Abstract
C-phycocyanin (C-PC) is a blue colored accessory photosynthetic pigment found in cyanobacteria. Some of the medicinal properties of Spirulina have been attributed to this pigment, which includes anticancer, antioxidant, and anti-inflammatory activity. We have screened cyanobacteria isolated from freshwater habitats in Florida for their high content of C-PC. Of 125 strains tested, one filamentous strain identified as Limnothrix sp. was selected for further research. This strain produced 18% C-PC of total dry biomass. Here we describe a simple method for obtaining C-PC of high purity without the use of ion exchange chromatography. The procedure is based on pigment precipitation from the cell lysate with an appropriate concentration of ammonium sulfate, then purification with activated carbon and chitosan, followed by a sample concentration using tangential flow filtration. We have shown that when the lower concentration of ammonium sulfate was used, C-PC with higher purity index was recovered. Characterization of C-PC from Limnothrix showed that it had an absorbance maximum at 620 nm and fluorescence at 639 nm. The molecular mass of intact C-PC was estimated to be ~50 kDa with α and β subunits forming dimmers. When C-PC content per unit biomass was compared to that of marketed Spirulina powder, we found that Limnothrix was superior. C-phycocyanin from Limnothrix had an antioxidative activity on DPPH free radicals similar to that found in a natural antioxidant – rutin.
Keywords: Cyanobacteria, C-phycocyanin, Purification, Antioxidative activity
1. Introduction
C-phycocyanin is a blue colored photosynthetic accessory pigment that absorbs light at about 620 nm and emits fluorescence at about 640 nm. It is a phycobiliprotein with a molecular mass of about 110 kDa containing two subunits (Glazer and Stryer, 1984). Unlike other phycobiliproteins (phycoerythrin and allophycocyanin), C-PC is a major accessory pigment and it is present in all cyanobacteria. This pigment is traditionally isolated from Spirulina (Boussiba and Richmond, 1979; Herrera et al., 1989; Patil et al., 2006; Bhaskar et al., 2005) however, some other cyanobacterial genera have also been used (Santiago-Santos et al., 2004).
Isolation and purification of C-PC is a multistep process that includes fractional precipitation with ammonium sulfate, ion exchange chromatography and gel filtration (Herrera et al., 1989; Zhang and Chen, 1999; Bhaskar et al., 2005). Besides this general procedure, attempts were made to optimize C-PC purification by including the use of rivanol (Minkova et al., 2003), chitosan and charcoal (Patil et al., 2006), and hydrophobic interaction chromatography (Soni et al., 2008).
Spirulina has been reported to have a number of medicinal properties (Belay et al., 1993; Gantar and Svircev, 2008), some of which have been attributed to C-PC. One of the first reports on beneficial effect of C-PC (Belay et al., 1993) cites the Japanese patent #58-65216 (Dainippon Ink & Chemicals, 1983), which claims that this blue pigment from Spirulina significantly increases the survival rates of mice that had been injected with liver tumour cells. It has been further suggested that stimulation of the immune system by C-PC was a mechanism that inhibited growth of tumour cells. In fact, a number of authors have described the induction of apoptosis as a mechanism of C-PC activity (Reddy et al., 2003; Bobbili et al., 2003).
C-PC was shown to suppress allergic inflammation reactions in different animal models. This pigment reduces inflammation of the small intestine in mice by suppressing the level of antigen specific IgE antibody (Nemoto-Kawamura et al., 2004), prevents colonic damage in acetic acid-induced colitis in rats (Gonzales et al., 1999), and inhibits the induced mouse ear oedema by reducing PGE2 (prostaglandin E2 levels) (Romay et al., 1998). Remirez et al. (2002) suggest that the inhibition of allergic inflammatory response by C-PC is mediated, at least in part, by inhibition of histamine release from mast cells. Hypocholesterolemic effect of Spirulina platensis concentrate was attributed to C-PC and its effect on inhibition of both jejunal cholesterol absorption and ileal bile acid reabsorption (Nagaoka et al., 2005).
Besides a potential use for therapeutic purposes, C-PC is being used as a fluorescent marker in biomedical research (Glazer, 1994). Apparently, there is a great interest for C-PC research and therefore productive organisms and new technologies for its isolation and purification are needed. In this work, we assess the ability of different cyanobacterial strains to produce C-PC in amounts equal or higher to those obtained from traditionally used Spirulina. We also describe a new and simple technique for isolation of this pigment. In addition, we have characterized the pigment obtained from the novel strain of Limnothrix and assessed its antioxidative activity.
2. Materials and methods
2.1. Screening of organisms
We screened 125 cyanobacteria collected from freshwater habitats in Florida from our culture collection for the isolates with high C-PC content. Taxonomic identity of the isolates was based on morphological characteristics (Anagnostidis and Komarek, 1988). The taxonomic identity of the strain that showed the highest content of C-PC, was confirmed by 16S rRNA gene sequencing and BLASTN search. Isolation of the total genomic DNA, 16S rRNA gene amplification, and its sequencing were performed as described elsewhere (Myers et al., 2007). The cyanobacteria specific primers CYA359F and CYA781R(b) (Nubel et al., 1997) were used for PCR amplification and the sequencing of the 16S rRNA gene.
