Abstract
Pulmonary arterial hypertension (PAH) consists of a group of heterogeneous but distinct disorders characterized by complex proliferation of the pulmonary vascular endothelium and progressive pulmonary vascular remodeling that leads to right ventricular failure and death. Over the past two decades, significant advances in our understanding of the pathobiology of PAH have led to the development of several therapeutic targets in this disease. Besides conservative therapeutic strategies such as anticoagulation and diuretics, the current treatment paradigm for PAH targets the mediators of the three main biologic pathways that are critical for its pathogenesis and progression: endothelin receptor antagonists inhibit the upregulated endothelin pathway by blocking the biologic activity of endothelin-1; phosphodiesterase-5 inhibitors prevent breakdown and increase the endogenous availability of cyclic guanosine monophosphate, which signals the vasorelaxing effects of the downregulated mediator nitric oxide; and prostacyclin derivatives provide an exogenous supply of the deficient mediator prostacyclin. In addition to these established current therapeutic options, a large number of potential therapeutic targets are being investigated. These novel therapeutic targets include soluble guanylyl cyclase, phosphodiesterases, tetrahydrobiopterin, 5-hydroxytryptamine (serotonin) receptor 2B, vasoactive intestinal peptide, receptor tyrosine kinases, adrenomedullin, rho kinase, elastases, endogenous steroids, endothelial progenitor cells, immune cells, bone morphogenetic protein and its receptors, potassium channels, metabolic pathways, and nuclear factor of activated T cells. This review provides an overview of the current therapeutic options and potential therapeutic targets for PAH.
Keywords: pulmonary hypertension, endothelin-1, vascular smooth muscle, vascular endothelium
Introduction
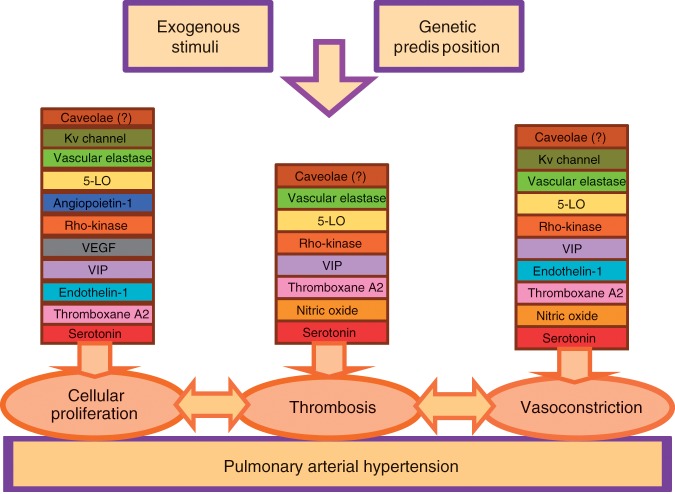
Pulmonary arterial hypertension (PAH) refers to a clinical syndrome of vascular disease with a stereotyped pattern of histopathology and is related to a variety of secondary disease states. It has become increasingly clear that the development of PAH entails a complex, multifactorial pathophysiology. Over the past two decades, increasing insight into the pathobiology of PAH has led to the emergence of a ‘multiple hit’ hypothesis to explain the development and progression of clinical PAH. A complex interplay of genetic mutations, exogenous exposures, and acquired disease states can predispose to PAH. Downstream of the genetic and acquired triggers of PAH, the histopathologic processes that predominate in the later stages of the disease include vasoconstriction, cellular proliferation, and thrombosis. These processes are influenced by a complex and dysregulated balance of vascular effectors controlling vasodilatation and vasoconstriction, growth suppressors and growth factors, and prothrombotic versus antithrombotic mediators (Figure 1).
Figure 1.
Pathogenesis and pathobiology of pulmonary arterial hypertension. 5-LO, 5-lipoxygenase; VEGF, vascular endothelial growth factor; VIP, vasoactive intestinal peptide.
There is currently no cure for PAH. However, the past two decades have seen significant advances with the development and clinical implementation of a number of medications that specifically target the aberrant regulatory and structural changes in the pulmonary arterial bed [McLaughlin et al. 2009; Farber and Loscalzo, 2004]. In addition to chronic adjunctive therapy, three classes of drugs have been developed and approved for the treatment of PAH: endothelin-1 (ET-1) receptor antagonists (ERAs), prostanoids, and phosphodiesterase type 5 (PDE-5) inhibitors. All three classes of medication have been shown to favorably affect hemodynamic parameters and to improve functional capacity and exercise tolerance [McLaughlin et al. 2009]. Furthermore, a variety of other substances that play roles as mediators through a final common pathway of pulmonary angiogenesis have emerged as appealing therapeutic targets and are currently the subject of intensive laboratory and clinical research. This review article provides an overview of these current therapeutic options and future potential targets.
Chronic adjuvant therapy
Chronic adjuvant therapies include digoxin, diuretics, supplemental oxygen and anticoagulation. There are no prospective, randomized clinical trials evaluating the chronic use of digoxin in PAH patients. Short-term use of digoxin in one small, uncontrolled study was beneficial and reduced circulating catecholamines [Rich et al. 1998]. Diuretics are recommended for alleviating systemic congestion. The response to diuretic therapy is variable and doses should be individualized. Renal function and electrolyte balance should be monitored, as excessive diuresis can cause serious hypotension and renal failure by impairing right ventricular function. Supplemental oxygen should be used to correct hypoxemia, which can aggravate pulmonary vasoconstriction. Chronic anticoagulation with warfarin is controversial in PAH patients. There are no prospective data supporting its routine use, although there is retrospective evidence demonstrating improved outcomes in idiopathic PAH patients who receive chronic anticoagulation [Frank et al. 1997].
Calcium channel blockers benefit only idiopathic PAH patients who demonstrate acute reduction (>20%) in mean pulmonary artery pressure and pulmonary vascular resistance during vasoreactivity testing [Rich et al. 1992]. Acute vasoreactivity to this degree is observed in only 12% of patients, however, and a sustained long-term response to calcium channel blockers is seen only in patients in whom mean pulmonary artery pressure falls to <40 mmHg during acute vasodilator challenge (about 6.8% of patients) [Sitbon et al. 2005].
Only high doses of calcium channel blockers have demonstrated efficacy, and their use is not recommended in World Health Organization (WHO) class IV patients and patients with PAH associated with other conditions.
