Abstract
To provide site-specific delivery and targeted release of growth factors to implanted urine-derived stem cells (USCs), we prepared microbeads of alginate containing growth factors. The growth factors included VEGF, IGF-1, FGF-1, PDGF, HGF and NGF. Radiolabeled growth factors were loaded separately and used to access the in vitro release from the microbeads with a gamma counter over 4 weeks. In vitro endothelial differentiation of USCs by the released VEGF from the microbeads in a separate experiment confirmed that the released growth factors from the microbeads were bioactive. USCs and microbeads were mixed with the collagen gel type 1 (2 mg/ml) and used for in vivo studies through subcutaneous injection into nude mice. Four weeks after subcutaneous injection, we found that grafted cell survival was improved and more cells expressed myogenic and endothelial cell transcripts and markers compared to controls. More vessel formation and innervations were observed in USCs combined with six growth factors cocktail incorporated in microbeads compared to controls. In conclusion, a combination of growth factors released locally from the alginate microbeads induced USCs to differentiate into a myogenic lineage, enhanced revascularization and innervation, and stimulated resident cell growth in vivo. This approach could potentially be used for cell therapy in the treatment of stress urinary incontinence.
Keywords: Drug delivery, Stem cells, Alginate microbeads, Controlled release, Growth factors
1. Introduction
Stress urinary incontinence (SUI) is most common in people older than 50 years of age; these are primarily women, but there are an increasing number of male patients as well [1, 2]. Urinary incontinence affects up to 13 million people in the United States and 200 million worldwide. The cost of treating urinary incontinence in United States alone is $16.3 billion annually [3]. SUI is associated with the loss of various amounts of urine when intra-abdominal pressure increases due to dysfunction of the urethral sphincter or the pelvic floor muscles. Besides pharmacotherapy [4], several invasive surgical therapies, including sling surgical procedures [5] and injection of bulking agents [6], have been commonly used to treat SUI. Sub-urethral slings, such as the transvaginal or transobturator tape procedures, have about 71 to 72.9% success rates [5]. Although the sling procedure can enforce the weakness of pelvic floor muscles, the urethral sphincter deficiency remains [7]. Bulking procedures are particularly useful for treating SUI in patients who wish to avoid open surgical procedures [6]. A variety of biomaterials, such as bovine collagen [8], calcium hydroxyapatite, silicone [9], carbon beads [10] polydimethylsiloxane (Macroplastique), and polytetrafluoroethylene (PTFE; Teflon) [11], have been used to insert bulk around the urethra and thereby raise its outlet resistance. This provides closure of the sphincter without obstructing it, and is most effective in patients with a relatively fixed urethra. Although injection of bulking agents has provided encouraging outcomes, over time these agents are absorbed and can cause several complications, such aschronic inflammation, periurethral abscess, foreign body giant cell responses, erosion of the urinary bladder or the urethra, migration to inner organs, obstruction of the lower urinary tract with resultant urinary retention, severe voiding dysfunction, and even pulmonary embolism [6, 12–14].
Cell-based therapy is an alternative to restore deficient urethral sphincter function in the treatment of SUI. Several investigations have focused on autologous stem cells derived from skeletal muscle [15], bone marrow [16] or fat tissues [17], with success rates ranging from 12 to 79 % [18]. To obtain these stem cells, invasive tissue biopsy procedures are usually involved, with an attendant risk of complications. We recently demonstrated that stem cells exist in human voided urine or urine drained from upper urinary tract. These cells, termed urine-derived stem cells (USCs), possess stem cell characteristics with robust proliferative potential and multi-potential differentiation [19–21]. These cells can be obtained using simple, safe, non-invasive and low-cost procedures, thus avoiding the adverse events associated with obtaining cells from other sources. Our recent studies demonstrated that adding exogenous angiogenic factors, such as transfection of the VEGF gene, significantly promoted myogenic differentiation of USCs and induced angiogenesis and innervation. However, VEGF delivered by virus caused several side effects in our animal model, including hyperemia, hemorrhage, and even animal death [22]. Thus, it is desirable to employ a safer approach in stem cell therapy to increase angiogenesis and promote muscle regeneration.
Biodegradable polymers, specifically hydrogels that deliver molecules in a controlled fashion, can be beneficial as delivery vehicles to promote regeneration and tissue healing [23]. Alginate is one of the most commonly-used natural hydrogels as an aqueous drug carrier for encapsulation because of its mild gelling conditions and tunable microbead characteristics. Since alginate is a hydrophilic and negatively-charged polymer, alginate microspheres also resist protein adsorption thus making them attractive for in vivo studies [24]. Alginate microbeads have been shown to stably release active FGF-1 for at least 3 weeks in vitro, and this sustained release of FGF-1 promoted neovascularization in vivo without any side effects [25–27].
Our more recent data showed that USCs display myogenic and endothelial differentiation capacity when cultured in media containing the associated growth factors [28, 29]. Our hypothesis was that skeletal myogenic, anigogenic, and neurogenic growth factors released from alginate microbeads can induce USCs to give rise to a skeletal myogenic lineage, improve revascularization and innervations, and recruit resident cells to take part in tissue repair. Therefore, in the present study, we examined whether a synergistic mixture of growth factors could be released efficiently in a controlled manner from alginate microbeads, thus guiding USCs to cell differentiation and enhancing tissue regeneration for potential use in cell therapy of SUI.
2. Materials and Methods
2.1 Preparation of alginate microbeads
A low-viscosity (<20 m Pas) ultrapure alginate with high guluronic acid (LVG) content (minimum 60% guluronate monomer units) was used for this study (Nova Matrix, Sandvika, Norway). LVG (1.5 wt %) was prepared in calcium free minimum essential medium (MEM) and stored at 4°C till further use. The LVG microbeads were generated using an eight nozzle flow-focusing device at the flow rate of 1.4 ml/min and 1.5 psi air pressure. These microbeads were collected in a calcium chloride solution (1.1 wt %) and allowed to cross-link for 15 min. These microbeads were washed three times with calcium containing Hank’s buffered salt solution (HBSS). The amounts of growth factors to be loaded in alginate beads were determined according to the effective dose (ED 50) provided by the manufacturer. A solution of 100 ug/ml PDGF-BB (4 µg) and 100 ug/ml HGF (10 µg) served as a skeletal myogenic promoter; 100 ug/ml VEGF (7 µg) as the angiogenesis inducer; and a combination of 1 mg/ml IGF (14 µg), 10 ug/ml NGF (0.5 µg), 300 ug/ml FGF-1 (1 ug) to promote innervation. Five units/ml heparin was added to the initial growth factor solutions. To preload the microbeads with growth factors, about 0.5 g of capsules was incubated overnight (24 h) with 0.5 ml of growth factor solutions in an Eppendorf tube on a shaker at 4°C. The supernatant was removed and the microbeads were washed three times with HBSS (with Ca2+) to remove non-incorporated growth factors. To control the release of growth factors from the microbeads we coated a semi-permeable membrane of poly-L-ornithine (PLO). Just washed growth factor loaded microbeads were incubated in 0.1 wt% PLO solution in HBSS (with Ca2+) for 10 min at 4°C followed by triple wash. Finally we incubated the microbeads in 0.2 wt% ultrapure alginate with high mannuronic acid (LVM, minimum 60% mannuronate monomer units) for 5 min at 4°C followed by triple wash, to get alginate-PLO-alginate (APA) growth factor loaded microbeads. These will be addressed as just alginate microbeads throughout this manuscript. Each growth factor mixture was decreased to one-third of the original amount when these three parts were combined, to document synergistic effects (Table 1).
Table 1.
Research Design
| Groups (G) | Function groups | Injections of Cell–Microsphere Beads in Collagen I gel |
Doses of growth factors/injection | Number of Grafts/ Number of Animals |
|---|---|---|---|---|
| G1 | Control 1 | Cell-free/empty beads | 0 | 8/2 |
| G2 | Control 2 | Human Myoblasts/empty beads | 0 | 8/2 |
| G3 | Control 3 | USCs/empty beads | 0 | 8/2 |
| G4 | Control 4 | USCs-EC/empty beads | 0 | 8/2 |
| G5 | Control 5 | Cell-free combine with IGF/NGF/FGF-1+VEGF+PDGF/HGF beads | IGF (43.75ng), NGF(7.8 ng), FGF-1(15.625ng), VEGF (21.875 ng), PDGF-BB (12.5ng), HGF (62.5ng) | 8/2 |
| G6 | Myogenic | USCs combine with PDGF-BB/HGF beads | PDGF-BB (37.5ng), HGF (187.5ng) | 8/2 |
| G7 | Angiogenic | USCs combine with VEGF beads | VEGF (65.6 ng) | 8/2 |
| G8 | Neurogenic | USCs combine with IGF/NGF/FGF-1 beads | IGF (131.25ng), NGF(23.44 ng), FGF-1(46.875ng) | 8/2 |
| G9 | Synergetic 1 | USCs combine with IGF/NGF/FGF-1 + VEGF + PDGF/HGF beads | IGF (43.75ng), NGF(7.8 ng), FGF-1(15.625ng), VEGF (21.875 ng), PDGF-BB (12.5ng), HGF (62.5ng) | 16/4 |
| G10 | Synergetic 2 | USCs-ECs combine with IGF/NGF/FGF-1 + VEGF+ PDGF/HGF beads | IGF (43.75ng), NGF(7.8 ng), FGF-1(15.625ng), VEGF (21.875 ng), PDGF-BB (12.5ng), HGF (62.5ng) | 16/4 |
2.2 Measurement of growth factor release in vitro
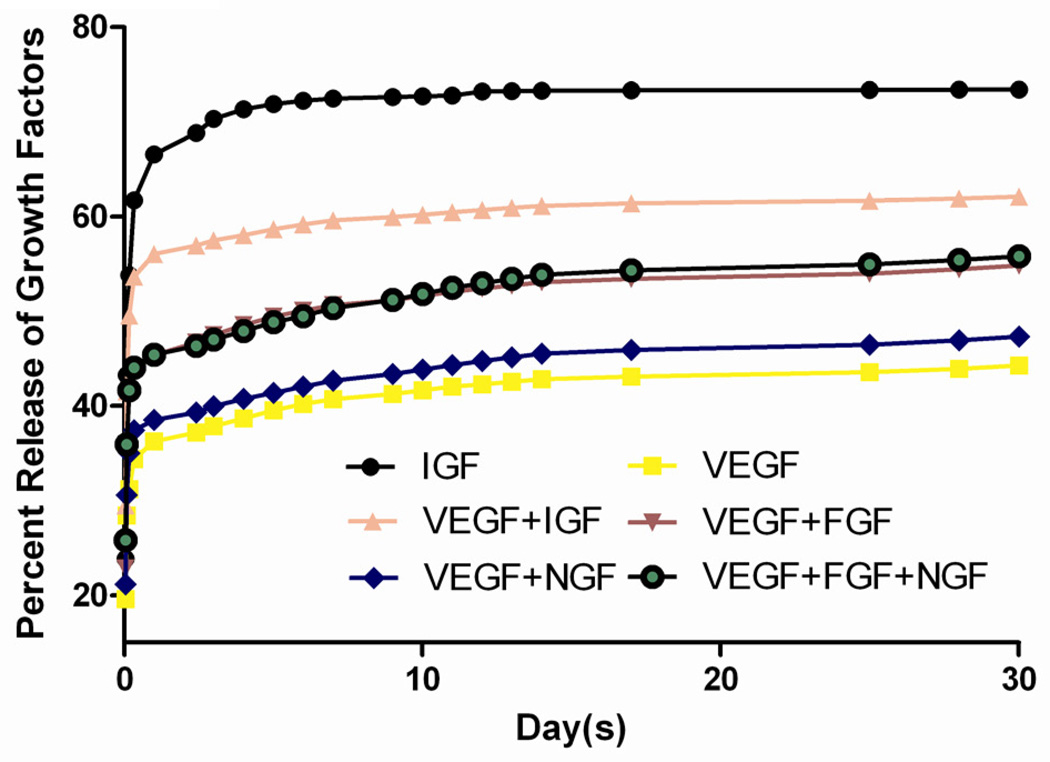
The growth factor release efficiency was evaluated in vitro when single, bi- or multi- combined growth factors were loaded within alginate microbeads. I-125 labeled (VEGF and IGF, Phoenix Pharmaceuticals, Inc.) and unlabeled NGF and FGF-1 (Protech) growth factors were loaded in the microbeads to investigate the in vitro release of growth factors. To measure the release kinetics of I-125-labeled growth factors incorporated in alginate microbeads, the microbeads were suspended in 0.5 ml of HBSS (with Ca2+) and incubated at 37°C. The supernatant was replaced fully at pre-determined time points (Fig. 1) and radioactivity between two consecutive time points was read in a gamma counter (Model 2470, PerkinElmer) to determine the growth factor release. Counts per minute (CPM) were measured and corrected for radioactivity decay.