To determine the C-PC content in the individual strains, all strains were grown in 3-liter cultures in BG11 medium (Rippka et al., 1979) under cool-white light (50 µE m−1 s−1), temperature of 25 °C, and aeration with sterile air. The biomass was harvested by centrifugation, freeze dried, and kept in a freezer until further use. In order to obtain the cell extract, 100 mg of dry biomass was suspended in 10 ml of 0.1 M phosphate buffer pH 7.0 and left in refrigerator (4 °C) for overnight extraction. Those cell extracts that showed blue color, were centrifuged and filtered through 0.45 µm pore size filters (Millipore, Billerica, MA) and the amount of CPC was determined spectrophotometrically (Bennett and Bogorad, 1973).
2.2. Isolation and purification of phycocyanin
For isolating larger amounts of C-PC and for the purpose of optimizing the purification process, the selected strain Limnotrhrix sp. 37-2-1 was grown in a 30-liter bioreactor under the same condition as described above. The harvested biomass was suspended in distilled water (Silveira et al., 2007) and repeatedly freeze–thawed (Soni et al., 2006; Zhang and Chen, 1999) at least three times for the purpose of lyzing the cells. The crude cell lysate, which had an intense blue color, was sequentially filtered through Whatman 43, Whatman GF/B (Maidstone, UK), and 0.47 µm Millipore filters (Billerica, MA).
This crude extract was stirred on a magnetic stirrer with activated carbon (1%, w/v) (Darco, 20–40 mesh granulated, Sigma, St. Louis, MO, USA) and chitosan (Sigma, St. Louis, MO, USA) to a final concentration of 0.01 g l−1. After 15 min of stirring, the crude extract was centrifuged at 4000 × g and the supernatant was precipitated with different concentrations of ammonium sulfate, ranging from 20% to 60% (with increments of 5%). The precipitation was done overnight at 4 °C. The precipitate was separated by centrifugation at 14,000 × g and temperature of 15 °C. The precipitate was re-suspended in 0.1 M phosphate buffer pH 7.0. Desalting and concentrating the pigment was performed by a tangential flow filtration system (Labscale TFF System, Millipore, Billerica, MA, USA) using a membrane with the pore size of 30 kDa (Pellicon XL, PLCTK 30, Millipore, Billerica, MA, USA). The purity of the pigment was assessed by calculating the ratio of absorbencies at 620/280 (Boussiba and Richmond, 1979) and with using SDS-PAGE, which was supposed to reveal the presence of contaminating proteins. Pigment concentration and its purity (620/280 ratio) was determined in each individual step during the purification process (Table 2). The purified phycocyanin was freeze-dried and kept in a refrigerator until further use. The presented data are the means of measurements from three different experiments by using the same batch of cyanobacterial biomass.
Table 2.
Recovery of C-PC from different sources of dried biomass and its purity (620/280 ratio) in different stages of purification process. The presented data are the means obtained from three experiments.
| Crude extract |
After A.C.a and chitosan |
After precipitation with A.S.b |
After TTFc |
|||||
|---|---|---|---|---|---|---|---|---|
| C-PC content (%) | 620/280 ratio | C-PC content (%)d | 620/280 ratio | C-PC content (%)d | 620/280 ratio | C-PC content (%)d | 620/280 ratio | |
| Limnothrix | 18 ± 1.9 | 2.0 | 15.3 ± 1.2 | 3.6 | 10.9 ± 0.9 | 4.2 | 8 ± 0.7 | 4.3 |
| Spirulina 1 | 7 ± 0.8 | 0.5 | N/A | N/A | 6.1 | 1.1 | 3.2 | 1.5 |
| Spirulina 2 | 1 ± 0.01 | 0.2 | N/A | N/A | 0.9 | 0.5 | 0.6 | 0.8 |
A.C. – activated carbon.
A.S. – ammonium sulfate.
TTF – tangential flow filtration.
C-PC content – percent of C-PC in dry biomass.
For comparisons reasons, the phycocyanin was also isolated from powdered Spirulina samples manufactured and marketed by two different companies (designated as Spirulina #1 and Spirulina #2) by using the same procedure. For this experiment, the Limnothrix biomass was freeze dried and used for extraction. The Spirulina samples were purchased in health food stores.
2.3. Spectroscopic measurements
During the purification process, the absorption of C-PC in the visible and UV range was measured on the 96-well plate reader Synergy 2 (BioTek, Winooski, VT, US). Fluorescence emission and excitation spectra were determined on the PC1 fluorometer (ISS, Champaign IL) and UV-vis absorption spectra were measured on a single beam spectrophotometer (Cary-50 Varian). Samples for fluorescence or absorbance measurements were prepared by dissolving 30 µg mL−1 of C-PC in a 50 mM phosphate buffer pH 7.0. Excitation spectra were measured through a 700 nm cut-off filter and the emission spectra were recorded using 620 nm excitation and 0.5 mm width slits.
2.4. Polyacrylamide gel electrophoresis
To check the purity of the isolated pigment, and to estimate molecular mass, sodium dodecyl sulfate–polyacrylamid gel electrophoresis (SDS–PAGE) of purified phycocyanin from Limnothrix sp. 37-2-1 was performed. For that purpose, PAGEr®Gold precats gels (Lonza, Rockland, ME, USA) were used.
