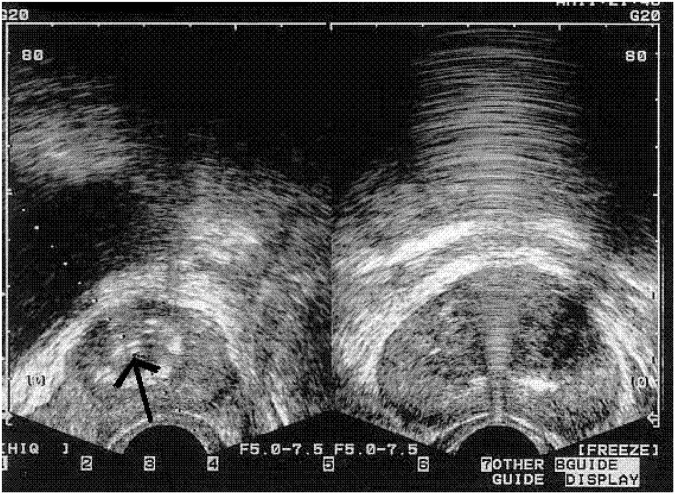
NX-1207 has been administered as an office-based procedure by a urologist. Patients receive antibiotic prophylaxis for 3 days before the procedure and for 1 week after. NX-1207 0.25 mg/ml is administered by transrectal intraprostatic injection with ultrasound guidance (Figure 2) using a no. 22 gauge needle. A total of 5 ml is injected into each lobe of the transition zone of the prostate. The procedure is of brief duration (5–10 min in total) and does not require local anesthesia, intravenous sedation, or urethral catheterization. Patients have reported minimal discomfort from the injection procedure itself. No specialized training for the urologist is required, assuming familiarity with other TRUS-guided procedures such as TRUS-guided prostate biopsy.
Figure 2.
Ultrasound image showing NX-1207 injection (arrow on left) in a subject with benign prostatic hyperplasia. (Nymox Pharmaceutical Corporation, Hasbrouck Heights, NJ, USA).
Clinical efficacy
Completed phase II studies
Two completed US phase II trials provided positive evidence of the safety and efficacy of NX-1207 for the treatment of BPH, confirming the results from two earlier US phase I/II studies [Nymox Pharmaceutical Corporation, data on file; Shore et al. 2007, 2008; Cowan et al. 2007]. The first phase II trial (0014) was a multicenter, randomized, double-blind, placebo-controlled study involving 43 clinical trial sites in the USA. A total of 175 men with BPH were enrolled in the study and randomized to receive a single TRUS-guided intraprostatic injection of one of three doses of NX-1207 (2.5, 5.0, or 10 mg), or a saline vehicle placebo control. The primary endpoint was improvement in AUASI score after 90 days. All three doses of NX-1207 showed therapeutic effect at the study’s primary endpoint (90 days after treatment) with the mean improvement in AUASI score ranging from 11.0 (2.5 mg; p = 0.008 when compared with placebo) to 8.7 (5.0 mg; p = 0.08) to 8.1 (10 mg; p = 0.17) with a total pooled improvement of 9.35 (p = 0.017). Subjects receiving NX-1207 had mean prostate glandular volume (PGV) reduction of 6.8 ml in the transition zone (p < 0.05, compared with placebo). The second phase II trial (0016) was designed as a multicenter, randomized, non-inferiority study involving 32 clinical US sites. The 2.5 mg dose of NX-1207 was chosen for comparison with an active open-label comparator (finasteride) because it was the lowest dose showing clinically significant therapeutic effect. A lower dose of NX-1207 (0.125 mg) was also employed in the trial for dose-response efficacy. There were 85 subjects randomized to either a therapeutic dose of NX-1207 (2.5 mg), the active comparator (finasteride), or a low dose of NX-1207 (0.125 mg). Subjects and investigators were blinded to the dosage delivered. The study’s primary endpoint was change in AUASI at 90 and 180 days for a single injection of NX-1207 compared with finasteride, powered to achieve a non-inferiority comparison. The mean AUASI score improvement after 90 days in the intent-to-treat group was 9.71 points for NX-1207 2.5 mg (n = 48) versus 4.13 points for finasteride (n = 24; p = 0.001), and 4.29 for NX-1207 0.125 mg (n = 7; p = 0.034). Subjects receiving full-dose (2.5 mg) NX-1207 had a mean PGV reduction of 4.6 g (p < 0.001), compared with the 0.125 mg dose. The 180-day results showed 7.51 points in mean reduction in AUASI score (p < 0.01, compared with the 0.125 mg dose, non-inferior to open-label finasteride). There has been no as yet discernible significant difference in results between patients with moderate and severe LUTS.
Pharmacokinetic measurements of NX-1207 in serum postinjection for the phase I and phase II trials showed no detectable levels of NX-1207 in any patients during any time interval [Nymox Pharmaceutical Corporation, data on file]. There were no significant changes in serum testosterone or serum PSA levels in the NX-1207 cohorts and no reported adverse effects on sexual function in the NX-1207 subjects.
