Abstract
The effect of shortening the pulse width of the electrical stimulus when administering electroconvulsive therapy (ECT) has recently been systematically studied with promising results. This review examines reported outcomes from three randomized controlled trials which compared ultrabrief (≤0.3 ms) with brief (0.5–1.5 ms) pulse width ECT, and other recent clinical trials of ultrabrief pulse width ECT. The emerging evidence for ultrabrief pulse right unilateral (RUL) ECT suggests clinically meaningful efficacy and substantially reduced neuropsychological side effects compared with standard (brief) pulse ECT; this may represent a generational advance in the ECT technique. However, it is unclear if patients receiving ultrabrief pulse RUL ECT may have a slower speed of response and require additional treatments compared with brief pulse ECT. Therefore, until further data are available, clinicians may be well advised to use brief pulse ECT in situations requiring an urgent clinical response. The evidence base for ultrabrief bilateral ECT is limited, with findings that efficacy may be reduced compared with brief pulse width ECT. Thus ultrabrief bilateral ECT should not be used outside the research setting.
Keywords: efficacy, electroconvulsive therapy, neuropsychological, pulse width, review, ultrabrief
Introduction
Electroconvulsive therapy (ECT) remains the most effective clinical treatment for depression, particularly for its most severe forms: psychotic, melancholic and treatment-resistant depression. Unfortunately, many patients continue to experience distressing cognitive and memory side effects and this significantly limits its use. There is a critical need to develop modes of application for this life-saving procedure that improve its tolerability while preserving its high efficacy. A new approach in ECT delivery is to reduce the pulse width of the electrical stimulus, ‘ultrabrief pulse width’ ECT (see Figure 1). Initial studies suggest that this approach has the potential to dramatically reduce cognitive side effects while delivering meaningful efficacy – that is, potentially a generational advance in ECT technique.
Figure 1.
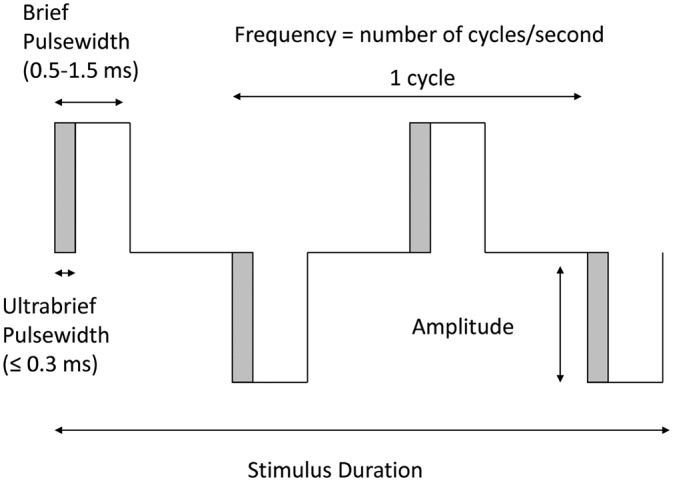
Schematic diagram illustrating parameters of the electroconvulsive therapy (ECT) stimulus.
This paper provides an introduction to the significance of pulse width in the ECT stimulus, and a review of clinical trials which have compared the outcomes of ultrabrief and brief pulse width ECT, as well as recent trials reporting clinical outcomes with ultrabrief pulse width ECT.
Pulse width
Since its inception in 1938, one of the most important advances in ECT technique has been the modification of the electrical stimulus, from a long pulse width (8 ms), sine-wave stimulus to a ‘brief’ pulse width (0.5–1.5 ms), square-wave stimulus. With this development efficacy was preserved (see meta-analysis by Kho and colleagues [Kho et al. 2003]) while cognitive side effects (confusion, retrograde amnesia) were markedly reduced [Carney et al. 1976; Valentine et al. 1968; Weiner et al. 1986]. Thus brief pulse widths (typically 1.0 ms) are now standard in modern clinical ECT practice. Neurophysiological observations indicate, however, that the ideal pulse width for neuronal stimulation is in the order of 0.1–0.2 ms. With this shorter pulse width, unnecessary stimulation during the refractory period of the neuron is avoided [Ranck, 1975]. This review focuses on studies which utilised pulse widths of less than 0.5 ms (‘ultrabrief’ in contrast to ‘brief’).
Mechanisms
It has been hypothesized that shortening the pulse width of the ECT stimulus results in less cognitive impairment as a narrower band of tissue is stimulated and areas such as the hippocampus and other temporal lobe structures may be relatively spared [Sackeim, 2004]. Computer simulations of both current density and active neuronal depolarization testing two stimuli in the typical ECT dose range have confirmed this prediction [Bai et al. 2010; Peterchev et al. 2010]. This factor, and the avoidance of excitotoxicity through excessive neuronal stimulation during the refractory period, may explain the reduced cognitive side effects observed with an ultrabrief pulse width.
Clinical trials of ultrabrief pulse width ECT
Studies that have examined ultrabrief pulse ECT are summarized in Table 1. Apart from the early study by Pisvejc and colleagues [Pisvejc et al. 1998], these have focused exclusively on the treatment of depression, which will be the subject of this review. The discussion below will focus on unilateral ultrabrief pulse ECT, for which there is the most evidence; the latter section focuses on bilateral (bitemporal and bifrontal) ultrabrief pulse ECT.
Table 1.
Studies which have examined ultrabrief pulse electroconvulsive therapy (ECT).
