Abstract
Growing evidence suggests that polyphenols could be serious candidates to explain the protective effects of plant-derived foods and beverages. Based on current studies, a general consensus has been achieved to sustain the hypothesis that the specific intake of foods and beverages containing relatively high concentrations of flavonoids may play a meaningful role in reducing cardiovascular disease (CVD) risk through an improvement in vascular function and a modulation of inflammation. This review aims at providing an update on the effects of the consumption of polyphenols-rich foods on intermediate clinical markers of CVD in humans, namely cholesterolemia, blood pressure, endothelial function and platelet function. To date, on the basis of clinical studies, the demonstration is particularly convincing for flavonoids from cocoa-derived products and to a lesser extent for those of tea. While additional studies in this area are clearly needed, incorporating plant foods that are rich in flavanols in the diet of healthy individuals could help to reduce CVD risk. For flavonoids from fruits such as berries, pomegranate, grapes or citrus fruits and those from beverages such as red wine or coffee, the evidence is so far inconclusive. This is primarily due to the limited number and the weakness of experimental designs of the studies performed with these dietary sources. Future long-term well-designed investigations with polyphenols-rich foods but also with isolated phenolic compounds would provide valuable information to establish public health recommendations on polyphenols, taking into account both the nature of the compounds and the optimal dose, for cardiovascular health protection.
Keywords: blood lipids, blood pressure, dietary polyphenols, flavonoids, endothelial function, nutritional prevention of cardiovascular diseases, platelet function, randomized controlled clinical trials
Introduction
The public health burden of cardiovascular diseases (CVDs) is substantial. CVD events remain the leading cause of mortality and a major cause of morbidity and disability in both genders worldwide [World Health Organization, 2008]. An estimated 83 million US adults suffer from CVDs [Roger et al. 2011]. In the 53 member states of the World Health Organization European Region, CVDs account for over 4.35 million deaths [Allender et al. 2008]. CVDs cover a collection of various heart and vascular diseases, but the main public health problems are hypertension, coronary heart and cerebrovascular diseases.
The evidence that nutritional factors are central to the aetiology of cardiovascular disorders is compelling [O’Toole et al. 2008]. CVDs have multiple causes, but the majority of CVD events originate from the complications of atherosclerosis, a pathophysiological process that can be prevented by nutrition (Figure 1) [Wheatcroft et al. 2005]. Atherosclerosis is primarily a disease of the innermost layer of arterial wall, the intima, which comprises smooth muscle cells lined by a monolayer of endothelial cells. The importance of endothelium exceeds its role as a physical barrier as endothelial cells also maintain vascular homeostasis by secreting a number of locally acting mediators. The early phase of atherosclerosis is characterized by the accumulation of cholesterol deposits in macrophages in the intima of the arteries. These foam cells can aggregate to form fatty streaks that progressively mature into a fibrous plaque which is a hallmark of established atherosclerosis. Fibrous plaque is covered by a connective tissue cap with embedded smooth muscle cells. If the plaque continues to grow, it gradually restricts the blood flow causing ischaemia. The clinically important complications of atheroma usually involve rupture of the fibrous cap, causing exposure of highly thrombogenic collagen and resulting intraluminal thrombus. Activated platelets trigger vasoconstriction and further propagation of the thrombus, which may result in total cease in blood flow, and subsequent clinical event.
Figure 1.
The majority of cardiovascular disease (CVD) events originate from the complications of atherosclerosis, a pathophysiological process that can be prevented by nutrition.
Evidence from epidemiological studies indicates a positive association between reduction in the incidence of CVD and consumption of plant-based foods such as fruit and vegetables, nuts, whole grains, plant-derived beverages [Dauchet et al. 2005; Hu and Willett, 2002]. In 2009, Dauchet and collaborators offered a review on the evidence for a relationship between fruit and vegetable consumption and the occurrence of CVD events [Dauchet et al. 2009]. The existing data indicate that the role of fruits and their associated nutrients in the cardiovascular prevention could be stronger than that of vegetables. For years, it has been considered that this beneficial effect was largely due to vitamins and carotenoids present in plant foods and known for their antioxidant properties. However, due to the disappointing results of a number of large intervention studies performed with these micronutrients, showing no reduction in overall mortality and even an increased cardiovascular risk [Bjelakovic et al. 2007; Vivekananthan et al. 2003], scientists were led to consider other potentially beneficial compounds present in fruits and vegetables. During the last 10 years, special attention was paid to polyphenols, a large family of phytochemicals which are the most abundant antioxidants in our diet [Scalbert et al. 2005]. Many studies have emphasized their ability to protect various cellular constituents against oxidation [Collins, 2005]. However, the evaluation of their effects on human health is complicated particularly by their structural diversity and wide distribution in foods as well as by limitations of experimental studies, usually conducted at nonnutritionally achievable doses.
Polyphenols in foods
Polyphenols are a complex group of secondary plant metabolites. The most common groups of phenolic compounds in our diet are phenolic acids and flavonoids (Table 1) [Manach et al. 2004]. Phenolic acids can be further distinguished into two groups, hydroxybenzoic acids (C6–C1) and hydroxycinnamic acids (C6–C3) (Table 2). The hydroxybenzoic acid content of edible plants (gallic, salicylic and vanillic acids) is generally very low, with the exception of certain red fruits, black radish and onions. The hydroxycinnamic acids constitute the most abundant polyphenols in our diet because they are present at high levels in many foods widely consumed (coffee, cereals, fruits). They consist chiefly of p-coumaric, caffeic, ferulic and sinapic acids that are rarely found in the free form except in processed food. Flavonoids represent the second group of polyphenols largely present in the human diet. The structure of flavonoids consists of two or more aromatic rings connected with three carbons (C6–C3–C6) (Table 1). The basic structure of a flavonoid allows a wide variety of different substitution in the A, B and C rings, resulting in multiple subclasses. Classification of flavonoids into subclasses is based on the functional groups in the C ring (Table 3). Subclasses include isoflavones (soyabean), anthocyanidins (berries and wine), flavones (herbs), flavonols (onion, broccoli, tomato, tea), flavanones (citrus fruits and juices) and flavan-3-ols (fruits, cocoa, tea, wine) [Manach et al. 2004]. Oligomeric or polymeric forms of flavanols are referred to as proanthocyanidins or condensed tannins. Other polyphenol structures occur in plants, such as stilbenes (C6–C2–C6, e.g. resveratrol in wine and nuts), lignans (C6–C3–C3–C6, particularly in flaxseeds) and curcuminoids (C6–C3–C1–C3–C6, e.g. curcumin in spices) (Table 1). However, due to both the low quantities consumed and the limited food sources, the contribution of these latter categories to the total polyphenol intake is generally minor.
Table 1.
Backbone structures of the main classes of dietary polyphenols.
| Classes of polyphenols | Chemical structures |
|---|---|
| Phenolic acids (C6–C1 and C6–C3) | 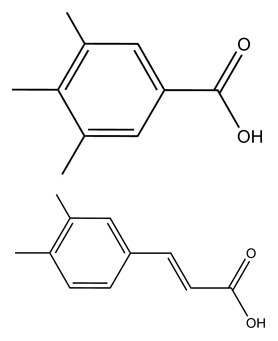 |
| Flavonoids (C6–C3–C6) | 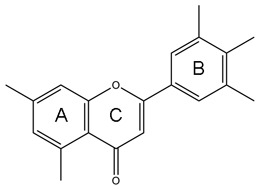 |
| Stilbenes (C6–C2–C6) |  |
| Lignans (C6–C3–C3–C6) |  |
| Curcuminoids (C6–C3–C1–C3–C6) | 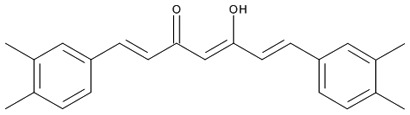 |
Table 2.
Dietary phenolic acids and major food sources.
| Phenolic acids | Representative compounds | Major food sources |
|---|---|---|
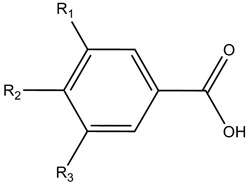
| Hydroxybenzoic acids | ||
 |
|
|
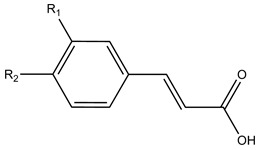
| Hydroxycinnamic acids | ||
 |
|
|
| Example of an ester of hydroxycinnamic acid: |
|
Large variety of fruits: kiwis, blueberries, plums, cherries, apples, etc. |
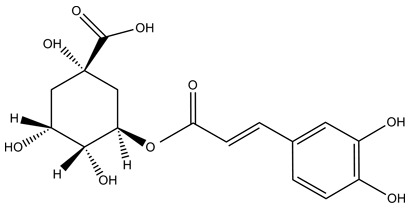 |
Chlorogenic acid (5-caffeoyl quinic acid) |
|
Table 3.
