Abstract
Purpose
To determine in-vivo effects of modifications to core and epitendinous suture techniques in a canine intrasynovial flexor tendon repair model using clinically relevant rehabilitation. Our null hypothesis was that gap formation and rupture rates would remain consistent across repair techniques.
Methods
We evaluated gap formation and rupture in 75 adult mongrel dogs that underwent repair of intrasynovial flexor tendon lacerations followed by standardized post-operative therapy. The current suture technique was a 4-0, 8-strand core suture with a purchase of 1.2 cm and a 5-0, epitendinous suture repair with a 2 mm purchase length and depth. Gap and failure were compared by chi-squared analysis to a historical group of in-vivo repairs (n=76) from the same canine model using 8-strand core suture repair with purchase of 0.75 cm and 6-0 epitendinous suture with a 1 mm purchase length and depth.
Results
Ninety-three percent of tendons (n = 70) demonstrated gapping of <3 mm using the current suture technique. Five percent of tendons (n = 4) had a gap of 3 mm or greater, and there was 1 repair site failure. This was significantly improved over the comparison group of historical 8-strand core repair technique which resulted in 82% (n = 62) and 81% (n=112) of repairs with a gap of <3 mm and 7 failures (9%).
Conclusions
In an in-vivo model, current modifications of suture techniques for intrasynovial flexor tendon repair demonstrated significant improvements in gap formation and rupture compared to a similar technique using shorter purchase lengths and shallower purchase depth.
Clinical Relevance
Suggested repair modifications for the treatment of Zone 2 flexor tendon transections demonstrate improvements in gap formation and tendon rupture in-vivo.
Keywords: Tendon, repair, intrasynovial, zone 2, suture
INTRODUCTION
Over the last 3 decades, experimental studies have demonstrated conclusively that early, protected postoperative digital motion is a critical factor to achieving adhesion free intrasynovial tendon repair and improved functional outcomes. Tempering the enthusiasm for early motion, however, is the observation that repair site deformation occurs regularly between the tendon stumps in the first 3 to 4 weeks following repair and that gap formation greater than 3 mm can prevent healing tendons from accruing tensile strength, contributing to catastrophic repair site failure [1-4]. Multi-strand repairs combined with early post operative mobilization protocols significantly improve the tensile properties of repair, with 6- and 8- strand constructs providing substantially higher values for ultimate failure load compared to 2-strand or 4-strand [5]. Increasing the caliber of core suture [6] and the depth and purchase of the epitendinous suture [7] has been shown to have a substantial positive effect on the tensile properties of the repair in ex-vivo models.
Over the past 15 years, we have consistently used a clinically relevant canine in-vivo model for flexor tendon repair research [5, 15-18]. During this time, technical advances associated with greatest capacity to reduce gap formation have been identified, including increasing the number of strands crossing the repair site, the length of core suture purchase, suture caliber, and the configuration of the epitendinous suture [15]. In response to these advances, we have modified our tendon repair techniques and now have 6 years of experience with an 8-strand core suture method with a 1.2cm core suture purchase technique and a modified 2mm deep epitendinous suture in a series of intrasynovial flexor tendon repairs [9-12, 15]. As most prior studies demonstrating the biomechanical advantages of these modifications have been performed in ex-vivo models [2, 6, 7, 16-18] we recognized the need to evaluate the effect of such modifications in-vivo. Therefore, we performed the current investigation to evaluate the gap formation and rupture rates of tendons repaired by this current suture technique in an established animal model treated with early post-operative controlled mobilization.
Our primary objective was to determine whether or not the implementation of modified suture repair methods had a positive effect on gap formation and repair rupture rates in-vivo. To accomplish this aim, we compared gap formation and rupture using current repair methods to an internal historical control set of tendons from the same animal model repaired with an 8-strand core suture method that used a shallower core and epitendinous suture. Our null hypothesis was that gap formation and rupture rate would remain consistent across repair techniques.
MATERIALS AND METHODS
Seventy-five canine flexor tendons from 75 adult mongrel dogs were examined. These tendons were repaired by our current suture technique described below. Gap formation and rupture rates of these tendons were compared to a group of internal historical controls which used shallower core and epitendinous suture techniques. The same post-operative rehabilitation protocol was used for both the current and historical control tendon repairs. All studies were approved by the (Blinded for Review) Animal Studies Committee. The historical comparisons consisted of 76 tendons reported previously in studies examining the effect of postoperative mobilization protocols on tendon repair tensile strength [13]. The 75 tendons examined in this study all served as control specimens for experimental tendon repairs that investigated the effect of repair site augmentation with growth factors [9-12]. As control specimens, the tendons did not have any growth factor treatment.
