Abstract
Imaging of chlorophyll autofluorescence by confocal microscopy in intact whole petals of Arabidopsis thaliana has been used to analyze chloroplast development and redifferentiation during petal development. Young petals dissected from unopened buds contained green chloroplasts throughout their structure, but as the upper part of the petal lamina developed and expanded, plastids lost their chlorophyll and redifferentiated into leukoplasts, resulting in a white petal blade. Normal green chloroplasts remained in the stalk of the mature petal. In epidermal cells the chloroplasts were normal and green, in stark contrast with leaf epidermal cell plastids. In addition, the majority of these chloroplasts had dumbbell shapes, typical of dividing chloroplasts, and we suggest that the rapid expansion of petal epidermal cells may be a trigger for the initiation of chloroplast division. In petals of the Arabidopsis plastid division mutant arc6, the conversion of chloroplasts into leukoplasts was unaffected in spite of the greatly enlarged size and reduced number of arc6 chloroplasts in cells in the petal base, resulting in few enlarged leukoplasts in cells from the white lamina of arc6 petals.
Studies of the molecular genetic control of plastid differentiation in the model plant Arabidopsis have focused primarily on the proplastid-to-chloroplast transition during the development of leaves (Susek et al., 1993; Reiter et al., 1994). However, plastids can undergo several other differentiation pathways, the nature of which is dependent on the cell type in which the plastid resides. Whereas several of these differentiation pathways can arise directly from proplastids in meristem cells, interconversion and redifferentiation of different plastid types can also occur (Marano et al., 1993). Since the Arabidopsis model system offers great potential for unraveling the genetic basis of developmental processes, we have embarked on a study analyzing a major plastid redifferentiation event that occurs in Arabidopsis, namely the conversion of chloroplasts into leukoplasts during petal development.
A major route for chromoplast and leukoplast formation in plants is by the redifferentiation of chloroplasts, although other pathways of chromoplast formation directly from proplastids or amyloplasts have been reported (Marano et al., 1993). The molecular genetic controls of these processes are poorly understood, although the chromoplast differentiation pathway, which occurs primarily in ripening fruits of Solanaceae and Citrus species, has been the most studied (Mayfield and Huff, 1986; Lawrence et al., 1997). Plastid differentiation during petal development has been studied with only a few species, primarily those with yellow petals: Tropaeolum majus (Falk, 1976; Winkenbach et al., 1976), Ranunculus sp. (Brett and Sommerard, 1986), and Caltha palustris (Whatley, 1984). Studies of the yellow corollas of cucumber (Cucumis sativus L.) flowers (Smith and Butler, 1971) have given rise to a molecular analysis of chromoplast biogenesis in cucumber (Smirra et al., 1993; Vainstein et al., 1994; Vishnevetsky et al., 1996). Both pink corollas of petunia (Petunia hybrida) containing anthocyanin pigment and white petals of Dianthus caryophyllus have been reported to contain chloroplasts with low levels of chlorophyll capable of photosynthesis (Weiss et al., 1990; Weiss and Halevy, 1991; Vainstein and Sharon, 1993).
Although the petals of Arabidopsis thaliana and cresses in general are white, several other Brassica species have yellow petals, and in the wallflower (Cheiranthus spp.) a wide range of red and yellow petal colors exist. The plastids in the lamina of white petals are colorless, and in this study we have named them leukoplasts after the terminology of Kirk and Tilney-Bassett (1978). We also wished to investigate whether mutations that dramatically affect plastid division and expansion in Arabidopsis leaf cells interfere with plastid redifferentiation. Foremost among these is the arc6 mutant (Pyke et al., 1994; Robertson et al., 1995), in which the leaf mesophyll cells contain only two greatly enlarged chloroplasts and in which proplastid division in meristematic cells is also perturbed. Mesophyll chloroplasts are approximately 20-fold greater in size than the wild type, with highly variable morphology, and we wanted to determine whether this drastic change in chloroplast number and morphology has any significant affect on the plastid redifferentiation pathway in arc6 petals.