Fig. 1. Controllable release curve of alginate beads in vitro.
The microbeads loaded growth factors, including I-125 radio-labeled VEGF, IGF and unlabeled FGF-1, NGF, were released quickly in the first few days after overnight incubation, regardless of the radiolabel. When two or more growth factors were incorporated, no significant change in the release kinetics was seen.
2.3 Cell culture
Fresh human urine were obtained from healthy donors, umbilical cord, and human skeletal muscle specimens were collected from Wake Forest Baptist Hospital for this study have been approved by the Wake Forest University Health Sciences Institutional Review Board. Fifteen voided urine samples (100–400 ml) from two healthy men (25 and 40 years old, respectively) were collected and immediately transferred to the laboratory for isolation and culture, as reported previously [7]. Briefly, urine specimens were centrifuged at 500×g for 5 min and the supernatant was removed. The cell pellet was gently re-suspended in mixed media composed of embryo fibroblast medium (EFM) and keratinocyte serum free medium (KSFM) (1:1 ratio) and plated in 24-well plates (p0). Individual clones appeared 3–5 days after plating. It took 7–10 days for a single USC clone to reach confluence in the initial culture (p0) and then the cells were serially passaged at every 3–4 days after. Each single cell clone was trypsinized and transferred into 6-well dishes when the cells reached a confluence of 70–80% (p1). Finally, cell cultures were transferred to a 150 mm culture dish (p2) for expansion; USCs (< p5) were used for all experiments as passaged adult stem cells have higher differentiation capacity.
Human umbilical cord endothelial cells (HUVECs) were isolated by brief perfusion of 0.1 U/ml collagenase, 0.8 U/ml dispase prepared in Hank’s balanced salt solution into umbilical cord veins [30]. HUVECs were then cultured on plates coated with fibronectin (Millipore, Billerica, MA) using Endothelial Growth Medium-2 (EGM2) (Lonza Biologics, Portsmouth, NH) containing 2% fetal bovine serum (FBS) at 37°C in a 5% CO2 cell incubator. Cultured HUVECs were used as positive control in the assessment of angiogenesis.
Human skeletal muscle cells were isolated from chopped muscle tissue (1 mm × 1 mm) by incubation in 10 ml of collagenase-II (0.1% w/v)-dispase (4 mg/ml) solution prepared in DMEM for 1 hour at 37°C with constant shaking (60 rpm). The liberated cells were collected (400×g) and washed with DMEM medium containing 10% horse serum and plated into a 6-well tissue culture dish. After 2 hours, the supernatant from the dish was transferred to another well and the process repeated. After 5 days in culture, the media was changed to SkGM2 (Lonza, Biologics, Portsmouth, NH) containing 10% FBS at 37°C in a 5% CO2 cell incubator. Cultured human skeletal muscle cells were used as control.
2.4 Endothelial differentiation of USCs in vitro
To assay the effects of growth factor releases on angiogenic differentiation of USCs, cells were cultured with alginate microbeads loaded with VEGF. USCs at passage 3 were seeded in a 24-well plate (1,000 cells/cm2) and microbeads containing VEGF were added to the cell inserts (Millipore, Billerica, MA) on the top of wells. We intentionally excluded VEGF from the EBM-2 media, so microbeads were the sole source of VEGF in the solution if any endothelial induction was to occur. EGM-2 containing 10 ng/ml VEGF was used as a positive control, and Dulbecco's modified Eagle’s medium (DMEM) containing 10% FBS and 1% antibiotics penicillin and streptomycin (P/S) only or combined with VEGF microbeads was used as a negative control. Media were changed every three days. mRNA was collected for real-time PCR analysis of endothelial markers (CD31 and vWF) at 14 days from these samples, as previously reported [31].
2.5 In vivo implantation
A total of 24 male athymic mice at 6 weeks old (Harlan Laboratories, Indianapolis, IN) divided into ten groups, as listed in Table 1. Cell-free or/and cytokine-free preparations were used in Groups 1–5 (controls). USCs mixed with microbeads containing six growth factors were divided into five groups: myogenic (PDGF-BB plus HGF), angiogenic (VEGF), neurogenic (IGF, NGF, and FGF-1), synergistic 1 (six growth factors cocktail) and synergistic 2 (growth factor cocktail plus endothelial cells). When six growth factors were all delivered together in (Groups 9 and 10), the growth factor doses in these groups were reduced to one-third of those used in Groups 6–8. USCs at p3, HUVECs, and human skeletal muscle cells (as controls) at p5 were used for cell injection.
A total of 5×106 cells (human skeletal muscle cells or USCs alone, or USCs plus ECs (4:1)) were embedded in 0.5 ml collagen-I gel (2 mg/ml) combined with various alginate beads according to group assignment. These cell-bead-collagen gel preparations were subcutaneously injected into 4 sites (right and left flanks in front and rear areas) per animal. All experiments with nude mice were approved by the Wake Forest University Health Sciences Institutional Animal Care and Use Committee.
2.6 Gross inspection and microhistologic analysis
Graft appearance was grossly assessed at day 28, at which point the mice were sacrificed. After harvesting, each implanted graft specimen was photographed and weighed. Half of the tissue specimen was frozen immediately in liquid nitrogen for real-time PCR measurements. The remaining half sample was embedded in optimal cutting temperature (O.C.T.) for immunofluorescence or fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin for histology. Five-µm graft sections were cut and mounted using anti-fade mounting media (Vector Laboratories). For visualization of cell density, 4',6-diamidino-2-phenylindole (DAPI) staining was performed. For histological evaluation, routine hematoxylin and eosin (H&E) and Masson’s trichrome staining were performed.
To monitor the fate and differentiation of human USCs in vivo, we conducted immunofluorescent triple staining using DAPI and human nuclei antibodies combined with either endothelial-, muscle-, or nerve fiber-specific markers (Table 2). Slides were visualized under a fluorescent microscope (Leica-DM 4000B, Germany) and the images were recorded for analysis. For semi-quantitative analyses of new nerve fibers, sections stained with specific immunofluorescent markers and Masson’s trichrome were evaluated by two independent and blinded observers using images captured by the microscope. The average total number of targeted cells was counted by semi-quantitative assessment in 10 separate fields under 200 X magnification.
Table 2.
Antibodies used in this study
| Cell markers | Host | Dilution | Company | |
|---|---|---|---|---|
| CD31 | Endothelial cells | Goat | 1:100 | Santa Cruz SC-1506 |
| vWF | Endothelial cells | Rab | 1:200 | Dako A0086 |
| Desmin | Skeletal muscle cells | Goat | 1:100 | Santa Cruz SC-7559 |
| Myf-5 | Skeletal muscle cells | Goat | 1:100 | Santa Cruz SC-12117 |
| Myo D | Skeletal muscle cells | Rab | 1:100 | Santa Cruz SC-304 |
| Human nuclei | Human nuclear | Mouse | 1:50 | Millipore MAB1281 |
| S-100 | Peripheral nerve | Rab | 1:100 | Abcam ab868 |
| NF | Peripheral nerve | Rab | 1:1000 | Abcam ab82259 |
2.7 Real-time PCR
The mRNA was extracted from two sources 1) endothelial differentiated USCs, induced in vitro with VEGF released from microbeads in endothelial differentiation medium; and 2) implanted grafts. With an RNA isolation kit (5 PRIME, Gaithersburg, MD) according to the manufacturer’s instructions, 5 µg RNA was converted to cDNA in a reaction containing random primers, nucleotides, and reverse transcriptase enzyme using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). One-tenth of the cDNA was then used for real-time analysis along with Taqman Universal PCR master mix and Taqman gene expression probes according to the manufacturer’s instructions, using a 7300 Real Time PCR system (Applied Biosystems, Foster City, CA). Reagents used for real-time RT PCR analysis were purchased from ABI (Applied Biosystems, Foster City, CA). The primer pairs used in this study are listed in Table 3.
Table 3.
Primers for real-time PCR used in this study*
| Primary antibody | Cell markers | Catalog # |
|---|---|---|
| CD31 | Endothelial cells | Hs01065279_m1 |
| vWF | Endothelial cells | Hs00169795_m1 |
| Desmin | Skeletal muscle cells | Hs01090875 |
| Myf-5 | Skeletal muscle cells | Hs00224610 |
| MyoD | Skeletal muscle cells | Hs00159528_m1 |
| GAPDH | Housekeeping gene | NM_002046.3 |
All primers obtained from Applied Biosystems, Foster City, CA.
2.8 Statistical analyses
Values are expressed as mean ± standard deviation (SD). Comparisons of the graft weight, human nuclei/DAPI ratio, real-time PCR analysis for endothelial and muscle transcripts, and numbers of neuron fibers among ten groups were performed by using one-way ANOVA (SPSS 16.0), followed by a Student-Newman-Keuls post hoc test for multiple comparisons when appropriate. P values ≤ 0.05 were considered as statistically significant.
3. Results
3.1 Release of I-125-labeled growth factors from microbeads in vitro
Alginate microbeads appeared stable and uniformly spherical after their preparation. No broken or damaged capsules were detected. The size of microbeads were about 400–500 um and the pore size after PLO coating was about 70–80 kDa. In this study, we chose to assess the release kinetics of IGF a smaller peptide and VEGF a bigger peptide separately and in combination with other growth factors in order to determine how molecular size of the growth factors may affect their release kinetics when encapsulated in the presence of others. The imbedded growth factors, including I-125-labeled VEGF, IGF and unlabeled FGF-1, NGF, were released quickly in the first few days of incubation followed by a steady rate of release for a month. As expected, the release rate of IGF-1 (mw ~7.6 KDa) was higher when present in the microbeads alone than its release rate when combined with VEGF (mw ~45 KDa) in the microbeads (Fig. 1). In contrast, the release of VEGF when present alone in the microbeads was similar to its release when VEGF combined with other growth factors (Fig. 1). The same steady state (VEGF) of slow release trend was consistent over 30 days, regardless of growth factor groups. After the sampling was stopped, no remaining growth factors were detected in the microbeads, indicating the cytokines were all successfully released from the microbeads.