Animal Model and Current Surgical Technique
Since 1995, we have used a canine digital flexor tendon model due to the similarity of the canine flexor tendon structures to those of humans [8]. We used adult mongrel dogs (Covance, Denver, Pennsylvania) weighing 20-30 kg in all cases. Animals are maintained in a licensed animal-care facility. For the surgical procedure, the animals were anesthetized with an initial intravenous dose of thiopental sodium (0.5 mL/kg) supplemented by intermittent injections of atropine (0.5 mL) and acepromazine (0.2 mL). They were intubated and maintained on 1% halothane or isofluorane anesthesia. The forelimb was shaved and washed with povidone-iodine. The forelimb was exsanguinated, and a tourniquet was applied to the forelimb.
Each forelimb (n=75) underwent a digital flexor tendon repair in the second and fifth digits. We selected the second and fifth digits due to ease and reproducibility of the surgical approach to the border digits. One repair served as the control and the other as the experimental repair. The control repairs were the specimens reported in the present study. The control tendon was chosen by consecutively alternating between the second and fifth digits to ensure an equal distribution. The sheath of the second or fifth digit was approached through a midlateral incision in the region between the annular pulleys proximal and distal to the proximal interphalangeal joint. The tendon was cut sharply in a transverse fashion using a scalpel blade at the level of the proximal interphalangeal joint. In 59 of 75 cases, due to standardization of the control tendon with the experimental tendon, a small defect, oriented transversely to the tendon and within the free tendon stumps, was created using the entire width of a 64-blade scalpel for standardization with the repair taking place in the other tendon of the same forelimb [11]. No trough was created in the other 16 tendons. Tendon repair was then immediately performed using the Winters-Gelberman core suture technique [5], which employs an 8-strand core-suture technique consisting of 2 orthogonally placed, 4-strand repairs with 1.2cm purchase length performed using a continuous loop of 4-0 Supramid suture (4-0 Supramid; S. Jackson, Alexandria, VA) (Figure 1). Following core suture repair, a 5-0 Prolene suture (Ethicon, Sommerville, NJ) was used in a continuous, non-locking circumferential fashion as an epitendinous repair. Epitendinous suture was placed to capture a 2mm purchase from the tendon edge and achieve a depth of 2mm on each pass. The flexor sheaths were not repaired.
Figure 1.
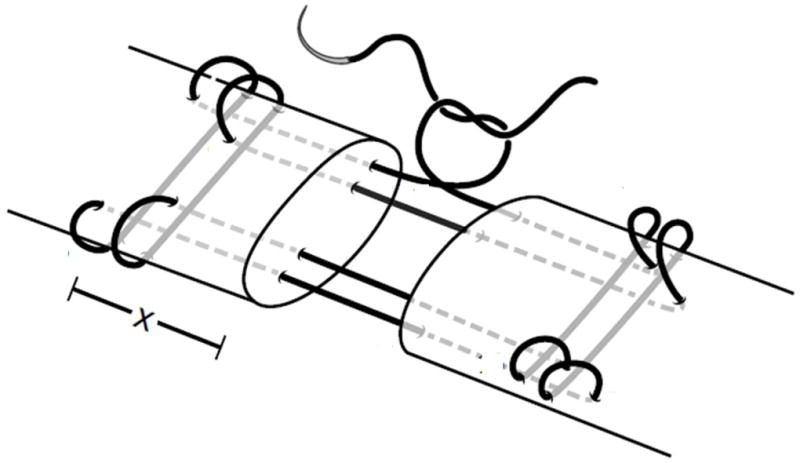
Core suture repair technique. The current 8-strand suture repair technique used 4-0 caliber looped suture where x = 1.2 cm. Historical 8-strand suture repair technique used 4-0 looped caliber suture where x = 0.75 cm. The solid line depicts a looped suture, and therefore this is an 8-strand repair.
For purposes of previous investigations, the other tendon (either the second or fifth in the same forelimb) was repaired in a similar fashion, but with the use of a local growth factor delivery system. The details of these studies have been previously described [9-12], and growth factor effect and administration was localized to the experimental digit. The results of these experimental tendons are not presented in this study.
Historical Surgical Techniques
The tendons examined as historical controls were prepared for tendon repair in identical fashion. In a total of 76 tendons, the core and epitendinous repairs were performed as described above with the exception of a 0.75 cm purchase length for the core suture and a 1 mm purchase length and depth on the running epitendinous suture. The flexor sheaths were not repaired.