Among vasodilator responders, calcium channel blocker therapy can be initiated with nifedipine (30 mg/day) or diltiazem (120 mg/day) and then increased to the maximal tolerated dose. Close follow up for continued benefit is required, because only 50% of patients maintain long-term responses [Chin and Rubin, 2008].
Endothelin receptor antagonists
ET-1 is a very potent vasoconstrictor; its use results in increased pulmonary vascular resistance [Raja, 2010a]. It also has proliferative effects on vascular smooth muscle cells [Raja and Dreyfus, 2008]. Blockade of the endothelin receptor has been used in the treatment of PAH. Several endothelin receptor antagonists (ERAs) have been identified and differ in their selectivity toward the ET-A and ET-B receptors. Currently, both selective and nonselective ERAs are approved and available for treating PAH.
Bosentan (Tracleer®; Actelion Pharmaceuticals Ltd, Allschwil, Switzerland) shows 20:1 ET-A/ET-B selectivity, ambrisentan (Letairis®; Gilead Sciences, Foster City, CA, USA) has 100:1 selectivity, and sitaxsentan (Thelin®; Encysive Pharmaceuticals, Houston, TX, USA) has 6500:1 selectivity.
The Bosentan Trial of Endothelin Antagonist Therapy (BREATHE-1) study revealed the ability of oral bosentan in patients with PAH to improve 6 min walk test distance, improve Borg dyspnea scores and time to clinical worsening as early as 16 weeks after initiating therapy. A 44 m placebo-adjusted difference in 6 min walk test distance in the BREATHE-1 patient population treated with bosentan was noted [Rubin et al. 2002]. The recommended treatment dosage is 125 mg twice daily after careful uptitration. Bosentan can increase transaminase levels in a reversible pattern. As a result of the potential for elevated transaminases, patients receiving bosentan require monthly monitoring of liver function during continuous therapy. It is recommended that if the transaminase levels are increased to at least three times the upper limit of normal, the dosage either needs to be held or decreased until the transaminase levels return to a normal range before resuming therapy.
Ambrisentan is a more selective ET-A receptor antagonist and is currently clinically available. The Ambrisentan in Pulmonary Artery Hypertension, Randomized, Double-Blinded, Placebo-Controlled, Multicenter, Efficacy Study I and II (ARIES I and II) demonstrated the efficacy of ambrisentan in improving 6 min walk test distance and time to clinical worsening, and the drug has received Food and Drug Administration (FDA) approval for functional class II and III patients. Although ambrisentan is in the same pharmacologic class as bosentan and its use requires transaminase monitoring, elevation of transaminases was not seen in any patient treated with ambrisentan. Side effects more commonly seen were nasal congestion, peripheral edema, and headaches [Galiè et al. 2008].
Sitaxsentan, the most selective ET-A receptor antagonist clinically available, which has been approved for use in Europe, Canada, and Australia but not in the USA, was recently voluntarily withdrawn by Pfizer. Even though the Sitaxsentan to Relieve Impaired Exercise trials (STRIDE 1 and 2) did show 6 min walk test distance improvement and functional class improvement, there had been reported prior cases of fatal hepatitis at higher doses in the STRIDE 2 patient population comparable with placebo [Barst et al. 2006, 2004]. This withdrawal was prompted by a review of emerging safety information from clinical trials and postmarketing reports regarding concerns about idiosyncratic, fatal hepatic failure.
All three drugs are contraindicated in conjunction with cyclosporine A and glyburide [Treiber et al. 2007; van Giersbergen et al. 2002]. Bosentan induces warfarin metabolism and requires an increase in the warfarin dose [Murphey and Hood, 2003], while sitaxsentan decreases warfarin metabolism, requiring a drop in the warfarin dose [Barst et al. 2006]. There are no known interactions between ambrisentan and warfarin.
It is important to mention that a clinically meaningful difference between the three approved ERAs with respect to their endothelin receptor selectivity has not been demonstrated to date. In clinical practice, therefore, other features are likely to be of greater relevance when considering treatment, such as the potential for serious drug–drug interactions, convenience of the dosing schedule, and rates of limiting side effects. These characteristics bear more relation to the chemical or pharmacologic properties of the drug than to receptor selectivity itself.
A recently published robust meta-analysis confirmed the therapeutic benefit of ERAs but concluded that they have no proven effect on mortality [Ryerson et al. 2010]. It is important, however, to highlight that there has been no definitive study proving a survival benefit for any ERA, owing to the fact that long-term, placebo-controlled studies are perceived as ethically unjustifiable and randomized, controlled trials are not powered to detect a mortality benefit.
Phosphodiesterase-5 inhibitors
Sildenafil (Viagra®, Revatio; Pfizer, New York, NY, USA) is a PDE-5 inhibitor initially approved for erectile dysfunction but subsequently shown to have effects on the pulmonary vasculature [Raja and Nayak, 2004]. Sildenafil increases the effects of locally produced nitric oxide (NO) by inhibiting the breakdown of NO’s second messenger, cyclic guanosine monophosphate. This results in pulmonary vasodilation and inhibition of smooth muscle cell growth [Raja et al. 2006]. Sildenafil is currently approved for the treatment of WHO class II and III patients. The Sildenafil Use in Pulmonary Arterial Hypertension (SUPER-1) trial showed improvement in 6 min walk test distances in patients receiving sildenafil at 20, 40, or 80 mg three times daily compared with patients receiving placebo as early as 4 weeks and extending to 12 weeks. No significant improvements were noted at dosages above 20 mg three times daily in the short-term trial [Galiè et al. 2005]. Therefore, it is recommended that only the lower dose be used in clinical practice.
Tadalafil (Adcirca®; Eli Lilly and Company, Indianapolis, IN, USA) is the second PDE-5 inhibitor that has recently been approved by the FDA. The results of the Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST-1) study showed a 33 m improvement in 6 min walk test distance adjusted for placebo at 16 weeks in naïve treatment plus combined therapy with bosentan, and a 44 m improvement in patients on monotherapy [Galiè et al. 2009]. Tadalafil has a longer half-life than sildenafil, thus allowing once daily dosing. The recommended daily dose is 40 mg [Falk et al. 2010].
Vardenafil (Levitra®; Bayer Health Care Pharmaceuticals, West Haven, CT, USA) is a new PDE-5 inhibitor that has shown some efficacy in the treatment of PAH. In a multicenter, open-label study of 1 year’s duration that recruited 45 patients with PAH to determine the long-term safety and efficacy of vardenafil (5 mg once daily for the first 4 weeks, then 5 mg twice daily), treatment with vardenafil was associated with improvements in 6 min walk distance, hemodynamic parameters, and WHO functional class [Jing et al. 2009]. Overall, vardenafil treatment was well tolerated. No patients were withdrawn owing to adverse events and none died during the course of the study.