MATERIALS AND METHODS
Seeds of wild-type Arabidopsis thaliana cv Landsberg erecta and the arc6 mutant (Pyke et al., 1994; Robertson et al., 1995) were sown as previously described (Pyke et al., 1991) and grown under greenhouse conditions, with a daytime temperature of 20°C, and supplementary lighting to give a photoperiod of 16 h of light and 8 h of darkness. When the plants flowered, buds of different developmental stages were removed from the main inflorescence and the petals were dissected from the flower bud under a binocular microscope. Plants of the arc6–1 mutant grow normally and have normal flower and petal morphology, so petals from arc6 plants were harvested in the same manner.
Ultrastructural Analysis of Petals
For analysis of petal structure and plastid ultrastructure within petal cells, petals were fixed in 3% glutaraldehyde and 4% formaldehyde in 0.1 m Pipes buffer (N,N′-bis[2-ethanesulfonic acid], pH 7.2), postfixed in 1% buffered osmium tetroxide, and dehydrated in an ethanol series prior to embedding in Spurr's resin. To minimize damage to young petals at early stages of development prior to bud opening, the entire bud was fixed and sectioned. For analysis of petal-tissue structure, transverse 0.5-μm-thick sections were cut along the length of the petal on an ultramicrotome (Cambridge Huxley II), stained with 1% toluidine blue for 5 s at 60°C, and observed using an Optiphot microscope (Nikon). For the ultrastructural analysis of plastids, silver sections were cut and stained with uranyl acetate and Reynold's lead stain and examined using a transmission electron microscope (model EM109, Zeiss). Anatomical measurements of the petal structure were carried out directly from mounted sections using a Lucia image analysis system (Nikon) linked to the Optiphot microscope. A measurement of cell shape was made using the ratio of the maximum Feret diameter of the cell transect to the minimum Feret diameter of the cell transect. Feret's diameters of a convex object are the projected lengths of an object at angle α. Lucia measures Feret diameters at 10o intervals through 180o, and maximum and minimum Feret diameters are the largest and smallest of these diameter values, respectively. Thus, cell transects tending toward circularity will have similar values for maximum and minimum Feret diameters and will have a cell shape value of approximately 1. For long, thin cell transects, the maximum Feret diameter will approximate cell length and the minimum Feret diameter will approximate cell width.
Confocal Microscopy
Whole, intact petals were dissected from buds at different developmental stages, mounted in Vectashield (Vector Laboratories, Peterborough, UK), and examined using a confocal laser-scanning microscope (model TCS 4D, Leica). Red chlorophyll autofluorescence was visualized in chloroplasts using the tetramethylrhodamine B isothiocyanate excitation channel. Confocal images were exported to a PowerPC and montaged using Adobe Photoshop software.
RESULTS
Anatomy of Petal Development
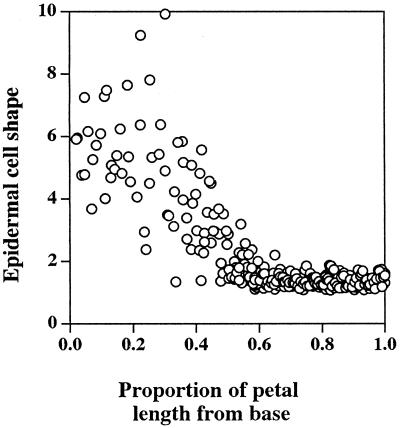
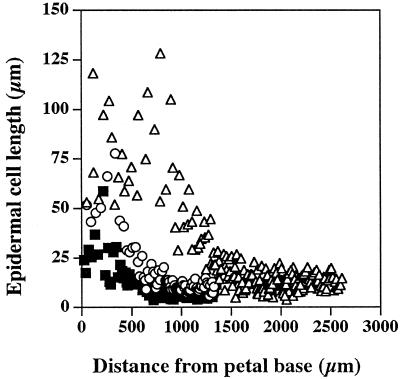