2.5. Determination of molecular mass
Estimation of the molecular mass of C-PC was based on rotational correlation time determined in time-resolved anisotropy measurements. For that purpose, a Chrono fluorometer (ISS, IL USA) equipped with a xenon lamp as an excitation source was used. The steady-state anisotropy data were collected using a 650 nm long-pass filter. C-PC concentration was determined using an extinction coefficient of ε620 nm = 0.00256 µg mL−1. C-PC samples for anisotropy measurements were freshly prepared from lyophilized protein, by dissolving C-PC in 50 mM phosphate buffer pH 7.0 at final concentration of 30 µg mL−1. To favor the formation of the (αβ) monomer, C-PC was dissolved in 50 mM phosphate buffer pH 7.0 and 0.9 M KSCN to – a final concentration of 30 µg mL−1 (Pizarro and Sauer, 2001). Fluorescence lifetimes and time resolved anisotropy data were measured in frequency domain using the ChronosFD fluorometer (ISS, IL, USA), equipped with a 470 nm laser diode and the emitted light was collected through a 600 nm long pass filter (Andover Corp, USA). Rhodamine B solubilized in water was used as a lifetime standard (τ = 1.68 ns). Samples were placed in a quartz cuvette with 1 cm excitation and 0.1 cm emission path and measurements were recorded at room temperature. Time resolved anisotropy data were analyzed with a double exponential model using Globals WE software (LFD, Irvine, USA). From rotational correlation times, the molecular mass of protein was estimated using the Stokes–Einstein relationship (Φ = ηV/RT), where η is the viscosity of water (0.00089 Pa s), R is the gas constant, T is the temperature and V is the molecular volume of the protein.
2.6. Antioxidative activity of C-phycocyanin
Antioxidant activity of C-PC from Limnothrix and two Spirulina samples was determined by electron spin resonance (ESR) spectroscopy (Čanadanović-Brunet et al., 2009). All ESR spectra were recorded on an ESR spectrometer Bruker 300E (Rheinstetten, Germany). The concentrations of investigated samples were in the range of 0.05–0.3 mg ml−1. As a standard, DPPH (2,2-diphenyl-1-picrylhydrazyl) a synthetic stable free radical was used. ESR measurements were performed under the following conditions: field modulation 100 kHz, modulation amplitude 0.256 G, receiver gain 2 × 104, time constant 40.96 ms, conversion time 327.68 ms, center field 3440.00 G, sweep width 100.00 G, x-band frequency 9.45 GHz, power 7.96 mW, and temperature 23 °C. The antioxidative activity of C-PC was defined as: AADPPH = 100 × (ho − hx)/ho [%], where ho and hx are the height of the second peak in the ESR spectrum of DPPH radicals of the blank and the probe, respectively.
3. Results
3.1. Screening for a productive strain
Among the 125 cyanobacteria tested, 10 strains produced a distinctive blue-colored extract which was indicative of high C-PC content. The highest C-PC content (Table 1) was found in Limnothrix sp. strain 37-2-1. The second highest was Anabaena sp. strain 66-2 which had less than half of the content of Limnothrix sp. strain 37-2-1. In addition, Limnothrix sp. strain 37-2-1 had the highest purity index of the crude extract and the highest growth yield under applied laboratory conditions. Taxonomic identity of this organism was confirmed by 16S rDNA gene sequencing and by BLASTN search, which indeed showed that the closest relative was Limnothrix redekei M2-7 (GenBank accession number EF634458.1).
Table 1.
Cyanobacterial strains that showed high content of phycocyanin, their origin, biomass yield, and phycocyanin purity in a crude extract, based on A615/280 ratio. Percent of C-PC content in biomass was calculated in a crude extract by using spectrophotometric method and it is based on measurements of three separate extractions.
| Genus | Strain | Origin | Biomass yield (g L−1) | % PC in biomass | 615/280 ratio |
|---|---|---|---|---|---|
| Aphanotheca | 80–12a | Lake Ontario, NY | 1.05 ± 0.10 | 4.0 | 0.49 |
| Anabaena | 40-3 | Lake Seminole, FL | 0.70 ± 0.06 | 1.0 | 0.46 |
| Anabaena | 66-2 | Lake Seminole, FL | 0.60 ± 0.05 | 8.0 | 1.10 |
| Limnothrix | 39-1 | Lake Dora, FL | 1.00 ± 0.08 | 4.7 | 0.71 |
| Limnothrix | 37-2-1 | Crescent Lake, FL | 1.20 ± 0.10 | 18.0 | 2.08 |
| Limnothrix | 48-1 | Lake Ariana, FL | 0.59 ± 0.06 | 2.5 | 0.35 |
| Limnothrix | 21-2-2 | STA-1Wa | 0.50 ± 0.04 | 5.6 | 0.50 |
| Lyngbya | 15-2 | C111b | 0.60 ± 0.04 | 5.0 | 0.61 |
| Nostoc | 47-3 | Lake Placid, FL | 0.45 ± 0.05 | 4.0 | 0.35 |
| Synechococcus | 21-11 | STA-1 W | 0.35 ± 0.02 | 5.2 | 0.40 |
Storm water treatment area 1 W, FL.
Everglades C111 canal, FL.