| Study | Study design | Study groups | Response* | Remission* | Mean number of ECT treatments | Cognitive tests | Cognitive outcomes |
|---|---|---|---|---|---|---|---|
| [Cronholm and Ottosson, 1963a] (I) | RCT | UB-BT (N = 24) BT (N =24) BT + lidocaine (N = 24) Dosing: BT – age based UB-BT – age based (×2) Dose increases not specified |
N/A | N/A | N/A Study assessed effects of single ECT session |
Immediate and delayed recall: 20 figure test, 30 personal data test, 30 word-pair test, combined test Tested day prior to and day of ECT |
Between groups$: UB-BT > BT 30 word-pair test, combined test Within UB group‡: Not reported |
| [Cronholm and Ottosson, 1963b] (II) | RCT | UB-BT (N = 21) BT (N = 20) Dosing: BT – age based UB-BT – age based (×2) Dose increases not specified |
8-item scale: no significant differences Global rating: BT > UB-BT Recovered/ pronounced improvement§: UB-BT: 13/21, BT: 18/20 |
Recovered§: UB-BT: 8/21, BT: 13/20 |
UB-BT: 7.3 BT: 6.1 |
Immediate and delayed recall: 30 word-pair test, 30 figure test, 30 personal data test, combined test Tested at baseline and 1 week after last treatment |
Between groups: NS Within UB group: NS |
| [Pisvejc et al. 1998] | RCT | UB-RUL (N = 21; schizophrenia: 19, MDD: 2) RUL (N = 27; schizophrenia: 23, MDD: 4) Dosing: algorithm‖ Dose increase if seizures < 30s (UB-RUL: 395%; RUL: 51%) |
No significant differences in BPRS | No significant differences in BPRS | Set protocol of 8 treatments | WAL, SSQM, BCRT Tested at baseline, after 8 ECT treatments, and 1 month after final ECT |
Between groups: NS Within UB group: NS |
| [Loo et al. 2007] | Retrospective; age and gender matched. (UB group overlaps with 2008 study sample) | UB-RUL (N = 30) RUL (N = 30) Dosing: thresholds established by empirical titration. UB-RUL at 6 × ST; RUL at 3.5 × ST Subsequent dose increases based on seizure quality (UB-RUL: 95%; RUL: 61%) |
Clinical response§: UB-RUL: 56.67% RUL: 50% |
UB-RUL 13% RUL N/A |
UB-RUL: 11.8 RUL: 8.8 Significantly different at p<0.05 |
CFT, RAVLT, COWAT, Digit Span, Stroop test, AMI (10 items) Tested baseline and after 6 ECT treatments |
Between groups:
Not reported Within UB group: Significant decreases in RAVLT, COWAT |
| [Sackeim et al. 2008] | RCT | UB-RUL (N = 22) UB-BT (N = 23) RUL (N = 22) BT (N = 23) Dosing: thresholds established by empirical titration BT at 2.5 × ST, RUL at 6 × ST Dose increase not permitted |
UB-RUL 77% ¶UB-BT 48%¶ RUL 73%¶BT = 70%¶Significantly different at p<0.05 (UB-BT less than other groups) |
UB-RUL 77% UB-BT 43% RUL 73%BT = 70% Significantly different at p<0.05 (UB-BT less than other groups) |
UB-RUL 8.7 UB-BT 8.9 RUL 8.5 BT 6.2 Significantly different at p<0.05 (BT less than other groups) |
Immediate effects (after each ECT session): orientation, retrograde memory, anterograde memory, cancellation task, COWAT, language. Main battery (baseline, after 6–7 treatments, 1 week after randomized and crossover phases (short-term effects), 2 months later, and 6 months later (long-term effects): MMSE, CFT, BCRT, Randt, AMI, Goldberg, subjective cognitive evaluation |
Between groups: Immediate effects: Ultrabrief>brief: Orientation, retrograde memory, sentence recognition (from anterograde memory), cancellation task, COWAT category subtest, visual confrontation naming (from language) After 6–7 treatments: UB-RUL>RUL: MMSE, BCRT Short-term effects: UB-RUL>RUL MMSE, CFT, BCRT, AMI, subjective cognitive evaluation. Long-term effects: UB-RUL> RUL AMI, memory for public events. Within UB group: Not reported |
| [Loo et al. 2008] | Open label, prospective | UB-RUL (N = 74) RUL (N = 22) Dosing: thresholds established by empirical titration. UB-RUL at 6 × ST; RUL at 5 × ST Subsequent doses increased by 50% if poor seizure quality (UB-RUL: 91%; RUL: 32%) |
UB-RUL: 43.24% RUL: 50% |
UB-RUL: 27.03% RUL: 36.36% |
UB-RUL: 10.3 RUL: 7.6 Significantly different at p<0.05 |
CFT, RAVLT, COWAT, Digit Span, Stroop task, AMI-SF Tested at baseline, after 6 ECT treatments, and at end of the treatment course (post) |
Between groups: UB-RUL > RUL: CFT copy (baseline); CFT copy and delayed recall (after 6 ECT); RAVLT delayed recall (after 6 ECT); CFT delayed recall (post), and AMI-SF (post) Within UB group: Significant decreases in RAVLT learning, RAVLT immediate recall, RAVLT delayed recall, COWAT letters subtest, COWAT category subtest, AMI-SF (post) |
| [McCormick et al. 2009] | Retrospective | UB-RUL (N = 26) BT (N = 30) Dosing: thresholds established by empirical titration BT at 2.5 × ST, UB-RUL at 6 × ST Dose increase not specified |
N/A | N/A | UB-RUL: 7.2 (not including those who switched to BT); 9.4 (including those who switched to BT) BT: 7.7 Significantly different at p<0.05 |
N/A | N/A |
| [Sienaert et al 2009a, 2010] | RCT | UB-RUL (N = 32) UB-BF (N = 32) Dosing: thresholds established by empirical titration UB-BF at 1.5 × ST, UB-RUL at 6 × ST Subsequent doses increased (UB-RUL: 35%, UB-BF: 59%) |
UB-RUL 78.13% UB-BF 78.13% |
UB-RUL 71.88% UB-BF 59.38% |
UB-RUL: required for response 7.76; required for remission 9.83 UB-BF: required for response 10.08; required for remission 11.84 Required for response –significant at p<0.05 |
MMSE, RAVLT, CPT, LNS, TMT, WCST, AMI, SSQM Tested at baseline and at 1 and 6 weeks after finishing the treatment course |
Between groups: NS Within UB group: Improvements in MMSE, RAVLT, CPT, WCST, SSQM |
| [Roepke et al. 2011] | RCT | UB-RUL at 40 Hz (N = 20) UB-RUL at 100 Hz (N = 20) Dosing: thresholds established by empirical titration. RUL at 2.5 × ST Subsequent doses increased by 20% if poor seizure quality (UB-RUL 40 Hz: 104%, UB-RUL 100 Hz: 58%) |
UB-RUL at 40 Hz 45% UB-RUL at 100 Hz 20% |
UB-RUL at 40 Hz 35% UB-RUL at 100 Hz 25% |
Set protocol of 9 treatments | Digit span, word fluency, VLMT Tested at baseline and at end of treatment course |
Between groups: NS Within UB group: Not reported |