The four petals of the Arabidopsis flower are initiated at stage 5 of bud development (Smyth et al., 1990), and by stage 9 they have formed small, flat lamina, which reach to about one-third of stamen height. Stage 9 was the earliest stage in petal development used in this study. The structure of the fully expanded petals was relatively simple, consisting of two single-celled epidermal layers and some internal mesophyll-like parenchyma cells (Fig. 1). Image analysis of the internal anatomical structure of mature petals, determined from entire longitudinal sections of five individual petals, gave a volume proportion for the epidermis, mesophyll, vascular tissue, and airspace of 42:30:1:27%, respectively. A simple vascular array was apparent in fully expanded petals (Fig. 1c), with traces of chlorophyll fluorescence associated with the vascular strands in the white, flattened petal lamina. In the flattened upper part of the petal, the epidermal cells, particularly those on the upper petal surface, were highly specialized and had a distinct, columnar shape perpendicular to the axis of the petal (Fig. 1a). In contrast, the epidermal cells toward the base of the petal were thin and elongated (Fig. 1b). Analysis of epidermal cell shape in relation to position in the mature petal (Fig. 2) showed that these two types of epidermal cells each occupied about 50% of the petal length and that the point of transition of epidermal cell shape is quite marked. The elongate morphology of the basal epidermal cells relates to their rapid expansion, which pushes the petal out of the bud as it opens. The upper specialized epidermal cells did not expand significantly during the latter stages of petal development (stage 10 to bud opening), whereas the basal epidermal cells increased on average approximately 3-fold in length (Fig. 3). From our observations and measurements of petal anatomy between stage 9 and full expansion, there was no evidence for cell division within petals during this time, and the increase in petal size during this period appears to be solely a result of cell expansion, primarily of the basal epidermal and mesophyll cells.
Figure 1.
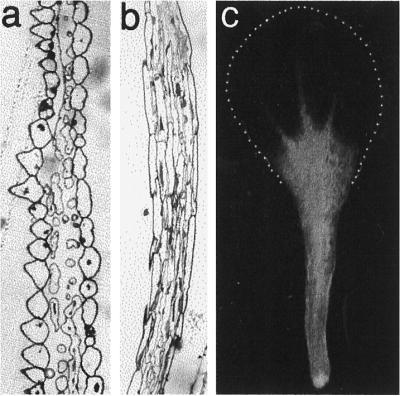
Structure of mature Arabidopsis petals. a, Transverse section through the upper white petal lamina. b, Transverse section through the stalk at the basal part of the petal. c, Entire intact petal viewed by conventional fluorescence microscopy showing chlorophyll autofluorescence in the petal stalk and the lack of fluorescence in the upper white petal lamina. The extent of the white petal lamina is shown by the dotted line.
Figure 2.
The relationship between the shape of petal epidermal cells viewed in transverse section and the relative position of the epidermal cell along the length of mature Arabidopsis petals. Data were collected from three different petals and pooled, since variability between individual petals was very low. Values of cell shape were maximum. Feret diameter/minimum Feret diameter, see Methods.
Figure 3.
The relationship between the length of epidermal cells viewed in transverse section and their distance from the petal base for petals at different developmental stages: ▪, stage 10; ○, stage 12; and ▵, mature.
Plastid Development during Petal Expansion
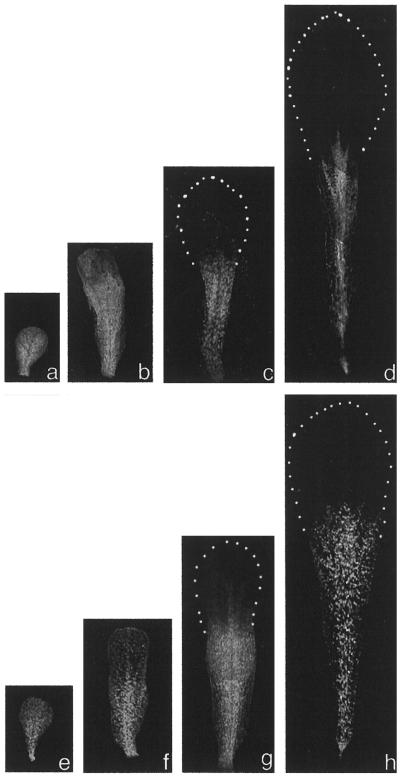
By using chlorophyll autofluorescence and confocal laser-scanning microscopy on intact petals, we have developed a simple method to observe individual chloroplasts within petal cells during development. Young petals at stage 9 dissected from buds contained green chloroplasts throughout the petal (Fig. 4a). As the petal elongated and expanded, plastids in the upper portion of the petal, which becomes the flattened petal lamina, lost their chlorophyll and underwent redifferentiation to become colorless plastids and were no longer visible in confocal images (Fig. 4, b–d). Green autofluorescent chloroplasts were maintained in the lower part of the petal stalk into maturity (Fig. 4d). This green stalk was maintained largely within the bud, and only the white, flattened petal lamina was normally observed in intact flowers.