Dietary flavonoids subclasses and major food sources
| Flavonoids | Representative compounds | Major food sources |
|---|---|---|
| Flavanols | Monomers | |
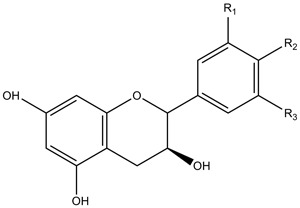 |
|
|
 |
|
|
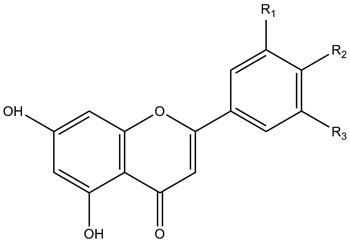
| Flavonols |
|
|
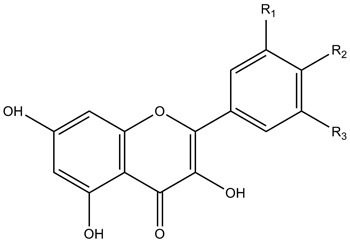 | ||
| Flavanones |
|
|
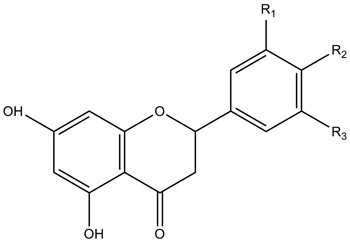 | ||
| Anthocyanidins |
|
|
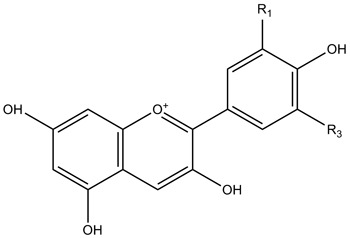 | ||
| Isoflavones |
|
|
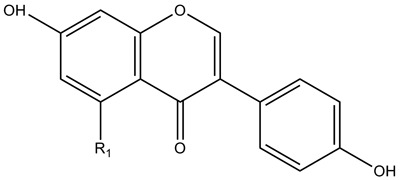 | ||
| Flavones |
|
Vegetables: parsley, celery, sweet peppers |
 | ||
The diversity of the chemical structures of dietary polyphenols makes quite difficult the estimation of their total content in foods. Thus, until very recently, the composition tables for polyphenols were very incomplete. A typical diet rich in fruits, vegetables and plant beverages has been estimated to provide about 1 g of polyphenols/day, with significant variations depending on the extent of consumption of drinks rich in polyphenols (tea, wine, coffee, fruit juices) [Ovaskainen et al. 2008]. Recently, a new database on polyphenol content in foods has been developed, and is now available online [Neveu et al. 2010]. The Phenol-Explorer database (available at http://www.phenol-explorer.eu) allows retrieval of information on the content of 502 polyphenols (including glycosides, esters and aglycones) in 452 foods (fruits, vegetables, beverages, cereals and spices). In an applied work of the Phenol-Explorer database, the richest sources of polyphenols were identified as various spices and dried herbs, cocoa products, some darkly coloured berries, some seeds (flaxseed) and nuts (chestnut, hazelnut) and some vegetables, including olive and artichoke [Pérez-Jiménez et al. 2010]. This database has also been used to analyse the food frequency questionnaires collected over an 8-year period in a French cohort (SUVIMAX2, 4950 men and women, 50–65 years old) and to determine the mean daily intake of polyphenols [Pérez-Jiménez et al. 2011]. The estimated total daily intake of polyphenols was about 1.2 g/day (40% of flavonoids, 60% of phenolic acids). The main food contributors to the polyphenols intake were nonalcoholic beverages and fruits.
Most of the polyphenols rarely occur in fresh foods as unconjugated aglycones but rather as conjugates with sugars or organic acids or as polymers for flavonoids [Clifford and Brown, 2006]. Only a small proportion of the polyphenols ingested is absorbed and the rate and extent of absorption vary greatly depending on the structure of the molecule [Manach et al. 2005]. During absorption, dietary polyphenols are extensively metabolized so that the predominant (and very often exclusive) forms that reach the blood and tissues beyond the gut are conjugated metabolites, chemically distinct from the parent compounds found in plant foods. The process of conjugation, which occurs both in the small intestine and later in the liver, mainly includes methylation, sulfation and glucuronidation. Thus, the circulating forms of polyphenols are mainly glucuronidated, sulfated and methylated derivatives. Maximum concentrations of flavonoids in plasma are usually reached between 1 and 6 h after consumption. Circulating levels range between 0.1 and 7 µM for flavonols, flavanones, isoflavones and flavanols, and do not exceed 0.15 µM for anthocyanins [Manach et al. 2005]. So far, there is no convincing evidence for long-term accumulation of water-soluble metabolites even when high doses of polyphenols are consumed repeatedly [Clifford and Brown, 2006]. Thus, the low bioavailability of phenolic compounds and their rapid clearance imply that a repeated and regular consumption of plant foods is required to sustain the plasma levels of potential bioactive molecules.
Polyphenols and cardiovascular protection
Several epidemiological studies that have examined the relationship between the extent of polyphenol-rich food consumption (fruit and fruit juices, tea, wine, cocoa) and chronic diseases support a protective effect of the compounds upon CVD [Ghosh and Scheepens, 2009; Kuriyama et al. 2006; Mink et al. 2007; Szmitko and Verma, 2005]. Some observational studies have also shown an inverse association between the consumption of some classes of polyphenols and overall mortality or risk of CVD [Arts and Hollman, 2005; Hollman et al. 2010; Mink et al. 2007]. This evidence is supported by results from numerous studies conducted in animal models with nutritionally realistic levels of isolated flavonoids [Auclair et al. 2009; Mauray et al. 2009; Norata et al. 2007] and in humans with flavonoid-rich foods [Hooper et al. 2008]. For a long time, based on the results from experimental studies carried out in vitro, the preventive effect of phenolics on age-related chronic diseases such as CVD was attributed to their antioxidant capacity [Kris-Etherton and Keen, 2002]. However, substantial data indicate that the hypothesis of a direct antioxidant action of polyphenols is unlikely. First, the poor bioavailability of phenolic compounds leads to relatively low plasma concentrations compared with that of other exogenous or endogenous antioxidants [Halliwell, 2008]. Second, data obtained from animal experiments and human intervention studies offer conflicting results [Mladenka et al. 2010]. Furthermore, recently, experts from an International Life Sciences Institute Europe working group found limited evidence concerning the impact of polyphenol-rich products on biomarkers of oxidative stress in humans. This group concluded that the biological relevance of direct antioxidant effects of polyphenols for cardiovascular health could not be established [Hollman et al. 2011]. According to the recent literature, the mechanisms by which polyphenols express their beneficial properties appear to involve their interaction with molecular signalling pathways and related machinery that regulate cellular processes such as inflammation [Gonzalez-Gallego et al. 2010; Gonzalez et al. 2011].
Most of the human clinical studies designed to demonstrate the cardiovascular protective effects of polyphenols have used polyphenol-rich foods and only few of them have used isolated compounds. So far, whether the effect of these food sources could be specifically related to polyphenols is largely unknown. For this update on the effects of the consumption of flavonoid-rich foods on well-identified intermediate markers for cardiovascular diseases (namely cholesterolemia, blood pressure (BP), endothelial function and platelet function), we considered randomized controlled trials performed during the latest years. Some interesting meta-analyses are also explored in this review.
Blood lipids
Lipoproteins are substrates essential to the formation of atherosclerotic plaque as the first step begins with the infiltration and accumulation of low-density lipoproteins (LDLs) in the intima. This phase of lipid infiltration is followed by oxidative modifications of LDL. LDL, recognized as the major atherogenic lipoprotein, makes up approximately 70% of total serum cholesterol. In contrast to this, levels of high-density lipoproteins (HDLs), which contain 20–30% of the total serum cholesterol, are considered as protective against atherosclerosis [Tsompanidi et al. 2010]. Abnormalities of the blood lipid profile, particularly an increased plasma concentration of LDL cholesterol, are a key risk factor for the development of CVD. Reducing LDL levels and improving LDL/HDL cholesterol ratios, notably by diet, are fundamental goals of CVD risk management.
Blood lipoproteins were the most frequently determined factors in the human intervention trials investigating the impact of polyphenols or polyphenols-rich foods on CVD risk markers. In the meta-analysis published in 2008 by Hooper and co-authors, the heterogeneity of the effects on lipoproteins between the flavonoid classes was highlighted [Hooper et al. 2008]. Only soy protein isolates, but not other soy products or components, providing isoflavones and green tea, which is a major dietary source of flavanols, were found to significantly reduce LDL cholesterol (−0.19 mmol/l and −0.23 mmol/l, respectively). Green tea and its content in catechins, particularly (–)-epigallocatechin gallate, are suspected to be effective lipid-lowering agents by inhibiting critical steps in the intestinal absorption of dietary fat, cholesterol and other lipids [Koo and Noh, 2007]. From clinical trials considered by Hooper and colleagues [Hooper et al. 2008], anthocyanins extracts, black tea, chocolate and cocoa, red wine or grapes were polyphenols-rich sources which had no effect on plasma LDL concentrations. Similarly, the global impact on HDL levels was also reported to be minimal. Although changes in blood lipids induced by the consumption of cocoa products remain controversial, another recent meta-analysis involving 215 participants from 8 clinical trials clearly indicates that the short-term consumption of flavanols-rich cocoa could significantly reduce plasma LDL cholesterol without affecting HDL concentrations [Jia et al. 2010]. The meta-analysis of Tokede and colleagues, carried out to examine the effects of flavanol-rich cocoa products on the lipid profile, led to consistent conclusions [Tokede et al. 2011]. Although cocoa contains saturated fat and is a source of dietary calories, short-term intervention trials (2–12 weeks of intervention) with flavonoid-rich cocoa products or dark chocolate provide beneficial effects on total and LDL cholesterol and induce no major effects on HDL and triglycerides [Tokede et al. 2011]. Interestingly, it appears that responses to blood lipids were dependent on the dose of cocoa regularly consumed and could be influenced by the individual’s health status. The latter observation was noted for other classes of polyphenols. For example, in middle-aged subjects with cardiovascular risk factors, a significant increase in HDL cholesterol (+5.2%) was observed after a 8-week consumption of a combination of berries (50 g/day of bilberries, 25 g/day of lingonberries, 50 g/day of blackcurrant and strawberry puree, 35 g/day of chokeberry and raspberry juice) [Erlund et al. 2008]. In this study, the total intake of polyphenols from the berry products was estimated at 837 mg/day and the main classes represented were anthocyanidins and procyanidins. Similarly, a daily consumption of a low-calorie cranberry juice (250 ml/day), a source of flavonols, anthocyanins and proanthocyanidins, was shown to be associated with an increase in plasma HDL cholesterol concentrations (+8.6%) in abdominally obese men [Ruel et al. 2006]. However, the consumption of a flavonoid-rich sea buckthorn berry (mainly flavonol glycosides and proanthocyanidins) was reported to not affect the circulating concentrations of lipids in healthy normolipidaemic adults [Larmo et al. 2009]. Citrus flavonoids such as naringin and hesperidin have been proposed in the literature as potential blood cholesterol-lowering agents [Benavente-Garcia and Castillo, 2008]. However, in a population of healthy middle-aged moderately overweight men, our research group found that total cholesterol, LDL cholesterol, HDL cholesterol were not significantly different in groups consuming orange juice (500 ml/day providing 292 mg of hesperidin) or a control drink supplemented with pure hesperidin (equivalent dose), compared with placebo group [Morand et al. 2011]. In another trial enrolling healthy moderately hypercholesterolemic men and women, a 4-week supplementation with citrus flavonoids also failed to significantly affect blood lipids, even with doses as high as 800 mg of hesperidin or 500 mg of naringin [Demonty et al. 2010].