3.2. Isolation of phycocyanin
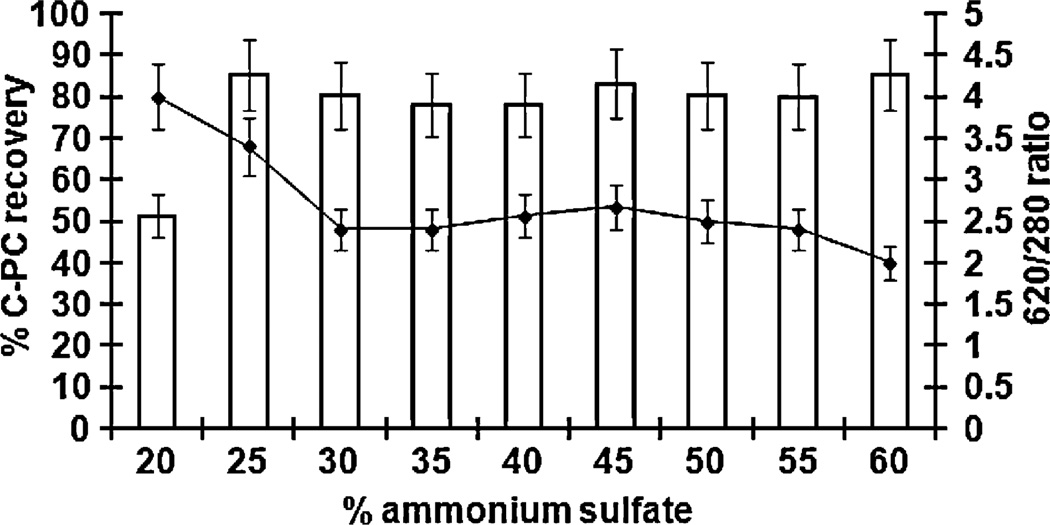
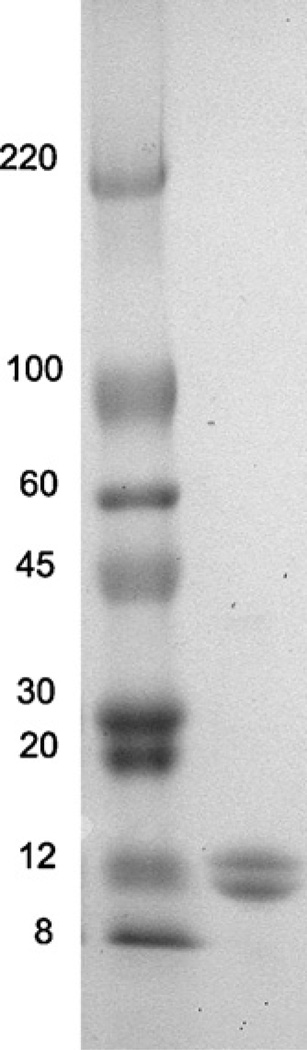
Phycocyanin was purified from the selected strain Limnotrix sp. 37-2-1 as well as from two commercially available samples of Spirulina. Introduction of the treatment with the activated carbon and chitosan, significantly improved the purity of the pigment in the crude extract. This step increased the 620/280 ratio from 2.0 to 3.6 but also contributed to reduction of the pigment yield (Table 1). Efficacy of C-PC precipitation with different ammonium sulfate concentrations (done with Limnothrix sp. 37-2-1 only) showed that increasing the ammonium sulfate concentration from 20% to 25% significantly increased the recovery of C-PC (Fig. 1). However, further increase of the ammonium sulfate concentration did not affect the recovery of C-PC, but on the other hand, it did reduced the purity of the pigment (according to 620/280 ratio) (Fig. 1). Therefore, in our further work, C-PC was purified with 25% ammonium sulfate. The final step, which included tangential flow filtration, yielded 8% C-PC of dry biomass with the purity index (620/280 ratio) of 4.3. The purity of the C-PC was also tested with SDS-PAGE, which showed two bands representing α and β subunits (Fig. 4).
Fig. 1.
C-phycocyanin recovery (line) and purity index (bars) obtained by precipitation with different ammonium sulfate concentrations. The bars indicate the standard error of three measurements.
Fig. 4.
SDS-PAGE of C-PC; lane 1 – prestained protein standards (ColorBurst, Sigma, St. Louise, MI, USA); lane 2 – C-PC from Limnothrix sp. 37-2-1 after purification.
When the C-PC yield of Limnothrix was compared to that of two commercially available Spirulina samples by using the same procedure, it was shown that the crude extract of Limnothrix 37-2-1 contained 18% of C-PC while the samples of two Spirulina had a considerably lower yield (Table 2). Similarly, Limnothrix crude extract had an initial 620/280 ratio of 2.0, compared to 0.5 and 0.2 of two tested Spirulina biomass respectively (Table 2).