Figure 4.
Developing Arabidopsis petals of the wild type (a–d) and the arc6 mutant (e–h) viewed by confocal microscopy. Red chlorophyll autofluorescence is shown as gray. The outline of the white lamina lacking fluorescing plastids is outlined by dotted lines. a and e, Stage 9; b and f, stage 10; c and g, stage 12; and d and h, mature.
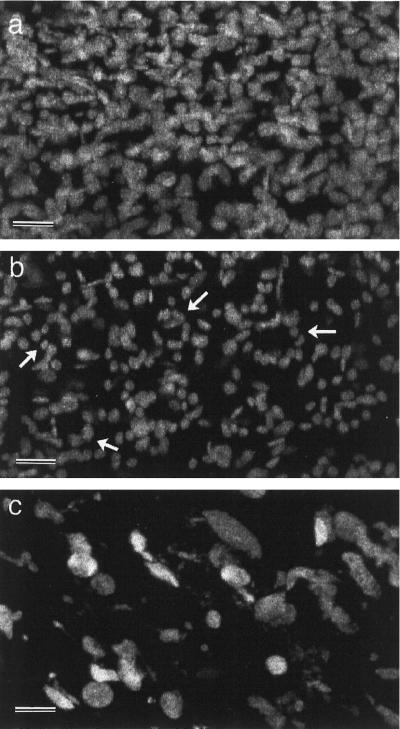
In the arc6 mutant, petal development and the spatial pattern of plastid development were similar to the wild type (Fig. 4), although it can be seen that even at this low magnification individual chloroplasts in the mature petal stalk were larger in arc6 petals than in wild-type petals (Fig. 4, e–h). The density of chloroplasts in cells from the petal stalk was much lower than that in leaf mesophyll cells, such that at higher magnification confocal images showed populations of discrete fluorescent chloroplasts (Fig. 5). Since the petal cell walls were not autofluorescent, it was difficult to count precisely the number of plastids per cell, but we estimated that the petal cells each contained 15 to 20 fluorescent plastids. Confocal optical sectioning allowed the chloroplasts in the epidermal and mesophyll cell layers to be viewed separately (Fig. 5, a and b, respectively). Epidermal cell chloroplasts were slightly smaller than chloroplasts within the internal parenchyma cells and could be seen in circular arrays (Fig. 5b). These circular arrays relate to the spatial arrangement of chloroplasts around the periphery of the conically shaped epidermal cells. In arc6 petals greatly enlarged fluorescent plastids were observed (Fig. 5c), confirming that this mutant plastid phenotype is maintained during petal development. Confocal imaging of partly dissected entire buds revealed that large arc6 plastids were also observed in all other green floral organs, including the sepals and the style wall (data not shown).
Figure 5.
Confocal images of fluorescing chloroplasts from the basal part of mature Arabidopsis petals. a, Wild-type mesophyll parenchyma chloroplasts. b, Wild-type epidermal chloroplasts. c, arc6 chloroplasts from both epidermal and mesophyll cells. Arrows in b indicate circular arrays of chloroplasts. Bars = 5 μm.