Blood pressure
High BP is defined as systolic blood pressure (SBP) greater than 140 mmHg or diastolic blood pressure (DBP) greater than 90 mmHg. Because risk of CVD is linear throughout the entire range of BP, a large segment of the population, who is not classically defined as ‘hypertensive’, may still be at risk [MacMahon et al. 1990]. Therefore, lowering BP, even in the ‘normal’ range, through dietary means may decrease the rate of end-organ damage caused by hypertension [Cook et al. 1995].
There is evidence suggesting that the consumption of specific flavonoid-rich foods could lead to lower BP and may provide protection against CVD and stroke [Moline et al. 2000]. The main food sources of flavonoids studied for their potential BP-lowering properties are cocoa products. Currently, interest in the biological activities of cocoa and its content in flavanols is growing. In addition to epidemiological evidence, several short-term randomized controlled intervention studies indicate that the ingestion of flavonoid-rich cocoa products such as dark chocolate has interest in the control of both SBP and DBP not only in hypertensive patients [Grassi et al. 2005b, 2008], but also in healthy individuals [Grassi et al. 2005a]. More evidence on potential antihypertensive properties of cocoa polyphenols derives from an intervention study comparing the medium-term effect (18 weeks) of dark (6.3 g/day providing 30 mg of polyphenols) versus white chocolate consumption in patients with prehypertension or stage I hypertension [Taubert et al. 2007a]. In this study, the introduction of a relatively small amount of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced mean SBP by 2.9 ± 1.6 mmHg and DBP by 1.9 ± 1.0 mmHg. Further, hypertension prevalence declined from 86% to 68%. These changes were accompanied by a sustained increase in plasma markers of nitric oxide (NO), suggesting an improved formation of this vasodilative agent as a potential mechanism of the reduction in BP following dark chocolate consumption [Taubert et al. 2007a]. The positive effect of flavonols-rich cocoa on BP has also emerged from the meta-analysis of Hooper and colleagues in which a chronic consumption of chocolate and cocoa beverages was found to globally reduced SBP by 5.88 mmHg (95% confidence interval [CI] −9.55 to −2.21; 5 studies) and DBP by 3.30 mmHg (95% CI −5.77 to −0.83; 4 studies) [Hooper et al. 2008]. More recently, the meta-analysis of Desch and colleagues [Desch et al. 2010] has confirmed the BP-lowering capacity of flavanol-rich cocoa products in a large set of trials (n = 10), with treatment duration ranging from 2–18 weeks. The selected randomized controlled trials included healthy normotensive or hypertensive patients (n = 297). The mean SBP change in the active treatment arms across all the trials was −4.5 mmHg (95% CI −5.9 to −3.2, p < 0.001) and −2.5 mmHg (95% CI −3.9 to −1.2, p < 0.001) for DBP.
In addition, a few studies have investigated the acute effect of flavonoids-rich cocoa products on BP control. Of clinical relevance, the study of Davison and colleagues [Davison et al. 2010], aiming at evaluating the effect of the intake of different doses of cocoa flavanols (33, 372, 712 or 1052 mg/day during 6 weeks) on 24-h mean arterial BP in untreated mild hypertensive patients, reported significant reductions in 24-h SBP (−5.3 ± 5.1 mmHg; p = 0.001), DBP (−3 ± 3.2 mmHg; p = 0.002) and mean arterial BP (−3.8 ± 3.2 mmHg; p = 0.0004) only at the highest dose studied. It has been estimated that a 3 mmHg reduction in SBP would reduce the relative risk of stroke mortality by 8%, of coronary artery disease (CAD) mortality by 5% and of all-cause mortality by 4% [Whelton et al. 2002]. Thus, the BP changes in response to the consumption of flavanol-rich cocoa in healthy subjects as well as in prehypertensive and hypertensive patients might interestingly suggest the inclusion of moderate amounts of flavanol-rich cocoa or chocolate in daily diet to potentially delay the onset of hypertension.
Surprisingly, two meta-analyses pointed out the absence of effect on BP for alternative rich sources of flavanols such as tea [Hooper et al. 2008; Taubert et al. 2007b]. However, results of population studies suggest that the long-term regular ingestion of tea may lower BP [Hodgson and Croft, 2010]. With regard to the specific components of tea that could be responsible for hypotensive effects, a few data are available. In acute studies, both flavonoids (mainly flavanols and flavonols such as quercetin) and caffeine present in tea can cause a transient increase of BP [Hodgson et al. 2005]. Nagao and colleagues completed a study on green tea extract (583 mg of flavanols) in volunteers with abdominal obesity [Nagao et al. 2007]. In the subgroup of participants with baseline SBP ≥ 130 mmHg, tea extracts significantly reduced BP compared with placebo. In a randomized placebo-controlled trial performed in overweight or obese men receiving epigallocatechine gallate (400 mg administrated twice daily for 8 weeks), Brown and colleagues observed a 2.7 mmHg reduction in DBP [Brown et al. 2009]. Nantz and colleagues published the results of a double-blind trial in 111 healthy volunteers instructed to consume a standardized capsule containing 200 mg of decaffeinated catechin green tea extract or a placebo [Nantz et al. 2009]. They observed a 5 mmHg decrease in SBP in the group treated with catechin green tea extract. The observed discrepancy between the results of intervention trials conducted with tea and those obtained from tea extracts or purified phenolic compounds, suggest that other tea constituents (in particular caffeine) could mask the effects on BP [Deka and Vita, 2011].
Until now, the effects of fruit and vegetable polyphenols on BP in human intervention studies were less investigated. Some promising results were obtained with pomegranate juice [Stowe, 2011] which constitutes a particular rich source of tannins and anthocyanins [Gil et al. 2000]. Notably, two studies have reported a significant reduction of SBP (5% and 21%) in hypertensive subjects and patients with carotid artery stenosis consuming pomegranate juice (50 ml/day) for respectively 2 weeks and 1 year [Aviram and Dornfeld, 2001; Aviram et al. 2004]. In recent years, no more clinical research with robust methodologies, large sample sizes and a well-characterized composition of polyphenols in studied products have been undertaken to confirm the preliminary observations of Aviram and colleagues. To date, limited evidence is available concerning a potential hypotensive role of berries. In the study of Erlund and colleagues investigating the effect of consuming, for 8 weeks, 160 g/day of a combination of berries providing 837 mg/day of polyphenols of which 60% were anthocyanins, a significant decrease in SBP was observed. The decrease mostly occurred in subjects with high baseline BP (−7.3 mmHg for SBP in the highest tertile) [Erlund et al. 2008]. The only other relevant study reporting a significant effect of berries on BP was a parallel trial investigating the effect of a chokeberry flavonoid extract (Aronia melanocarpa E; 255 mg/day; about 25% of anthocyanins, 50% of monomeric and polymeric procyanidins and 9% of phenolic acids) or a placebo for a period of 6 weeks in CAD patients [Naruszewicz et al. 2007]. It was found that SBP and DBP were significantly reduced by a mean average of 11 and 7.2 mmHg, respectively.
In few studies the main goal was to evaluate the direct impact of the consumption of isolated polyphenols on BP. Quercetin is one of the most ubiquitous flavonoids found in fruits and vegetables and probably the best studied molecule because of its large spectrum of biological activities. A small panel of clinical trials has analyzed the effects of pure quercetin on BP. The first investigation was carried out in healthy volunteers (1 g/day of quercetin for 28 days) in which no significant modifications of BP was observed, despite the marked increases in quercetin concentrations in plasma [Conquer et al. 1998]. More recently it has been shown that BP was not altered in prehypertensive patients after quercetin supplementation (730 mg/day for 28 days), whereas reductions in SBP (−7 ± 2 mmHg) and DBP (−5 ± 2 mmHg) were observed in stage 1 hypertensive patients after quercetin treatment [Edwards et al. 2007]. Similarly, in overweight–obese subjects with metabolic syndrome traits in relation to apoE3 genotype who received 150 mg/day of quercetin for 5–6 weeks, the SBP was significantly reduced by 3.4 mmHg [Egert et al. 2009, 2010]. Recently, our research group showed that hesperidin, the major flavonoid in orange products, was highly involved in the BP-lowering effect induced by orange juice consumption [Morand et al. 2011]. In this randomized crossover study with healthy volunteers, we found that 4-week consumption of orange juice (500 ml/day) as well as of control drink plus hesperidin resulted in a significantly lower DBP (−3.2 ± 1.5 mmHg and -5.5 ± 1.8 mmHg, respectively) compared with that measured after consumption of control drink plus placebo (p = 0.023) [Morand et al. 2011]. A recent clinical trial has also considered the putative antihypertensive action of dietary anthocyanins [Hassellund et al. 2011]. In this trial, middle-aged healthy men with screening BP >140/90 mmHg, not on antihypertensive or lipid-lowering medication, were randomized in a double-blind crossover study to placebo versus 320 mg anthocyanins twice daily during 4 weeks. No differences in BP values were observed between the two treatment periods. Whereas flavonoids are largely studied in the field of CVD prevention, few data are available concerning the impact of phenolic acids. An interesting study on chlorogenic acid has to be mentioned [Watanabe et al. 2006]. In this double-blind placebo-controlled study, mild hypertensive subjects treated for 12 weeks with 140 mg/day of chlorogenic acid from green coffee bean extract experienced a significant lowering in systolic BP and diastolic BP by 10 and 7 mmHg, respectively.