| [Loo et al. 2011] | Retrospective analysis of open-label trial (includes some of 2008 study sample) | UB-RUL (N = 75) Dosing: thresholds established by empirical titration. UB-RUL at 6 × ST Subsequent doses increased by 50% if poor seizure quality |
UB-RUL 61.3% | UB-RUL 36% | UB-RUL: 9.69 |
N/A | N/A |
| [Niemantsverdriet et al. 2011] | Retrospective | UB-BT (0.25 ms; N = 19) versus BT (0.5 ms; N = 46) Dosing: thresholds established by empirical titration. UB-BT and BT at 1.5 × ST Subsequent doses increased until seizure duration ≥ 25 s |
UB-BT 73.6% BT 75.6% |
UB-BT 42.1%# BT 45.6%# |
UB-BT: 17.6 BT: 13.7 |
N/A | N/A |
| [Quante et al. 2011] | RCT | UB-RUL at 4 × ST (N = 14) UB-RUL at 7 × ST (N = 15) UB-RUL at 10 × ST (N = 12) Dosing: thresholds established by empirical titration. Randomized to dose Subsequent doses increased by 25% if seizures <25 s |
UB-RUL at 4 × ST 39% UB-RUL at 7 × ST 35% UB-RUL at 10 × ST 55% |
UB-RUL at 4 × ST 14% UB-RUL at 7 × ST 6% UB-RUL at 10 × ST 8% |
Set protocol of 9 treatments | VLMT, digit span, word fluency Tested at baseline and at end of treatment course |
Between groups: NS Within UB group: 7 × ST – decrease in VLMT recognition 10 × ST – decrease in VLMT recognition |
>denotes significantly better outcomes than; AMI (10 items) – Autobiographical Memory Interview – abbreviated to 10 items [Kopelman et al. 1989]; AMI-SF, Autobiographical Memory Interview – Short Form [McElhiney et al. 2001]; BCRT, Buschke Cued Recall Test [Buschke, 1984]; BPRS, Brief Psychiatric Rating Scale [Ventura et al. 1993]; BT, bitemporal; CFT, Rey-Osterrieth and Taylor Complex Figure Test [Osterrieth, 1944]; COWAT = Controlled Oral Word Association Test [Benton and Hamsher, 1976]; CPT, Continuous Performance Task [Rosveld et al. 1956]; Digit Span, Digit Span subtest from Wechsler Memory Scale [Wechsler, 1987]; Goldberg, Goldberg Remote Memory Test [Goldberg and Barnett, 1985]; HDRS, Hamilton Depression Rating Scale [Hamilton, 1967]; LNS, Letter number sequencing [Wechsler, 1997]; MADRS, Montgomery-Asberg Depression Scale [Montgomery and Asberg, 1979]; MMSE, Mini Mental Status Examination [Folstein et al. 1975]; NS, not significant; Randt, Randt Memory Test [Randt and Brown, 1983]; RAVLT, Rey Auditory Verbal Learning Task [Rey, 1964]; RUL, right unilateral; SSMQ, Squire Subjective Memory Questionnaire [Squire et al. 1979]; ST, seizure threshold; Stroop, Stroop test [Stroop, 1935]; TMT, Trail Making Test [Spreen and Strauss, 1998]; UB-BF, ultrabrief bifrontal; UB-BT, ultrabrief bitemporal; UB-RUL, ultrabrief right unilateral; VLMT, Verbal Learning Recognition Memory Test [Helmstaedter and Durwen, 1990]; WAL, Weschler Memory Scale subtest of Associated Learning [Wechsler, 1945]; WCST = Wisconsin Card Sorting Test [Spreen and Strauss, 1998]; word fluency, phonemic verbal fluency performance as part from Rogensburger Wortflüssigkeits Test [Aschenbrenner et al. 2000].
Unless otherwise indicated, response based on 50% or more reduction in MADRS or HDRS score and remission based on MADRS or HDRS score < 10.
Only reported for ultrabrief versus brief pulse width comparisons.
Only reported for ultrabrief pulse width.
As judged by clinical observation.
Based on patient’s age, gender and electrode placement.
Response ≥ 60% decrease from baseline in HDRS score.
Remission ≤ 7 on HDRS.
Efficacy of ultrabrief pulse right unilateral ECT – comparisons with brief pulse ECT
Only two randomized controlled trials (RCTs) have directly compared brief and ultrabrief pulse ECT [Pisvejc et al. 1998; Sackeim et al. 2008].
Although remarkable for its time, the study by Pisvejc and colleagues [Pisvejc et al. 1998] had numerous methodological shortcomings, making it very difficult to interpret their results. It is unclear if the treatment groups (also further divided into patients with schizophrenia and depression) were equivalent in illness severity, and important aspects of ECT treatment were not standardized between the groups. The ECT machines used and criteria for determining initial dose and dose increments were not standardized between treatment groups, and the use of very high stimulus frequencies (200–270 Hz) in the brief pulse group may have compromised efficacy in this group. Though the study reported no difference in efficacy and cognitive outcomes between the groups, these confounding factors (in particular the different dosing regimens) make it difficult to draw any conclusions from their results.
The study by Sackeim and colleagues [Sackeim et al. 2008] was a methodologically rigorous trial performed at a research centre. It demonstrated a large advantage for ultrabrief pulse right unilateral (RUL) ECT in cognitive outcomes with no difference in efficacy compared with brief pulse RUL ECT. This is a seminal study and currently forms the main evidence base for the comparison of ultrabrief and brief pulse ECT. Though between-group differences were significant and convincing, limitations include a relatively small sample (N = 22 in each RUL group), and choice of 1.5 ms (at the higher end of the brief pulse width range and not commonly used in clinical practice) for the brief pulse comparison group. Thus, further work comparing ultrabrief RUL ECT at 0.3 ms pulse width to the more commonly used brief pulse width of 1.0 ms is needed to extend these findings.
The largest sample of ultrabrief [6 × seizure threshold (ST)] and brief pulse (5 × ST) RUL ECT reported to date [Loo et al. 2008] suggested that efficacy may be slightly reduced in the former, particularly when taking the number of treatments required into account (mean number 10.3 and 7.6, respectively). Although ratings of mood and cognition in this study were done prospectively by research staff blinded to treatment condition, it was a nonrandomized effectiveness trial. Treatment assignment and the number of ECT treatments given were clinically judged by their supervising psychiatrists. This pragmatic methodological approach means the comparison of ultrabrief and brief pulse ECT cannot be considered definitive, although the study reflects treatment outcomes in typical clinical settings.