3.3. Characterization of C-phycocyanin
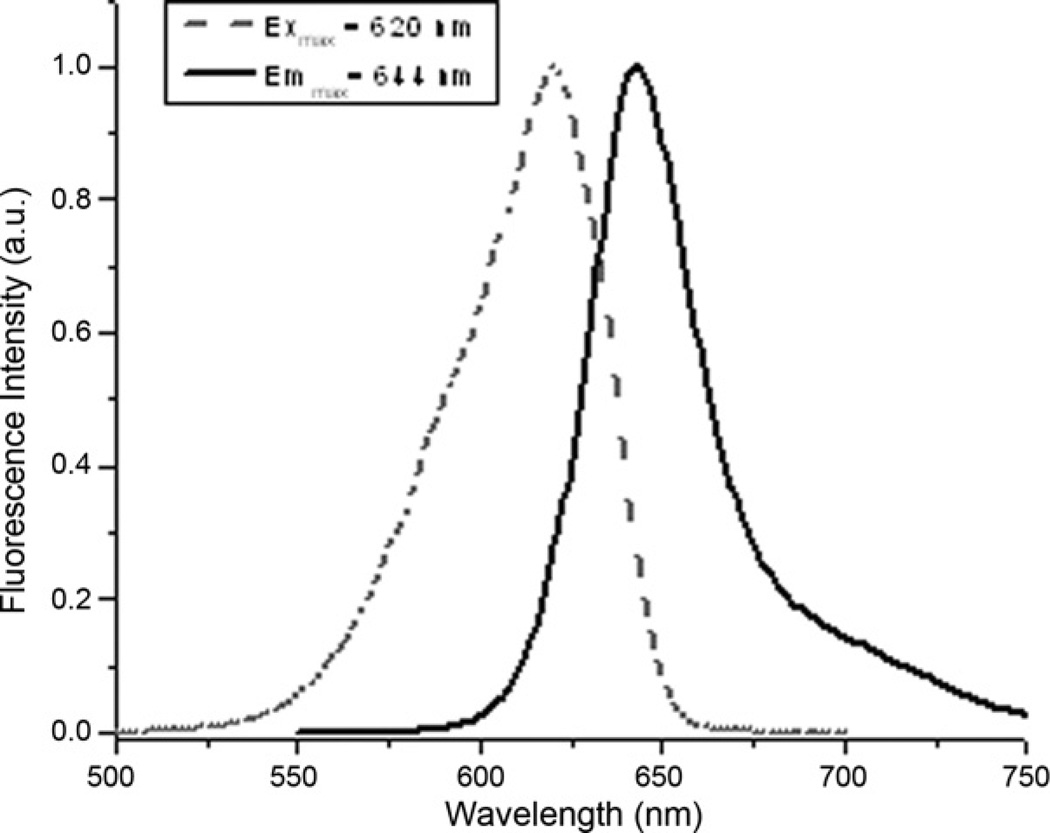
Absorbance spectra of C-PC showed a pick on 620 nm while the fluorescence spectra had a maximum at 644 nm (Fig. 3). The molecular mass of C-PC in the (αβ) monomeric form and C-PC oligomer was estimated based on time-resolved anisotropy data. Time-resolved anisotropy data provided two rotational correlation times. The shorter correlation time of 0.46 ± 0.05 ns and 0.2 ± 0.10 ns observed for the monomeric and oligomeric form of C-PC, respectively, can be associated with the local motion of the fluorophore. While, the longer correlation time of 6.9 ± 0.8 ns and 13.0 ± 2.1 ns for the monomeric and oligomeric form, respectively, reflects to the global motion of the protein. Based on the correlation time and using Stokes–Einstein equation we have estimated the molecular mass of C-PC in the monomeric form to be 26 ± 3 kDa and 50 ± 8 kDa for the oligomer. The estimated molecular mass of the (αβ) monomer is in agreement with the molecular mass determined using SDS–PAGE electrophoresis (Fig. 4), ~13 kDa for the α-subunit and ~11 kDa for the β-subunit. The estimated molecular mass of the oligomeric form of ~50 kDa, suggest that C-PC subunits form dimmers (αβ)2. The detailed characterization of C-PC fluorescence lifetime and time-resolved anisotropy will be published separately.
Fig. 3.
Excitation and emission spectra of phycocyanin from Limnothrix sp. strain 37-1-2 after purification with the described procedure.
3.4. Antioxidative activity
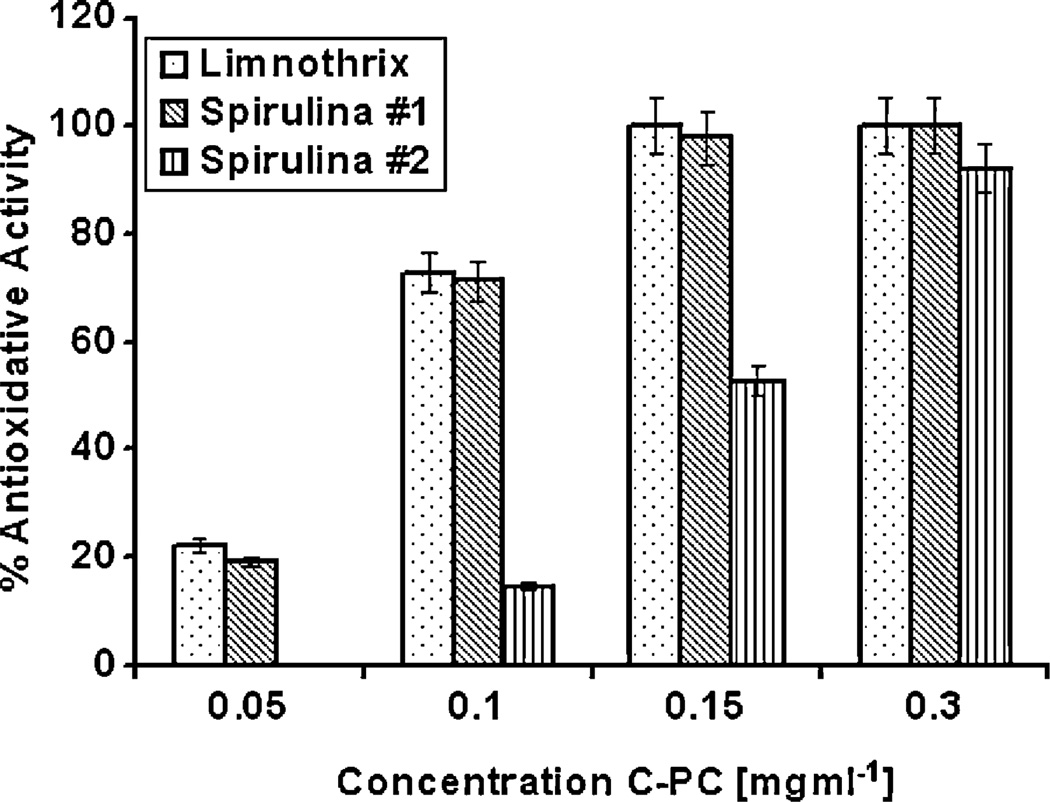
The highest antioxidative activity was obtained with C-PC purified from Limnothrix sp. 37-2-1, which exhibited 100% activity at the concentration of 0.15 mg ml−1 (Fig. 2). Spirulina #1 had the activity that was similar to that of Limnothrix however, C-PC that was isolated from Spirulina #2, did not have any activity at the lowest tested concentration. The half maximal effective concentration (EC50) value of C-PC from Limnothrix and Spirulina #1 was about 0.08 mg ml−1 which is somewhat higher, but still comparable to antioxidant activity of rutin at a EC50 value of 0.055 mg ml−1 (Čanadanović-Brunet et al., 2009).