A recently published randomized, double-blind, placebo-controlled study confirmed that vardenafil is effective and well tolerated in patients with PAH at a dose of 5 mg twice daily [Jing et al. 2011]. In this study, 66 patients with PAH were randomized 2:1 to vardenafil (5 mg once daily for 4 weeks then 5 mg twice daily, n = 44) or placebo (n = 22) for 12 weeks. Patients completing this phase were then treated with open-label vardenafil (5 mg twice daily) for a further 12 weeks. At week 12, the mean placebo-corrected 6 min walking distance was increased with vardenafil (69 m, p < 0.001), and this improvement was maintained for at least 24 weeks. Vardenafil also increased the mean placebo-corrected cardiac index (0.39 l/min/m2, p = 0.005) and decreased mean pulmonary arterial pressure and pulmonary vascular resistance (5.3 mmHg, p = 0.047; and 4.7 Wood units, p = 0.003, respectively) at week 12. Four patients in the placebo group (20.0%) and one in the vardenafil group (2.3%) had clinical worsening events (hazard ratio 0.105, 95% confidence interval 0.012–0.938, p = 0.044). Vardenafil was associated with only mild and transient adverse events.
Like ERAs, PDE-5 inhibitors have no proven effect on mortality [Ryerson et al. 2010].
Prostanoids
Prostacyclin synthase levels are low in patients with PAH; the production of prostacyclin is diminished, preventing adequate vasodilation and loss of the antiproliferative effects on the smooth muscle cells in the vascular wall. Through their actions as potent pulmonary artery vasodilators, prostanoid medications have been used in the treatment of PAH for over 15 years. Epoprostenol (synthetic prostacyclin, Flolan®; Gilead Sciences) was the first FDA-approved therapy for PAH. Because of its short half-life, it requires delivery through a continuous portable infusion pump and an indwelling central venous catheter. Epoprostenol remains the treatment of choice for patients with the most advanced disease and leads to improvements in exercise capacity, hemodynamics, and quality of life. It also appears to improve survival, based on a 12-week randomized but unblinded study [Barst et al. 1996] and two longer-term open-label studies [McLaughlin et al. 2002; Sitbon et al. 2002].
Longer-acting prostacyclin analogs have also been developed for intravenous, inhaled, and oral use, with variable effectiveness and tolerability. These analogs include treprostinil (Remodulin®; United Therapeutics, Silver Spring, MD, USA), approved for subcutaneous and intravenous use in the USA; iloprost (Ventavis®; Actelion), approved for inhaled use in the USA and for intravenous and inhaled use in Europe; and Beraprost® (Toray Industries, New York, NY, USA), an oral medication approved in Japan and several other countries in the Far East [Barst et al. 2003].
Epoprostenol is the most comprehensively studied of all the prostanoids. It has been shown to have favorable hemodynamic effects, including lowering mean pulmonary artery pressure and pulmonary vascular resistance and increasing cardiac output. Originally, epoprostenol was used as a bridge for patients waiting for lung transplantation. Its effects were ultimately found to be so favorable that many clinicians and patients preferred to remain on chronic epoprostenol therapy rather than be listed for lung transplantation. Clinical trials have shown beneficial improvements in 6 min walk test distances up to 60 m at 12 weeks when adjusted for patients receiving only conventional therapy. In addition, survival has been shown to be improved in epoprostenol-treated patients even after adjustment for changes in stroke volume and systemic vascular resistance [Barst et al. 1996]. Difficulties in administration include the need for permanent intravenous access, resulting in infected catheters, flushing, thrombocytopenia, gastrointestinal disturbances, headaches, and pain on mastication.
Iloprost is available as an inhaled prostanoid agent through a nebulizer device. In a placebo-controlled trial of 203 patients, inhaled iloprost significantly improved the combined endpoint of change in New York Heart Association (NYHA) functional class and 10% improvement in 6 min walk distance (p = 0.007) [Olschewski et al. 2002]. Small, short-term clinical trials demonstrated hemodynamic benefits for inhaled iloprost alone and in combination with other pulmonary vasodilating agents [Baker and Hockman, 2005]. The aerosolized delivery route and low incidence of adverse events are positive attributes of inhaled iloprost, while the frequency of administration and lack of comparative data limit its role in PAH. Long-term effects of iloprost on survival are not known at this time. Common side effects include flushing, cough, headache, and jaw pain on mastication. Iloprost is currently FDA approved for functional class II and III patients.
Treprostinil is stable at room temperature and has the pharmacologic profile of epoprostenol with a much longer half-life, permitting subcutaneous rather than intravenous administration. In 470 patients randomized in a placebo-controlled study, subcutaneous treprostinil improved placebo-adjusted 6 min walk test distance by 16 m in a dose-dependent manner [Simonneau et al. 2002]. Side effects are similar to those of other prostanoid agents.
Beraprost is absorbed rapidly after the administration of an oral dose of tablets under fasting conditions; it reaches a peak concentration within 30 min and has an elimination half-life of 35–40 min. In Europe, 130 NYHA functional class II and III subjects were enrolled in a 12-week randomized, double-blind, placebo-controlled trial of beraprost [Galiè et al. 2002]. Subjects had PAH of idiopathic origin or caused by connective tissue diseases, congenital left-to-right shunts, portal hypertension and human immunodeficiency virus. At a median dosage of 80 µg four times daily, subjects had a mean increase of 25 m in 6 min walk distance (p = 0.04). Subjects with idiopathic PAH had a mean increase of 46 m (p = 0.04), whereas subjects with PAH from other causes experienced no significant improvement. In the USA, a similar trial was designed but it included NYHA functional class II and III patients and had a study duration of 12 months. This study demonstrated improved 6 min walk distance at 3 and 6 months compared with placebo, which was not sustained at 9 and 12 months [Barst et al. 2003].
Of all the currently available therapeutic options for PAH, epoprostenol is the only drug that has a significant mortality benefit [Ryerson et al. 2010].
Future prospects
Increasing insight into the pathobiology and pathogenesis of PAH has led to the discovery of several potential novel therapeutic targets. These include soluble guanylyl cyclase, phosphodiesterases, tetrahydrobiopterin, 5-hydroxytryptamine (serotonin) receptor 2B, vasoactive intestinal peptide, receptor tyrosine kinases, adrenomedullin, rho kinase (ROCK), elastases, endogenous steroids, endothelial progenitor cells, immune cells, bone morphogenetic protein and its receptors, potassium channels, metabolic pathways, and nuclear factor of activated T cells. This section summarizes the currently available evidence for the potential role of these vascular effectors, immune cells, growth factors and cytokines as novel therapeutic targets.