Postoperative Care and Rehabilitation
After surgery, the operative forelimb was immobilized with the use of a fiberglass shoulder spica cast with the elbow flexed to 90 degrees and the wrist flexed to 70 degrees. The distal ends of the casts were removable to allow for controlled passive mobilization during two 5-minute rehabilitation sessions performed 5 days a week starting on the first postoperative day [12].
Evaluation of Gap Formation
In both the historical and current cohorts, dogs were killed at designated time points (5, 7, 10, 14, 21, or 42 days) after repair. These time points were selected based on the purposes of each study for which the tendons served as a control, but all were designed to evaluate early stages of flexor tendon healing. At the time of sacrifice, the operative forelimb was disarticulated at the elbow and frozen until gap measurement. The limbs were thawed to room temperature and the experimental digit was disarticulated at the metacarpophalangeal joint. The flexor digitorum profundus tendons were transected proximally at the musculotendinous junction. Each repair site was exposed through identical midlateral incisions. The repaired tendon was removed from the surrounding tendon sheath to allow for measurement of gap formation. Gaps were measured using a digital caliper (Max-Cal; Fowler, Newton, MA). Measurements were recorded to the nearest tenth of a millimeter. Consistent with prior investigations, we defined repair failures as cases of complete repair discontinuity or gap formation > 8 mm. All surgeries, tendon harvests, and measurements in the current technique cohort were made by the same experienced hand surgeon (RHG). For the historical surgical technique, surgeries were performed either by the senior author or by one of 2 other experienced hand surgeons who performed all initial surgeries under direct supervision of the senior author and only became independent when surgical technique was deemed technically equivalent.
Statistical Analysis
Descriptive statistics were produced to characterize our set of tendons repaired with current methods. A chi square test was used to compare rates of acceptable and unacceptable repair between repair techniques using 3mm of gap formation as the cut-off [2]. We did not perform an a priori power analysis but predicted that a 10 percent difference in gap formation of <3mm or 50% reduction in the rupture rate would represent a clinically substantial improvement between repair groups.
RESULTS
In our sample of 75 tendons repaired with a current 8-strand technique, there was 1 tendon gap > 8mm, meeting criteria for repair site failure (1%). The distribution of gap formation is presented in Figure 2. Overall, 70/75 tendons (93%) demonstrated gap formation <3mm. This rate was significantly (P=0.047) improved over historical control 8-strand repair tendons in which 82% (62/76) maintained acceptable levels of gapping (Table 1). When examining repair site failures (complete discontinuity or >8 mm gapping), the historical 8-strand repairs failed more frequently with a rate of 9% (7/76). Time to harvest was distributed in a statistically similar manner across repair techniques for current and historical 8-strand (chi square P = 0.7).
Figure 2.
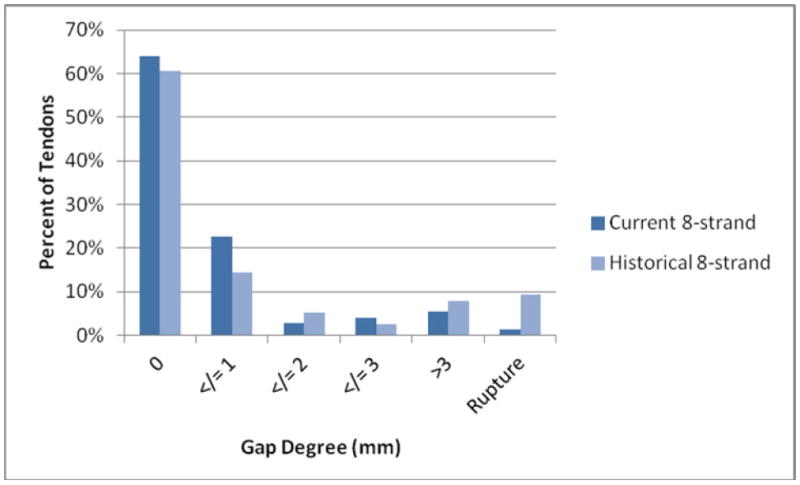
Distribution of gap formation among tendons with current repair technique.
Table 1.
Gapping according to repair technique
| Repair | Gap <3mm | Gap >3mm | Failure |
|---|---|---|---|
| Current 8-strand | 70/75 (93%)* | 4 (5%) | 1 (1%) |
| Historical 8-strand | 62/76 (82%) | 7 (9%) | 7 (9%) |
P = 0.047 compared to historical 8-strand.