In a retrospective report of outcomes of patients who had received brief pulse bitemporal and ultrabrief pulse RUL ECT in a clinical service, McCormick and colleagues [McCormick et al. 2009] concluded that ultrabrief RUL ECT was less efficient and cost effective. Forty-six percent of the ultrabrief sample were switched to bitemporal ECT due to lack of response or poor seizure induction, meaning that more ECT treatments overall were received. This study was unable to comment on efficacy as such as no mood outcome measures were done.
Efficacy of ultrabrief pulse right unilateral ECT: response and remission rates
A wide range of response and remission rates have been reported in studies of ultrabrief pulse RUL ECT so far (see Table 1), from 77% and 77% [Sackeim et al. 2008] to 35% and 6% [Quante et al. 2011] (treatment given at 7 × ST). While initially the discrepancies seem puzzling, on closer examination it can be observed that the studies differed in several methodological aspects that are likely to have affected efficacy, including the number of ECT treatments, the method of data analysis [completers or the intention-to-treat (ITT) sample], presence or absence of concurrent medication, patient characteristics, ECT dose and variation over the treatment course (see the section on ECT dose below).
Two studies [Roepke et al. 2011; Quante et al. 2011] examined the effects of a fixed course of nine ECT treatments whereas other studies permitted a flexible number of treatments depending on treatment response. Thus, it would be expected that response, and particularly remission rates, would be lower in the above studies, given that other studies reported mean number of ECT treatments required in a course of ultrabrief pulse RUL ECT to be 10.3 [Loo et al. 2008], 8.7 [Sackeim et al. 2008] and 7.8–10 [Sienaert et al. 2009a]. Similarly, the analysis of outcomes in completers or those who received a minimum number of treatments rather than an ITT sample is likely to find higher response rates. Loo and colleagues [Loo et al. 2008] reported response rates of only 43% in an ITT analysis. However, in a later analysis focusing on predictors of response, restricted to patients who had received at least six treatments (extracted from a larger sample which included some of the patients from the 2008 report), the response rate was 61%. This factor may partly account for the high response and remission rates reported by Sienaert and colleagues [Sienaert et al. 2009a] who only analysed completers.
Outcomes from the Sackeim study [Sackeim et al. 2008] are remarkable given that response and remission rates were calculated in an ITT sample. A factor which may have increased the gains seen with ECT in the Sackeim (and Sienaert) studies compared with other studies is that patients were withdrawn from psychotropic medications for 3–5 days prior to commencing ECT, whereas patients were allowed to continue on psychotropic medications throughout the course of ECT in the other studies. In a meta-analysis of another brain stimulation therapy, repetitive transcranial magnetic stimulation (rTMS), the effect size (ES) was almost twice as great when rTMS was given as monotherapy (that is, antidepressant medications were withdrawn prior to trial entry, ES 0.96) than when it was given during continuation of pre-existing antidepressant treatment (ES 0.51) [Slotema et al. 2010]. Thus, the Sackeim and Sienaert studies were conducted under research conditions which optimized the effects seen with ultrabrief pulse RUL ECT, but it may be unrealistic to expect efficacy of this magnitude when the same treatment is given in typical clinical settings.
Patient illness characteristics, such as treatment resistance, presence of psychosis and bipolarity, have been shown to affect therapeutic outcomes with ultrabrief pulse RUL ECT [Loo et al. 2011 (discussed further below) and it is likely that the variation between studies in efficacy outcomes substantially reflect clinical differences in the samples treated. Note that in the Sackeim study [Sackeim et al. 2008] there were significantly more patients with psychotic depression in the ultrabrief pulse RUL group (5 of 22) than the brief RUL group (2 of 22), and the finding of equivalent efficacy between groups must be interpreted with this in mind. Though the authors commented that psychotic features were not a predictor of response in their study (including bitemporal treatment groups), the number of patients involved was so small that this cannot be viewed as a reliable finding.
Predictors of response
In a recent analysis of 75 patients who received at least six treatments of ultrabrief pulse RUL ECT, we found that predictors of response were similar to those previously reported for brief pulse ECT [Loo et al. 2011]. A regression analysis found that episode duration of greater than a year, less treatment resistance, prior treatment with ECT, presence of psychotic features and higher baseline depression severity were predictors of response or remission. Likewise, Sienaert and colleagues [Sienaert et al. 2009a] reported that psychosis (present in 4 of 32 in the ultrabrief pulse RUL group and 13 of 32 in the ultrabrief pulse bifrontal group) and greater depression severity at baseline were positive predictors of response. Bipolarity was not found in any of these studies to be a predictor of response [Loo et al. 2011; Sienaert et al. 2009b], but was associated with faster onset of response [Loo et al. 2011; Sienaert et al. 2009b].
Treatment considerations
Dosage relative to seizure threshold
The optimal dosage relative to seizure threshold (DRST) for ultrabrief pulse RUL ECT is yet to be determined. (Note that data reported in studies and examined for this review are in terms of DRST and total charge in milliCoulombs. While the latter is not an ideal metric for ECT ‘dose’ as subtleties in stimulus parameters are likely to affect therapeutic and cognitive outcomes [Peterchev et al. 2010], it is used here as a convenient comparator as data on stimulus parameter combinations are not reported.) Most studies to date have used 6 × ST with good overall response and remission rates, notwithstanding the caveats discussed above. Roepke and colleagues [Roepke et al. 2011] used 2.5 × ST but allowed subsequent dose increases, with rises of 104% (40 Hz group) and 58% (100 Hz group) over the ECT course, possibly accounting for the respectable response and remission rates found. The only study to compare ultrabrief pulse RUL ECT at different DRST is that of Quante and colleagues [Quante et al. 2011]. Surprisingly, no significant differences in efficacy were found between dosing at 4, 7 and 10 × ST (in contrast to studies of brief pulse RUL ECT [McCall et al. 2000; Sackeim et al. 2000]) though the study was underpowered to find a difference (total N = 41). The highest dose group was more impaired in one neuropsychological test aspect (verbal learning – recognition), reminiscent of earlier findings with brief pulse RUL ECT that increments in cognitive impairment may be steeper than increments in efficacy as DRST increases [McCall et al. 2000]. Our group is currently comparing ultrabrief pulse RUL ECT at 8 × ST and brief RUL ECT at 5 × ST, following earlier observations that ultrabrief pulse RUL ECT may have lesser efficacy to brief RUL ECT when both are given at similar DRST [Loo et al. 2008].