3.2 Endothelial differentiation of USCs in vitro
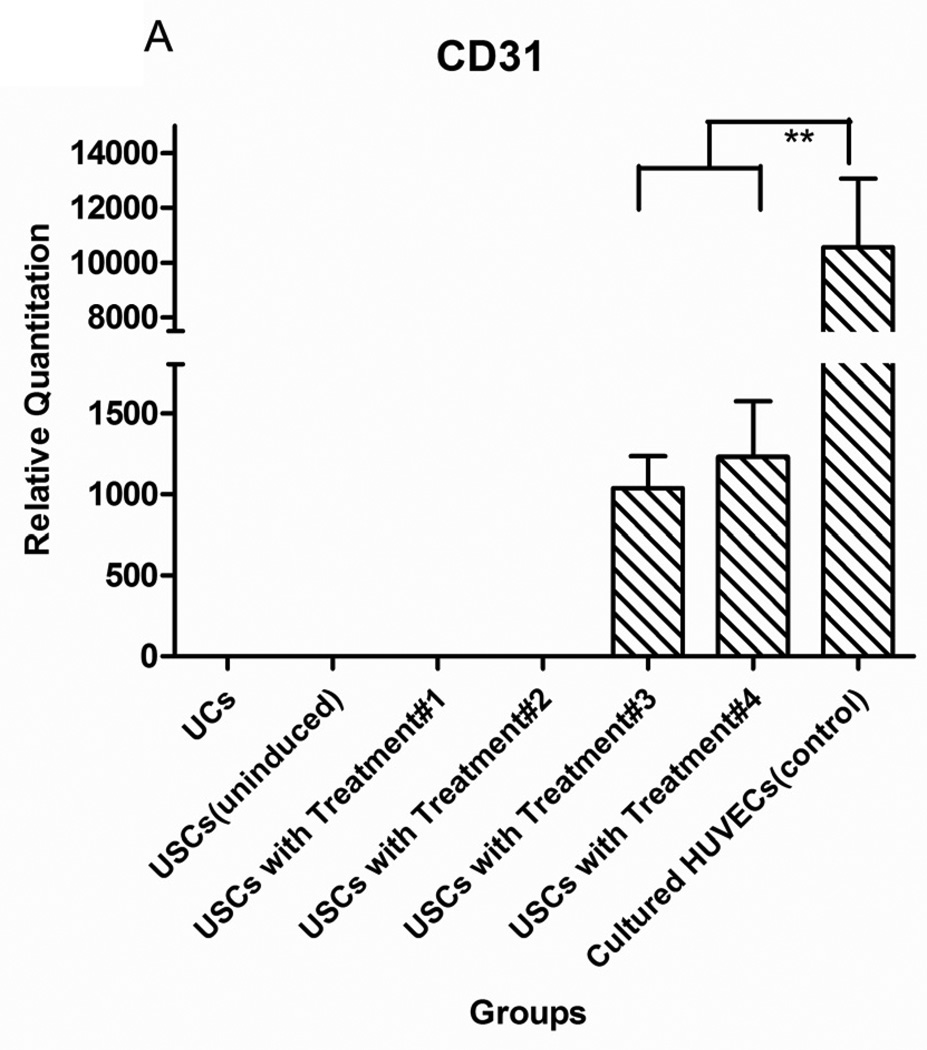
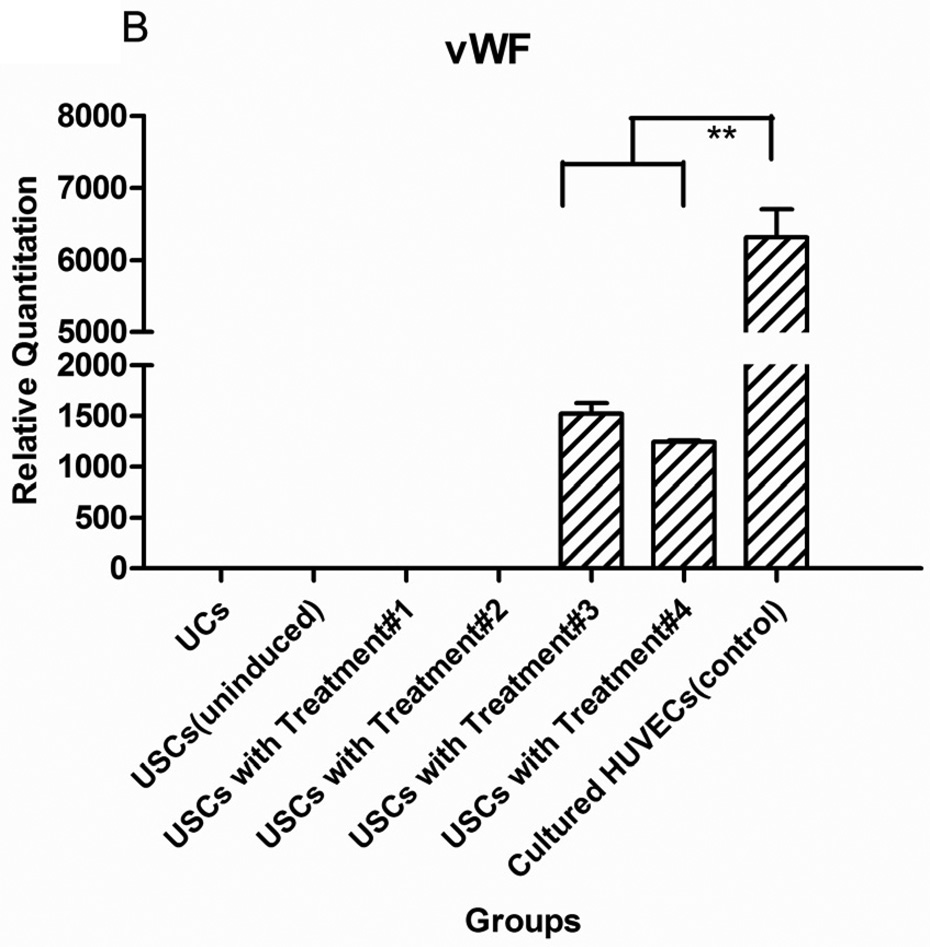
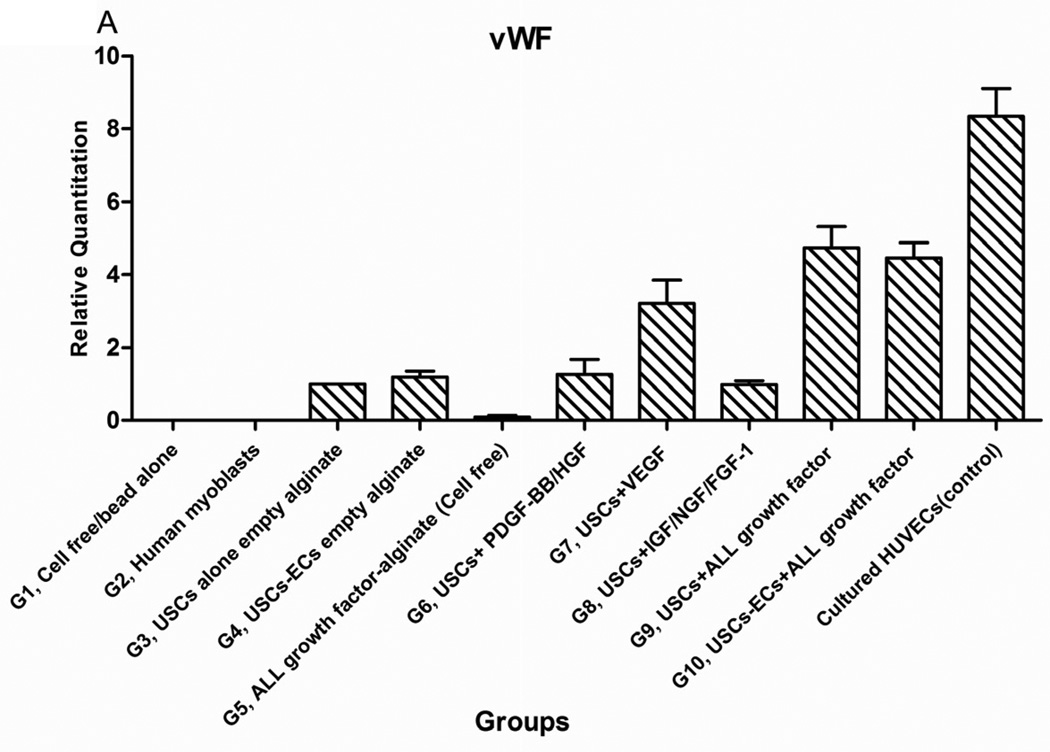
After USCs were cultured in the induced medium with VEGF-loaded microbeads for 14 days, gene expression of endothelial markers (CD31 and vWF) was significantly increased compared to negative controls (non-induced USCs and urothelial cells) (p<0.05) (Fig.2). Moreover, endothelial gene expression of USCs induced in the medium with VEGF-beads was similar to expression in USCs treated with medium with VEGF, indicating that VEGF release from the microbeads was bioactive.
Fig. 2. Endothelial gene expression of USCs in vitro.
USC (P3) were seeded at 1,000 cells/cm2 and induced by endothelial differentiation media as follows: Treatment#1= DMEM (10%FBS) with VEGF alginate beads located in transwell; Treatment#2= DMEM (10%FBS+1%P/S); Treatment#3=EC induced medium (EGM-2) plus alginate microbeads loaded with VEGF; Treatment#4= Endothelial cell-induced medium including VEGF solution (10 ng/ml). Significant increase of endothelial cell-specific gene expression CD31 (A) and vWF (B) could be detected in both Treatments #3 and 4, regardless of whether VEGF was added directly to the medium or delivered by the alginate beads. Urothelial cells (UC) were the negative control and human umbilical vein endothelial cells (HUVEC) were the positive control. **p<0.01.
3.3 Gross assessment of implanted grafts
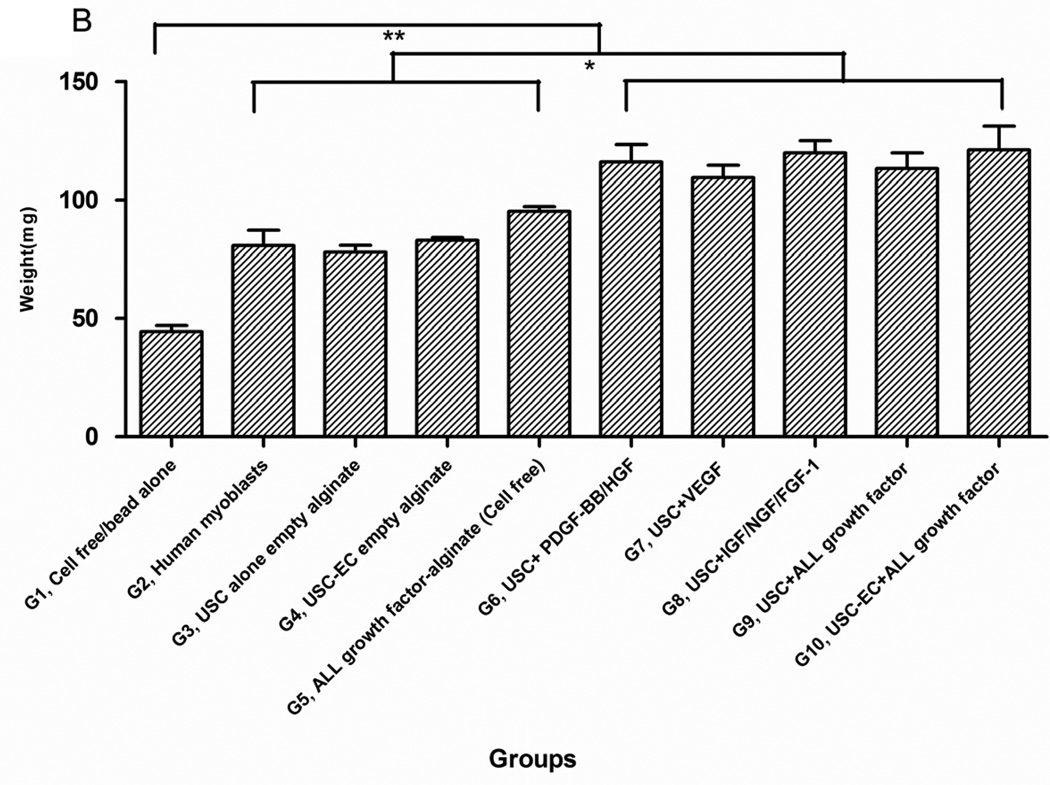
Although implant sizes were similar among 9 of the 10 groups (the graft sizes in Group 1 were 1/3 smaller than other groups, Fig. 3A), the weight of implanted grafts in USCs combined with the microbeads containing growth factors (Groups 6–10) was significantly higher compared to those in Groups 2–5 (p<0.05) (Fig. 3B). No significant differences in graft weight were seen among Groups 6–10. This was also true among the control groups (Groups 2–5). Importantly, no tumors were found, and no animals died during the 28 days of subcutaneous implantation.