DISCUSSION
Ex-vivo experimental studies have demonstrated improvements in the tensile properties of repaired flexor tendons with the implementation of specific modifications to suture technique. Tang et al. showed that progressively increasing core suture purchase from 0.4 to 1.2 mm led to heightened resistance to gap formation and increased ultimate strength [16]. Diao et al. demonstrated that evolving from a superficial to a deeper penetration of the epitendinous suture provided an increase in mean load to failure of repaired intrasynovial tendons [17]. Recently, Nelson et al. studied the combined effect of these previously reported technical modifications ex-vivo [15]. They demonstrated that the factors having the most significant effect on repair strength were the number of core strands extending across the repair site and the length of epitendinous suture purchase. Core suture purchase and epitendinous suture caliber had the smallest relative effect on repair site tensile properties. These studies provided the rationale behind our repair technique modifications over time and prompted our interest in examining the effects of these suture modifications in-vivo in the current study.
In historical experiments from our laboratory, an 8-strand flexor tendon repair technique was used with a 0.75cm core suture purchase and a superficial invagination of free tendon ends by an epitenon suture. Gaps less than 3mm were achieved in 82% of tendons. The rate of gap formation <3mm observed in the tendons following current suture techniques (93%) demonstrated superior reduction in gap formation. Comparison of these data suggests that the modifications of extended core suture purchase (from 0.75 cm to 1.2 cm) and deeper epitendinous suture repair improved in-vivo repairs by reducing incidence of gap formation and tendon rupture.
We previously demonstrated that tendons will uniformly accrue tensile strength as expected over time if the gap remains <3mm [2]. While smaller gaps (<3mm) likely have a detrimental effect on tendon repair characteristics, we do not know the natural history of the small gaps seen in this study. It has not been proven, either biomechanically or clinically, that such small gap formation has substantial effects on repair strength. In distinction, gaps over 3 mm have been documented to prevent tensile strength accumulation in the first 6 weeks and thus represent a critical condition in which tendon healing is impaired. In the presence of 3 mm gap formation, tendon repairs are presumed to remain dependent on suture properties and remain at risk for delayed rupture.
As we introduced 3 modifications to the experimental repair technique, ascribing differences in the rates of gap formation and rupture to any single factor, such as the depth of the epitenon suture, is not possible. In an ex-vivo study, Kim et al. described the modifications to the 8-strand Winters-Gelberman repair which included: 1) increasing core suture purchase from 0.75 cm to 1.2cm, 2) use of a 5-0 as opposed to 6-0 Prolene epitendinous suture, and 3) anatomic epitendinous repair using a 2 mm purchase and 2 mm deep stitch [14]. Each of these modifications was implemented in the current study. Kim’s study suggested that the increased epitenon suture depth and purchase would likely be the most important of the modifications. Increasing suture purchase, to a degree, necessarily increases depth. While it is technically challenging to functionally measure a circumferential epitendinous stitch with a 2 mm depth in small tendons in-vivo, the 2 mm purchase would likely be reproducible.
A limitation inherent to our study is that this investigational cohort was not originally designed to answer our current hypothesis. The animals used in this study were initially analyzed to answer separate but related research questions. Thus, our data were collected prospectively but examined retrospectively to evaluate a clinical hypothesis based on a growing body of ex-vivo data. While this approach is not typical in basic science research, it is similar to clinical investigations conducted on administrative datasets or registry data that capitalize on existing data for novel hypothesis testing. Acknowledging this limitation, we also note several strengths of our data.
First, the current flexor tendon repairs were all performed by a single surgeon who was experienced with the in-vivo canine model. Although 2 other surgeons performed some of the surgeries in the historical control group, each of the 2 surgeons were directly trained by the senior author. In this costly, large animal model, our protocols were designed to allow for meaningful biomechanical comparison. Towards that end, every effort was made to ensure that the technical standards of tendon repair and gap measurement remain consistent over time. Second, the current tendon repairs analyzed in this study, which served as controls in prior studies, were not previously reported in regard to gap formation and repair site failure. Thus the data presented from these tendons following current repair technique have not been previously published. Third, the opportunity to study the combined effect of several recent modifications to flexor tendon repair in an animal model is quite valuable. While prior efforts have been made to assess these modifications ex-vivo, this study now quantifies the effect of such modifications in a clinically relevant, large animal in-vivo model.