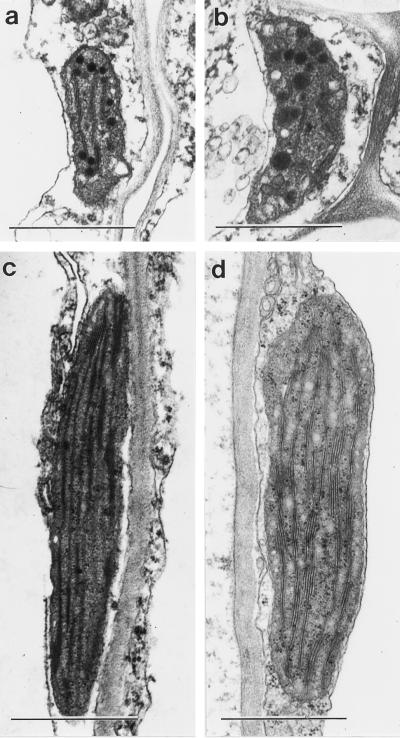
To determine how plastids redifferentiate in the white petal blade, the ultrastructure of plastids in the basal and upper parts of petals was observed by transmission EM. The redifferentiation of chloroplasts into leukoplasts in mature wild-type petals is shown in Figure 6. Chloroplasts from cells toward the petal base had a normal internal thylakoid membrane, similar to mesophyll chloroplasts, but lacked substantial starch grains (Fig. 6, c and d). In the upper cells from the white petal blade, the leukoplasts were greatly reduced in size and had only remnants of the internal thylakoid membrane system (Fig. 6, a and b). They also contained several osmophilic bodies. This redifferentiation process occurred simultaneously in both mesophyll parenchyma and epidermal cells (Fig. 6). It is especially noteworthy that the epidermal cell chloroplasts in petals were normal and of a similar size and morphology to leaf mesophyll chloroplasts, unlike the epidermal cell chloroplasts in leaves, which are greatly retarded in development and have a much reduced thylakoid membrane containing low levels of chlorophyll (Dupree et al., 1991).
Figure 6.
Electron micrographs of leukoplasts from the upper petal lamina (a and b) and chloroplasts from the green stalk of mature wild-type Arabidopsis petals (c and d) in mesophyll parenchyma cells (a and c) and epidermal cells (b and d). Bars = 1 μm.
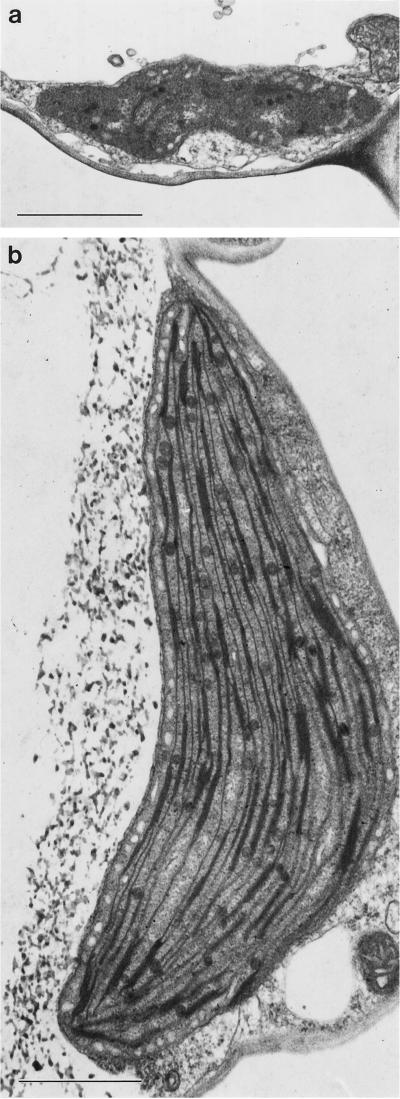
In arc6 petals the redifferentiation of chloroplasts occurred in a similar way to the wild type (Fig. 7), except that the initial chloroplasts were greatly enlarged (Fig. 7b) and the resulting leukoplasts were also greatly enlarged compared with the wild-type (Fig. 7a) and yet appeared to have a similar structure, with a residual amount of thylakoid membrane and some osmophilic bodies. This development was similar in both arc6 epidermal and mesophyll parenchyma cells. Thus, in spite of the greatly increased plastid size of arc6 chloroplasts, the redifferentiation of these plastids into petal leukoplasts was unaffected, resulting in enlarged leukoplasts. Examination of sectioned arc6 white petal tissues by transmission EM also showed that these arc6 leukoplasts were rare and difficult to find within cells. Thus, it appears that a reduced number of leukoplasts exist in cells in the upper regions of arc6 petals compared with the equivalent cells in the wild type, in which leukoplasts were more readily observed.
Figure 7.
Electron micrographs of a leukoplast from the upper white petal lamina (a) and chloroplasts from the green stalk of a mature Arabidopsis petal (b) of the mutant arc6. Bars = 1 μm, the same magnification as in Figure 6.