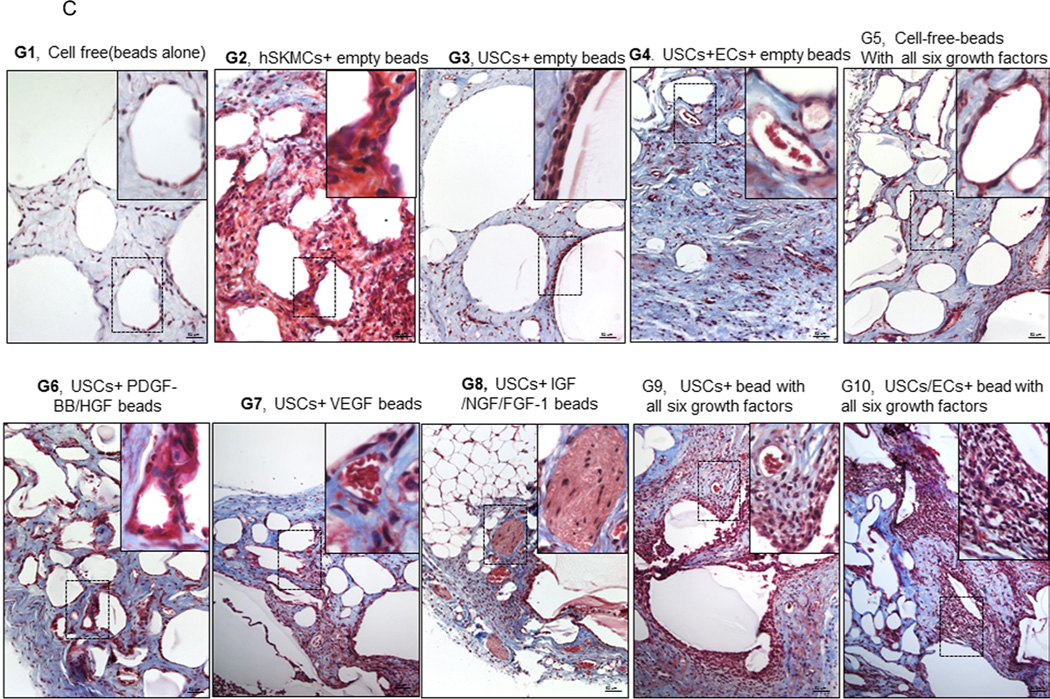
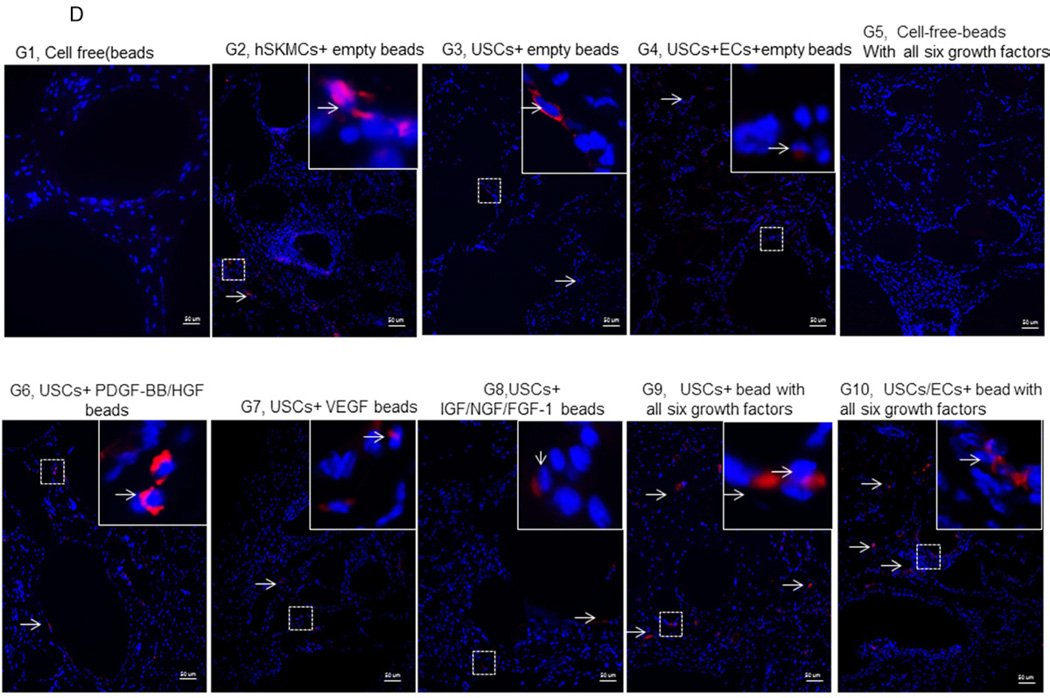
Fig. 3. Gross appearance, graft weight, histochemical staining, and immunostaining of the implanted grafts after 4 weeks in vivo.
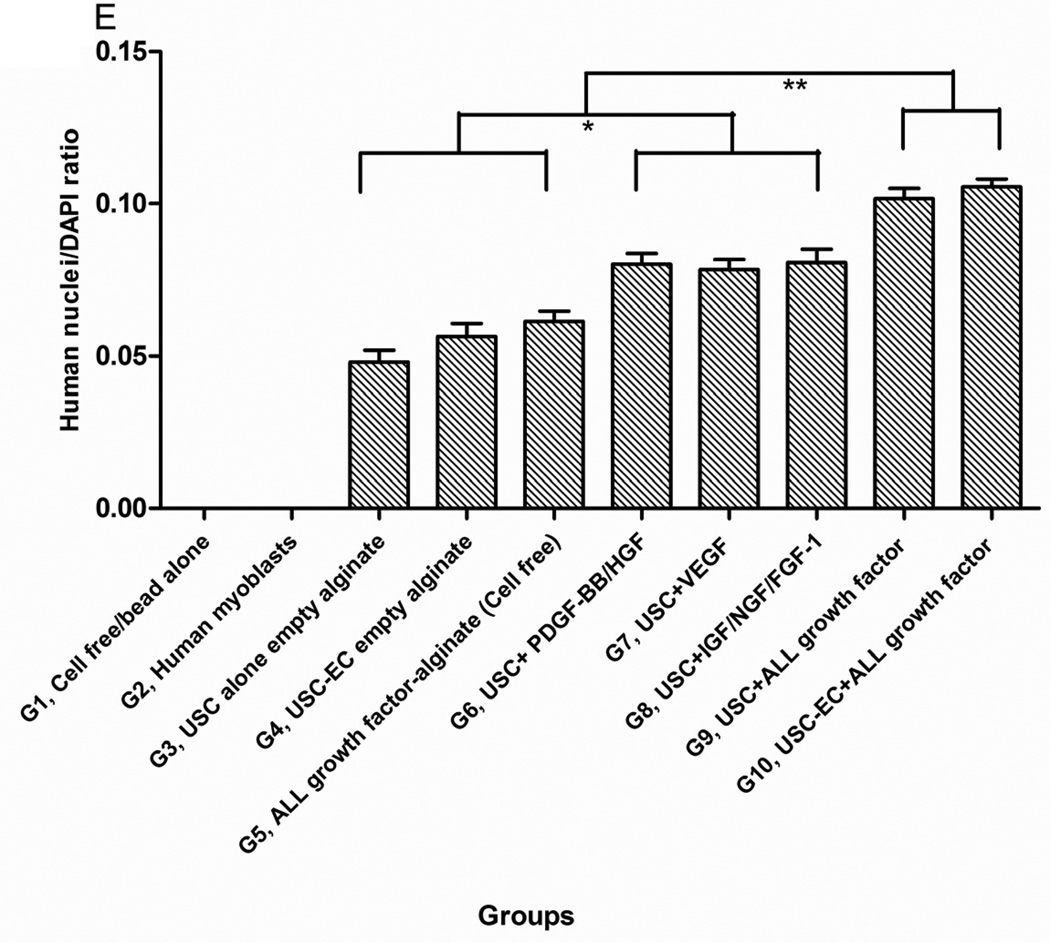
A) Gross morphology of the implants showing details of vascularization and implant size. Neovascularization of grafts was observed in G9 and G10 compared to the poor vascularization of the G1 grafts (Gel alone+ empty beads). B) Weight changes among implanted grafts after 4 weeks in vivo. *p<0.05, **p<0.01. C) Histochemical analyses of implanted grafts after 4 weeks in vivo. Masson’s Trichrome staining on the grafts depicts cells (red) more abundant in G9 and G10. D) Implanted cells were detected in vivo by immunofluorescent labeling using human specific nuclear mitotic apparatus antibody (right upper corner), stained in red. Specific staining appears reddish-purple (arrows) due to colocalization with DAPI (blue) stained nucleus. Scale bar = 50 µm. E) Semi-quantitative analyses human nuclei/DAPI staining ratio in implanted grafts. *p<0.05, **p<0.01.
3.4 Real-time PCR analysis, histology, and immunocytochemistry
More implanted cells with human nuclei protein expression and resident cells were found surrounding the microbeads in Groups 6–10, especially in Groups 9–10, based on the high ratio of human nuclei/DAPI staining, compared to the other groups after 28 days of subcutaneous implantation (Figs. 3C–E).
3.4.1 Myogenic differentiation of USCs
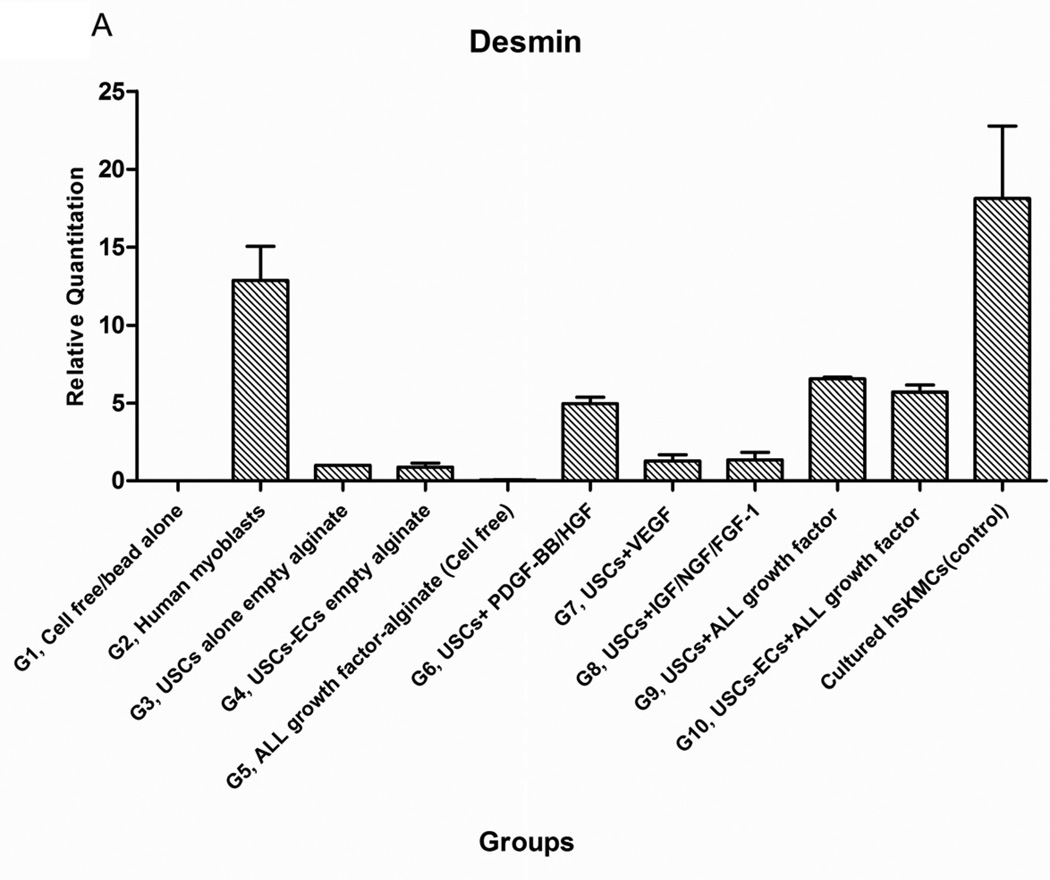
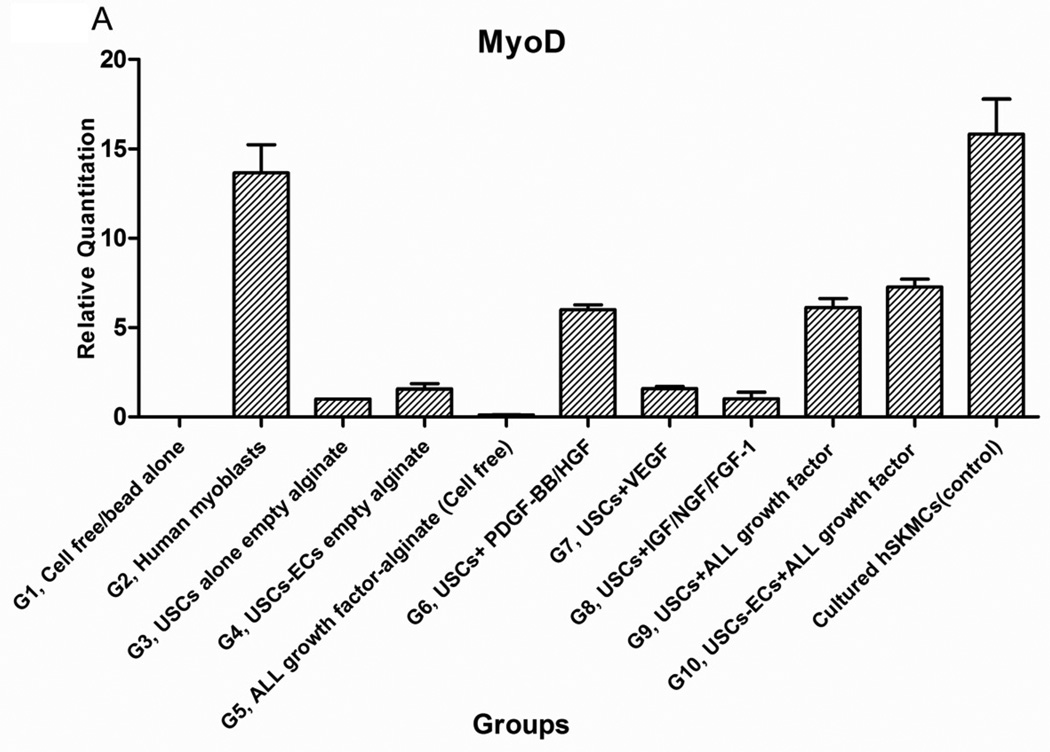