Long-term, blinded, follow-up studies
Long-term follow-up studies of available, blinded subjects from the phase I and phase II studies of NX-1207 have provided efficacy of durable benefit from NX-1207 treatment. These studies have excluded subjects who were unavailable (e.g. had moved, unwilling), and investigational sites or clinics that had subsequently closed or did not wish to participate. Therefore, there are limitations to the interpretation of follow-up data. Accessible follow-up subjects provided symptom scores and reports of any subsequent BPH medical or surgical treatments. Follow up of 103 unselected blinded subjects from the 0014 phase II study at 16–27 months revealed that 52% of subjects treated with NX-1207 were not on any BPH medication and had not required surgical intervention since their initial NX-1207 treatment, and had a mean decrease of 10.2 points in AUASI. A blinded follow-up study at 22–35 months in 97 unselected subjects found that subjects who had not received any other subsequent treatment for BPH continued to demonstrate a decrease in AUASI from baseline in the NX-1207 group (overall two-sided t-test p < 0.001; 2.5 mg [p = 0.003], 5 mg [p = 0.001], 10 mg [p = 0.02]), while subjects in the placebo group did not. At the 5-year follow-up (48–60 months) 37% of patients who had received NX-1207 2.5 mg had not received any endoscopic BPH treatments, were on no BPH medications, and had a mean improvement of 10.1 points in their symptom scores. The improvement from baseline reached statistical significance (p < 0.001). At the 6.5-year follow up, 36% of subjects in phase I studies had received no other medical or surgical treatments in that interval, and 55% reported no surgical treatment for BPH at any time and no current BPH medications with mean AUASI improvement of 14.3 points. A further 3-year follow-up study of patients who received NX-1207 (2.5 mg) in the second 0016 phase II study found that over 50% of subjects required no further medical or endoscopic treatments and had a mean improvement of 11.8 points in their symptom scores (p < 0.001). At the 5-year follow up, 3.6% of available placebo subjects had required no further medical or endoscopic treatment for their BPH.
Safety and tolerability
Preclinical animal toxicology and safety studies showed no evidence of toxicity or other safety concerns for NX-1207 [Shore, 2010]. The clinical trials to date have not identified any significant safety issues relating to NX-1207 itself. Adverse events reported were attributable to the transrectal procedure (e.g. transient minimal hematuria and dysuria usually ≤1–2 days, occasional mild infections). There was no difference in overall incidence of these events between placebo and drug, and the overall rate of adverse events was significantly less than that reported in the medical literature for comparable transrectal procedures such as transrectal prostate biopsy and for other approved BPH procedures, such as TURP and MIST. The clinical studies have not shown any dose-related safety effect of NX-1207 administration. The 0014 phase II study provided evidence that the safety profile of NX 1207 at all three doses was comparable to that of the control intraprostatic saline injection (10 ml of PBS).
Conclusion and clinical experience
Intraprostatic injection of NX-1207 produced dramatic prostate gland volume reduction in animal studies and safety studies showed no toxic effect of high dosages on other tissues and no risk from repeated systemic administration of high dosages.
Phase I and II clinical trials have now demonstrated a significant treatment effect of NX-1207 for patients with LUTS attributable to BPH. AUASI (IPSS) questionnaires have shown marked improvement in subjects receiving NX-1207 in comparison to placebo in double-blind, randomized, controlled clinical trials. Positive results in long-term follow up of the treatment effect in nonselected subjects have been consistently found in retrospective questionnaires requested of all participants. Safety findings appear to be minor with adverse events primarily related to the transrectal delivery and not associated with any specific drug toxicity. Two large phase III trials are ongoing to further confirm efficacy, safety and tolerability for this catheter and anesthetic-free, clinic-based procedure for the treatment of BPH.
The authors have both participated as clinical investigators in the NX-1207 trials, and have found the administration of the drug to be a quick and uncomplicated procedure that takes a only few minutes, and which can be easily done by urologists, most of whom do very similar injections on a routine basis (e.g. during prostate biopsy and prostate infiltrations). There is no need for any new or special equipment, and no special training is required.
Compared with existing oral drug therapies (alpha blockers and 5ARIs), the use of NX-1207 does not have daily and lifelong compliance issues or the concerns of polypharmacy facing the aging population as well as the risk for drug–drug interactions. In addition, NX-1207 has not shown any of the bothersome and limiting sexual side effects of the oral therapies (such as impotence, loss of libido, or retrograde ejaculation). In comparison to MIST options, the transrectal injection approach of NX-1207, which is anesthetic/analgesic and catheter free, may be compelling for many patients. If the ongoing large phase III trials give similar results to the earlier phase trials, for both efficacy and safety, then the administration of NX-1207 could be expected to impact significantly the treatment algorithm for symptomatic patients with BPH.