While advances in repair technique were demonstrated to be beneficial in our in-vivo examination, gap formation and repair rupture have not been completely eliminated. This finding suggests that there may be other factors contributing to gap formation and repair site failure that have not been fully addressed by these technical modifications. In our experimental protocol, we did not over-tension our repairs but instead attempted to obtain neutral tension at the repair site. Some reports have investigated other factors contributing to repair site failure including tensioning of the core suture and resistance to flexor tendon excursion caused by post-operative edema [18, 19, 20]. Our investigation did not examine these factors and future in-vivo studies may benefit from investigation of the effect of these factors on flexor tendon repair characteristics.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) R01-AR062947 and the Washington University Musculoskeletal Research Center (NIH P30-AR057235)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hagberg L, Selvik G. Tendon excursion and dehiscence during early controlled mobilization after flexor tendon repair in zone II: An x-ray stereophotogrammetric analysis. J Hand Surg. 1991;16A:669–680. doi: 10.1016/0363-5023(91)90193-f. [DOI] [PubMed] [Google Scholar]
- 2.Gelberman RH, Boyer MI, Brodt MD, Winters SC, Sliva MJ. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. J Bone Joint Surg. 1999;81A:975–982. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Lindsay WK, Thomson HG, Walker FG. Digital flexor tendon: An experimental study: part II: The significance of a gap occurring at the line of suture. Br J Plast Surg. 1960;12:289–316. doi: 10.1016/s0007-1226(60)80003-3. [DOI] [PubMed] [Google Scholar]
- 4.Aoki M, Kubota H, Pruitt DL, Manske PR. Biomechanical and histological characteristics of canine flexor tendon repair using early postoperative mobilization. J Hand Surg. 1997;22A:107–114. doi: 10.1016/S0363-5023(05)80189-3. [DOI] [PubMed] [Google Scholar]
- 5.Winters SC, Gelberman RH, Woo SL, Chan SS, Grewal R, Seiler JG., III The effects of multiple strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg. 1998;23A:97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- 6.Taras JS, Raphael JS, Marczyk SC, Bauerle WB. Evaluation of suture caliber in flexor tendon repair. J Hand Surg. 2001;26A:1100–1104. doi: 10.1053/jhsu.2001.28946. [DOI] [PubMed] [Google Scholar]
- 7.Diao E, Hariharan JS, Soejima O, Lotz JC. Effect of peripheral suture depth on strength of flexor tendon repairs. J Hand Surg. 1996;21A:234–239. doi: 10.1016/S0363-5023(96)80106-7. [DOI] [PubMed] [Google Scholar]
- 8.Potenza AD. Tendon healing within the flexor digital sheath in the dog. J Bone and Joint Surg. 1962;44A:49–64. [PubMed] [Google Scholar]
- 9.Thomopoulos S, Zaegel M, Das R, Harwood FL, Silva MJ, Amiel D, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–1368. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 10.Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27:1209–1215. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomopoulos S, Kim M, Das D, Silva MJ, Sakiyama-Elbert S, Amiel D, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg. 2010;92A:2285–2293. doi: 10.2106/JBJS.I.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelberman RH, Thomopoulos S, Sakiyama-Elber SE, Das R, Silva M. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: An in-vivo biomechanical study at 3 weeks in canines. J Hand Surg. 2007;32A:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair: An experimental study comparing low and high levels of in-vivo force during rehabilitation in canines. J Bone Joint Surg. 2001;83A:891–899. [PubMed] [Google Scholar]
- 14.Kim HM, Nelson G, Thomopoulos S, Silva MJ, Das R, Gelberman RH. Technical and biological modifications for enhanced flexor tendon repair. J Hand Surg. 2010;35A:1031–1037. doi: 10.1016/j.jhsa.2009.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson GN, Potter RM, Ntouvali E, Silva MJ, Boyer MI, Gelberman RH, et al. Intrasynovial flexor tendon repair: A biomechanical study of variations in suture application in human cadavera. J Orthop Res. 2012 Mar 27; doi: 10.1002/jor.22108. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang JB, Zhang Y, Cao Y, Xie RG. Core suture purchase affects strength of tendon repairs. J Hand Surg. 2005;30A:1262–1266. doi: 10.1016/j.jhsa.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Diao E, Harihara JS, Soejima O, Lotz JC. Effect of peripheral suture depth on strength of tendon repairs. J Hand Surg. 1996;21A:234–239. doi: 10.1016/S0363-5023(96)80106-7. [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Zhao C, Amadio PC, Tanaka T, Zhao KD, An KN. Total and intrasynovial work of flexion of human cadaver flexor digitorum profundus tendons after modified Kessler and MGH repair techniques. J Hand Surg. 2005;30A:466–470. doi: 10.1016/j.jhsa.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Zhao C, Amadio PC, Paillard P, Tanaka T, Zobitz ME, Larson DR, An KD. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in-vivo. J Bone Joint Surg. 2004;86A:320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Wu YF, Tang JB. Effects of tension across the tendon repair site on tendon gap and ultimate strength. J Hand Surgery. 2012;37A:906–912. doi: 10.1016/j.jhsa.2012.01.004. [DOI] [PubMed] [Google Scholar]