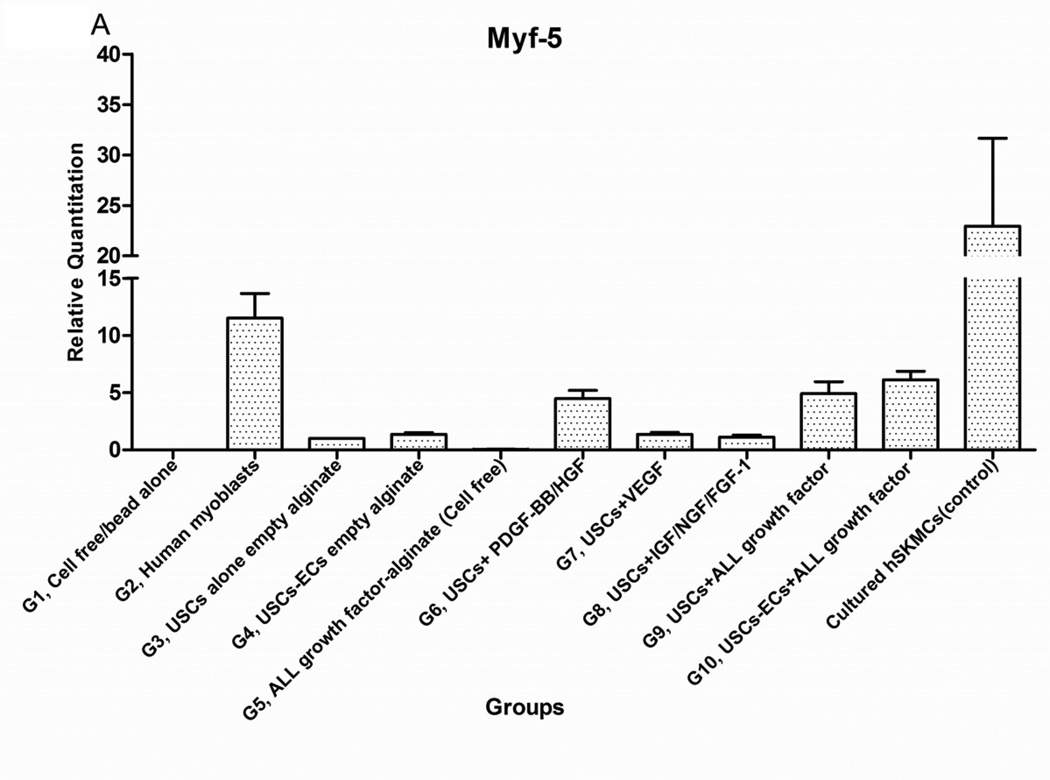
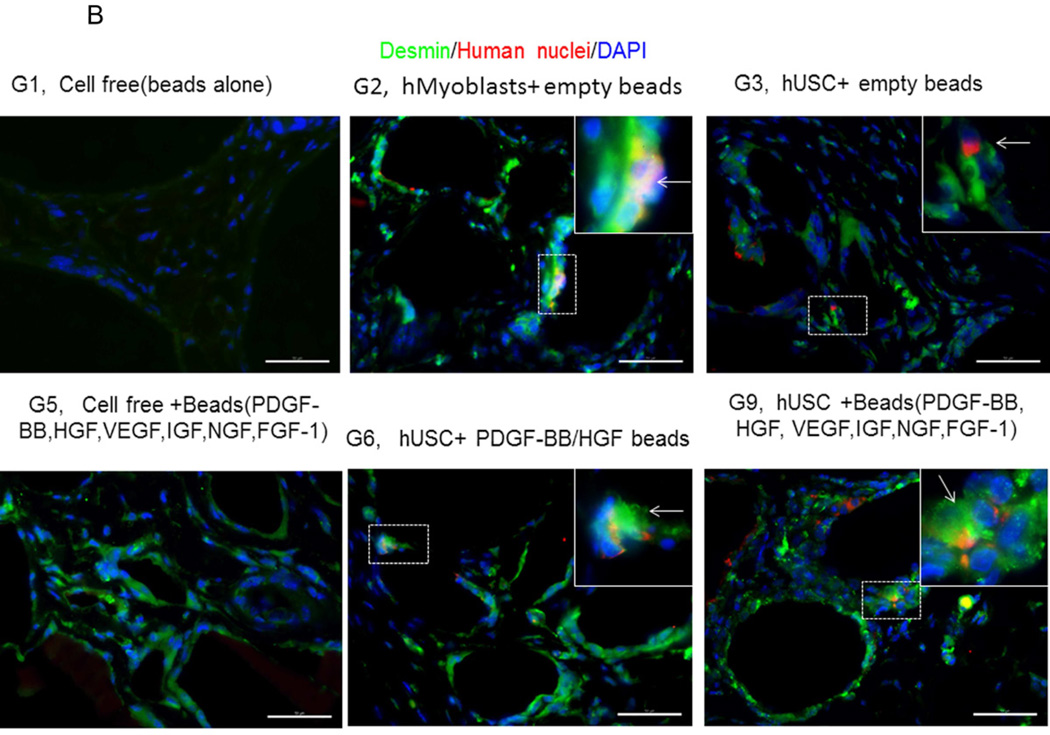
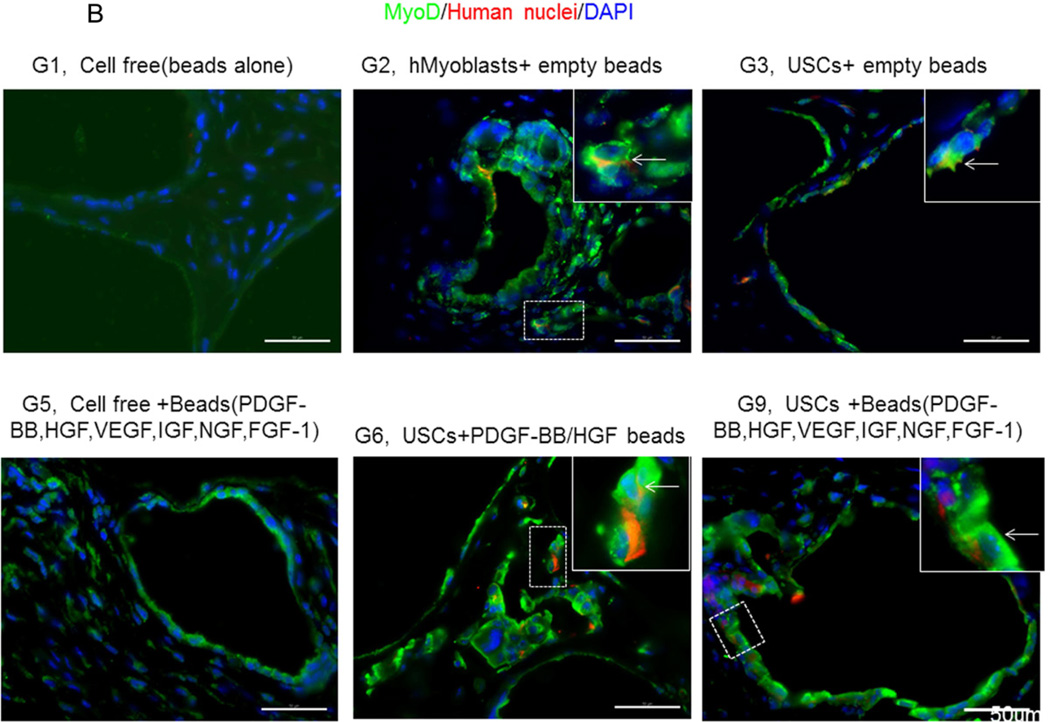
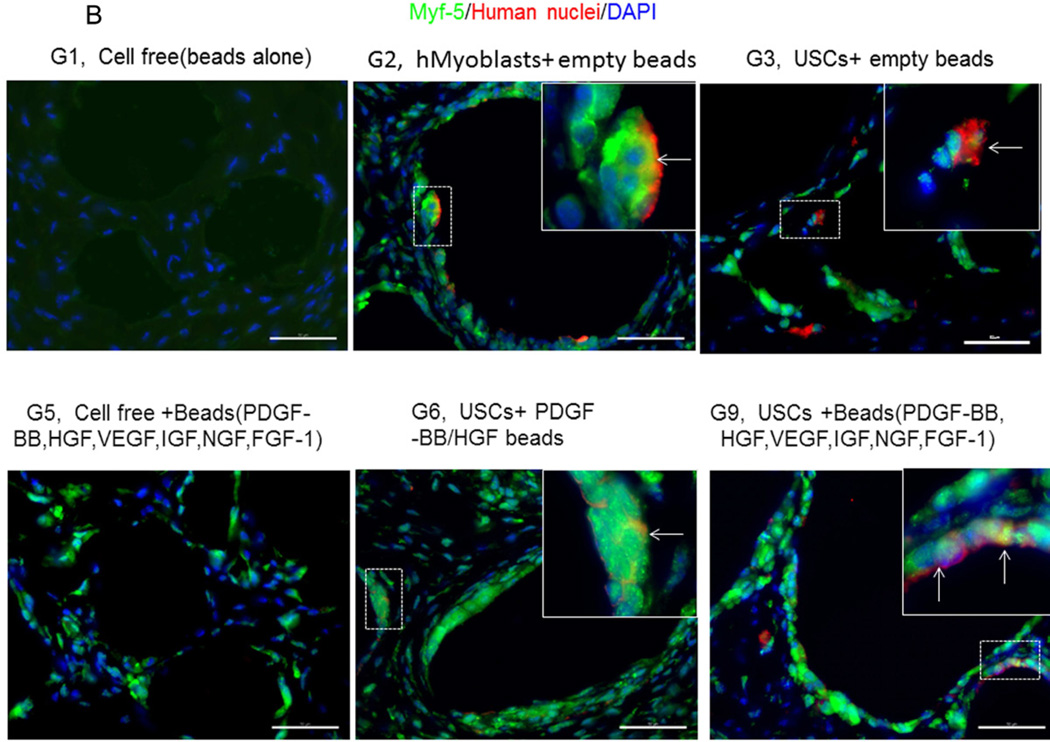
Expression of skeletal muscle-specific transcripts (Myo-D, desmin, and myf-5) were about 4-5-fold greater in USCs with myogenic growth factors (Group 6), all growth factors cocktail (Group 9), or plus ECs (Group 10) (Fig. 4A). The highest levels of the transcript were in cultured human skeletal muscle cells (control) and in the graft with skeletal muscle cells plus empty microbeads (Group 2). No human muscle transcripts expression was detected in the gel-alone group (Group 1) and growth factors alone (Group 5) because these primers were species-restricted transcripts for human cells. Immunofluorescent triple staining of myogenic markers, human nuclei, and DAPI showed that numbers of implanted cells expressing the skeletal muscle markers significantly increased in Groups 2, 9, and 10; the next was Group 6, consistent with real-time PCR data. Many cells without human nuclei expression also displayed myogenic markers in Groups 6, 9, 10 compared to the empty microbead groups (Groups 1 and 3), indicating that the resident muscle cells or resident stem cells migrated from the host into the graft tissue. The resident stem cells might be differentiated into muscle lineage cells induced by the growth factors released from microbeads (Fig. 4B).
Fig. 4. Expression of skeletal myogenic-specific marker on induced USCs in vivo assessed with quantitative PCR and immunofluorescent staining.