Macitentan
Macitentan (ACT-064992), under development by Actelion in collaboration with Japanese licensee Nippon Shinyaku Co. Ltd, is an orally active, non-peptide dual endothelin (ET-A and ET-B) receptor antagonist for the potential treatment of idiopathic pulmonary fibrosis (IPF) and PAH. Macitentan, because of its ability to target the tissues and to block both ET-A and ET-B receptors, is emerging as a new agent to treat IPF and PAH [Raja, 2010b].
The dual ET-A and ET-B receptor antagonism of macitentan was examined in rats by measuring plasma ET-1 concentrations [Iglarz et al. 2008]. When administered to normotensive rats, macitentan, like bosentan, augmented plasma ET-1 levels, confirming that both ET-A and ET-B receptors were blocked. The increase in plasma ET-1 levels occurred with a 10-fold lower dose of macitentan than of bosentan, indicating that macitentan displays more potent pharmacologic activity in vivo. In hypertensive deoxycorticosterone acetate salt rats, macitentan reduced mean arterial blood pressure with an ED50 value of 1 mg/kg, compared with 10 mg/kg for bosentan. With the maximal effective dose of macitentan (10 mg/kg), the decrease in blood pressure was maintained for ~40 h, whereas with bosentan (100 mg/kg) the response duration was 20 h [Iglarz et al. 2008].
The monocrotaline rat model of pulmonary hypertension was used to assess the effect of macitentan (0.3–100 mg/kg/day orally for 4 weeks) on cardiac hypertrophy and survival [Iglarz et al. 2008]. Both macitentan and bosentan dose-dependently prevented the development of pulmonary hypertension and, in addition, both drugs prevented the development of right ventricle hypertrophy, with maximal effective doses of 30 mg/kg for macitentan and 300 mg/kg for bosentan. After administration of macitentan (30 mg/kg/day; p < 0.002), the 42-day mortality rate was reduced by 66% compared with vehicle-treated rats [Iglarz et al. 2008].
The streptozotocin-induced diabetic rat model was used to evaluate the effect of macitentan on end-organ damage, in particular renal toxicity [Iglarz et al. 2008]. Compared with untreated control diabetic rats, animals treated with macitentan (30 mg/kg orally for 24 weeks) showed partial prevention of the development of renal vasoconstriction, increased renal blood flow and an enhanced glomerular filtration rate (p < 0.05). Chronic macitentan treatment reduced vascular and tubulointerstitial lesion formation and glomerular damage, and proteinuria was partially prevented. Also, an increase in vascular endothelial growth factor (VEGF) expression in the retina of diabetic rats was prevented by macitentan treatment [Iglarz et al. 2008].
The phase I and II clinical trials conducted to date have demonstrated that macitentan increases plasma levels of ET-1, displays dose-dependent pharmacokinetics, and is well tolerated in healthy volunteers and patients. At the time of writing, a phase III trial in patients with PAH was ongoing. It is expected that the results of this trial will validate the safety and efficacy of macitentan [Raja, 2010b].
Rho kinase inhibitors
The ROCK inhibitors are a new class of agents that may be beneficial in the treatment of PAH. The first-generation agents, fasudil and Y-27632, have been widely studied, fasudil showing efficacy in human studies in Japan and the USA [Fujita et al. 2010; Ishikura et al. 2006]. SB-772077-B is a recently developed aminofurazan-based ROCK inhibitor with strong anti-inflammatory properties [Doe et al. 2007].
Multiple cell types in the vascular wall rely upon the ROCK signaling pathway for homeostatic function and response to injury [Shimokawa and Takeshita, 2005]. These cell types include endothelial and vascular smooth muscle cells, inflammatory cells, and fibroblasts. Rho is a guanosine triphosphate binding protein that activates its downstream target, ROCK, in response to activation of a variety of G protein-coupled receptors. When activated, ROCK inhibits myosin phosphatase and conversely upregulates the ezrin–radixin–moesin (ERM) family of kinases. In vitro activation of these signaling cascades results in modulation of multiple cellular processes, including enhanced vasoconstriction, proliferation, impaired endothelial response to vasodilators, chronic pulmonary remodeling, and upregulation of vasoactive cytokines via the Nuclear factor-κB transcription pathway. ROCK activity has also been linked specifically to a number of known effectors of PAH, including ET-1 [Weigand et al. 2006], serotonin [Li et al. 2007], and endothelial NO synthase (eNOS) [Takemoto et al. 2002], among others. Recently, elevated ROCK activity has been demonstrated in animal models of PAH [Nagaoka et al. 2004], ROCK inhibitors being associated with pulmonary vasodilatation and regression of PAH in various animal models [Murthy et al. 2010].
Emerging evidence from both animal and human studies suggests that the three different ROCK inhibitors can promote vasodilation independently of the mechanism that induces vasoconstriction and will be useful in conditions in which endothelial function is impaired, including PAH.
Riociguat
Cyclic guanosine monophosphate (cGMP) is an important mediator of the preferential perfusion of well-ventilated regions throughout the lung. Drugs that increase cGMP levels could promote pulmonary vasorelaxation while maintaining optimal gas exchange. cGMP is generated by soluble guanylate cyclase (sGC), which can be stimulated by NO [Schermuly et al. 2011].
Riociguat, a potent sGC stimulator, has an improved pharmacokinetic profile and has positive effects on pulmonary hemodynamics and exercise capacity in patients with PAH [Grimminger et al. 2009]. Riociguat is currently being investigated in phase III clinical trials for the oral treatment of PAH. The results of these studies show that pulmonary vascular resistance is decreased and cardiac function is improved in patients with moderate to severe PAH [Ghofrani et al. 2010a]. Riociguat offers great therapeutic potential as a treatment for patients with pulmonary vascular disorders, but does not have selective pulmonary vasodilator activity [Murthy et al. 2010].