Endothelial function
The endothelium plays an integral role in the regulation of vascular tone, leucocytes adhesion, platelet activity and thrombosis and it is intimately involved in the development of atherosclerosis [Dharmashankar and Widlansky, 2010]. A variety of risk factors (including age, smoking, hypercholesterolemia, hypertension and diabetes) are recognized to adversely affect endothelial function resulting in reduced vasodilation as well as proinflammatory and prothrombotic states [Balakumar et al. 2008]. Increasing evidence indicates that these alterations in the functional properties of the vascular endothelium are highly involved in the initiation, progression and clinical complications of atherosclerotic vascular diseases [Vanhoutte, 2009]. Endothelial function has been largely assessed as endothelium-dependent vasomotion, based on the assumption that impaired endothelium-dependent vasodilation also reflects the alteration of the other important functions of endothelium [Landmesser et al. 2004]. The most straightforward, valid, noninvasive and reliable measure of endothelial function is flow-mediated dilation (FMD). FMD corresponds to a physiological response measured as the increase in vascular diameter subsequent to resumed blood flow after transient ischaemia, generally applied on the brachial artery. FMD, which reflects endothelium-dependent and largely NO-mediated arterial function, has been validated as a prognostic parameter of CVD events; therefore this parameter is used as a clinical surrogate marker of vascular health [Thijssen et al. 2011].
Several intervention studies have suggested that the consumption of flavonoid-rich foods such as tea, red wine, cocoa and soya can improve endothelial function in patients with manifest cardiovascular and cerebrovascular disease as well as in healthy volunteers with or without cardiovascular risk factors [Vita, 2005; Zuchi et al. 2010]. Teragawa and colleagues offered an overview of the studies investigating the effect of isoflavone supplementation, using soy protein isolates and isoflavone extract, on endothelial function, as determined by assessing brachial FMD [Teragawa et al. 2008]. Owing to the oestrogen-like nature of isoflavones, the subjects involved in most of these studies were menopausal women, known to have FMD values ranging from ‘severely reduced’ to ‘normal’ [Corretti et al. 2002]. As underlined by Teragawa and colleagues [Teragawa et al. 2008], an improved endothelial function in response to isoflavones or isoflavones-rich products intake was only found in subjects with a baseline reduced FMD [Cuevas et al. 2003]. Furthermore, in a randomized, double-blind, placebo-controlled trial, Chan and colleagues demonstrated that oral isoflavone supplements (80 mg/day), taken over 12 weeks, moderately but significantly restored FMD levels in patients with prior ischaemic stroke [Chan et al. 2008]. This result suggests that isoflavones could ameliorate vascular function especially in individuals with a severe impaired endothelial function.
Although there are several epidemiological studies investigating the short-term effects of red wine and its main constituents (notably resveratrol) on cardiovascular health, there are very few clinical studies [Opie and Lecour, 2007]. In their meta-analysis, Hooper and colleagues also suggest a minimal vascular action for the chronic consumption of red wine or grape [Hooper et al. 2008]. Recently, Karatzi and colleagues have reviewed the studies investigating acute and short-term effects of red wine on endothelial function [Karatzi et al. 2009]. Except two studies demonstrating a positive effect of the consumption of purple grape juice (containing phenolic compounds also found in red wine) on endothelial function in patients with CAD [Chou et al. 2001; Stein et al. 1999], the majority of other short-term clinical studies with red wine or dealcoholized red wine showed conflicting results [Cuevas et al. 2000; Guarda et al. 2005; Zilkens et al. 2005]. From Karatzi and colleagues the analysis of all clinical data about the acute effects of red wine constituents on endothelial function is inconclusive [Karatzi et al. 2009]. Pending further studies, the authors concluded that physicians and nutritionists should be very careful in suggesting red wine consumption in high-risk populations, as its acute postprandial effect is not yet clear.
The consumption of tea has been shown to improve endothelial function. Indeed, in their meta-analysis, Hooper and colleagues [Hooper et al. 2008] suggested a beneficial effect of black tea on FMD of +3.40% (95% CI 1.85–4.95%) after chronic consumption, and +1.70% (95% CI −0.17–3.57%) after acute intake. However, these data were based on the analysis of a limited number of studies (n = 5), and several other trials on black and green tea and FMD have been published since. Recently, Ras and colleagues have analyzed nine intervention studies on the effect of tea consumption on FMD [Ras et al. 2011]. Seven of these trials had a crossover design [Alexopoulos et al. 2008; Ardalan et al. 2007; Duffy et al. 2001a; Grassi et al. 2009; Hodgson et al. 2005; Jochmann et al. 2008; Lorenz et al. 2007] and two had a parallel design [Hodgson et al. 2002; Park et al. 2010]. In five out of nine studies, subjects were healthy or mildly hypercholesterolemic; the other studies included renal transplant recipients, chronic kidney disease patients or CAD patients. The majority of the studies investigated the effect of black tea; only three trials interested in the consumption of green tea [Alexopoulos et al. 2008; Jochmann et al. 2008; Park et al. 2010]. From the trials analysed by Ras and colleagues a median daily intake of 500 ml of tea (2–3 cups) induced approximately a 40% increased of FMD when compared with the average basal FMD (6.3%) measured under placebo or baseline conditions [Ras et al. 2011].
Undoubtedly, cocoa and dark chocolate, rich in flavanols, represent the only flavonoid-rich sources (especially flavanols) that show consistent significant effects on FMD, both acutely and chronically [Galleano et al. 2009]. Hooper and colleagues reported that chocolate or cocoa increased FMD after both acute (3.99%; 95% CI: 2.86, 5.12; six studies) and chronic (1.45%; 0.62, 2.28; two studies) intakes [Hooper et al. 2008]. Consumption of a flavanol-rich cocoa drink (containing 176 to 185 mg/dl of flavanols) by patients with cardiovascular risk factors was shown to increase the bioavailability of NO and enhance FMD [Heiss et al. 2003, 2005, 2007]. These parallel effects were reversed by the infusion of a NO synthesis inhibitor. In addition, Wang-Polagruto and colleagues published the first study identifying beneficial vascular effects of flavanol-rich cocoa consumption in hypercholesterolemic postmenopausal women [Wang-Polagruto et al. 2006]. In this study, brachial artery hyperaemic blood flow increased significantly after a 6-week intervention with a high-flavanol cocoa beverage (providing 446 mg of total flavanols) compared with a low-flavanol cocoa beverage (providing only 43 mg of total flavanols). In healthy humans, the consumption of flavanol-rich cocoa (821 mg of flavanols/day during 5 days) also induced an improved vasodilation via activation of the NO system [Fisher et al. 2003]. Similarly, dark chocolate (46 to 100 g/day) has been described to have an acute and short-term beneficial effect on endothelial function [Engler et al. 2004; Grassi et al. 2005b; Vlachopoulos et al. 2005].
Because these clinical trials have been performed with complex foods or beverages, it cannot be completely excluded that the observed effects on endothelial function may involved compounds other than flavonoids contained in these foods/extracts [Heiss et al. 2006]. Information ensuing from investigations in humans using specific, chemically pure flavonoids is rare. Schroeter and colleagues demonstrated improved FMD up to four hours after administration of pure (–)-epicatechin (a monomeric flavanol found in cocoa-derived products and in tea) [Schroeter et al. 2006]. In this study, the effects of pure epicatechin mimicked the effects of flavanol-rich cocoa or tea and the circulating epicatechin metabolites levels correlated with the degree of improvement in endothelial function. The authors concluded that the intake of the flavanol epicatechin accounts, at least in part, for the reported vascular effects observed after the consumption of flavanol-rich cocoa or tea in humans. Moreover, in patients with CAD, Widlansky and colleagues observed an improvement in brachial artery FMD from 7.1 ± 4.1% to 8.6 ± 4.7% 2 hours after the administration of a dose of 300 mg of epigallocatechine gallate (EGCG), a flavonoid abundant in green tea [Widlansky et al. 2007]. Furthermore, Loke and colleagues investigated, in 12 healthy men, the acute effects of the oral administration of 200 mg of quercetin, (–)-epicatechin or EGCG on NO status and on endothelin-1 (ET-1) production, which have ultimate implications for endothelial function [Loke et al. 2008]. They found that that acute treatment with quercetin and (–)-epicatechin, but not EGCG, augmented endogenous NO. Quercetin was also shown to reduce ET-1 production.
Recently, we and others have demonstrated a positive effect of other isolated phenolic compounds on endothelial function. First, citrus hesperidin when administered to healthy subjects (292 mg) or to patients with metabolic syndrome (500 mg) for 3–4 weeks significantly improved vascular reactivity [Morand et al. 2011; Rizza et al. 2011]. Our study also showed that the improved postprandial microvascular endothelial reactivity induced by hesperidin positively correlates to its plasma concentrations and that this flavonoid directly contributes to the vascular protective effect of orange juice [Morand et al. 2011]. Another recent trial has assessed the acute effect of resveratrol (30, 90 and 270 mg) in overweight/obese individuals with mildly elevated BP [Wong et al. 2010]. This study showed a significant dose effect of resveratrol on plasma resveratrol concentration and on FMD, which increased from 4.1 ± 0.8% (placebo) to 7.7 ± 1.5% after 270 mg resveratrol.
