Abstract
Despite increasing rates of opioid abuse by human adolescents, few laboratory experiments address adolescent vulnerability to opiates. We examined intravenous morphine self-administration after adolescent- vs. adult-onset, followed by extinction and cue-induced reinstatement. Adolescent male Sprague-Dawley rats [postnatal day (P) 35 at start] and adults (P91) acquired lever pressing maintained by 0.375 mg/kg/infusion morphine on a fixed ratio one schedule of reinforcement. Subjects were subsequently divided into short or long daily access conditions (ShAcc, 1-hr vs. LgAcc, 8-hr; 18 sessions). After extinction, cue-induced reinstatement was recorded over 1 hr. During the first six 1-hr acquisition sessions and continuing throughout ShAcc conditions, adolescent-onset rats self-administered less morphine than adults, an effect commonly interpreted as higher drug sensitivity. In contrast under LgAcc conditions, escalation of morphine intake was similar across ages. Extinction of drug-seeking was similar across ages, although rats from LgAcc conditions pressed more than ShAcc conditions. Notably, cue-induced reinstatement was less robust in rats that began morphine self-administration during adolescence vs. adulthood. Although increased sensitivity of younger rats to morphine reinforcement under ShAcc conditions might help explain opioid abuse by human adolescents, lower rates of reinstatement in younger rats might suggest that adolescent development includes some protective factors that dampen the long-term impact of early drug intake.
Keywords: narcotic, periadolescent, ontogeny, relapse, extended access, limited access, drug loading, in vivo
Introduction
Adolescent and young adult humans aged 12–25 years old are more likely than any other age group to abuse pain relievers (SAMHSA, 2006). Specifically, opioid narcotics are the third most frequently abused drugs among 12th graders (behind alcohol and marijuana), and adolescent use of two synthetic opioids (Oxycontin and Vicodin) has increased dramatically since 2002 (Johnston et al., 2006). Drug use and abuse among adolescents is particularly alarming because initiation of drug use during adolescence may increase the propensity for addiction in adulthood (Anthony and Petronis, 1995; Clark et al., 1998; Kandel et al., 1992). Moreover, opioidergic pain killers are prescribed for pediatric patients, despite a lack of knowledge about potential long-term detrimental effects in young people (Carlezon and Konradi, 2004; Duedahl and Hansen, 2007). These human use trends call for basic research on adolescent vulnerability to drugs of abuse, particularly opioid narcotics.
Rodent models have good face validity for studying the behavioral and physiological changes associated with human adolescence (Adriani and Laviola, 2004; Smith, 2003; Spear, 2000). Adolescence in rodents, often termed periadolescence because a precise definition is elusive, may be limited to approximately two weeks between postnatal days (P) 28 and 42 (Spear, 2000; Spear and Brake, 1983) In many species, including both primates and rodents, transition from youth to adulthood is characterized by robust behavioral, morphologic, metabolic, hormonal, and neurochemical changes (Spear, 2000). For example, adolescents exhibit high levels of social interaction and play (Brown, 1990; Panksepp, 1981), high levels of risk-taking, sensation-seeking, or novelty-seeking (Adriani et al., 1998; Douglas et al., 2003; Zuckerman, 1992), and perhaps elevated basal or novelty-stimulated motor activity (Spear and Brake, 1983; Stansfield and Kirstein, 2006), although not all studies confirm the latter effect (Bolanos et al., 1998; Frantz et al., 2007; Frantz and Van Hartesveldt, 1999). When some of these characteristics are displayed by adult rodents, they are associated with a higher propensity to self-administer drugs of abuse (Ambrosio et al., 1995; Belin et al., 2008; Piazza et al., 1989). Coupled with high levels of drug use in humans during adolescence, these findings suggest that adolescence could be a critical period of heightened vulnerability to the reinforcing effects of drugs, perhaps including opioid narcotics (Adriani and Laviola, 2004; Crews et al., 2007; Laviola et al., 1999; Spear, 2000).
Indeed adolescent rats are differentially sensitive, compared with adults, to some physiological effects of the prototypical opiate morphine. For example, weanling and adolescent male rats (P21 and P28-35) become tolerant to the analgesic effects of repeated morphine injections more quickly than older males (Ingram et al., 2007; Wang et al., 2005). With regard to motor activity, acute morphine injections stimulate more locomotion in adolescent (P35) vs. adult male rats (Spear et al., 1982), and repeated morphine injections induce more motor sensitization among P30-32 male rats than P65-67 males (White and Holtzman, 2005). Results are mixed on age differences in morphine conditioned place preference; P35 male rats completely failed to show a preference in one study (Bolanos et al., 1996), whereas adolescent and adult, male and female rats all showed similar levels of morphine place preference in another (Campbell et al., 2000). Together these results suggest that responsivity to opiates changes during development, and that specific tests on the reinforcing effects of opiates are necessary.