Fig. 2.
Antioxidative activity of C-phycocyanin from Limnothrix sp. 37-2-1 and two commercially available Spirulina powder. The bars indicate the standard error of three measurements.
4. Discussion
Of 125 freshwater strains that we screened, only 8% showed high level of C-PC cell content. Interestingly, of 10 strains that had high C-PC content, four strains belonged to genus Limnothrix, which might indicate that this is characteristic of the genus. Among those strains, Limnothrix sp. strain 37-2-1 produced the highest level of C-PC, which amounted to 18% of biomass dry weight in a crude extract. Traditionally C-PC is extracted and purified from Spirulina, however some other organisms such as Calothrix (Santiago-Santos et al., 2004), Aphanizomenon flos-aquae (Benedetty et al., 2004), Phormidium and Lyngbya (Patel et al., 2005) were also used. To our knowledge, this is the first report on Limnothrix as a potential novel source of C-PC.
There are number of different procedures describing the isolation and purification of C-PC (Boussiba and Richmond, 1979; Bhaskar et al., 2005; Minkova et al., 2003). Most of them are based on ion exchange chromatography techniques, which are known to be time-consuming and not able to produce large amounts of the purified product. Here we describe a simple and efficient technique for obtaining C-PC of high quality. Our method is based on (i) obtaining a crude extract from fresh biomass; (ii) purification of the crude extract with chitosan and activated carbon, (iii) selective saturation with ammonium sulfate, (iv) and further purification and concentration by using tangential flow filtration. Introducing a step of chitosan/activated carbon treatment greatly improved the quality of the C-PC, as already reported by Patil et al. (2006).
We have shown that the concentration of 20% of ammonium sulfate provided C-PC with the highest purity index of (620/280 absorbance ratio = 4) without the use ion exchange chromatography. Even though the lower ammonium sulfate concentration provided a purer C-PC (Fig. 1), higher recovery of the pigment was obtained with the higher concentration of ammonium sulfate (25%). This amounted in 72% increase in recovery, and therefore, we consider that the concentration of 25% of ammonium sulfate should be used as a compromise between high yield and high quality of the recovered C-PC. Interestingly, in their process of C-PC purification from Spirulina, Patel et al. (2005) discarded the 25% ammonium sulfate saturation fraction as the one that contained “other proteins” and used the 50% saturation fraction. Similarly, Zhang and Chen (1999) also discarded the fraction precipitated with 30% ammonium sulfate, while those from 50% contained mainly C-PC. One possible explanation for this discrepancy is that there is a difference in physico-chemical properties of C-PC obtained from Limnothrix and that from Spirulina. This is supported by observation of Silva et al. (2009) that “each biomass shows a different behavior with the respect to the different purification processes”.
It is generally accepted that C-PC with the purity ratio (A620/280) of four and above is of analytical grade (Bhaskar et al., 2005). Our procedure produced C-PC from Limnothrix sp. 37-2-1 with the purity ratio of 4.3, which satisfies the standard for analytical grade. It appears that obtaining high quality C-PC from Limnothrix without the use of ion exchange chromatography can be attributed to the fact that the initial material (the crude extract) had relatively high purity ratio (620/280 = 2.08) compared to those obtained from Spirulina which ranges between 0.45 (Doke, 2005) and 1.14 (Zhang and Chen, 1999). SDS-PAGE electrophoresis showed that the described procedure yielded a product without protein contamination. The tangential flow filtration was used to eliminate the salts however the quantity of residual salt in the final product was not determined.