Aviptadil
Downregulation of vasoactive intestinal peptide (VIP) may also play a pathogenic role. VIP is a pulmonary vasodilator, an inhibitor of proliferation of vascular smooth muscle cells, and an inhibitor of platelet aggregation [Maruno et al. 1995; Cox et al. 1984]. Decreased concentrations of VIP have been reported in serum and lung tissue of patients with PAH [Petkov et al. 2003]. VIP-null mice suffer from moderate pulmonary hypertension [Said et al. 2007]. They also show moderate right ventricular hypertension and right ventricular hypertrophy confirmed by an increased ratio of right ventricle to left ventricle plus septum weight, and an enlarged, thickened pulmonary artery and smaller branches with increased muscularization and narrowed lumen. Furthermore, both pulmonary arterial pressure and pulmonary vascular resistance decrease after treatment with VIP [Haydar et al. 2007].
Aviptadil, a synthetically produced version of VIP, has been used in inhaled form in a small study recruiting humans to test the acute effects on hemodynamics and blood gases, and the safety, of a single dose [Leuchte et al. 2008]. A total of 20 patients with pulmonary hypertension (PAH in nine, pulmonary hypertension in lung disease in eight and chronic thromboembolic pulmonary hypertension in three) inhaled a single 100 µg dose of aviptadil during right heart catheterization. Hemodynamics and blood gases were measured. The aviptadil aerosol caused a small and temporary but significant selective pulmonary vasodilation, an improved stroke volume and mixed venous oxygen saturation. Overall, six patients experienced a pulmonary vascular resistance reduction greater than 20%. In patients with significant lung disease, aviptadil tended to improve oxygenation. The pulmonary vasodilating effect of the aviptadil aerosol was modest and short-lived, did not cause any side effects, and led to a reduced workload of the right ventricle without affecting systemic blood pressure. However, further studies are needed to evaluate the full therapeutic potential of aviptadil aerosol, including higher doses and chronic treatment.
Adrenomedullin
Adrenomedullin is a potent vasodilator peptide that was originally isolated from human pheochromocytoma [Kitamura et al. 1993]. Immunoreactive adrenomedullin has subsequently been detected in plasma and a variety of tissues, including vessels, heart, and lungs [Ichiki et al. 1994]. Owji and colleagues reported that there are specific receptors for adrenomedullin in the lungs [Owji et al. 1995]. It has been shown that the plasma adrenomedullin concentration increases in proportion to the severity of pulmonary hypertension, and that circulating adrenomedullin is partially metabolized in the lungs [Kakishita et al. 1999]. These findings suggest that adrenomedullin plays an important role in the regulation of pulmonary vascular tone.
Recently, experimental studies have shown that exogenously administered adrenomedullin induces pulmonary vasodilatation in rats and cats [Nossaman et al. 1996, 1995]. It has been shown that short-term infusion of adrenomedullin ameliorates pulmonary hypertension secondary to congestive heart failure [Nagaya et al. 2000]. Adrenomedullin has also been shown to inhibit the migration and proliferation of vascular smooth muscle cells [Kano et al. 1996; Horio et al. 1995]. Unfortunately, however, intravenously administered adrenomedullin induced systemic hypotension in such patients because of nonselective vasodilation in the pulmonary and systemic vascular beds.
More recently, inhalation of aerosolized adrenomedullin has been shown to have beneficial effects on pulmonary hemodynamics and exercise capacity, without systemic hypotension, in patients with idiopathic PAH [Nagaya et al. 2000]. In this study, acute hemodynamic responses to inhalation of aerosolized adrenomedullin (10 µg/kg body weight) were examined in 11 patients with idiopathic PAH during cardiac catheterization. Cardiopulmonary exercise testing was performed immediately after inhalation of aerosolized adrenomedullin or placebo. The work rate was increased by 15 W/min until the symptom-limited maximum, with breath-by-breath gas analysis. Inhalation of adrenomedullin produced a 13% decrease in mean pulmonary arterial pressure (from 54 ± 3 to 47 ± 3 mmHg, p < 0.05) and a 22% decrease in pulmonary vascular resistance (from 12.6 ± 1.5 to 9.8 ± 1.3 Wood units, p < 0.05). However, neither systemic arterial pressure nor heart rate was altered. Inhalation of adrenomedullin significantly increased peak oxygen consumption during exercise (peak O2, from 14.6 ± 0.6 to 15.7 ± 0.6 ml/kg/min, p < 0.05) and the ratio of change in oxygen uptake to that in work rate (ΔO2/ΔW ratio, from 6.3 ± 0.4 to 7.0 ± 0.5 ml/min/W, p < 0.05). These parameters remained unchanged during placebo inhalation. There is a need for a multicenter, randomized, controlled trial with well defined endpoints to verify the long-term safety and efficacy of adrenomedullin.
Imatinib mesylate
Imatinib mesylate (formerly CGP 57148B or STI571; Gleevec® in the USA and a few other countries; Glivec® in the rest of the world, including Europe) is a first-generation receptor tyrosine kinase inhibitor drug marketed by Novartis (Frimley, Surrey, UK) and approved by the FDA in 2001 for the treatment of chronic myelogenous leukemia. Imatinib selectively inhibits the platelet-derived growth factor (PDGF) receptor kinase as well the cKIT and Abl kinases, among other targets. Various studies have implicated the PDGF pathway in the pathogenesis of PAH [Chhina et al. 2010].
The potential of imatinib mesylate in PAH has been described in a number of case reports, in which it was usually administered in conjunction with and after an inadequate response to conventional therapy [Patterson et al. 2006; Souza et al. 2006; Ghofrani et al. 2005]. The results of these case reports are mixed; while some report salutary results, others report no benefit from imatinib mesylate exposure.
A multicenter phase II, double-blind, placebo-controlled trial evaluating the safety and efficacy of imatinib mesylate in PAH was recently completed and positive preliminary results were presented at the European Respiratory Society Congress in Berlin, Germany, in 2008 and again at the American Thoracic Society meeting in San Diego, CA, USA, in May 2009. This study included 59 patients with PAH, who remained symptomatic on one or more standard PAH therapies (prostanoids, endothelin antagonists, or PDE-5 inhibitors), and in whom the safety and tolerability of a 400 mg dose of imatinib was evaluated. However, this study did not meet the prespecified efficacy endpoint of change in 6 min walk distance. Although the placebo-adjusted difference was 23 m in favor of the imatinib group, the small number of patients in the study meant this result was not statistically significant (22 ± 63 versus −1.0 ± 53 m; mean ± SD). Nonetheless, the potential of the drug was reaffirmed on this basis and there were statistically significant improvements in hemodynamic endpoints, including both a significant decrease in pulmonary vascular resistance (imatinib −300 ± 346 compared with −78 ± 269 dynes; p < 0.01) and a significant increase in cardiac output (0.7 ± 1.6 compared with 0.1 ± 1.2 l/min; p < 0.05) [Ghofrani et al. 2009]. A subgroup analysis of the most severely affected patients (pulmonary vascular resistance >1000 dynes/cm2) suggested even more robust improvements in exercise and hemodynamic variables [Ghofrani et al. 2010b]. These results are awaiting validation in a larger follow-up phase III, randomized, controlled trial, which is in the early stages of implementation.