Plastid Division during Petal Development
During observation of wild-type petal plastids in the basal part of mature petals, it became obvious that a large proportion of these chloroplasts showed dumbbell shapes characteristic of dividing chloroplasts. In high-magnification confocal images, these dumbbell-shaped plastids were clearly observed (Fig. 8, arrowheads) and made up more than 50% of the total plastid population. Such dividing plastids were seen in only these basal cells of the petal stalk, and images of green chloroplasts at earlier stages of petal development did not show large numbers of the dumbbell shapes (data not shown). Our observations of older petals did not show a higher density of distinct chloroplasts in these cells nor were extended dumbbell-shaped, thin isthmuses observed, representative of the latter stages of plastid division. Consequently, it seems unlikely that these chloroplasts complete their division process to become separate daughter plastids.
Figure 8.
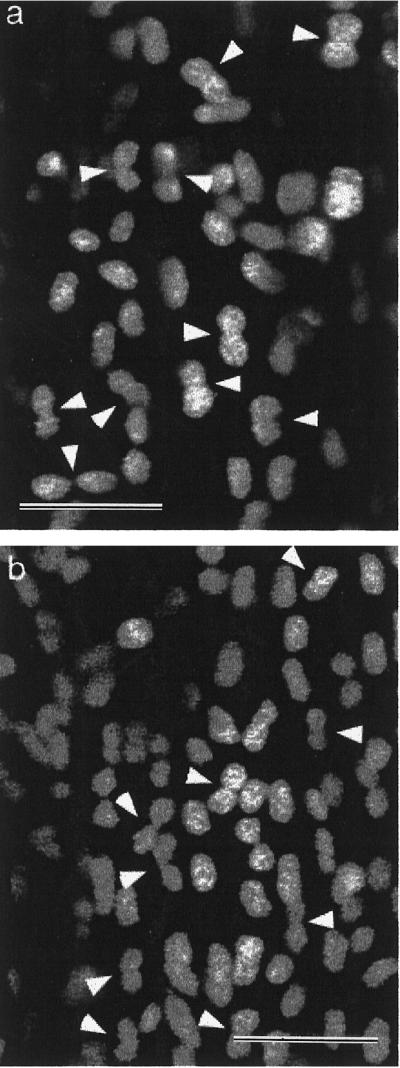
Confocal images of fluorescent chloroplasts from the base of different mature wild-type petals (a and b) showing the large proportion of the chloroplast population with dumbbell phenotypes typical of chloroplasts in division (arrowheads). Bars = 5 μm.
DISCUSSION
We have described, for the first time to our knowledge, the nature of plastids and their redifferentiation, which occurs during petal development in Arabidopsis and results in the mature petal having a white upper lamina that contains colorless leukoplasts and a green stalk that still contains green chloroplasts. The application of scanning confocal laser microscopy to analyze plastid development in petals has proven to be an extremely easy and useful tool. The relatively low density of green chloroplasts in petal cells compared with leaf mesophyll cells allows individual chloroplasts to be imaged with ease. We estimate that in young petal cells there are 15 to 20 chloroplasts per cell, suggesting that little plastid division occurs in these cells. The redifferentiation into leukoplasts appears to result in a reduction in plastid number, since at the EM level leukoplasts are considerably harder to observe in section, although this may be also partly due to the reduction in size that occurs when chloroplasts redifferentiate into leukoplasts. A similar reduction in plastid size also occurs during chloroplast-to-chromoplast redifferentiation in ripening tomato fruit (Pyke, 1997).
A particularly interesting observation is that epidermal cells contain fully differentiated green chloroplasts with typical internal morphology. This is in marked contrast to leaf epidermal cells in Arabidopsis and many other species in which epidermal chloroplasts are small and poorly developed and contain only low levels of chlorophyll and other thylakoid-associated proteins (Dupree et al., 1991). All epidermal cells in the plant arise from the same cell lineage, derived from the L1 layer in the shoot apical meristem. Petal epidermal cells are obviously a specialized cell type, in which the repression of chloroplast development, which occurs in leaf epidermal cells, has been released. Authentic chloroplast development in petal epidermis is not confined to the elongate basal epidermal cells but is also observed at the top of the stalk in mature petals in the specialized conical epidermal cells, which form primarily on the upper surface of the petal blade. From a functional point of view, presumably, true chloroplasts exist in petal epidermal cells so that the potential for chromoplast differentiation and the production of colored petals is maintained. In contrast, in the leaf epidermis the presence of mature chloroplasts in epidermal cells would interfere with light penetration into the leaf and reduce light-capture efficiency.