A) Quantitative real-time PCR performed on total RNA for all groups using human myogenic-specific primers (desmin, MyoD, and Myf-5). B) Implants of different treatment groups of USC (p3) were harvested after 4 weeks in vivo. Groups 1,2, 3, 5, 6, and 9 were subjected to immunofluorescent staining using skeletal myogenic markers (desmin, MyoD, and Myf-5) and human nuclei specific marker. Specific areas of staining (depicted by white arrows) appear green (desmin, MyoD, and Myf-5) and red (Human nuclei) nuclei were counterstained with DAPI (blue). Scale bar = 50 µm.
Interestingly, a few cells were positive for myogenic marker and human nuclei staining in the USC-alone group (Group 3), suggesting that some implanted USCs could be induced to give rise to muscle-lineage cells in vivo (Fig. 4B, Table 4).
Table 4.
Summary of USC subcutaneous injection in vivo for 28 days
| Groups | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell-free + empty beads |
Human Myoblasts +empty beads |
USC+ empty beads |
USC/EC+ empty beads |
Cell free +all growth factor beads |
USC+ PDGF/HGF beads |
USC+ VEGF Beads |
USC+IGF/ FGF/NGF Beads |
USC+All growth factor containing beads |
USC/EC+All growth factor containing beads |
|
| Gross measurement | ||||||||||
| Graft size | 2+ | 3+˜4+ | 3+ | 3+ | 3+ | 4+ | 3+˜4+ | 4+ | 3+˜4+ | 4+ |
| Blood supply | − | +˜2+ | 2+ | 2+ | 2+˜3+ | 3+ | 3+˜4+ | 2+˜3+ | 4+ | 4+ |
| Cell density | ||||||||||
| DAPI staining | + | 3+˜4+ | 2+˜3+ | 2+˜3+ | 2+ | 3+ | 4+ | 3+˜4+ | 4+ | 4+ |
| Human nuclei staining | − | 3+ | 2+ | 2+˜3+ | − | 3+ | 3+˜4+ | 3+ | 3+˜4+ | 4+ |
| Muscle regeneration | ||||||||||
| MyoD | −˜+ | 3+˜4+ | 2+ | 2+ | 2+ | 3+˜4+ | 3+ | 2+˜3+ | 3+˜4+ | 4+ |
| Desmin | − | 4+ | 2+ | +˜2+ | 2+ | 4+ | 2+˜3+ | 3+ | 4+ | 4+ |
| Myf-5 | − | 4+ | +˜2+ | 2+ | 2+˜3+ | 3+ | 2+˜3+ | 2+ | 3+˜4+ | 4+ |
| Angiogenesis | ||||||||||
| Trichorome observation | + | 2+˜3+ | 2+ | 2+ | +˜2+ | 3+ | 3+˜4+ | 3+ | 4+ | 4+ |
| CD 31 | −˜+ | 2+ | 2+˜3+ | 2+ | 2+˜3+ | 3+ | 3+˜4+ | 3+ | 3+˜4+ | 4+ |
| vWF | −˜+ | +˜2+ | 2+ | 2+˜3+ | 2+˜3+ | 2+˜3+ | 3+ | 3+ | 4+ | 4+ |
| Nerve regeneration | ||||||||||
| S-100 | −˜+ | +˜2+ | +˜2+ | + | 2+ | +˜2+ | 2+ | 3+ | 4+ | 4+ |
| NF | −˜+ | 2+ | 2+ | +˜2+ | 2+ | 2+ | 2+˜3+ | 3+˜4+ | 3+˜4+ | 4+ |
Notes: All growth factors including PDGF-BB/HGF/VEGF/IGF/FGF-1/NGF.
− no targeted cell or capillary; + 1%–25% of G10 changes; ++ 25%–50% of G10 changes; +++ 50%–75% of G10 changes; ++++ G10 changes as standard
3.4.2 Angiogenesis and neo-vessel formation
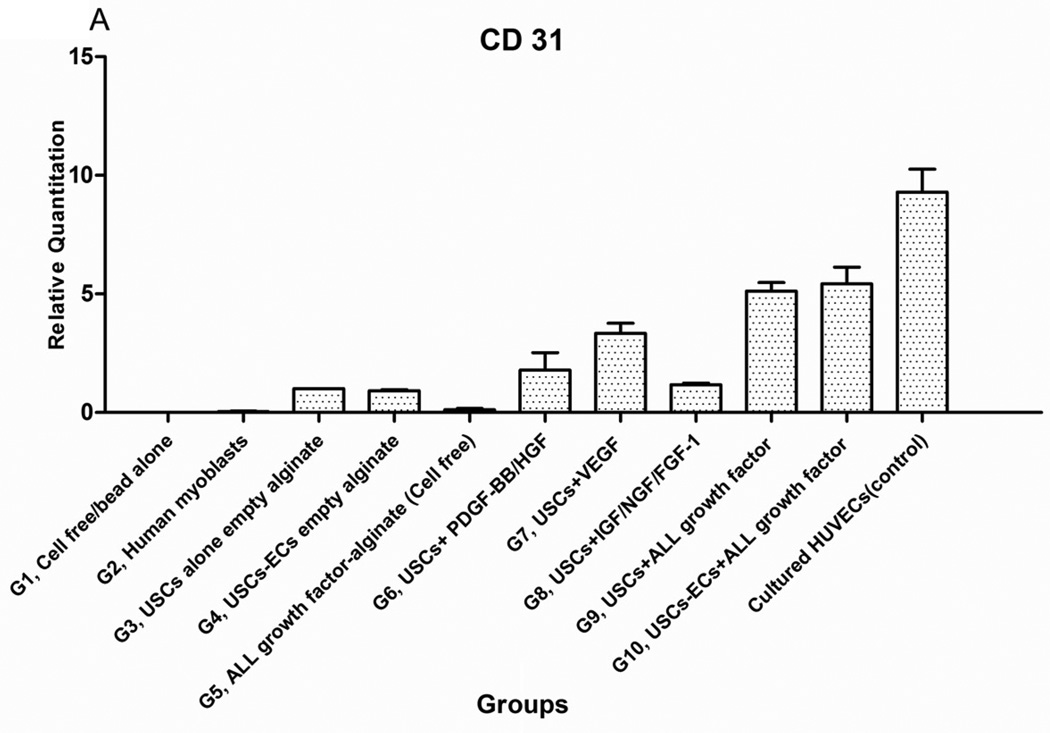
Expression level of endothelial cell transcripts (CD31 and vWF) was significantly higher, in Groups 9 and 10, compared to the other groups (P<0.05) (Fig. 5A) except positive control (cultured HUVECs). No human endothelial transcripts expression was detected in the gel-alone group (Group 1) and growth factors alone (Group 5) as these primers were species-restricted transcripts for human cells. No significant differences in transcript expression were found between Groups 9 and 10. The same pattern was shown in triple staining. More cells around the microbeads were positive for endothelial cell markers compared to the cells further away from the microbeads. In addition, beside the cells with human nuclei expression, many cells without human nuclei expression were also positive for endothelial markers in all groups, particularly, more cells were positive for endothelial cell markers and more neo-vessels formed in the groups with growth factors microbeads alone (Group 5) compared to the group with skeletal myocytes (Group 2) or gel alone (Group 1) (Fig. 5B, Table 4). These observations suggest that resident endothelial cells migrated from the host to participate in angiogenesis. It is also possible that the resident stem cells migrated from the host into the graft tissue and then differentiated into endothelial lineage cells induced by the growth factors released from microbeads.
Fig. 5. Endothelial differentiation of USCs and angiogenesis of implanted grafts 28 days after implantation in vivo.
A) Quantitative real-time PCR was performed on total RNA from all groups using endothelial-specific primers (CD31, vWF). B) Groups 1, 3, 4, 5, 7, and 9 were subjected to immunofluorescent staining using the epithelial markers CD31 and von Willebrand factor (vWF) with Human nuclei specific marker. Specific staining (shown by arrows) appears green with nuclear staining in red (human nuclei) and blue (DAPI) indicating these were implanted cells that successfully differentiated to endothelial cells in vivo. Scale bar = 50 µm.
Interestingly, a few cells in the USC-alone group (Group 3) were positive for both human nuclei and endothelial markers, implying that implanted USCs may participate in angiogenesis in vivo even without growth factor delivery. In addition, the amount of neo vessel formation in microbeads loaded with agiogenic growth factors (Groups 8–10) significantly increased compared to microbeads with growth factor cocktail alone (Group 5), indicating that USCs may play a role in revascularization within the implanted grafts.
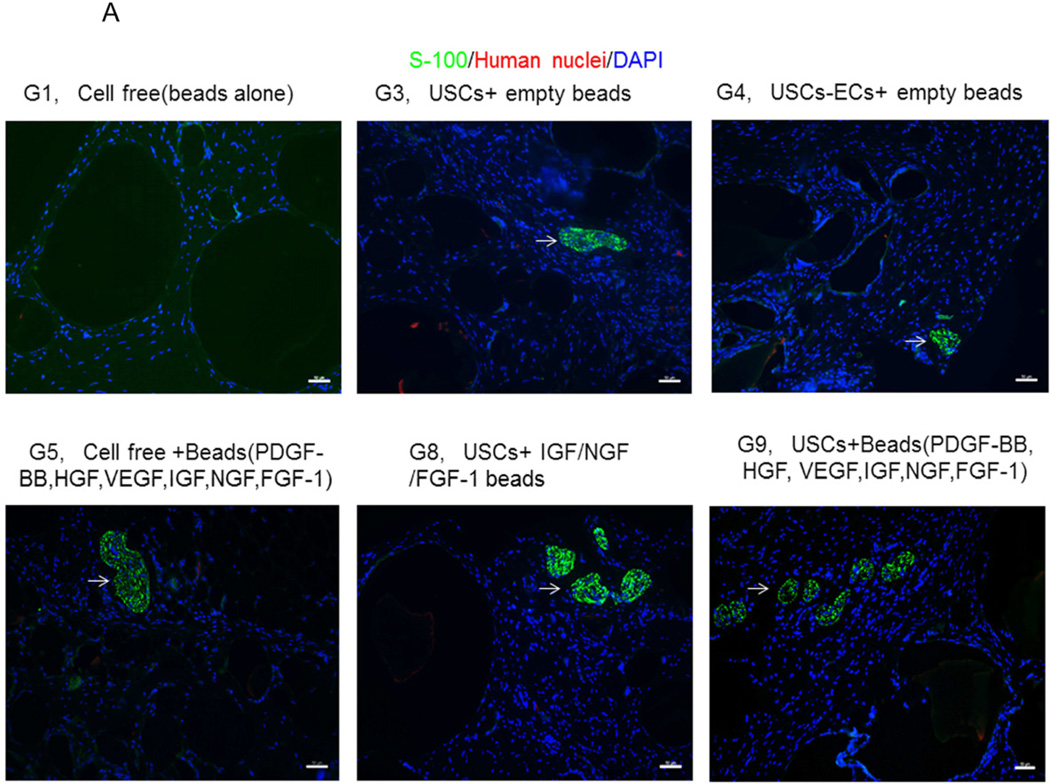
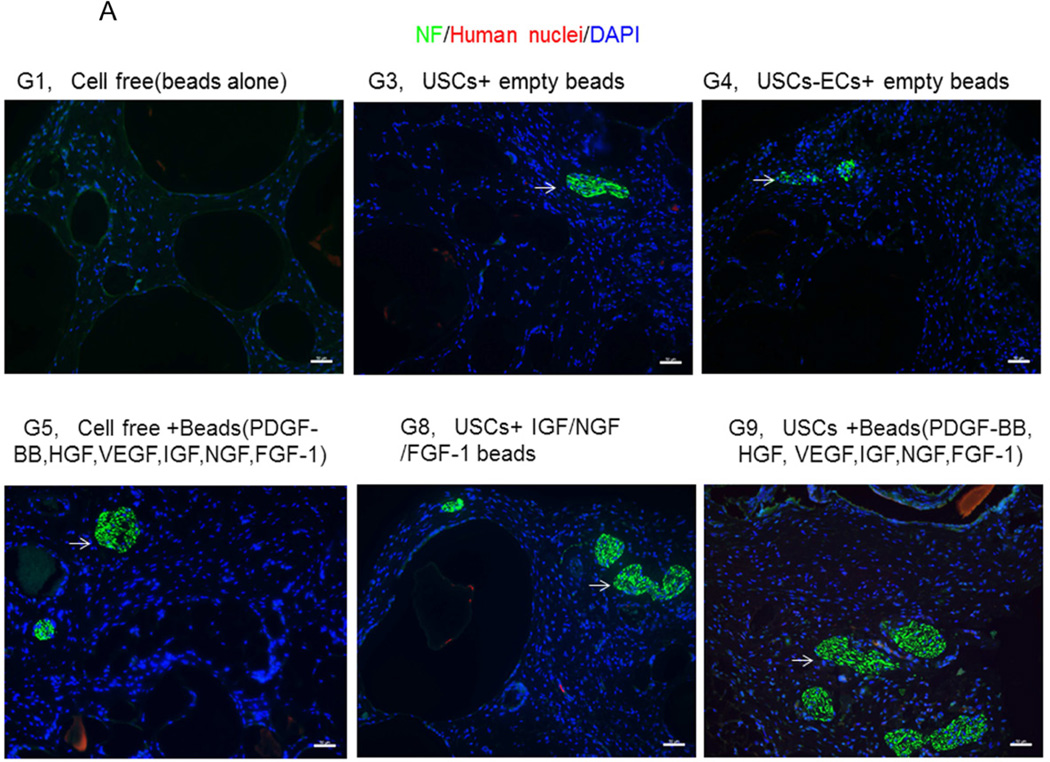
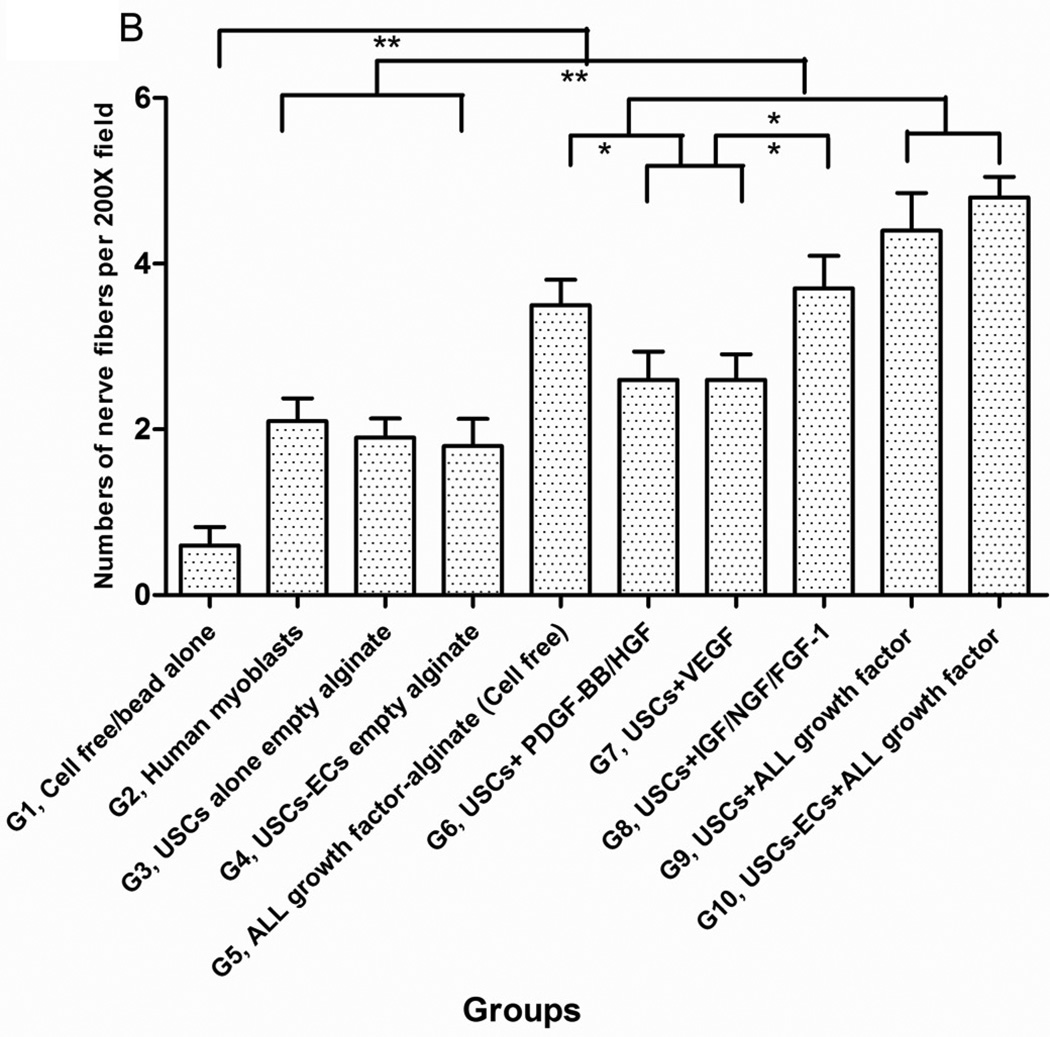
3.4.3 Innervation
The numbers of neuron fibers within the grafts significantly increased in Groups 9 and 10 as identified by semi-quantitative analysis based on triple immunofluorescence staining (Fig.6A, 6B), including peripheral nerve cell markers (neurofilament and S-100) combined with human nuclei and DAPI. The least amount of innervation was in the gel-alone group (Fig. 6B, Table 4). Most new nerve fibers were around the edges of grafts, but few cells expressing human nuclear markers were found in new nerve fibers, indicating that nerve fibers were derived from the host tissue, not the implanted cells. In addition, significantly increasing amount of innervation in microbeads loaded with neurogenic growth factors (Group 8) or growth factors cocktail with USCs (Group 9) or plus ECs (Group 10) compared to microbeads with growth factor cocktail alone (Group 5), similar results being observed in USCs alone (Group 3) verse empty microbeads (Group 1) (Fig. 6B), suggesting that USCs may possess indirect neurogenic and neuron rescue properties.
Fig. 6. Innervation of implanted grafts 28 days after implantation.
A) Cross-sections of samples from Groups 1, 3, 4, 5, 8, and 9 were subjected to immunofluorescent staining using DAPI (blue), human nuclear (red) and nerve cell antibodies (green)-S-100 and Neurofilament (NF). Scale bar= 50 µm. B) Semiquantitative analyses of nerve fibers in the implanted grafts. *p<0.05, **p<0.01.
4. Discussion
Two potential treatments have been investigated to accelerate tissue repair from sites of chronic injury or ischemia, growth factor therapy [32] and stem cell therapy [33]. A new approach combining both therapies has been recently studied to induce stem cell differentiation and increase cell differentiation efficiency for tissue repair [34]. The present study documents a series of experiments aimed at demonstrating potential treatments for patients with SUI. Using a feasible delivery system with synergistic growth factors, we report that implanted autologous USCs were induced to differentiate into a myogenic lineage, and that the growth factor combinations enhanced angiogenesis and innervation, and stimulated resident cells to participate in regeneration of urethra sphincter tissue.