Other potential therapeutic targets
VEGF is a well studied endothelial cell mitogen and angiogenic factor. In the pulmonary circulation it binds endothelial cells via tyrosine kinase receptors (two subtypes: VEGFR-1/KDR and VEGFR-2/Flt). The production of VEGF and its receptors is upregulated in human pulmonary tissue in both acute and chronic hypoxia [Tuder et al. 1995]. Increased VEGF expression has also been observed in PAH, accompanied by elevated levels of VEGFR-1 in the affected pulmonary endothelium and specifically elevated levels of VEGFR-2 in plexiform lesions [Tuder et al. 2001]. Evidence from animal studies suggests that VEGF is a potential therapeutic target in PAH [Wanstall et al. 2002]. Correlation of these findings with in vivo human disease is awaited.
Other potential therapeutic targets include phosphodiesterase I [Schermuly et al. 2007], survivin [McMurtry et al. 2005], the calcium-binding protein S100A4/Mts1 [Greenway et al. 2004], the transient receptor potential channels [Yu et al. 2004], and angiopoietin-1 [Brindle et al. 2006]. These represent important but at present incompletely described pathogenic contributors. Furthermore, other vascular growth factors, such as basic fibroblast growth factor, insulin-like growth factor-1, and epidermal growth factor, may play downstream roles in later stages of PAH and provide avenues that need further exploration.
Conclusion
PAH is a progressive, potentially fatal disease that is not curable with the currently approved therapies. These available pharmaceutical therapies target three specific effector pathways – the prostacyclin pathway, the endothelin pathway, and the PDE-5 pathway – through the augmentation of their downstream effector molecules. The therapeutic endpoint for all three of these classes of approved drugs is vasodilation in the PAH lung vasculature, which is achieved by targeting the imbalance between vasodilators and vasoconstrictors. At the same time, the imbalance between the proliferative and apoptotic rates of the cell types in the vessel walls, which leads to vascular remodeling, is a potential therapeutic target. Intuitively, it makes sense that both of these imbalanced mechanisms should be corrected and optimized to achieve molecular and physiologic homeostasis in PAH.
Increasing understanding of the pathobiology of PAH has resulted in the emergence of multiple potential targets that could halt and/or reverse the vascular remodeling and the progression of PAH. Some of the new drugs on the horizon for PAH therapy affecting these potential targets, including macitentan, ROCK inhibitors, aviptadil, riociguat, and imatinib, are undergoing rigorous clinical testing to verify their safety and efficacy. It is expected that in the near future some of these potential therapeutic options can be utilized in combination with established pharmacologic therapies to treat PAH aggressively by targeting multiple effector pathways, thereby offering both symptomatic and survival benefits to the sufferers of this progressively fatal disease.
Footnotes
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The author declares no conflict of interest in preparing this article.
References
- Baker S.E., Hockman R.H. (2005) Inhaled iloprost in pulmonary hypertension. Ann Pharmacother 39: 1265–1274 [DOI] [PubMed] [Google Scholar]
- Barst R.J., Langleben D., Badesch D., Frost A., Lawrence E.C., Shapiro S., et al. for the STRIDE-2 Study Group (2006) Treatment of pulmonary arterial hypertension with the selective endothelin: a receptor antagonist sitaxsentan. J Am Coll Cardiol 47: 2049–2056 [DOI] [PubMed] [Google Scholar]
- Barst R.J., Langleben D., Frost A., Horn E.M., Oudiz R., Shapiro S., et al. for the STRIDE-1 Study Group (2004) Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med 169: 441–447 [DOI] [PubMed] [Google Scholar]
- Barst R.J., McGoon M., McLaughlin V., Tapson V., Rich S., Rubin L., et al. for the Beraprost Study Group (2003) Beraprost for pulmonary arterial hypertension. J Am Coll Cardiol 41: 2119–2125 [DOI] [PubMed] [Google Scholar]
- Barst R.J., Rubin L.J., Long W.A., McGoon M.D., Rich S., Badesch D.B., et al. (1996) A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 334: 296–302 [DOI] [PubMed] [Google Scholar]
- Brindle N.P., Saharinen P., Alitalo K. (2006) Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98: 1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhina M.K., Nargues W., Grant G.M., Nathan S.D. (2010) Evaluation of imatinib mesylate in the treatment of pulmonary arterial hypertension. Future Cardiol 6: 19–35 [DOI] [PubMed] [Google Scholar]
- Chin K.M., Rubin L.J. (2008) Pulmonary arterial hypertension. J Am Coll Cardiol 51: 1527–1538 [DOI] [PubMed] [Google Scholar]
- Cox C.P., Linden J., Said S.I. (1984) VIP elevates platelet cyclic AMP (cAMP) levels and inhibits in vitro platelet activation induced by platelet-activating factor (PAF). Peptides 5: 325–328 [DOI] [PubMed] [Google Scholar]
- Doe C., Bentley R., Behm D.J., Lafferty R., Stavenger R., Jung D., et al. (2007) Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther 320: 89–98 [DOI] [PubMed] [Google Scholar]
- Falk J.A., Philip K.J., Schwarz E.R. (2010) The emergence of oral tadalafil as a once-daily treatment for pulmonary arterial hypertension. Vasc Health Risk Manag 6: 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber H.W., Loscalzo J. (2004) Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665 [DOI] [PubMed] [Google Scholar]
- Frank H., Mlczoch J., Huber K., Schuster E., Gurtner H.P., Kneussl M. (1997) The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest 112: 714–721 [DOI] [PubMed] [Google Scholar]
- Fujita H., Fukumoto Y., Saji K., Sugimura K., Demachi J., Nawata J., et al. (2010) Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels Vol. 25: 144–149 [DOI] [PubMed] [Google Scholar]
- Galiè N., Brundage B.H., Ghofrani H.A., Oudiz R.J., Simonneau G., Safdar Z., et al. for the Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group (2009) Tadalafil therapy for pulmonary hypertension. Circulation 119: 2894–2903 [DOI] [PubMed] [Google Scholar]