Footnotes
References
- American Urological Association (2003) Guideline on the Management of Benign Prostatic Hyperplasia, [Available from: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph]. [PubMed]
- American Urological Association (2003) Guideline on the Management of Benign Prostatic Hyperplasia. Chapter 3: Results of the Treatment Outcomes Analyses 3-33 to 2-34. [Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph] [PubMed]
- Berry S.J., Coffey D.S., Walsh P.C., Ewing L.L. (1984) The development of human benign prostatic hyperplasia with age. J Urol 132: 474–479 [DOI] [PubMed] [Google Scholar]
- Chute C.G., Panser L.A., Girman C.J., Oesterling J.E., Guess H.A., Jacobsen C.J., et al. (1993) The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol 150: 85–89 [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov Clinical Evaluation of NX-1207 for the Treatment of Benign Prostatic Hyperplasia (BPH) NX02-0017. Identifier: NCT00918983, , [http://clinicaltrials.gov/ct2/show/NCT00918983]
- ClinicalTrials.gov Clinical Evaluation of NX-1207 for the Treatment of Benign Prostatic Hyperplasia (BPH) NX02-0018. Identifier: NCT00945490, , [http://clinicaltrials.gov/ct2/show/NCT00945490]
- Cowan B., Cline K., Wachs B., Freedman S., Kalota S., Shore N. (2007) Outcome Analysis 8-19 Months After Single Treatment with Transrectal NX-1207 in Multi-center Prospective Blinded Randomized Placebo Controlled Study of Men With Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia, Annual Meeting of the Mid-Atlantic Section of the American Urological Association: Bermuda, 18 October 2007 [Google Scholar]
- Cowan B., Wachs B., Wurzel R., Bailen J., Freedman S., Threatt C., et al. (2008) Prospective Randomized Phase 2 Trial Single-injection Transrectal Intraprostatic NX-1207 in Men with Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia, Annual Meeting of the Western Section of the American Urological Association: Monterey, 30 October 2008 [Google Scholar]
- European Association of Urology (2004) Guidelines on Benign Prostatic Hyperplasia, [http://www.uroweb.org/fileadmin/tx_eauguidelines/2009/Full/BPH.pdf].
- Fitzpatrick J.M. (2006) The natural history of benign prostatic hyperplasia. BJU Int 97(Suppl 2): 3–6; discussion 21–22 [DOI] [PubMed] [Google Scholar]
- Issa M.M., Fenter T.C., Black L., Grogg A.L., Kruep E.J. (2006) An assessment of the diagnosed prevalence of diseases in men 50 years of age or older. Am J Manag Care 12(Suppl 4): S83–S89 [PubMed] [Google Scholar]
- National Clinical Guideline Centre (2010) The Management of Lower Urinary Tract Symptoms in Men. Chapter 4: Diagnosis of Men with Lower Urinary Tract Symptoms. [Available at: http://www.nice.org.uk/nicemedia/live/12984/48554/48554.pdf]
- Nichol M.B., Knight T.K., Wu J., Barron R., Penson D.F. (2009) Evaluating use patterns of and adherence to medications for benign prostatic hyperplasia. J Urol 181: 2214–2222 [DOI] [PubMed] [Google Scholar]
- Nymox Pharmaceutical Corporation Data on file, Hasbrouck Heights, NJ, USA [Google Scholar]
- Roehrborn C.G. (2008) Currently available treatment guidelines for men with lower urinary tract symptoms. BJU Int 102(Suppl 2): 18–23 [DOI] [PubMed] [Google Scholar]
- Shore N. (2010) NX-1207: a novel investigational drug for the treatment of benign prostatic hyperplasia. Expert Opin Investig Drugs 19: 305–310 [DOI] [PubMed] [Google Scholar]
- Shore N., Freedman S., Kalota S., Wachs B., Cowan B., Cline K. (2007) Multi-center Prospective Randomized Double-blind Study of Single-injection Transrectal NX-1207 in Men with Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia, Annual Meeting of the South Central Section of the American Urological Association: Boston, 10 September 2007 [Google Scholar]
- Shore N., Wachs B., Wurzel R., Bailen J., Freedman S., Cline K., et al. (2008) A Prospective Randomized Two Dose Level Comparison of Single-injection Transrectal Intraprostatic NX-1207 and Finasteride in Men with Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia, Annual Meeting of the North Central Section of the American Urological Association: Chicago, 25 September 2008 [Google Scholar]
- Verhamme K.M., Dieleman J.P., Bleumink G.S., Bosch J.L., Stricker B.M., Sturkenboom M.C. (2003) Treatment strategies, patterns of drug use and treatment discontinuation in men with LUTS suggestive of benign prostatic hyperplasia: the Triumph project. Eur Urol 44: 539–545 [DOI] [PubMed] [Google Scholar]
- Wei J.T., Calhoun E.A., Jacobsen S.J. (2007) In: Benign prostatic hyperplasia, Litwin M.S., Saigal C.S. (eds). Urologic Diseases in America, US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. US Government Printing Office: NIH Publication No. 07-5512 Washington, DC, 48–53 [Google Scholar]