The sphincter muscle unit of the urethra has both internal and external sphincter muscles. The internal sphincter is the extension of the detrusor muscle (the primary muscle for forcing urine out of the bladder), is made of smooth muscle under involuntary or autonomic control. By contrast, the external sphincter is made of skeletal muscle under voluntary control of the somatic nervous system. Other connective tissues around the urethra, including vessels and peripheral nerves, also play important roles in control of micturition. Urinary incontinence occurs in three types of softy tissue injures, i.e. muscle weakness, nerve damage, or vascular (blood supply) changes, all of which are potential targets for stem cell therapies. Unlike using bulking materials to mechanically squeeze the urethra, a long-term strategy to treat SUI is to repair defects of both skeletal and smooth muscle, and to improve the blood supply and innervation in the mid-urethral segment [35]. Several clinical trials have demonstrated that MSCs isolated from skeletal muscle or fat tissue injected into the middle urethra restored the damaged contractile function of the striated muscles and rhabdosphincter [36, 37]. The rationale of stem cell therapy is based on the multi-potent differentiation capability and trophic properties of these cells [38]. Stem cells can give rise to the target cells and secrete paracrine factors, such as angiogenic, neurogenic and cytoprotective factors, to prolong cell survival and facilitate vascularization and innervation. In the present study, we have demonstrated that USCs efficiently gave rise to skeletal myogenic or endothelial lineage cells, and had neuro rescue effect in vivo via synergistic activity of growth factors released from microbeads vehicles. The growth factors not only improved the environment for the implanted cells by creating angiogenesis and innervation, but also recruited resident cells into the graft site for tissue repair. In addition, the combination of growth factors that facilitated myogenesis, angiogenesis, and innervation was more effective in in vivo tissue regeneration than the growth factors applied individually. Furthermore, this therapeutic approach would not require a preconditioning for in vitro stem cell differentiation; thus it shortens the process and increases cell differentiation efficiency.
An adequate blood supply is crucial for survival of cells in cell therapy, particularly in the central core of the implants [39, 40]. Our previous studies demonstrated that modifying USCs by exposing them to the angiogenic gene VEGF remarkably improved the cell survival rate and myogenic differentiation of USCs by promoting angiogenesis in vivo [19]. However, angiogenic gene manipulation causes potential side effects, such as extensive hemorrhaging within the liver [41] and tumorigenesis [42, 43] in implanted sites. Except for gene transfection, growth factor injection therapy including cytokines such as VEGF, HGF, IGF, NGF, PDGF, FGF, BMP, and EGF also acted as powerful therapeutic agents in tissue engineering [44]. However, most growth factors are soluble and disappear quickly due to their short half-life time in vivo. This growth factor injection approach also requires multiple injections of large doses of proteins that results in several potential side effects, including only transient improvements [42] or abnormal vascular structure, resulting in insufficient therapeutic effect [44]. Thus, several growth factor delivery systems, such as chemical conjugation of the growth factor to the matrix, or physical encapsulation of growth factors in the delivery system [45], have been designed to overcome these disadvantages.
Different types of biomaterials have been used to achieve cytokine or drug delivery, including biologics, polymers, silicon-based materials, carbon-based materials, or metals [46]. Among those delivery vehicles, alginate hydrogel microbeads are an excellent candidate for cytokine delivery, since they retain the bioactivity of the growth factors as cross-linking occurs under physiological conditions. The alginate microbeads can be easily modified; higher concentrations of alginate yield a tightly cross-linked matrix, resulting in lower porosity and hence slower release of growth factors. Alginate-encapsulated proteins such as FGF-1 [27], PDGF, and VEGF [47] have demonstrated a slow, low-level consistent release of growth factors, and the efficacy of the delivery conduit was demonstrated both in vitro and in vivo. Unlike gene delivery or protein injection, the effective delivery of proteins, safety, and biocompatibility of microbeads provide promising benefits for angiogenesis [25–27].
Our previous study showed heparin binding to FGF-1 could increase its half-life and retain the normal mitogenic properties of FGF-1. Release time was prolonged when alginate microbeads were combined with the heparin-binding growth factors [48].The loading efficiency for all growth factors in this study was between 36–40%, which is very comparable to other loading methods [23]. As alginate beads have a porosity of about 600 kDa, we applied a semi-permeable membrane of PLO coating which reduces the porosity to about 70–80 kDa. This semi-permeable membrane allowed us to control the release of the growth factors from these microbeads. No significant difference in the loading efficiency was observed when the growth factors were loaded into microbeads between 24 to 48 h. As is the case with hydrophilic drug carriers with hydrophilic payload, there is usually an initial burst release that is followed by a sustained release of smaller levels of the encapsulated substance [25], which explains why about 40–60% of the growth factors were released in one day. Previous studies had shown that this release profile consisting of a high growth factor concentration initially, followed by a decreasing concentration over time was found to result in optimal angiogenic effect [49]. Thus, it was desirable for such burst release to occur for the enhancement of the bioeffect of the growth factors. In our experiments, we observed a steady and consistent release of smaller levels after the initial burst release during the first day. Although certain variation in release profile was noted when multiple growth factors were combined, the growth factors were still consistently released from the microbeads. The growth factors release efficiency depends on their molecular weights because of their release competition effect. Our data confirmed that biologically-active VEGF was efficiently released from the alginate microbeads, leading endothelial differentiation of USC in vitro.
Increasing evidence has shown that cytokine combinations are better than a single cytokine in tissue repair [50]. In the present study, we investigated the impact of growth factors on angiogenesis with single, dual, or multiple delivery patterns in vivo. When given together, the growth factors had a synergistic effect that improved implanted cell survival, muscle tissue regeneration, neo-vacuolization, and innervations in vivo. Although IGF/NGF/FGF acts on innervation, VEGF on angiogenesis, and PDGF/HGF on myogensis, most of them have cross-cutting properties. For example, PDGF not only induces myogenic differentiation of stem cells, but also promote angiogenesis [51]; furthermore, IGF can enhance innervation as well as promote myogenic differentiation of stem cells [52]. In addition, FGF-1 also promotes angiogenesis [26].