- Galiè N., Ghofrani H.A., Torbicki A., Barst R.J., Rubin L.J., Badesch D., et al. (2005) Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 353: 2148–2157 [DOI] [PubMed] [Google Scholar]
- Galiè N., Humbert M., Vachiéry J.L., Vizza C.D., Kneussl M., Manes A., et al. for the Arterial Pulmonary Hypertension and Beraprost European (ALPHABET) Study Group (2002) Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 39: 1496–1502 [DOI] [PubMed] [Google Scholar]
- Galiè N., Olschewski H., Oudiz R.J., Torres F., Frost A., Ghofrani H.A., et al. for the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) Group (2008) Ambrisentan for the treatment of pulmonary arterial hypertension: results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-blinded, Placebo-controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation 117: 3010–3019 [DOI] [PubMed] [Google Scholar]
- Ghofrani H.A., Hoeper M.M., Halank M., Meyer F.J., Staehler G., Behr J., et al. (2010a) Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J 36: 792–799 [DOI] [PubMed] [Google Scholar]
- Ghofrani H.A., Morrell N.W., Hoeper M.M., Olschewski H., Peacock A.J., Barst R.J., et al. (2010b) Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med 182: 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghofrani H.A., Seeger W., Grimminger F. (2005) Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 353: 1412–1413 [DOI] [PubMed] [Google Scholar]
- Greenway S., van Suylen R.J., Du Marchie Sarvaas G., Kwan E., Ambartsumian N., Lukanidin E., et al. (2004) S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol 164: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimminger F., Weimann G., Frey R., Voswinckel R., Thamm M., Bölkow D., et al. (2009) First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J 33: 785–792 [DOI] [PubMed] [Google Scholar]
- Haydar S., Sarti J.F., Grisoni E.R. (2007) Intravenous vasoactive intestinal polypeptide lowers pulmonary-to-systemic vascular resistance ratio in a neonatal piglet model of pulmonary arterial hypertension. J Pediatr Surg 42: 758–764 [DOI] [PubMed] [Google Scholar]
- Horio T., Kohno M., Kano H., Ikeda M., Yasunari K., Yokokawa K., et al. (1995) Adrenomedullin as a novel antimigration factor of vascular smooth muscle cells. Circ Res 77: 660–664 [DOI] [PubMed] [Google Scholar]
- Ichiki Y., Kitamura K., Kangawa K., Kawamoto M., Matsuo H., Eto T. (1994) Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett 338: 6–10 [DOI] [PubMed] [Google Scholar]
- Iglarz M., Binkert C., Morrison K., Fischli W., Gatfield J., Treiber A., et al. (2008) Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther 327: 736–745 [DOI] [PubMed] [Google Scholar]
- Ishikura K., Yamada N., Ito M., Ota S., Nakamura M., Isaka N., et al. (2006) Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J 70: 174–178 [DOI] [PubMed] [Google Scholar]
- Jing Z.C., Jiang X., Wu B.X., Xu X.Q., Wu Y., Ma C.R., et al. (2009) Vardenafil treatment for patients with pulmonary arterial hypertension: a multicentre, open-label study. Heart 95: 1531–1536 [DOI] [PubMed] [Google Scholar]
- Jing Z.C., Yu Z.X., Shen J.Y., Wu B.X., Xu K.F., Zhu X.Y., et al. for the Efficacy and safety of VArdenafiL in the treatment of pUlmonary Arterial hyperTensION (EVALUATION) Study Group (2011) Vardenafil in pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 183: 1723–1729 [DOI] [PubMed] [Google Scholar]
- Kakishita M., Nishikimi T., Okano Y., Satoh T., Kyotani S., Nagaya N., et al. (1999) Increased plasma levels of adrenomedullin in patients with pulmonary hypertension. Clin Sci (Lond) 96: 33–39 [PubMed] [Google Scholar]
- Kano H., Kohno M., Yasunari K., Yokokawa K., Horio T., Ikeda M., et al. (1996) Adrenomedullin as a novel antiproliferative factor of vascular smooth muscle cells. J Hypertens 14: 209–213 [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kangawa K., Kawamoto M., Ichiki Y., Nakamura S., Matsuo H., et al. (1993) Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun 192: 553–560 [DOI] [PubMed] [Google Scholar]
- Leuchte H.H., Baezner C., Baumgartner R.A., Bevec D., Bacher G., Neurohr C., et al. (2008) Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J 32: 1289–1294 [DOI] [PubMed] [Google Scholar]
- Li M., Liu Y., Dutt P., Fanburg B.L., Toksoz D. (2007) Inhibition of serotonin-induced mitogenesis, migration, and ERK MAPK nuclear translocation in vascular smooth muscle cells by atorvastatin. Am J Physiol Lung Cell Mol Physiol 293: L463–L471 [DOI] [PubMed] [Google Scholar]
- Maruno K., Absood A., Said S.I. (1995) VIP inhibits basal and histamine-stimulated proliferation of human airway smooth muscle cells. Am J Physiol 268: L1047–L1051 [DOI] [PubMed] [Google Scholar]
- McLaughlin V.V., Archer S.L., Badesch D.B., Barst R.J., Farber H.W., Lindner J.R., et al. (2009) ACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 119: 2250–2294 [DOI] [PubMed] [Google Scholar]
- McLaughlin V.V., Shillington A., Rich S. (2002) Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 106: 1477–1482 [DOI] [PubMed] [Google Scholar]
- McMurtry M.S., Archer S.L., Altieri D.C., Bonnet S., Haromy A., Harry G., et al. (2005) Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 115: 1479–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey L.M., Hood E.H. (2003) Bosentan and warfarin interaction. Ann Pharmacother 37: 1028–1031 [DOI] [PubMed] [Google Scholar]
- Murthy S.N., Nossaman B.D., Kadowitz P.J. (2010) New approaches to the treatment of pulmonary hypertension: from bench to bedside. Cardiol Rev 18: 76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T., Morio Y., Casanova N., Bauer N., Gebb S., McMurtry I., et al. (2004) Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672 [DOI] [PubMed] [Google Scholar]
- Nagaya N., Satoh T., Nishikimi T., Uematsu M., Furuichi S., Sakamaki F., et al. (2000) Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation 101: 498–503 [DOI] [PubMed] [Google Scholar]