We have shown that the arc6 mutation, which results in one to three greatly enlarged chloroplasts in leaf mesophyll cells, shows a similar phenotype in young petal cells and the altered chloroplast morphology and number do not affect the redifferentiation of arc6 chloroplasts into arc6 leukoplasts. These observations, coupled with those for arc6 roots (Robertson et al., 1995), show that alterations in plastid size and morphology, as manifested by the arc6 mutation, do not affect plastid differentiation into other types of plastid. This raises the possibility for the manipulation of other plastid types in plants by genetic engineering using genes such as arc6 for modifying the potential for storage in larger plastids or identifying opportunities for modifying flower color in species with colored petals.
Although a few anatomical studies have been carried out on plastids during petal development, mainly in yellow petals, an understanding of the genetic basis of plastid redifferentiation during petal development is completely lacking. It should be possible to identify Arabidopsis mutants in which the pathway of plastid development during petal development is perturbed, although to our knowledge, no Arabidopsis mutants with either green or colored petals have been identified to date. This is surprising considering the extent of mutagenesis and screening. The production of colored Arabidopsis petals by virtue of chromoplast formation is likely to require metabolic pathways for the biosynthesis of pigment molecules, which presumably are lacking in Arabidopsis. Such pathways must exist in the related domesticated wallflower (Cheiranthus spp.), since wallflower petal color is based on variations in red, brown, orange, and yellow. One would anticipate that true green petals containing chloroplasts, in which the leukoplast differentiation event has failed to occur, would exist. The absence of such mutants may suggest that leukoplast differentiation is the default pathway in petal blade cells and that a gain-of-function mutation would be necessary for chloroplasts to be maintained in these cells.
An interesting feature of chloroplasts in the green petal stalk is the propensity of dumbbell-shaped profiles. Similar dumbbell-shaped plastids have been observed in other cell types in Arabidopsis (Robertson et al., 1996; Pyke, 1997) and are indicative of an early stage in plastid division. The biological purpose of dividing plastids in these cells is unclear, since there is little requirement for a large population of chloroplasts in this tissue, which has a poor light environment and initiates senescence within 48 h of full flower opening, followed rapidly by petal abscission (Smyth et al., 1990). We have hypothesized that rapid cell expansion and the resulting reduction in plastid density in cells such as those of the petal stalk may be a trigger that initiates the plastid division process but does not necessarily lead to completion (Pyke, 1997). From these studies, it is apparent that Arabidopsis petals may be a useful and easily accessible tissue in which to analyze the early stages of the plastid division process.
Abbreviation:
- EM
electron microscopy
Footnotes
This work was supported by the University of London Central Research Fund.