More new nerve fibers appeared in the grafts of USCs implanted with alginate microbeads containing neurogenic growth factors, compared to the other experimental groups. The nerve fibers may have originated from the host tissue, rather than from the implanted USCs, because these regenerated nerve cells were largely not positive for few human nuclei staining. The present study also suggests that revascularization via angiogenic factors released from the microbeads enhanced both muscle regeneration and innervation in vivo.
In summary, we demonstrated that the implanted USCs play an important role in cell survival, myogenesis, angiogenesis, innervation, and recruitment of resident cells. All groups of USCs with added growth factors achieved better outcomes than the groups with the same types of growth factors without USCs. Some USCs could give rise to endothelial cells without the addition of any growth factors, but these effects were strengthened by the presence of angiogenic growth factors in vivo. In addition, as USCs could differentiate into endothelial cells, endothelial differentiated stem cells could replace endothelial cells with no necessity for adding extraneous endothelial cells to implanted grafts. Furthermore, USCs appear the indirect neruogenic and neruoprotective effects during tissue regeneration.
5. Conclusions
The present study has demonstrated that an alginate microsphere delivery system is feasible to control local levels of myogenic, angiogenic, and neurogenic growth factors and efficiently release multiple growth factors in vitro for over 4 weeks. The synergism of growth factors embedded in alginate microbeads can prolong grafted stem cell survival, promote myogenic differentiation of stem cells, enhance peripheral nerve regeneration, and recruit resident cells in vivo. Further investigations into the impact of combining growth factors and USCs are needed in an SUI animal model to demonstrate potential use for the incontinent patients.
Acknowledgements
The authors would like to thank Ms. Karen Klein (Research Support Core, Wake Forest School of Medicine) for her editorial assistance with this manuscript. This study was supported in part by funds from NIH grant (R01DK080897) and the Vila Rosenfeld research gift to Dr. Opara.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sampselle CM, Miller JM, Mims BL, Delancey JO, Ashton-Miller JA, Antonakos CL. Effect of pelvic muscle exercise on transient incontinence during pregnancy and after birth. Obstet Gynecol. 1998;91:406–412. doi: 10.1016/s0029-7844(97)00672-8. [DOI] [PubMed] [Google Scholar]
- 2.Markland AD, Goode PS, Redden DT, Borrud LG, Burgio KL. Prevalence of urinary incontinence in men: results from the national health and nutrition examination survey. J Urol. 2010;184:1022–1027. doi: 10.1016/j.juro.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Wilson L, Brown JS, Shin GP, Luc KO, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 4.Caruso DJ, Gomez CS, Gousse AE. Medical management of stress urinary incontinence: is there a future? Curr Urol Rep. 2009;10:401–407. doi: 10.1007/s11934-009-0063-2. [DOI] [PubMed] [Google Scholar]
- 5.Wai CY. Surgical treatment for stress and urge urinary incontinence. Obstet Gynecol Clin North Am. 2009;36:509–519. doi: 10.1016/j.ogc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Kotb AF, Campeau L, Corcos J. Urethral bulking agents: techniques and outcomes. Curr Urol Rep. 2009;10:396–400. doi: 10.1007/s11934-009-0062-3. [DOI] [PubMed] [Google Scholar]
- 7.Novara G, Artibani W. Myoblasts and fibroblasts in stress urinary incontinence. Lancet. 2007;369:2139–2140. doi: 10.1016/S0140-6736(07)60990-8. [DOI] [PubMed] [Google Scholar]
- 8.Mayer RD, Dmochowski RR, Appell RA, Sand PK, Klimberg IW, Jacoby K, et al. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology. 2007;69:876–880. doi: 10.1016/j.urology.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 9.Smith DP, Kaplan WE, Oyasu R. Evaluation of polydimethylsiloxane as an alternative in the endoscopic treatment of vesicoureteral reflux. J Urol. 1994;152:1221–1224. doi: 10.1016/s0022-5347(17)32552-1. [DOI] [PubMed] [Google Scholar]
- 10.Chrouser KL, Fick F, Goel A, Itano NB, Sweat SD, Lightner DJ. Carbon coated zirconium beads in beta-glucan gel and bovine glutaraldehyde cross-linked collagen injections for intrinsic sphincter deficiency: continence and satisfaction after extended followup. J Urol. 2004;171:1152–1155. doi: 10.1097/01.ju.0000103688.83606.06. [DOI] [PubMed] [Google Scholar]
- 11.Herschorn S, Glazer AA. Early experience with small volume periurethral polytetrafluoroethylene for female stress urinary incontinence. J Urol. 2000;163:1838–1842. [PubMed] [Google Scholar]
- 12.Kiilholma PJ, Chancellor MB, Makinen J, Hirsch IH, Klemi PJ. Complications of Teflon injection for stress urinary incontinence. Neurourol Urodyn. 1993;12:131–137. doi: 10.1002/nau.1930120206. [DOI] [PubMed] [Google Scholar]
- 13.Gopinath D, Smith AR, Reid FM. Periurethral abscess following polyacrylamide hydrogel (Bulkamid(R)) for stress urinary incontinence. Int Urogynecol J. 2012 doi: 10.1007/s00192-012-1768-1. [in press]. [DOI] [PubMed] [Google Scholar]
- 14.Koski ME, Enemchukwu EA, Padmanabhan P, Kaufman MR, Scarpero HM, Dmochowski RR. Safety and efficacy of sling for persistent stress urinary incontinence after bulking injection. Urology. 2011;77:1076–1080. doi: 10.1016/j.urology.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Mitterberger M, Marksteiner R, Margreiter E, Pinggera GM, Frauscher F, Ulmer H, et al. Myoblast and fibroblast therapy for post-prostatectomy urinary incontinence: 1-year followup of 63 patients. J Urol. 2008;179:226–231. doi: 10.1016/j.juro.2007.08.154. [DOI] [PubMed] [Google Scholar]
- 16.Lin CS, Lue TF. Stem cell therapy for stress urinary incontinence: a critical review. Stem Cells Dev. 2012;21:834–843. doi: 10.1089/scd.2011.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boissier R, Karsenty G. Cellular therapy and urinary incontinence. Prog Urol. 2012;22:454–461. doi: 10.1016/j.purol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Bharadwaj S, Liu G, Shi Y, Markert C, Andersson KE, Atala A, et al. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng Part A. 2011;17:2123–2132. doi: 10.1089/ten.tea.2010.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodin A, Bharadwaj S, Wu S, Gatenholm P, Atala A, Zhang Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials. 2010;31:8889–8901. doi: 10.1016/j.biomaterials.2010.07.108. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Liu Y, Bharadwaj S, Atala A, Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317–1326. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Wu S, Bharadwaj S, Soker S, Atala A, Zhang Y. Promoting muscle and nerve regeneration after human urine-derived stem cells expression of vascular endothelial growth factor for potential use in treatment of stress urinary incontinency. J Urology; American Urological Association (AUA) 2011 Annual meeting; May 14–19; Washington, D.C. p. e462. [Google Scholar]
- 23.Camarata PJ, Suryanarayanan R, Turner DA, Parker RG, Ebner TJ. Sustained release of nerve growth factor from biodegradable polymer microspheres. Neurosurgery. 1992;30:313–319. doi: 10.1227/00006123-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Pareta RA, McQuilling JP, Farney AC, Opara EC. Bioartificial Pancreas: Evaluation of Crucial Barriers to Clinical Application. In: Randhawa G, editor. Organ Donation and Transplantation-Public Policy and Clinical Perspectives: In Tech. 2012. pp. 240–266. [Google Scholar]
- 25.Moya ML, Lucas S, Francis-Sedlak M, Liu X, Garfinkel MR, Huang JJ, et al. Sustained delivery of FGF-1 increases vascular density in comparison to bolus administration. Microvasc Res. 2009;78:142–147. doi: 10.1016/j.mvr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Moya ML, Garfinkel MR, Liu X, Lucas S, Opara EC, Greisler HP, et al. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. J Surg Res. 2010;160:208–212. doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moya ML, Cheng MH, Huang JJ, Francis-Sedlak ME, Kao SW, Opara EC, et al. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 2010;31:2816–2826. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharadwaj S, Liu G, Wu S, Andersson K-E, Atala A, Zhang Y. In vivo skeletal myogenic differentiation of autologous urine-derived stem cells for potential application in treatment of urinary incontinence. J Urology; American Urological Association (AUA) 2011 Annual meeting; May 14–19; Washington, D.C. p. e 72. [Google Scholar]
- 29.Liu G, Bharadwaj S, Atala A, Zhang Y. Endothelial differentiation of human urine derived stem cells for urological tissue regeneration. American Urological Association (AUA) 2012 Annual meeting; May 17–23; Atlanta, G.A. p. 203. [Google Scholar]
- 30.Lang I, Schweizer A, Hiden U, Ghaffari-Tabrizi N, Hagendorfer G, Bilban M, et al. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation. 2008;76:1031–1043. doi: 10.1111/j.1432-0436.2008.00302.x. [DOI] [PubMed] [Google Scholar]
- 31.Bharadwaj S, Wu S, Hodges S, Atala A, Zhang Y. Skeletal muscle differentiation of human urine-derived stem cells for injection therapy in the treatment of stress urinary incontinence. J Urology. 2011;184:E681. [Google Scholar]
- 32.Liu Y, Sun L, Huan Y, Zhao H, Deng J. Effects of basic fibroblast growth factor microspheres on angiogenesis in ischemic myocardium and cardiac function: analysis with dobutamine cardiovascular magnetic resonance tagging. Eur J Cardiothorac Surg. 2006;30:103–107. doi: 10.1016/j.ejcts.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 33.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Zhang H, Zhang X, Lu W, Huang X, Xie H, et al. Synergistic angiogenesis promoting effects of extracellular matrix scaffolds and adipose-derived stem cells during wound repair. Tissue Eng Part A. 2011;17:725–739. doi: 10.1089/ten.TEA.2010.0331. [DOI] [PubMed] [Google Scholar]
- 35.Stangel-Wojcikiewicz K, Malgorzata S, Nikolavsky D, Chancellor MB. Cellular therapy for treatment of stress urinary incontinence. Curr Stem Cell Res Ther. 2010;5:57–62. doi: 10.2174/157488810790442840. [DOI] [PubMed] [Google Scholar]
- 36.Becker C, Jakse G. Stem cells for regeneration of urological structures. Eur Urol. 2007;51:1217–1228. doi: 10.1016/j.eururo.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 37.Mitterberger M, Pinggera GM, Marksteiner R, Margreiter E, Fussenegger M, Frauscher F, et al. Adult stem cell therapy of female stress urinary incontinence. Eur Urol. 2008;53:169–175. doi: 10.1016/j.eururo.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Djouad F, Bouffi C, Ghannam S, Noel D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nature reviews Rheumatology. 2009;5:392–399. doi: 10.1038/nrrheum.2009.104. [DOI] [PubMed] [Google Scholar]
- 39.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10(Suppl):S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 40.Shudo Y, Miyagawa S, Fukushima S, Saito A, Shimizu T, Okano T, et al. Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J Thorac Cardiovasc Surg. 2011;142:1188–1196. doi: 10.1016/j.jtcvs.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Epstein SE, Kornowski R, Fuchs S, Dvorak HF. Angiogenesis therapy: amidst the hype, the neglected potential for serious side effects. Circulation. 2001;104:115–119. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
- 42.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 44.Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 45.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. Journal of the Royal Society, Interface / the Royal Society. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine. 2005;1:22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 48.Khanna O, Moya ML, Opara EC, Brey EM. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A. 2010;95:632–640. doi: 10.1002/jbm.a.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials. 2010;31:1235–1241. doi: 10.1016/j.biomaterials.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X, Su J, Bao J, Peng T, Zhang L, Zhang Y, et al. Cytokine combination therapy prediction for bone remodeling in tissue engineering based on the intracellular signaling pathway. Biomaterials. 2012;33:8265–8276. doi: 10.1016/j.biomaterials.2012.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heldin CH. Structural and functional studies on platelet-derived growth factor. EMBO J. 1992;11:4251–4259. doi: 10.1002/j.1460-2075.1992.tb05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabinovsky ED, Gelir E, Gelir S, Lui H, Kattash M, DeMayo FJ, et al. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J. 2003;17:53–55. doi: 10.1096/fj.02-0183fje. [DOI] [PubMed] [Google Scholar]