Should dose be increased over the treatment course?
The above studies differed in whether dose increments were allowed over the treatment course. Judging from the final doses reported, dosage (in milliCoulombs) was increased by 91% [Loo et al. 2008], 35% [Sienaert et al. 2009a], 104% (40 Hz group) and 58% (100 Hz group) [Roepke et al. 2011] and by an unknown amount [Quante et al. 2011] if seizure quality or duration declined over the treatment course. In contrast, no dose change over the course occurred in the Sackeim study [Sackeim et al. 2008], which reported the highest remission rate of all these studies. At present, it is uncertain if ECT dose should be increased over the course if seizure quality or duration declines, as recommended for brief pulse ECT [American Psychiatric Association, 2001; Scott, 2004]. However, as moderate to good response rates were reported by studies in which treatment protocols included dose increases, this suggests it is likely to be necessary in clinical practice; this question however needs further research.
How many treatments should be given prior to judging nonresponse and switching to another type of ECT?
For brief RUL ECT, a switch to bilateral ECT (or increase in dose) may be considered if no clinical response is seen after four to six treatments [American Psychiatric Association, 2001; Scott, 2004]. It is uncertain if this recommendation should apply to ultrabrief pulse RUL ECT. The speed of response may be slower with ultrabrief ECT (personal observations) and if so more treatments, such as six to eight, should be given prior to judging a failed response. Results from our nonrandomized effectiveness trial indicated that approximately 50% more treatments were required for an adequate course of treatment with ultrabrief pulse RUL ECT compared with brief RUL ECT [Loo et al. 2008]. Response rates (35%) after nine ultrabrief pulse RUL ECT treatments at 7 × ST in the Quante study [Quante et al. 2011] also suggest that it may be prudent to observe the results of six to eight treatments prior to considering a change of treatment approach. However, the mean number of treatments required for a course of ultrabrief pulse RUL ECT (8.7) did not differ from mean numbers required for brief RUL ECT in several studies: Sackeim and colleagues [Sackeim et al. 1993, 2000, 2008] and Sienaert and colleagues [Sienaert et al. 2009a] reported mean treatment numbers of 7.8 and 10.1 required for response and remission, respectively. Further, in a formal speed of response Cox regression analysis controlling for other factors known to affect efficacy, we found no significant difference between ultrabrief pulse RUL ECT (6 × ST) and brief RUL ECT (5 × ST) (Loo et al. in preparation). Thus, this issue needs further clarification.
How frequently should treatment sessions be spaced for ultrabrief pulse RUL ECT?
Ultrabrief pulse RUL ECT was given three times per week in all of the above studies, except the Sienaert study [Sienaert et al. 2009a], which treated twice per week, with good outcomes. As these two approaches (three versus two treatments per week) have not been directly compared within a RCT, implications with respect to overall efficacy, speed of response and side effects is unknown. However, given that the main advantage of giving brief pulse ECT twice per week is to minimize cognitive side effects [UK ECT Review, 2003; Loo et al. 2010] and minimal or no cognitive impairment has been reported with ultrabrief pulse RUL ECT, there is no compelling reason for restricting treatments to twice per week.
Effects of other stimulus parameters
Apart from pulse width, it has been cogently argued that variation of pulse frequency, amplitude and train duration are likely to have implications for efficacy and cognitive outcomes in ECT [Peterchev et al. 2010]. This has been minimally studied for brief pulse ECT and only one study has examined this for ultrabrief pulse ECT. The Roepke study [Roepke et al. 2011] reported higher response rates after nine treatments when ultrabrief pulse RUL ECT (2.5 × ST) was given at 40 Hz than at 100 Hz, though this finding is confounded by the greater increment in DRST in the 40 Hz group, despite the fact that final ECT doses did not differ significantly between groups, which may be a result of the small sample size. However, given theoretical reasons favouring the use of pulse frequencies in the 20–30 Hz range and preliminary support for this approach in studies of brief pulse ECT [Peterchev et al. 2010], optimization of pulse frequency and other stimulus parameters is likely to be important for ultrabrief pulse ECT, as for brief pulse ECT.
Effects of anaesthesia used
Whether the choice of anaesthetic agent influences overall efficacy and cognitive outcomes (over the course of treatment) for brief pulse ECT has only been minimally investigated [Bauer et al. 2009; MacPherson and Loo, 2008], though given the strong anticonvulsant effects of some of the major induction agents, this would not be a surprise. If the efficacy of ultrabrief pulse RUL ECT is less robust than that of brief pulse ECT (which is as yet uncertain – see above), then it is possible that treatment outcomes may be more susceptible to the influence of anaesthetic agent and dose. None of the studies of ultrabrief pulse ECT examined this issue directly. A retrospective analysis of results in the Sienaert study [Sienaert et al. 2009a] in which patients had received either methohexital or etomidate anaesthesia found significantly higher odds of attaining remission status with methohexital. In a RCT of ultrabrief pulse RUL ECT (at 6 × ST) in which patients were randomized to receive thiopentone or a reduced dose of thiopentone combined with ketamine (0.5 mg/kg), improvement in the first week of treatment was slightly greater in the ketamine group [Loo et al. in revision]. Thus, how critical the anaesthetic approach is for outcomes of ultrabrief pulse ECT warrants further examination.
Ictal electroencephalogram features
There have been no published reports to date of the comparative appearance of the ictal electroencephalogram (EEG) with ultrabrief compared with brief pulse ECT. In particular, it is uncertain whether features of the ictal EEG found to predict good response and hence used to guide the adequacy of dosing with brief pulse ECT apply equally to ultrabrief pulse ECT.
Ultrabrief pulse bilateral ECT
Only two RCTs have compared ultrabrief and brief pulse bitemporal ECT, both finding reduced efficacy for ultrabrief ECT. The early study by Cronholm and Ottosson [Cronholm and Ottosson, 1963b] is confounded by the fact that other key stimulus parameters, pulse shape (square versus chopped sine), frequency (15 Hz versus 50 Hz) and peak current amplitude (2.1 A versus 0.7–0.8 A) also varied between the two groups. From knowledge gained in later research [Peterchev et al. 2010], the variations in pulse frequency and current amplitude are likely to have enhanced relative efficacy outcomes in the ultrabrief group. Another major confound is that the dosing method was age based and the DRST for each group is unknown. Further, both pulse forms were given with a unidirectional stimulus, so the applicability of these results to outcomes when the stimulus is bidirectional (as in current ECT machines) is uncertain.