When the data on phycocyanin yield from Limnothrix were compared to those obtained from the traditionally used Spirulina, it was shown that the biomass of two tested commercially available Spirulina products contained considerably less pigment per unit dry biomass. Not only that, but the purity of phycocyanin from two Spirulina samples after purification had a 620/280 ratio of only 1.5 and 0.8 respectively. Considering the fact that most literature data show that the C-PC content in Spirulina ranges between 10% and 17.5% of dry biomass (Vonshak, 1990; Patel et al., 2005), our data were inconsistent. Nevertheless, when the same Spirulina samples were analyzed again by the same procedure six month later, we obtained even lesser amounts of C-PC per unit biomass. For example, when Spirulina #1 was analyzed right after being purchased from a health food store, it had a C-PC content of 7% of total biomass. When the same sample (kept in the dark bottle at the room temperature in the laboratory) was analyzed after six months and after two years (data not presented), the C-PC content amounted for 5% and 1.6% respectively. Apparently, the quantity of C-PC within the dry Spirulina biomass decreases over time. Based on these findings, we believe that additional research is needed to assess how the shelf life affects the quality of Spirulina products.
The reported molecular mass of C-PC in different cyanobacterial genera ranges for the oligomer between 81 and 215 kDa and that for individual subunits (αβ) between 15.2 and 24.4 kDa (Santiago-Santos et al., 2004; Patel et al., 2005; Soni et al., 2006, 2008). In most cases, the subunits are organized in trimers. While, in the case of the C-PC isolated from our novel strain of Limnothrix 37-2-1, oligomeric mass was estimated to be ~50 kDa, with the α and β subunits of 13 and 11 kDa, respectively, and were organized in dimmers. Therefore, it appears that Limnothix 37-2-1 has one of the smallest molecular masses of C-PC described so far.
We have shown that C-PC from Limnothrix has antioxidative activity, a property that was previously described by a number of authors (Benedetty et al., 2004; Romay et al., 2003; Patel et al., 2005; Hirata et al., 2004). For example, Romay et al. (2003) demonstrated that C-PC was able to scavenge alkoxyl, hydroxyl and peroxyl radicals and to react with peroxynitrite (ONOO–) and hypochlorous acid (HOCl). In addition, they showed that C-PC inhibited microsomal lipid peroxidation. It has been shown (Patel et al., 2005) that there are differences in aggregation between C-PC subunits in Spirulina and Lyngbya and it has been suggested that this may change the biochemical properties of C-PC obtained from different organisms. Therefore, specificity of biological activity of C-PC obtained from different cyanobacteria should be further investigated.
In conclusion, we have shown that cyanobacteria other than Spirulina can be a potentially superior source of C-PC. The applied method for C-PC purification from Limnothrix is simple and does not require gel filtration. The molecular mass of C-PC from Limnothrix is the smallest reported so far and therefore its biological activity might be different from other cyanobacteria. C-phycocyanin from Limnothrix has an antioxidative activity comparable to one found in rutin. In this study, we have also shown that the quality of Spirulina products marketed by different companies can vary significantly in terms of their C-PC content.
Acknowledgements
This work was in part supported by ARCH program NIH/NIEHS S11 ES 11181. We would like to thank Emily Broderick for editing our English.
References
- Anagnostidis K, Komarek J. Modern approach to the classification system of Cyanophytes. 3-Oscillatoriales. Arch. Hydrobiol. Suppl. 1988;80:327–472. [Google Scholar]
- Belay A, Ota Y, Miyakawa K, Shimamatsu H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993;5:235–241. [Google Scholar]
- Benedetty S, Benvenutti F, Pagliarani S, Francogli S, Scoglio S, Canestrari F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004;75:2353–2362. doi: 10.1016/j.lfs.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bennett A, Bogorad L. Complementary adaptation in a filamentous bluegreen alga. J. Cell Biol. 1973;58:419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar SU, Gopalaswamy G, Raghu R. A simple method for efficient extraction and purification of C-phycocyanin from Spirulina platensis Geitler. Indian J. Exp. Biol. 2005;43:277. [PubMed] [Google Scholar]
- Bobbili VV, Mubarak PA, Kumari AAL, Reddana P, Khar A. Phycocyaninmediated apoptosis in AK-5 tumor cells involves down-regulation of Bcl-2 and generation of ROS. Mol. Cancer Therap. 2003;2:1165–1170. [PubMed] [Google Scholar]
- Boussiba S, Richmond AE. Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch. Microbiol. 1979;120:155–159. [Google Scholar]
- Čanadanović-Brunet JM, Četković GS, Djilas SM, Tumbas VT, Savatović SS, Mandić AI, Markov SL, Cvetković DD. Radical scavenging and antimicrobial activity of horsetail (Equisetum arvense L.) extracts. Int. J. Food Sci. Technol. 2009;44:269–278. [Google Scholar]
- Dianippon Ink & Chemicals (DIC); Lijima N, Fujii N, Shimamatsu H, inventors. Dainippon Ink & Chemical Tokyo Stress Foundation, assignee. Antitumoral agents containing phycobillin. #58-65216. Japanese Patent. 1983 Apr 18;:6.