LITERATURE CITED
- Brett DW, Sommerard AP. Ultrastructural development of plastids in the epidermis and starch layer of glossy Ranunculus petals. Ann Bot. 1986;58:903–910. [Google Scholar]
- Dupree P, Pwee K-H, Gray JC. Expression of photosynthetic gene-promoter fusions in leaf epidermal cells of transgenic tobacco plants. Plant J. 1991;1:115–120. [Google Scholar]
- Falk H. Chromoplasts of Tropaeolum majus L.: structure and development. Planta. 1976;128:15–22. doi: 10.1007/BF00397173. [DOI] [PubMed] [Google Scholar]
- Kirk JTO, Tilney-Bassett RAE (1978) The Plastids. Elsevier/North Holland Biomedical Press, Amsterdam, The Netherlands
- Lawrence SD, Cline K, Moore GA. Chromoplast development in ripening tomato fruit: identification of cDNAs for chromoplast-targeted proteins and characterisation of a cDNA encoding a plastid-localised low-molecular weight heat shock protein. Plant Mol Biol. 1997;33:483–492. doi: 10.1023/a:1005785321165. [DOI] [PubMed] [Google Scholar]
- Marano MR, Serra EC, Orellano EG, Carrrillo N. The path of chromoplast development in fruits and flowers. Plant Sci. 1993;94:1–17. [Google Scholar]
- Mayfield SP, Huff A. Accumulation of chlorophyll, chloroplastic proteins, and thylakoid membranes during reversion of chromoplasts to chloroplasts in Citrus sinensis epicarp. Plant Physiol. 1986;81:30–35. doi: 10.1104/pp.81.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA. The genetic control of plastid division in higher plants. Am J Bot. 1997;84:1017–1027. [PubMed] [Google Scholar]
- Pyke KA, Marrison JL, Leech RM. Temporal and spatial development of the cells of the expanding first leaf of Arabidopsis thaliana (L.) Heynh. J Exp Bot. 1991;42:1407–1416. [Google Scholar]
- Pyke KA, Rutherford SM, Robertson EJ, Leech RM. arc6, a fertile Arabidopsis mutant with only two mesophyll cell chloroplasts. Plant Physiol. 1994;106:1169–1177. doi: 10.1104/pp.106.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RS, Coomber SA, Bourett TM, Bartley GE, Scolnik PA. Control of leaf and chloroplast development by the Arabidopsis gene pale cress. Plant Cell. 1994;6:1253–1264. doi: 10.1105/tpc.6.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EJ, Pyke KA, Leech RM. arc6, an extreme chloroplast division mutant of Arabidopsis also alters proplastid proliferation and morphology in shoot and root apices. J Cell Sci. 1995;108:2937–2944. doi: 10.1242/jcs.108.9.2937. [DOI] [PubMed] [Google Scholar]
- Robertson EJ, Rutherford SM, Leech RM. Characterization of chloroplast division using the Arabidopsis mutant arc5. Plant Physiol. 1996;112:149–159. doi: 10.1104/pp.112.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirra I, Halevy AH, Vainstein A. Isolation and characterization of a chromoplast-specific carotenoid-associated protein from Cucumis sativus corollas. Plant Physiol. 1993;102:491–496. doi: 10.1104/pp.102.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Butler RD. Ultrastructural aspects of petal development in Cucumis sativus with particular reference to the chromoplasts. Protoplasma. 1971;73:1–13. [Google Scholar]
- Smyth D, Bowman J, Meyerowitz E. Early flower development in Arabidopsis. Plant Cell. 1990;2:755–767. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek RE, Ausubel FM, Chory J. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell. 1993;74:787–799. doi: 10.1016/0092-8674(93)90459-4. [DOI] [PubMed] [Google Scholar]
- Vainstein A, Halevy AH, Smirra I, Vishnevetsky M. Chromoplast biogenesis in Cucumis sativus corollas. Plant Physiol. 1994;104:321–326. doi: 10.1104/pp.104.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainstein A, Sharon R. Biogenesis of petunia and carnation corolla chloroplasts: changes in the abundance of nuclear and plastid-encoded photosynthesis-specific gene products during flower development. Physiol Plant. 1993;89:192–198. [Google Scholar]
- Vishnevetsky M, Ovadis M, Itzhaki H, Levy M, LibalWeksler Y, Adam Z, Vainstein A. Molecular cloning of a carotenoid-associated protein from Cucumis sativa corollas: homologous genes involved in carotenoid biosynthesis. Plant J. 1996;10:1111–1118. doi: 10.1046/j.1365-313x.1996.10061111.x. [DOI] [PubMed] [Google Scholar]
- Weiss D, Halevy AH. The role of light reactions in the regulation of anthocyanin synthesis in Petunia corollas. Physiol Plant. 1991;81:127–133. [Google Scholar]
- Weiss D, Shomer-Ilan A, Vainstein A, Halevy AH. Photosynthetic carbon fixation in the corollas of Petunia hybrida. Physiol Plant. 1990;78:345–350. [Google Scholar]
- Whatley JM. The ultrastructure of plastids in the petals of Calutha palustris. New Phytol. 1984;97:227–231. [Google Scholar]
- Winkenbach F, Falk H, Liedvogel B, Sitte P. Chromoplasts of Tropaeolum majus L.: isolation and characterisation of lipoprotein elements. Planta. 1976;128:23–28. doi: 10.1007/BF00397174. [DOI] [PubMed] [Google Scholar]