In the Sackeim study [Sackeim et al. 2008], efficacy was clearly reduced in the ultrabrief pulse bitemporal group (2.5 × ST) compared with brief pulse bitemporal and RUL ECT, as well as ultrabrief pulse RUL ECT. The reason for this differential loss of efficacy when ultrabrief pulse stimulation is applied to bitemporal but not unilateral ECT is not understood at present. Ultrabrief pulse bitemporal ECT appeared not to be a useful treatment approach, given the greater cognitive side effects yet lesser efficacy compared with ultrabrief pulse RUL ECT.
In contrast, Sienaert and colleagues [Sienaert et al. 2009a] only found slightly lower efficacy for ultrabrief pulse bifrontal (1.5 × ST) compared with ultrabrief pulse RUL (6 × ST) ECT, manifested in more treatments required to attain response and lower odds of attaining response and remission. However, the response and remission rates reported for ultrabrief pulse bifrontal ECT are still high, findings that are hard to reconcile with those of Sackeim and colleagues [Sackeim et al. 2008]. While it is possible that the use of a bifrontal rather than bitemporal electrode placement accounted for this discrepancy, there are no theoretical reasons to support this interpretation and trials with brief pulse ECT do not suggest that bifrontal ECT has a significantly higher efficacy than bitemporal ECT [Kellner et al. 2010] (recent bifrontal review article). A more likely explanation is the high proportion of patients with psychotic depression in the ultrabrief bifrontal group (41%) compared with the ultrabrief RUL group (13%) in the Sienaert study, and the ultrabrief bitemporal (26%) and RUL (23%) groups in the Sackeim study, noting evidence presented above that the presence of psychosis is likely to be a positive predictor of response. Though the good response to ultrabrief pulse bifrontal appeared to be at the relative low dosage of 1.5 × ST, dosage over the treatment course was increased by 59%, such that dosing was probably closer to 2 × ST.
Niemantsverdriet and colleagues performed a retrospective comparison in a clinical service and reported high response and good remission rates for ultrabrief (0.25 ms) and brief (0.5 ms) pulse bitemporal ECT, both given at 1.5 × ST [Niemantsverdriet et al. 2011]. These outcomes need to be interpreted with several factors in mind: there was a high proportion of patients with psychotic depression (63% and 59%, respectively); medications were withdrawn a week prior to ECT (see discussion in efficacy section above); dose increases over the course were permitted; and both groups received a relatively high number of ECT treatments. This study suggests that good outcomes may be achieved with low pulse width ECT in a clinical service if long treatment courses can be given. Note that with treatments twice per week, the average course of ultrabrief bitemporal ECT lasted about 9 weeks.
The evidence base for ultrabrief pulse bilateral ECT is very limited and results of the Sienaert and Niemantsverdriet studies are difficult to reconcile with those of the Sackeim study. These two studies reported good efficacy in samples with high proportions of psychotically depressed patients and where dose increases were permitted over the treatment course.
Cognitive outcomes
Apart from the early studies which are confounded by several methodological issues, recent trials comparing ultrabrief and brief pulse ECT have demonstrated substantial reductions in impairments across several cognitive domains with the reduction in pulse width [Loo et al. 2008; Sackeim et al. 2008; Sienaert et al. 2010].
The lesser degree of cognitive impairment with ultrabrief ECT compared with brief pulse ECT has also been shown at several time frames across a treatment course. Shorter time to recovery of orientation and predominantly better performance on measures of retrograde amnesia and recognition of newly learned material were found after a single treatment session [Sackeim et al. 2008]. After six to seven ECT sessions, delayed recall of newly learned information and global cognition were superior [Loo et al. 2008; Sackeim et al. 2008]. Similar advantages in these domains as well as enhanced retrograde and subjective global memory were reported after a full treatment course [Loo et al. 2008; Sackeim et al. 2008],
However, reports of the degree of cognitive impairment after a course of ultrabrief ECT have been mixed, with some studies reporting no significant changes, whereas some impairment has been measured in others. After controlling for improved depression scores, significant increases in cognitive performance were found across several domains, including learning and delayed recall for verbal material, retrograde autobiographical memory, sustained attention and probabilistic category learning [Sienaert et al. 2010]. Notably, parallel test forms were not used. In contrast, Loo and colleagues [Loo et al. 2008] found significant decreases in performance on verbal learning and memory, verbal generativity and retrograde autobiographical memory. These results, however, were potentially confounded by increases in dosage over the treatment course, which necessitated the use of pulse widths beyond 0.3 ms. Notwithstanding, using a constant pulse width, decreased verbal recognition memory has been shown following a full course of ultrabrief pulse ECT, when given at 7–10 × ST [Quante et al. 2011]. These findings therefore suggest that while ultrabrief pulse ECT is associated with fewer cognitive side effects than brief pulse ECT, cognitive impairment may still occur, particularly at higher DRST levels.
Importantly, preliminary data further indicate that the cognitive benefits of ultrabrief pulse ECT are maintained over time, with Sackeim showing superior retrograde memory with ultrabrief ECT compared with brief pulse ECT at two separate follow-up assessments over 6 months [Sackeim et al. 2008]. Future studies with long-term follow-up cognitive assessments are needed to replicate these findings.
Taken together, these results therefore suggest that cognitive sparing with ultrabrief pulse ECT is a more robust and consistent finding than equivalence in efficacy. In particular, memory domains, including delayed recall for newly learned material and retrograde memory for autobiographical events, appear to benefit most.
Conclusions, recommendations and future directions
The evidence base to date remains relatively small for ultrabrief pulse RUL ECT and quite limited for ultrabrief pulse bitemporal and bifrontal ECT. More head-to-head comparisons of ultrabrief and brief pulse RUL and bilateral ECT, ideally in RCT study designs, are needed to clarify the relative efficacy and side-effect profiles of these treatment approaches. Though studies have varied in reports of efficacy, likely due to methodological and sampling differences between studies, overall the data suggest that meaningful efficacy can be obtained with ultrabrief RUL ECT, including in typical clinical populations. The speed of response and numbers of treatments needed for ultrabrief compared with brief pulse RUL ECT requires further clarification. Thus, ultrabrief RUL ECT can be used if there is a high priority for minimal memory effects but standard brief pulse ECT should be used when there is a high priority for a more rapid response, for example in situations where there is significant suicide risk or for a severely unwell older patient who is at risk of rapid medical deterioration.