- Doke JM. An improved and efficient method for the extraction of phycocyanin from Spirulina sp. Int. J. Food Engin. 2005;1:1–11. [Google Scholar]
- Gantar M, Svircev Z. Microalgae and cyanobacteria: food for thought. J. Phycol. 2008;44:260–268. doi: 10.1111/j.1529-8817.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- Glazer A, Stryer L. Phycofluor probes. TIBS. 1984;9:423–427. [Google Scholar]
- Glazer AN. Phycobiliproteins a family of valuable, widely used fluorophores. J. Appl. Phycol. 1994;6:105–112. [Google Scholar]
- Gonzales R, Rodriguez, Romay C, Ancheta O, Gonzales A, Armesto J, Remirez D, Merino N. Anti-inflammatory activity of phycocyanin extract in acetic acid-induced colitis in rats. Pharmacol. Res. 1999;39:55–59. [PubMed] [Google Scholar]
- Herrera A, Boussiba S, Napoleone V, Hohlberg A. Recovery of Cphycocyanin from the cyanobacterium Spirulina maxima . J. Appl. Phycol. 1989;1:325–331. [Google Scholar]
- Hirata T, Tanaka M, Ooike M, Tsunomura T, Sakaguchi M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis . J. Appl. Phycol. 2004;12:435–439. [Google Scholar]
- Minkova, Tchernov KM, Tchorbadjieva AA, Fournadjieva MI, Antova ST, Busheva RE, Ch M. Purification of C-phycocyanin from Spirulina (Athrospira) fusiformis. J. Biotechnol. 2003;102:55–59. doi: 10.1016/s0168-1656(03)00004-x. [DOI] [PubMed] [Google Scholar]
- Myers JL, Sekar R, Richardson LL. Molecular detection and ecological significance of the cyanobacterial genera Geitlerinema and Leptolyngbya in black band disease of corals. Appl. Environ. Microbiol. 2007;73:5173–5182. doi: 10.1128/AEM.00900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S, Shimizu K, Kaneko H, Shibayama F, Morikawa K, Kanamaru Y, Otsuka A, Hirahashi T, Kato T. A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J. Nutr. 2005;135:2425–2430. doi: 10.1093/jn/135.10.2425. [DOI] [PubMed] [Google Scholar]
- Nemoto-Kawamura C, Hirahashi T, Nagai T, Yamada H, Katoh T, Hayashi O. Phycocyanin enhances secretary IgA antibody response and suppresses allergic IgE antibody response in mice immunized with antigen-entrapped biodegradable microparticles. J. Nutr. Sci. Vitaminol. (Tokyo) 2004;50:129–136. doi: 10.3177/jnsv.50.129. [DOI] [PubMed] [Google Scholar]
- Nübel UF, Garcia-Pichel F, Muyzer G. PCR primers to amplify [16S] rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997;63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Mishra S, Pawar R, Ghosh RP. Purification and characterization of C-phycocyanin a from cyanobacterial species of marine and freshwater habitat. Protein Express. Purif. 2005;40:248–255. doi: 10.1016/j.pep.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Patil G, Chethana S, Sridevi AS, Raghavarao KSMS. Method to obtain C-phycocyanin of high purity. J. Chromatogr. A. 2006;1127:76–81. doi: 10.1016/j.chroma.2006.05.073. [DOI] [PubMed] [Google Scholar]
- Pizarro SA, Sauer K. Spectroscopic study if the light-harvesting protein Cphycocyanin associated with olorless linker peptides. Photochem. Photobiol. 2001;73:556–563. doi: 10.1562/0031-8655(2001)073<0556:ssotlh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reddy MC, Subhashini J, Mahipal SVK, Bhat VP, Reddy PS, Kiranmai G, Madyastha KM, Reddanna P. C-Phycocyanin, a selective cyclooxygenase-2 inhibitor, induces apoptosis in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2003;304:385–392. doi: 10.1016/s0006-291x(03)00586-2. [DOI] [PubMed] [Google Scholar]
- Remirez D, Ledon N, Gonzales R. Role of histamine in the inhibitory effects of phycocyanin in experimental models of allergic inflammatory response. Mediators Inflamm. 2002;11:81–85. doi: 10.1080/09629350220131926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979;111:1–61. [Google Scholar]
- Romay C, Armesto J, Remirez D, Gonzalez R, Ledon N, Garcis I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998;47:36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- Romay Ch, González R, Ledón1 N, Remirez1 D, Rimbau V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein. Pept. Sci. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- Santiago-Santos Ma.C, Ponce-Noyola T, Olivera-Ramirez R, Ortega-Lopez J, Canizares-Villanueva RO. Extraction and purification of phycocyanin from Calothrix sp. Proc. Biochem. 2004;39:2047–2052. [Google Scholar]
- Silva LA, Kuhn KR, Moraes CC, Burkert CAV, Kalil SJ. Experimental design as a tool for optimization of C-phycocyanin purification by precipitation from Spirulina platensis . J. Braz. Chem. Soc. 2009;20:5–12. [Google Scholar]
- Silveira ST, Burket JFM, Costa JAV, Burket CAV, Kalil SJ. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007;98:1629–1634. doi: 10.1016/j.biortech.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Soni B, Kalavadia B, Trivedi U, Madamwar D. Extraction, purification and characterization of phycocyanin from Oscillatoria quadripunctulata-isolated from the rocky shores of Bet-Dwarka, Gujarat, India. Process Biochem. 2006;41:2017–2023. [Google Scholar]
- Soni B, Trivedi U, Madamwar D. A novel method of single step hydrophobic interaction chromatography for the purification of phycocyanin from Phormidium fragile and its characterization for antioxidant property. Biores. Technol. 2008;99:188–194. doi: 10.1016/j.biortech.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Vonshak A. Recent advances in microalgal biotechnology. Biotech. Adv. 1990;8:709–727. doi: 10.1016/0734-9750(90)91993-q. [DOI] [PubMed] [Google Scholar]
- Zhang Y-M, Chen F. A simple method for efficient separation and purification of c-phycocyanin and allophycocyanin from Spirulina platensis . Biotech. Techniques. 1999;13:601–603. [Google Scholar]