Finally, the importance of the complexity of the phenolic composition of food sources regarding the impact on the vascular health was illustrated by the work of Clifton on the effect of grape seed extract (GSE) and quercetin on cardiovascular and endothelial parameters [Clifton, 2004]. Individually, GSE and quercetin were reported to cause an endothelium-dependent relaxation of blood vessels both in animals [Edirisinghe et al. 2008; Khoo et al. 2010] and in humans [Clifton, 2004; Loke et al. 2008]. However, when combined, quercetin appeared to nullify the positive effect of GSE on endothelial function in high-risk subjects [Clifton, 2004].
Platelet function
It is well established that platelet activation in response to endothelial injury leads to thrombus formation in damaged arteries [Linden and Jackson, 2010]. However, growing evidence suggests that platelets are also important mediators of inflammation and play a central role in atherogenesis itself. The development of drugs interfering with platelet–vessel wall interaction at various stages of thrombus formation is crucial to manage cardiovascular risk. In parallel, the promotion of dietary therapies providing consistent inhibition of platelet function could be of interest. Based on epidemiological studies suggesting that diets rich in flavonoids can reduce the risk of arterial thrombosis [Arts and Hollman, 2005], it seems interesting to investigate the ability of these bioactives to inhibit platelet activation.
Considering the impact of polyphenols-rich diets on human platelet function, a recent critical review has to be considered with attention [Ostertag et al. 2010]. This review summarized 25 well-controlled human intervention trials and revealed a wide heterogeneity between studies as for levels of polyphenol intake (18.6 mg to 2.20 g/day), study population (healthy, increased risk factors and/or clinical disease), study size (between 6 and 1535 subjects), length of supplementation (acute intake for 1 day to chronic intake for over 90 days) and variety of tests used to assess platelet function. Therefore, the authors of this review concluded that it remains difficult to deduce from a relatively small number of well-designed studies if and how polyphenols have preventive effects on platelet aggregation and activation. However, one consistent finding is that the moderate consumption of cocoa-derived products, especially dark chocolate, significantly improves platelet function [Bordeaux et al. 2007; Flammer et al. 2007; Hermann et al. 2006; Innes et al. 2003]. Other studies also found a significant inhibition of platelet aggregation and activation upon acute or chronic intake of dietary flavanols from cocoa [Heptinstall et al. 2006; Murphy et al. 2003; Wang-Polagruto et al. 2006]. From these studies, it seems that the intake of 100 mg of flavanols induced a 3–11% reduction in platelet aggregation. The physiological relevance of the beneficial effects on platelets of an exposure to flavanols from cocoa consumption (equivalent to 100 g of dark chocolate with 70% cocoa solids) has been estimated to be comparable to standard doses of aspirin (~80 mg) clinically used as anti-aggregant therapy [Pearson et al. 2002].
The few human studies examining the effects on platelet function of polyphenols-rich beverages such as tea, coffee and alcoholic beverages do not provide consensual results. Consumption of one litre per day of black tea rich in gallic acid, flavanols, tannins, theaflavins and thearubigins was shown to inhibit platelet activation by 4–10% [Steptoe et al. 2007]. Two older studies on tea consumption did not find significant effects on platelet function [Duffy et al. 2001b; Hodgson et al. 2001]. In 2008, Natella and colleagues demonstrated that the acute intake of 200 ml of coffee mainly providing chlorogenic acid as phenolic compound also had significant anti-aggregatory effects [Natella et al. 2008]. Consumption of red wine has led to conflicting results on platelet function [Saleem and Basha, 2010].
The effects of fruit polyphenols on platelet aggregation was recently reviewed by Chong and colleagues [Chong et al. 2010]. Globally, a few studies supported the hypothesis that purple, but not red, grape juice is effective in inhibiting platelet aggregation [Freedman et al. 2001; Keevil et al. 2000]. Other polyphenols-rich fruits which have been reported to have significant inhibitory effects on platelet aggregation included pomegranate juice (50 ml/day for 14 days) [Aviram et al. 2000], GSE (acute study) [Polagruto et al. 2007] and a combination of various berries [Erlund et al. 2008]. However, chronic intake of polyphenols from berries (whole fruits or juices) may result in only a relatively low inhibition of platelet aggregation under shear stress [Ostertag et al. 2010]. Other fruit juices such as orange juice, grapefruit juice and sea buckthorn juice taken daily for 7–10 days in healthy volunteers failed to reduce ex vivo platelet activity [Eccleston et al. 2002; Keevil et al. 2000].
Conclusion
There is supportive clinical evidence for the beneficial effects of some flavonoids-rich foods or supplements on multiple endpoints of cardiovascular risk, the more convincing being reduction in BP and improvement in endothelial function. Based on current data with flavanol-containing foods and plant extracts, a general consensus has been achieved to sustain the hypothesis that dietary flavanol intake may play a meaningful role in the prevention of CVD in humans [Schroeter et al. 2010]. The lack of clinical data on other polyphenols, especially those abundant in fruits and vegetables [Chong et al. 2010; Terao et al. 2008], does not allow us to conclude as to their effectiveness in preventing CVD. The aforementioned knowledge gaps partly reflect a delay in resources invested to value fruit and vegetables and their derived phenolic compounds beyond highly consumed flavonoids-rich beverages such as cocoa drinks and tea.
In addition to their identified beneficial impact on BP and endothelial function, flavonoid-rich dietary sources might also favourably modulate arterial stiffness, another important clinical marker of the cardiovascular health [Adji et al. 2011]. Indeed, some promising preliminary data suggest that lifetime fruit and vegetable consumption [Aatola et al. 2010] but also dietary intervention with polyphenols-rich sources [Dohadwala et al. 2011; Grassi et al. 2009; Naissides et al. 2006; Pase et al. 2011; Vlachopoulos et al. 2005] may provide an effective means of reducing arterial stiffness. However, long-term standardized studies dedicated to examine the role of polyphenols in the management of arterial stiffness, assessed by the noninvasive measure of pulse wave velocity (PWV), are highly recommended [Laurent et al. 2006].
The question of whether isolated polyphenols are the biologically active compounds responsible for the modulation of vascular health biomarkers is still unresolved. This is partly due to key limitations with regard to the experimental design generally proposed in the studies performed until now. The lack of a proper control, i.e. the absence of a standardized control product regarding the polyphenols content, is notably an issue that should be addressed in future trials. In addition, the studies displayed an inconsistent spectrum of designs in terms of the duration of study periods (from weeks to several months) but also regarding the dose of polyphenols investigated. Another barrier is the question of the specific populations studied. Studies that recruited subjects at risk of CVD or patients with diagnosed CVD brought results that are not expected to apply to the general population. To establish the effects of oral exposure to the most promising polyphenols, large-scale, long-term, well-controlled trials preferably with clinical endpoints including cardiovascular morbidity/mortality seem warranted. Moreover, further specific clinical studies are needed to determine the optimal dose of the long-term ingestion of flavonoids-rich sources or isolated phenolic compounds to ensure safe cardiovascular benefits. An excessive intake of polyphenols, made possible by marketed fortified foods or nutritional supplements, could lead to unknown adverse effects. To ensure the safety of consuming dietary polyphenols and until sufficient data are collected to establish a dietary reference intake for each class of compounds, the development of sustainable functional foods should only consider the same level of polyphenols as the best dietary source can deliver at dietary doses [Holst and Williamson, 2008].
Once a compound or food is proven to exert bioefficacy in vivo, then mechanisms have to be tested in vitro. Until now, there are few reported in vitro studies using nutritionally achievable concentrations of conjugated forms of polyphenols found in plasma after consumption of polyphenol-containing foods. Some emerging arguments show the importance to promote such physiological and nutritional approaches to elucidate the mechanisms sustaining the in vivo observations [Holst and Williamson, 2008]. The recent study of Kawai and colleagues is particularly relevant as it has identified activated macrophages as a specific target of a conjugated metabolite of quercetin (quercetin-3-O-β-D-glucuronide) during the development of atherosclerotic lesions [Kawai et al. 2008]. In the same way, in their position paper on the role of dietary flavanols in the vascular function, Schewe and colleagues addressed the question about the relationships between the fate and the mode of action of polyphenols [Schewe et al. 2008]. Briefly, they reported that exposure of human endothelial cells to the methylated metabolite of (-)-epicatechin increased the production of NO leading to an improvement of the endothelial vasomotricity. In the future, it is essential for the scientific community to strictly consider literature offering well-designed experiments (in terms of nature and concentrations of metabolites used) in order to definitively identify the compounds (parent compounds, mammalian metabolites or colonic metabolites) biologically active in target tissues as well as the molecular mechanisms involved.
In conclusion, this summary of current scientific evidence indicates that dietary flavonoids may show promising potential to reduce the risk of CVD, but much remains to be done to produce conclusive data on the value of these compounds in the context of public health or clinical practice. Challenges for future research in the field of cardiovascular health effects of polyphenols will be to consider the multifaceted bioactivity of these compounds.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflict of interest in preparing this article.