The optimal treatment approach for ultrabrief RUL ECT needs further investigation. The DRST, frequency of sessions, dosage changes over the treatment course, impact of other stimulus parameters, anaesthetic agent, number of treatments required before nonresponse is judged, and the significance of ictal EEG features all require further investigation. Findings from research with brief ECT cannot be simply extrapolated and applied to ultrabrief ECT. ECT practitioners need to carefully familiarize themselves with the evidence available, including current gaps in knowledge, before applying ultrabrief pulse RUL ECT to their patients.
There are fewer data on ultrabrief pulse bilateral ECT than RUL ECT and outcomes reported to date are more variable. Thus, the use of pulse widths up to 0.3 ms in bilateral ECT should be considered experimental and should not be used in routine clinical practice.
The finding of substantially reduced cognitive side effects is consistent across studies, and heralds a promising future for ultrabrief pulse RUL ECT.
Footnotes
This work was funded by the Australian National Health and Medical Research Council Project Grant number 568678.
The authors declare that there is no conflict of interest.
References
- Aschenbrenner S., Tucha O., Lange K.W. (2000) Regensburger Wortflüssigkeits-Test. Göttingen, Germany: Hogrefe [Google Scholar]
- American Psychiatric Association (2001) The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging. 2nd edn. Washington, DC: American Psychiatric Publishing [Google Scholar]
- Bai S., Loo C., Dokos S. (2010) A computational model of direct brain stimulation by electroconvulsive therapy. In: Proceedings of the Annual Conference of the IEEE Engineering in Medicine and Biology Society, Vol. 1 [DOI] [PubMed] [Google Scholar]
- Bauer J., Hageman I., Dam H., Baez A., Bolwig T., Roed J., et al. (2009) Comparison of propofol and thiopental as anesthetic agents for electroconvulsive therapy: a randomized, blinded comparison of seizure duration, stimulus charge, clinical effect, and cognitive side effects. J ECT 25(2): 85–90 [DOI] [PubMed] [Google Scholar]
- Benton A.L., Hamsher K. (1976) Multilingual aphasia examination. Iowa City: University of Iowa [Google Scholar]
- Buschke H. (1984) Cued recall in amnesia. J Clin Neuropsychol 6: 433–440 [DOI] [PubMed] [Google Scholar]
- Carney M.W., Rogan P.A., Sebastian J., Sheffield B. (1976) A controlled comparative trial of unilateral and bilateral sinusoidal and pulse E.C.T. in endogenous depression. PDM 7–8: 77–79 [PubMed] [Google Scholar]
- Cronholm B., Ottosson J.O. (1963a) Ultrabrief stimulus technique in electroconvulsive therapy. I. Influence on retrograde amnesia of treatments with the elther es electroschock apparatus, siemens konvulsator III and of lidocaine-modified treatment. J Nerv Ment Dis 137: 117–123 [PubMed] [Google Scholar]
- Cronholm B., Ottosson J.O. (1963b) Ultrabrief stimulus technique in electroconvulsive therapy. II. Comparative studies of therapeutic effects and memory disturbances in treatment of endogenous depression with the Elther ES Electroshock Apparatus and Siemens Konvulsator III. J Nerv Ment Dis 137: 268–276 [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. (1975) ‘MIni-mental State’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Rese 12: 189–198 [DOI] [PubMed] [Google Scholar]
- Goldberg E., Barnett J. (1985) The Goldberg-Barnett Remote Memory Questionnaire. New York: Albert Einstein Medical College [Google Scholar]
- Hamilton M. (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6: 278–296 [DOI] [PubMed] [Google Scholar]
- Helmstaedter C., Durwen H.F. (1990) A useful and differentiated tool in evaluating verbal memory performance. Schweizer Archiv fuer Neurologie und Psychiatrie 141: 21–30 [PubMed] [Google Scholar]
- Kellner C.H., Knapp R., Husain M.M., Rasmussen K., Sampson S., Cullum M., et al. (2010) Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry 196: 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho K.H., van Vreeswijk M.F., Simpson S., Zwinderman A.H. (2003) A meta-analysis of electroconvulsive therapy efficacy in depression. J ECT 19: 139–147 [DOI] [PubMed] [Google Scholar]
- Kopelman M.D., Wilson B.A., Baddeley A.D. (1989) The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. J Clin Exp Neuropsychol 11: 724–744 [DOI] [PubMed] [Google Scholar]
- Loo C.K., Garfield J.B.B., Katalinic N., Schweitzer I., Smith D., Hadzi-Pavlovic D. (in preparation) Speed of response in ultrabrief and brief pulse width right unilateral ECT. [DOI] [PubMed]
- Loo C.K., Kaill A., Paton P., Simpson B. (2010) The difficult-to-treat electroconvulsive therapy patient – strategies for augmenting outcomes. J Affect Disord 124: 219–227 [DOI] [PubMed] [Google Scholar]
- Loo C., Katalinic N., Garfield J.B.B., Sainsbury K., Hadzi-Pavlovic D., MacPherson R.D. (in revision) Ketamine as an adjunct to electroconvulsive therapy: a randomised controlled trial. Eur Neuropsychopharm [Google Scholar]
- Loo C.K., Mahon M., Katalinic N., Lyndon B., Hadzi-Pavlovic D. (2011) Predictors of response to ultrabrief right unilateral electroconvulsive therapy. J Affect Disord 130: 192–197 [DOI] [PubMed] [Google Scholar]
- Loo C.K., Sainsbury K., Sheehan P., Lyndon B. (2008) A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. Int J Neuropsychopharmacol 11: 883–890 [DOI] [PubMed] [Google Scholar]
- Loo C., Sheehan P., Pigot M., Lyndon W. (2007) A report on mood and cognitive outcomes with right unilateral ultrabrief pulsewidth (0.3 ms) ECT and retrospective comparison with standard pulsewidth right unilateral ECT. J Affect Disord 103: 277–281 [DOI] [PubMed] [Google Scholar]
- MacPherson R.D., Loo C.K. (2008) Cognitive impairment following electroconvulsive therapy – does the choice of anesthetic agent make a difference? J ECT 24: 52–56 [DOI] [PubMed] [Google Scholar]