- Nossaman B.D., Feng C.J., Cheng D.Y., Dewitt B.J., Coy D.H., Murphy W.A., et al. (1995) Comparative effects of adrenomedullin, an adrenomedullin analog, and CGRP in the pulmonary vascular bed of the cat and rat. Life Sci 56: PL63–PL66 [PubMed] [Google Scholar]
- Nossaman B.D., Feng C.J., Kaye A.D., DeWitt B., Coy D.H., Murphy W.A., et al. (1996) Pulmonary vasodilator responses to adrenomedullin are reduced by NOS inhibitors in rats but not in cats. Am J Physiol 270: L782–L789 [DOI] [PubMed] [Google Scholar]
- Olschewski H., Simonneau G., Galiè N., Higenbottam T., Naeije R., Rubin L.J., et al. Aerosolized Iloprost Randomized Study Group (2002) Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 347: 322–329 [DOI] [PubMed] [Google Scholar]
- Owji A.A., Smith D.M., Coppock H.A., Morgan D.G., Bhogal R., Ghatei M.A., et al. (1995) An abundant and specific binding site for the novel vasodilator adrenomedullin in the rat. Endocrinology 136: 2127–2134 [DOI] [PubMed] [Google Scholar]
- Patterson K.C., Weissmann A., Ahmadi T., Farber H.W. (2006) Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med 145: 152–153 [DOI] [PubMed] [Google Scholar]
- Petkov V., Mosgoeller W., Ziesche R., Raderer M., Stiebellehner L., Vonbank K., et al. (2003) Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest 111: 1339–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja S.G. (2010a) Endothelin receptor antagonists for pulmonary arterial hypertension: an overview. Cardiovasc Ther 28: e65–e71 [DOI] [PubMed] [Google Scholar]
- Raja S.G. (2010b) Macitentan, a tissue-targeting endothelin receptor antagonist for the potential oral treatment of pulmonary arterial hypertension and idiopathic pulmonary fibrosis. Curr Opin Investig Drugs 11: 1066–1073 [PubMed] [Google Scholar]
- Raja S.G., Danton M.D., MacArthur K.J., Pollock J.C. (2006) Treatment of pulmonary arterial hypertension with sildenafil: from pathophysiology to clinical evidence. J Cardiothorac Vasc Anesth 20: 722–735 [DOI] [PubMed] [Google Scholar]
- Raja S.G., Dreyfus G.D. (2008) Current status of bosentan for treatment of pulmonary hypertension. Ann Card Anaesth 11: 6–14 [DOI] [PubMed] [Google Scholar]
- Raja S.G., Nayak S.H. (2004) Sildenafil: emerging cardiovascular indications. Ann Thorac Surg 78: 1496–1506 [DOI] [PubMed] [Google Scholar]
- Rich S., Kaufmann E., Levy P.S. (1992) The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 327: 76–81 [DOI] [PubMed] [Google Scholar]
- Rich S., Seidlitz M., Dodin E., Osimani D., Judd D., Genthner D., et al. (1998) The short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest 114: 787–792 [DOI] [PubMed] [Google Scholar]
- Rubin L.J., Badesch D.B., Barst R.J., Galie N., Black C.M., Keogh A., et al. (2002) Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 346: 896–903 [DOI] [PubMed] [Google Scholar]
- Ryerson C.J., Nayar S., Swiston J.R., Sin D.D. (2010) Pharmacotherapy in pulmonary arterial hypertension: a systematic review and meta-analysis. Respir Res 11: 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said S.I., Hamidi S.A., Dickman K.G., Szema A.M., Lyubsky S., Lin R.Z., et al. (2007) Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 115: 1260–1268 [DOI] [PubMed] [Google Scholar]
- Schermuly R.T., Janssen W., Weissmann N., Stasch J.P., Grimminger F., Ghofrani H.A. (2011) Riociguat for the treatment of pulmonary hypertension. Expert Opin Investig Drugs 20: 567–576 [DOI] [PubMed] [Google Scholar]
- Schermuly R.T., Pullamsetti S.S., Kwapiszewska G., Dumitrascu R., Tian X., Weissmann N., et al. (2007) Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation 115: 2331–2339 [DOI] [PubMed] [Google Scholar]
- Shimokawa H., Takeshita A. (2005) Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 25: 1767–1775 [DOI] [PubMed] [Google Scholar]
- Simonneau G., Barst R.J., Galie N., Naeije R., Rich S., Bourge R.C., et al. for the Treprostinil Study Group (2002) Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary artery hypertension. Am J Respir Crit Care Med 165: 800–804 [DOI] [PubMed] [Google Scholar]
- Sitbon O., Humbert M., Jaïs X., Ioos V., Hamid A.M., Provencher S., et al. (2005) Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 111: 3105–3111 [DOI] [PubMed] [Google Scholar]
- Sitbon O., Humbert M., Nunes H., Parent F., Garcia G., Hervé P., et al. (2002) Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 40: 780–788 [DOI] [PubMed] [Google Scholar]
- Souza R., Sitbon O., Parent F., Simonneau G., Humbert M. (2006) Long term imatinib treatment in pulmonary arterial hypertension. Thorax 61: 736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M., Sun J., Hiroki J., Shimokawa H., Liao J.K. (2002) Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 106: 57–62 [DOI] [PubMed] [Google Scholar]
- Treiber A., Schneiter R., Hausler S., Stieger B. (2007) Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab Dispos 35: 1400–1407 [DOI] [PubMed] [Google Scholar]
- Tuder R.M., Chacon M., Alger L., Wang J., Taraseviciene-Stewart L., Kasahara Y., et al. (2001) Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374 [DOI] [PubMed] [Google Scholar]
- Tuder R.M., Flook B.E., Voelkel N.F. (1995) Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest 95: 1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Giersbergen P.L., Treiber A., Clozel M., Bodin F., Dingemanse J. (2002) In vivo and in vitro studies exploring the pharmacokinetic interaction between bosentan, a dual endothelin receptor antagonist, and glyburide. Clin Pharmacol Ther 71: 253–262 [DOI] [PubMed] [Google Scholar]
- Wanstall J.C., Gambino A., Jeffery T.K., Cahill M.M., Bellomo D., Hayward N.K., et al. (2002) Vascular endothelial growth factor-B-deficient mice show impaired development of hypoxic pulmonary hypertension. Cardiovasc Res 55: 361–368 [DOI] [PubMed] [Google Scholar]
- Weigand L., Sylvester J.T., Shimoda L.A. (2006) Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 290: L284–L290 [DOI] [PubMed] [Google Scholar]
- Yu Y., Fantozzi I., Remillard C.V., Landsberg J.W., Kunichika N., Platoshyn O., et al. (2004) Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866 [DOI] [PMC free article] [PubMed] [Google Scholar]