References
- Aatola H., Koivistoinen T., Hutri-Kahonen N., Juonala M., Mikkila V., Lehtimaki T., et al. (2010) Lifetime fruit and vegetable consumption and arterial pulse wave velocity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation 122: 2521–2528 [DOI] [PubMed] [Google Scholar]
- Adji A., O’Rourke M.F., Namasivayam M. (2011) Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens 24: 5–17 [DOI] [PubMed] [Google Scholar]
- Alexopoulos N., Vlachopoulos C., Aznaouridis K., Baou K., Vasiliadou C., Pietri P., et al. (2008) The acute effect of green tea consumption on endothelial function in healthy individuals. Eur J Cardiovasc Prev Rehabil 15: 300–305 [DOI] [PubMed] [Google Scholar]
- Allender S., Scarborough P., Peto V., Rayner M., Leal J., Luengo-Fernandez R., et al. (2008) European cardiovascular disease statistics 2008. Brussels: European Heart Network [Google Scholar]
- Ardalan M.R., Tarzamni M.K., Shoja M.M., Tubbs R.S., Rahimi-Ardabili B., Ghabili K., et al. (2007) Black tea improves endothelial function in renal transplant recipients. Transplant Proc 39: 1139–1142 [DOI] [PubMed] [Google Scholar]
- Arts I.C., Hollman P.C. (2005) Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81(1 Suppl.): 317S-325S [DOI] [PubMed] [Google Scholar]
- Auclair S., Milenkovic D., Besson C., Chauvet S., Gueux E., Morand C., et al. (2009) Catechin reduces atherosclerotic lesion development in apo E-deficient mice: A transcriptomic study. Atherosclerosis 204: e21-e27 [DOI] [PubMed] [Google Scholar]
- Aviram M., Dornfeld L. (2001) Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 158: 195–198 [DOI] [PubMed] [Google Scholar]
- Aviram M., Dornfeld L., Rosenblat M., Volkova N., Kaplan M., Coleman R., et al. (2000) Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr 71: 1062–1076 [DOI] [PubMed] [Google Scholar]
- Aviram M., Rosenblat M., Gaitini D., Nitecki S., Hoffman A., Dornfeld L., et al. (2004) Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr 23: 423–433 [DOI] [PubMed] [Google Scholar]
- Balakumar P., Kaur T., Singh M. (2008) Potential target sites to modulate vascular endothelial dysfunction: current perspectives and future directions. Toxicology 245: 49–64 [DOI] [PubMed] [Google Scholar]
- Benavente-Garcia O., Castillo J. (2008) Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem 56: 6185–6205 [DOI] [PubMed] [Google Scholar]
- Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297: 842–857 [DOI] [PubMed] [Google Scholar]
- Bordeaux B., Yanek L.R., Moy T.F., White L.W., Becker L.C., Faraday N., et al. (2007) Casual chocolate consumption and inhibition of platelet function. Prev Cardiol 10: 175–180 [DOI] [PubMed] [Google Scholar]
- Brown A.L., Lane J., Coverly J., Stocks J., Jackson S., Stephen A., et al. (2009) Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr 101: 886–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y.H., Lau K.K., Yiu K.H., Li S.W., Chan H.T., Fong D.Y., et al. (2008) Reduction of C-reactive protein with isoflavone supplement reverses endothelial dysfunction in patients with ischaemic stroke. Eur Heart J 29: 2800–2807 [DOI] [PubMed] [Google Scholar]
- Chong M.F., Macdonald R., Lovegrove J.A. (2010) Fruit polyphenols and CVD risk: a review of human intervention studies. Br J Nutr 104(Suppl. 3): S28-S39 [DOI] [PubMed] [Google Scholar]
- Chou E.J., Keevil J.G., Aeschlimann S., Wiebe D.A., Folts J.D., Stein J.H. (2001) Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am J Cardiol 88: 553–555 [DOI] [PubMed] [Google Scholar]
- Clifford M.N., Brown J.E. (2006) Dietary flavonoids and health - broadening the perspective. In: Andersen O.M., Markham K.R. (eds), Flavonoids: Chemistry, Biochemistry and Applications. Boca Raton, FL: CRC Press [Google Scholar]
- Clifton P.M. (2004) Effect of grape seed extract and quercetin on cardiovascular and endothelial parameters in high-risk subjects. J Biomed Biotechnol 5: 272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.R. (2005) Assays for oxidative stress and antioxidant status: applications to research into the biological effectiveness of polyphenols. Am J Clin Nutr 81(1 Suppl.): 261S-267S [DOI] [PubMed] [Google Scholar]
- Conquer J.A., Maiani G., Azzini E., Raguzzini A., Holub B.J. (1998) Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr 128: 593–597 [DOI] [PubMed] [Google Scholar]
- Cook N.R., Cohen J., Hebert P.R., Taylor J.O., Hennekens C.H. (1995) Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 155: 701–709 [PubMed] [Google Scholar]
- Corretti M.C., Anderson T.J., Benjamin E.J., Celermajer D., Charbonneau F., Creager M.A., et al. (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265 [DOI] [PubMed] [Google Scholar]
- Cuevas A.M., Guasch V., Castillo O., Irribarra V., Mizon C., San Martin A., et al. (2000) A high-fat diet induces and red wine counteracts endothelial dysfunction in human volunteers. Lipids 35: 143–148 [DOI] [PubMed] [Google Scholar]
- Cuevas A.M., Irribarra V.L., Castillo O.A., Yanez M.D., Germain A.M. (2003) Isolated soy protein improves endothelial function in postmenopausal hypercholesterolemic women. Eur J Clin Nutr 57: 889–894 [DOI] [PubMed] [Google Scholar]
- Dauchet L., Amouyel P., Dallongeville J. (2005) Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology 65: 1193–1197 [DOI] [PubMed] [Google Scholar]
- Dauchet L., Amouyel P., Dallongeville J. (2009) Fruits, vegetables and coronary heart disease. Nat Rev Cardiol 6: 599–608 [DOI] [PubMed] [Google Scholar]
- Davison K., Berry N.M., Misan G., Coates A.M., Buckley J.D., Howe P.R. (2010) Dose-related effects of flavanol-rich cocoa on blood pressure. J Hum Hypertens 24: 568–576 [DOI] [PubMed] [Google Scholar]
- Deka A., Vita J.A. (2011) Tea and cardiovascular disease. Pharmacol Res, 64: 136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonty I., Lin Y., Zebregs Y.E., Vermeer M.A., van der Knaap H.C., Jakel M., et al. (2010) The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J Nutr 140: 1615–1620 [DOI] [PubMed] [Google Scholar]
- Desch S., Schmidt J., Kobler D., Sonnabend M., Eitel I., Sareban M., et al. (2010) Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens 23: 97–103 [DOI] [PubMed] [Google Scholar]
- Dharmashankar K., Widlansky M.E. (2010) Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep 12: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohadwala M.M., Holbrook M., Hamburg N.M., Shenouda S.M., Chung W.B., Titas M., et al. (2011) Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr 93: 934–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S.J., Keaney J.F., Jr, Holbrook M., Gokce N., Swerdloff P.L., Frei B., et al. (2001a) Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 104: 151–156 [DOI] [PubMed] [Google Scholar]
- Duffy S.J., Vita J.A., Holbrook M., Swerdloff P.L., Keaney J.F., Jr (2001b) Effect of acute and chronic tea consumption on platelet aggregation in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 21: 1084–1089 [DOI] [PubMed] [Google Scholar]
- Eccleston C., Baoru Y., Tahvonen R., Kallio H., Rimbach G.H., Minihane A.M. (2002) Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J Nutr Biochem 13: 346–354 [DOI] [PubMed] [Google Scholar]
- Edirisinghe I., Burton-Freeman B., Tissa Kappagoda C. (2008) Mechanism of the endothelium-dependent relaxation evoked by a grape seed extract. Clin Sci (Lond) 114: 331–337 [DOI] [PubMed] [Google Scholar]
- Edwards R.L., Lyon T., Litwin S.E., Rabovsky A., Symons J.D., Jalili T. (2007) Quercetin reduces blood pressure in hypertensive subjects. J Nutr 137: 2405–2411 [DOI] [PubMed] [Google Scholar]
- Egert S., Boesch-Saadatmandi C., Wolffram S., Rimbach G., Muller M.J. (2010) Serum lipid and blood pressure responses to quercetin vary in overweight patients by apolipoprotein E genotype. J Nutr 140: 278–284 [DOI] [PubMed] [Google Scholar]
- Egert S., Bosy-Westphal A., Seiberl J., Kurbitz C., Settler U., Plachta-Danielzik S., et al. (2009) Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr 102: 1065–1074 [DOI] [PubMed] [Google Scholar]
- Engler M.B., Engler M.M., Chen C.Y., Malloy M.J., Browne A., Chiu E.Y., et al. (2004) Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr 23: 197–204 [DOI] [PubMed] [Google Scholar]
- Erlund I., Koli R., Alfthan G., Marniemi J., Puukka P., Mustonen P., et al. (2008) Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr 87: 323–331 [DOI] [PubMed] [Google Scholar]
- Fisher N.D., Hughes M., Gerhard-Herman M., Hollenberg N.K. (2003) Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 21: 2281–2286 [DOI] [PubMed] [Google Scholar]
- Flammer A.J., Hermann F., Sudano I., Spieker L., Hermann M., Cooper K.A., et al. (2007) Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 116: 2376–2382 [DOI] [PubMed] [Google Scholar]
- Freedman J.E., Parker C., III, Li L., Perlman J.A., Frei B., Ivanov V., et al. (2001) Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 103: 2792–2798 [DOI] [PubMed] [Google Scholar]
- Galleano M., Oteiza P.I., Fraga C.G. (2009) Cocoa, chocolate, and cardiovascular disease. J Cardiovasc Pharmacol 54: 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Scheepens A. (2009) Vascular action of polyphenols. Mol Nutr Food Res 53: 322–331 [DOI] [PubMed] [Google Scholar]