- McCall W.V., Reboussin D.M., Weiner R.D., Sackeim H.A. (2000) Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Arch Gen Psychiatry 57: 438–444 [DOI] [PubMed] [Google Scholar]
- McCormick L.M., Brumm M.C., Benede A.K., Lewis J.L. (2009) Relative ineffectiveness of ultrabrief right unilateral versus bilateral electroconvulsive therapy in depression. J ECT 25: 238–242 [DOI] [PubMed] [Google Scholar]
- McElhiney M.C., Moody B.J., Sackeim H. (2001) The Autobiographical Memory Interview – Short Form: Manual for Administration and Scoring. New York: New York State Psychiatric Institute; Department of Psychiatry, College of Physicians and Surgeons; Department of Biological Psychiatry, Columbia University [Google Scholar]
- Montgomery S.A., Asberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389 [DOI] [PubMed] [Google Scholar]
- Niemantsverdriet L., Birkenhager T.K., van den Broek W.W. (2011) The efficacy of ultrabrief-pulse (0.25 millisecond) versus brief-pulse (0.50 millisecond) bilateral electroconvulsive therapy in major depression. J ECT 27: 55–58 [DOI] [PubMed] [Google Scholar]
- Osterrieth P.A. (1944) Le test du copie d’une figure complexe. Arch Psychol 28: 206–356 [Google Scholar]
- Peterchev A.V., Rosa M.A., Deng Z.D., Prudic J., Lisanby S.H. (2010) Electroconvulsive therapy stimulus parameters: rethinking dosage. J ECT 26: 159–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisvejc J., Hyrman V., Sikora J., Berankova A., Kobeda B., Auerova M., et al. (1998) A comparison of brief and ultrabrief pulse stimuli in unilateral ECT. J ECT 14: 68–75 [PubMed] [Google Scholar]
- Quante A., Luborzewski A., Brakemeier E.L., Merkl A., Danker-Hopfe H., Bajbouj M. (2011) Effects of 3 different stimulus intensities of ultrabrief stimuli in right unilateral electroconvulsive therapy in major depression: a randomized, double-blind pilot study. J Psychiatr Res 45: 174–178 [DOI] [PubMed] [Google Scholar]
- Ranck J.B., Jr (1975) Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417–440 [DOI] [PubMed] [Google Scholar]
- Randt C.T., Brown R.E. (1983) Randt memory test. Bayport, NY: Life Science [Google Scholar]
- Rey A. (1964) L’examen clinique en psychologie. Paris: Presses Universitaries de France [Google Scholar]
- Roepke S., Luborzewski A., Schindler F., Quante A., Anghelescu I., Heuser I., et al. (2011) Stimulus pulse-frequency-dependent efficacy and cognitive adverse effects of UB ECT in patients with major depression. J ECT 27: 109–113 [DOI] [PubMed] [Google Scholar]
- Rosveld H.E., Mirsky A.F., Sarason I., Bransome E.D., Jr, Beck L.H. (1956) A continuous performance test of brain damage. J Consult Psychol 20: 343–350 [DOI] [PubMed] [Google Scholar]
- Sackeim H. (2004) Convulsant and anticonvulsant properties of electroconvulsive therapy: towards a focal form of brain stimulation. Clin Neurosci Res 4: 39–57 [Google Scholar]
- Sackeim H.A., Prudic J., Devanand D.P., Kiersky J.E., Fitzsimons L., Moody B.J., et al. (1993) Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med 328: 839–846 [DOI] [PubMed] [Google Scholar]
- Sackeim H.A., Prudic J., Devanand D.P., Nobler M.S., Lisanby S.H., Peyser S., et al. (2000) A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry 57: 425–434 [DOI] [PubMed] [Google Scholar]
- Sackeim H.A., Prudic J., Nobler M.S., Fitzsimons L., Lisanby S.H., Payne N., et al. (2008) Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul 1: 71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A.I.F. (2004) The ECT Handbook, 2nd edn. Third report of the Royal College of Psychiatrists’ Special Committee on ECT London: Royal College of Psychiatrists [Google Scholar]
- Sienaert P., Vansteelandt K., Demyttenaere K., Peuskens J. (2009a) Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: clinical efficacy. J Affect Disord 116: 106–112 [DOI] [PubMed] [Google Scholar]
- Sienaert P., Vansteelandt K., Demyttenaere K., Peuskens J. (2009b) Ultra-brief pulse ECT in bipolar and unipolar depressive disorder: differences in speed of response. Bipolar Disord 11: 418–424 [DOI] [PubMed] [Google Scholar]
- Sienaert P., Vansteelandt K., Demyttenaere K., Peuskens J. (2010) Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: cognitive side-effects. J Affect Disord 122: 60–67 [DOI] [PubMed] [Google Scholar]
- Slotema C.W., Blom J.D., Hoek H.W., Sommer I.E. (2010) Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry 71: 873–884 [DOI] [PubMed] [Google Scholar]
- Spreen O., Strauss E.A. (1998) Compendium of Neuropsychological Tests, 2nd edn. New York: Oxford University Press [Google Scholar]
- Squire L.R., Wetzel C.D., Slater P.C. (1979) Memory complaint after electroconvulsive therapy: assessment with a new self-rating instrument. Biol Psychiatry 14: 791–801 [PubMed] [Google Scholar]
- Stroop J.R. (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18: 634–662 [Google Scholar]
- UK ECT Review (2003) Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 361: 799–808 [DOI] [PubMed] [Google Scholar]
- Valentine M., Keddie K.M., Dunne D. (1968) A comparison of techniques in electro-convulsive therapy. Br J Psychiatry 114: 989–996 [DOI] [PubMed] [Google Scholar]
- Ventura J., Lukoff D., Nuechterlein K.H., Liberman R.P., Green M.F., Shaner A. (1993) Brief Psychiatric Rating Scale Expanded Version (4.0) scales, anchor points, and administration manual. Int J Meth Psychiatr Res 3: 227–243 [Google Scholar]
- Wechsler D. (1945) A standardised memory scale for clinical use. J Psychol 19: 87–95 [Google Scholar]
- Wechsler D. (1987) Wechsler Memory Scale – Revised Manual. New York: Psychological Corporation [Google Scholar]
- Wechsler D. (1997) WAIS-III Administration and Scoring Manual. San Antonio: TX: The Psychological Corporation. [Google Scholar]
- Weiner R.D., Rogers H.J., Davidson J.R., Squire L.R. (1986) Effects of stimulus parameters on cognitive side effects. Ann N Y Acad Sci 462: 315–325 [DOI] [PubMed] [Google Scholar]