- Gil M.I., Tomas-Barberan F.A., Hess-Pierce B., Holcroft D.M., Kader A.A. (2000) Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 48: 4581–4589 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gallego J., Garcia-Mediavilla M.V., Sanchez-Campos S., Tunon M.J. (2010) Fruit polyphenols, immunity and inflammation. Br J Nutr 104(Suppl. 3): S15-S27 [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Ballester I., Lopez-Posadas R., Suarez M.D., Zarzuelo A., Martinez-Augustin O., et al. (2011) Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr 51: 331–362 [DOI] [PubMed] [Google Scholar]
- Grassi D., Desideri G., Necozione S., Lippi C., Casale R., Properzi G., et al. (2008) Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr 138: 1671–1676 [DOI] [PubMed] [Google Scholar]
- Grassi D., Lippi C., Necozione S., Desideri G., Ferri C. (2005a) Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr 81: 611–614 [DOI] [PubMed] [Google Scholar]
- Grassi D., Mulder T.P., Draijer R., Desideri G., Molhuizen H.O., Ferri C. (2009) Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J Hypertens 27: 774–781 [DOI] [PubMed] [Google Scholar]
- Grassi D., Necozione S., Lippi C., Croce G., Valeri L., Pasqualetti P., et al. (2005b) Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 46: 398–405 [DOI] [PubMed] [Google Scholar]
- Guarda E., Godoy I., Foncea R., Perez D.D., Romero C., Venegas R., et al. (2005) Red wine reduces oxidative stress in patients with acute coronary syndrome. Int J Cardiol 104: 35–38 [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2008) Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 476: 107–112 [DOI] [PubMed] [Google Scholar]
- Hassellund S.S., Flaa A., Sandvik L., Kjeldsen S.E., Rostrup M. (2011) Effects of anthocyanins on blood pressure and stress reactivity: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens, in press [DOI] [PubMed] [Google Scholar]
- Heiss C., Dejam A., Kleinbongard P., Schewe T., Sies H., Kelm M. (2003) Vascular effects of cocoa rich in flavan-3-ols. JAMA 290: 1030–1031 [DOI] [PubMed] [Google Scholar]
- Heiss C., Finis D., Kleinbongard P., Hoffmann A., Rassaf T., Kelm M., et al. (2007) Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J Cardiovasc Pharmacol 49: 74–80 [DOI] [PubMed] [Google Scholar]
- Heiss C., Kleinbongard P., Dejam A., Perre S., Schroeter H., Sies H., et al. (2005) Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 46: 1276–1283 [DOI] [PubMed] [Google Scholar]
- Heiss C., Schroeter H., Balzer J., Kleinbongard P., Matern S., Sies H., et al. (2006) Endothelial function, nitric oxide, and cocoa flavanols. J Cardiovasc Pharmacol 47(Suppl. 2): S128-S135; discussion S172-S126 [DOI] [PubMed] [Google Scholar]
- Heptinstall S., May J., Fox S., Kwik-Uribe C., Zhao L. (2006) Cocoa flavanols and platelet and leukocyte function: recent in vitro and ex vivo studies in healthy adults. J Cardiovasc Pharmacol 47(Suppl. 2): S197-S205; discussion S206-S199 [DOI] [PubMed] [Google Scholar]
- Hermann F., Spieker L.E., Ruschitzka F., Sudano I., Hermann M., Binggeli C., et al. (2006) Dark chocolate improves endothelial and platelet function. Heart 92: 119–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J.M., Burke V., Puddey I.B. (2005) Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J Hypertens 23: 47–54 [DOI] [PubMed] [Google Scholar]
- Hodgson J.M., Croft K.D. (2010) Tea flavonoids and cardiovascular health. Mol Aspects Med 31: 495–502 [DOI] [PubMed] [Google Scholar]
- Hodgson J.M., Puddey I.B., Burke V., Watts G.F., Beilin L.J. (2002) Regular ingestion of black tea improves brachial artery vasodilator function. Clin Sci (Lond) 102: 195–201 [PubMed] [Google Scholar]
- Hodgson J.M., Puddey I.B., Mori T.A., Burke V., Baker R.I., Beilin L.J. (2001) Effects of regular ingestion of black tea on haemostasis and cell adhesion molecules in humans. Eur J Clin Nutr 55: 881–886 [DOI] [PubMed] [Google Scholar]
- Hollman P.C., Cassidy A., Comte B., Heinonen M., Richelle M., Richling E., et al. (2011) The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr 141: 989S-1009S [DOI] [PubMed] [Google Scholar]
- Hollman P.C., Geelen A., Kromhout D. (2010) Dietary flavonol intake May lower stroke risk in men and women. J Nutr 140: 600–604 [DOI] [PubMed] [Google Scholar]
- Holst B., Williamson G. (2008) Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol 19: 73–82 [DOI] [PubMed] [Google Scholar]
- Hooper L., Kroon P.A., Rimm E.B., Cohn J.S., Harvey I., Le Cornu K.A., et al. (2008) Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 88: 38–50 [DOI] [PubMed] [Google Scholar]
- Hu F.B., Willett W.C. (2002) Optimal diets for prevention of coronary heart disease. JAMA 288: 2569–2578 [DOI] [PubMed] [Google Scholar]
- Innes A.J., Kennedy G., McLaren M., Bancroft A.J., Belch J.J. (2003) Dark chocolate inhibits platelet aggregation in healthy volunteers. Platelets 14: 325–327 [DOI] [PubMed] [Google Scholar]
- Jia L., Liu X., Bai Y.Y., Li S.H., Sun K., He C., et al. (2010) Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr 92: 218–225 [DOI] [PubMed] [Google Scholar]
- Jochmann N., Lorenz M., Krosigk A., Martus P., Bohm V., Baumann G., et al. (2008) The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br J Nutr 99: 863–868 [DOI] [PubMed] [Google Scholar]
- Karatzi K., Karatzis E., Papamichael C., Lekakis J., Zampelas A. (2009) Effects of red wine on endothelial function: postprandial studies vs clinical trials. Nutr Metab Cardiovasc Dis 19: 744–750 [DOI] [PubMed] [Google Scholar]
- Kawai Y., Nishikawa T., Shiba Y., Saito S., Murota K., Shibata N., et al. (2008) Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem 283: 9424–9434 [DOI] [PubMed] [Google Scholar]
- Keevil J.G., Osman H.E., Reed J.D., Folts J.D. (2000) Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J Nutr 130: 53–56 [DOI] [PubMed] [Google Scholar]
- Khoo N.K., White C.R., Pozzo-Miller L., Zhou F., Constance C., Inoue T., et al. (2010) Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic Biol Med 49: 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S.I., Noh S.K. (2007) Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem 18: 179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton P.M., Keen C.L. (2002) Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol 13: 41–49 [DOI] [PubMed] [Google Scholar]
- Kuriyama S., Shimazu T., Ohmori K., Kikuchi N., Nakaya N., Nishino Y., et al. (2006) Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 296: 1255–1265 [DOI] [PubMed] [Google Scholar]
- Landmesser U., Hornig B., Drexler H. (2004) Endothelial function: a critical determinant in atherosclerosis? Circulation 109(21 Suppl. 1): II27-II33 [DOI] [PubMed] [Google Scholar]
- Larmo P.S., Yang B., Hurme S.A., Alin J.A., Kallio H.P., Salminen E.K., et al. (2009) Effect of a low dose of sea buckthorn berries on circulating concentrations of cholesterol, triacylglycerols, and flavonols in healthy adults. Eur J Nutr 48: 277–282 [DOI] [PubMed] [Google Scholar]
- Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., et al. (2006) Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605 [DOI] [PubMed] [Google Scholar]
- Linden M.D., Jackson D.E. (2010) Platelets: pleiotropic roles in atherogenesis and atherothrombosis. Int J Biochem Cell Biol 42: 1762–1766 [DOI] [PubMed] [Google Scholar]
- Loke W.M., Hodgson J.M., Proudfoot J.M., McKinley A.J., Puddey I.B., Croft K.D. (2008) Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr 88: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Lorenz M., Jochmann N., von Krosigk A., Martus P., Baumann G., Stangl K., et al. (2007) Addition of milk prevents vascular protective effects of tea. Eur Heart J 28: 219–223 [DOI] [PubMed] [Google Scholar]
- MacMahon S., Peto R., Cutler J., Collins R., Sorlie P., Neaton J., et al. (1990) Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 335: 765–774 [DOI] [PubMed] [Google Scholar]
- Manach C., Scalbert A., Morand C., Rémésy C., Jimenez L. (2004) Polyphenols - Food sources and bioavailability. Am J Clin Nutr 79: 727–747 [DOI] [PubMed] [Google Scholar]
- Manach C., Williamson G., Morand C., Scalbert A., Remesy C. (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81(1 Suppl.): 230S–242S [DOI] [PubMed] [Google Scholar]
- Mauray A., Milenkovic D., Besson C., Caccia N., Morand C., Michel F., et al. (2009) Atheroprotective effects of bilberry extracts in apo E-deficient mice. J Agric Food Chem 57: 11106–11111 [DOI] [PubMed] [Google Scholar]
- Mink P.J., Scrafford C.G., Barraj L.M., Harnack L., Hong C.P., Nettleton J.A., et al. (2007) Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 85: 895–909 [DOI] [PubMed] [Google Scholar]
- Mladenka P., Zatloukalova L., Filipsky T., Hrdina R. (2010) Cardiovascular effects of flavonoids are not caused only by direct antioxidant activity. Free Radic Biol Med 49: 963–975 [DOI] [PubMed] [Google Scholar]
- Moline J., Bukharovich I.F., Wolff M.S., Phillips R. (2000) Dietary flavonoids and hypertension: is there a link? Med Hypotheses 55: 306–309 [DOI] [PubMed] [Google Scholar]
- Morand C., Dubray C., Milenkovic D., Lioger D., Martin J.F., Scalbert A., et al. (2011) Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr 93: 73–80 [DOI] [PubMed] [Google Scholar]
- Murphy K.J., Chronopoulos A.K., Singh I., Francis M.A., Moriarty H., Pike M.J., et al. (2003) Dietary flavanols and procyanidin oligomers from cocoa ( Theobroma cacao) inhibit platelet function. Am J Clin Nutr 77: 1466–1473 [DOI] [PubMed] [Google Scholar]
- Nagao T., Hase T., Tokimitsu I. (2007) A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 15: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Naissides M., Pal S., Mamo J.C., James A.P., Dhaliwal S. (2006) The effect of chronic consumption of red wine polyphenols on vascular function in postmenopausal women. Eur J Clin Nutr 60: 740–745 [DOI] [PubMed] [Google Scholar]
- Nantz M.P., Rowe C.A., Bukowski J.F., Percival S.S. (2009) Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition 25: 147–154 [DOI] [PubMed] [Google Scholar]
- Naruszewicz M., Laniewska I., Millo B., Dluzniewski M. (2007) Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infarction (MI). Atherosclerosis 194: e179–e184 [DOI] [PubMed] [Google Scholar]
- Natella F., Nardini M., Belelli F., Pignatelli P., Di Santo S., Ghiselli A., et al. (2008) Effect of coffee drinking on platelets: inhibition of aggregation and phenols incorporation. Br J Nutr 100: 1276–1282 [DOI] [PubMed] [Google Scholar]
- Neveu V., Perez-Jimenez J., Vos F., Crespy V., du Chaffaut L. Mennen, L. et al. (2010) Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database 2010: bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norata G.D., Marchesi P., Passamonti S., Pirillo A., Violi F., Catapano A.L. (2007) Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis 191: 265–271 [DOI] [PubMed] [Google Scholar]
- O’Toole T.E., Conklin D.J., Bhatnagar A. (2008) Environmental risk factors for heart disease. Rev Environ Health 23: 167–202 [DOI] [PubMed] [Google Scholar]
- Opie L.H., Lecour S. (2007) The red wine hypothesis: from concepts to protective signalling molecules. Eur Heart J 28: 1683–1693 [DOI] [PubMed] [Google Scholar]
- Ostertag L.M., O’Kennedy N., Kroon P.A., Duthie G.G., de Roos B. (2010) Impact of dietary polyphenols on human platelet function–a critical review of controlled dietary intervention studies. Mol Nutr Food Res 54: 60–81 [DOI] [PubMed] [Google Scholar]
- Ovaskainen M.L., Torronen R., Koponen J.M., Sinkko H., Hellstrom J., Reinivuo H., et al. (2008) Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr 138: 562–566 [DOI] [PubMed] [Google Scholar]
- Park C.S., Kim W., Woo J.S., Ha S.J., Kang W.Y., Hwang S.H., et al. (2010) Green tea consumption improves endothelial function but not circulating endothelial progenitor cells in patients with chronic renal failure. Int J Cardiol 145: 261–262 [DOI] [PubMed] [Google Scholar]
- Pase M.P., Grima N.A., Sarris J. (2011) The effects of dietary and nutrient interventions on arterial stiffness: a systematic review. Am J Clin Nutr 93: 446–454 [DOI] [PubMed] [Google Scholar]
- Pearson D.A., Paglieroni T.G., Rein D., Wun T., Schramm D.D., Wang J.F., et al. (2002) The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res 106: 191–197 [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez J., Fezeu L., Touvier M., Arnault N., Manach C., Hercberg S., et al. (2011) Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr, 93:1220–1228 [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez J., Neveu V., Vos F., Scalbert A. (2010) Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur J Clin Nutr 64(Suppl. 3): S112–S120 [DOI] [PubMed] [Google Scholar]
- Polagruto J.A., Gross H.B., Kamangar F., Kosuna K., Sun B., Fujii H., et al. (2007) Platelet reactivity in male smokers following the acute consumption of a flavanol-rich grapeseed extract. J Med Food 10: 725–730 [DOI] [PubMed] [Google Scholar]
- Ras R.T., Zock P.L., Draijer R. (2011) Tea consumption enhances endothelial-dependent vasodilation; a meta-analysis. PLoS One 6: e16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza S., Muniyappa R., Iantorno M., Kim J.A., Chen H., Pullikotil P., et al. (2011) Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab 96: E782–E792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger V.L., Go A.S., Lloyd-Jones D.M., Adams R.J., Berry J.D., Brown T.M., et al. (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123(4): e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel G., Pomerleau S., Couture P., Lemieux S., Lamarche B., Couillard C. (2006) Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br J Nutr 96: 357–364 [DOI] [PubMed] [Google Scholar]
- Saleem T.S., Basha S.D. (2010) Red wine: A drink to your heart. J Cardiovasc Dis Res 1: 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalbert A., Johnson I.T., Saltmarsh M. (2005) Polyphenols: antioxidants and beyond. Am J Clin Nutr 81(1 Suppl.): 215S–217S [DOI] [PubMed] [Google Scholar]
- Schewe T., Steffen Y., Sies H. (2008) How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys 476: 102–106 [DOI] [PubMed] [Google Scholar]
- Schroeter H., Heiss C., Balzer J., Kleinbongard P., Keen C.L., Hollenberg N.K., et al. (2006) (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A 103: 1024–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter H., Heiss C., Spencer J.P., Keen C.L., Lupton J.R., Schmitz H.H. (2010) Recommending flavanols and procyanidins for cardiovascular health: current knowledge and future needs. Mol Aspects Med 31: 546–557 [DOI] [PubMed] [Google Scholar]
- Stein J.H., Keevil J.G., Wiebe D.A., Aeschlimann S., Folts J.D. (1999) Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 100: 1050–1055 [DOI] [PubMed] [Google Scholar]
- Steptoe A., Gibson E.L., Vuononvirta R., Hamer M., Wardle J., Rycroft J.A., et al. (2007) The effects of chronic tea intake on platelet activation and inflammation: a double-blind placebo controlled trial. Atherosclerosis 193: 277–282 [DOI] [PubMed] [Google Scholar]
- Stowe C.B. (2011) The effects of pomegranate juice consumption on blood pressure and cardiovascular health. Complement Ther Clin Pract 17: 113–115 [DOI] [PubMed] [Google Scholar]
- Szmitko P.E., Verma S. (2005) Cardiology patient pages. Red wine and your heart. Circulation 111(2): e10–e11 [DOI] [PubMed] [Google Scholar]
- Taubert D., Roesen R., Lehmann C., Jung N., Schomig E. (2007a) Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA 298: 49–60 [DOI] [PubMed] [Google Scholar]
- Taubert D., Roesen R., Schomig E. (2007b) Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med 167: 626–634 [DOI] [PubMed] [Google Scholar]
- Teragawa H., Higashi Y., Kihara Y. (2008) Effect of isoflavone supplement on endothelial function: does efficacy vary with atherosclerotic burden? Eur Heart J 29: 2710–2712 [DOI] [PubMed] [Google Scholar]
- Terao J., Kawai Y., Murota K. (2008) Vegetable flavonoids and cardiovascular disease. Asia Pac J Clin Nutr 17(Suppl. 1): 291–293 [PubMed] [Google Scholar]
- Thijssen D.H., Black M.A., Pyke K.E., Padilla J., Atkinson G., Harris R.A., et al. (2011) Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokede O.A., Gaziano J.M., Djousse L. (2011) Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr, 65: 879–886 [DOI] [PubMed] [Google Scholar]
- Tsompanidi E.M., Brinkmeier M.S., Fotiadou E.H., Giakoumi S.M., Kypreos K.E. (2010) HDL biogenesis and functions: role of HDL quality and quantity in atherosclerosis. Atherosclerosis 208: 3–9 [DOI] [PubMed] [Google Scholar]
- Vanhoutte P.M. (2009) Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J 73: 595–601 [DOI] [PubMed] [Google Scholar]
- Vita J.A. (2005) Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr 81(1 Suppl.): 292S–297S [DOI] [PubMed] [Google Scholar]
- Vivekananthan D.P., Penn M.S., Sapp S.K., Hsu A., Topol E.J. (2003) Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361: 2017–2023 [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Aznaouridis K., Alexopoulos N., Economou E., Andreadou I., Stefanadis C. (2005) Effect of dark chocolate on arterial function in healthy individuals. Am J Hypertens 18: 785–791 [DOI] [PubMed] [Google Scholar]
- Wang-Polagruto J.F., Villablanca A.C., Polagruto J.A., Lee L., Holt R.R., Schrader H.R., et al. (2006) Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J Cardiovasc Pharmacol 47(Suppl. 2): S177-S186; discussion S206–S179 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Arai Y., Mitsui Y., Kusaura T., Okawa W., Kajihara Y., et al. (2006) The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin Exp Hypertens 28: 439–449 [DOI] [PubMed] [Google Scholar]
- Wheatcroft S., Noronha B., Kearney M. (2005) Heart and blood vessels. In: Gibney M.J., Elia M., Ljunqvist O. (eds), Clinical Nutrition. Oxford: Blackwell Science [Google Scholar]
- Whelton P.K., He J., Appel L.J., Cutler J.A., Havas S., Kotchen T.A., et al. (2002) Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 288: 1882–1888 [DOI] [PubMed] [Google Scholar]
- Widlansky M.E., Hamburg N.M., Anter E., Holbrook M., Kahn D.F., Elliott J.G., et al. (2007) Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr 26: 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.H., Howe P.R., Buckley J.D., Coates A.M., Kunz I., Berry N.M. (2010) Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr Metab Cardiovasc Dis, 21: 851–856 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2008) The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization; Available at: http://whqlibdoc.who.int/publications/2008/9789241563710_eng.pdf [Google Scholar]
- Zilkens R.R., Burke V., Hodgson J.M., Barden A., Beilin L.J., Puddey I.B. (2005) Red wine and beer elevate blood pressure in normotensive men. Hypertension 45: 874–879 [DOI] [PubMed] [Google Scholar]
- Zuchi C., Ambrosio G., Luscher T.F., Landmesser U. (2010) Nutraceuticals in cardiovascular prevention: lessons from studies on endothelial function. Cardiovasc Ther 28: 187–201 [DOI] [PubMed] [Google Scholar]