The first aim of the present study was to compare the reinforcing effects of morphine between adolescent and adult male Sprague-Dawley rats in the intravenous (i.v.) drug self-administration model. Therefore, we allowed adolescent rats (P35 at start) or adults (P91 at start) to acquire lever pressing maintained by morphine in 1-hr (ShAcc) daily sessions on a fixed ratio one (FR1) schedule of reinforcement (Kruzich et al., 2003).
The second aim of our study was to explore morphine self-administration in an escalation model that might mirror the transition from recreational drug use to compulsive addiction in humans (Ahmed et al., 2000). Thus, we compared morphine intake between conditions of short and long daily access to morphine (1-hr vs. 8-hr per day; ShAcc vs. LgAcc, respectively), in rats that acquired self-administration in adolescence or adulthood, generally following protocols from Ahmed et al. 2000 and Walker et al. 2003. In those studies, ShAcc conditions resulted in stable daily drug intake over several weeks, whereas LgAcc conditions of either 8- or 11-hrs per day produced gradual escalation to a new, higher rate of daily drug intake.
The third aim was to analyze the long-term effects of morphine intake using animal models of drug craving and relapse following abstinence (Shaham et al., 2003; Ahmed et al., 2000). Thus, extinction of drug-seeking in the absence of morphine, and cue-induced reinstatement of drug-seeking was compared across age and access (ShAcc vs. LgAcc) groups.
Based in part on the high rates of drug use during adolescence among humans (Johnston et al., 2006; SAMHSA, 2006), as well as experiments suggesting rapid physiological and behavioral adaptations to morphine among adolescent rats (Ingram et al., 2007; Spear et al., 1982; Wang et al., 2005; White and Holtzman, 2005), we hypothesized that rats that begin self-administration during adolescence are more sensitive than rats that begin in adulthood to both acute and long-term effects of morphine. Thus, adolescent-onset rats should take less morphine under ShAcc conditions, but should escalate faster and to higher levels of drug intake under LgAcc conditions than adult-onset rats. Rats that acquire morphine self-administration during adolescence should also take longer than older adults to extinguish drug-seeking in the absence of morphine, and should reinstate drug-seeking in the presence of morphine-associated cues to a higher level than older adults.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Zivic Miller; New Castle, PA) arrived in the laboratory at P22 (n=10) or P78 (n=16) for adolescent-onset or adult-onset age groups, respectively. Rats were housed in groups of two or three in a temperature and humidity controlled vivarium and maintained on a 12 hr light/dark cycle, with lights off at 0700 hr. All behavioral testing occurred at approximately the same time every day during the dark phase. Body weights were recorded daily to monitor health and to titrate drug doses. Food and water were freely available in home cages and self-administration chambers during long access conditions. All procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985) and approved by The Institutional Animal Care and Use Committee (IACUC) of Georgia State University.
Drugs
Morphine sulfate (Mallinckrodt, Inc.; Hobart, NY), methohexital sodium (1%, Brevital Sodium, King Pharmaceuticals, Inc.; Bristol, TN), and Timentin antibiotic (GlaxoSmithKline; Research Triangle Park, NC), all were dissolved in sterile saline and filtered through a 25 μm syringe filter (Fisher Scientific, Inc.; Pittsburgh, PA) before i.v. administration.
Equipment
Morphine self-administration was conducted in operant chambers housed in sound-attenuating cubicles (Med Associates, Inc.; St Albans, VT). Each chamber was equipped with two retractable levers. Only one lever was extended during morphine self-administration and reinstatement phases (see below). Pressing on the lever initiated a syringe pump with a 5 rpm motor (PVM-1000VS, Med Associates Inc.; St Albans, VT) to deliver an infusion via a stainless steel swivel and a polyethylene tube attached to the catheter portal on each subject’s back. Drug delivery and data collection were controlled by a computer system using Med Associates software (Med PC IV).
Intravenous (i.v.) Catheter Implantation
Intravenous catheters were constructed as described (Caine and Koob, 1993), with minor modifications (Shahbazi et al., 2008). Briefly, silastic tubing was fitted onto a guide cannula (Plastics One; Roanoke, VA) bent at a right angle and encased in dental cement anchored with a 2.5-cm circular mesh for subcutaneous, mid-scapular placement. The silastic tubing was 10 cm long for adolescent rats and 12 cm for adults.
Adolescent (P29-31) and adult (P84-86) rats were surgically catheterized in the right jugular vein, generally according to Caine et al. (1993), with minor modifications (Shahbazi et al., 2008). Briefly, rats were anesthetized with an isoflurane-oxygen vapor mixture (4–5% for initial anesthetization and 1.5–2.5% for the remainder of surgery). Catheter tubing was passed subcutaneously from the back and inserted into the right jugular vein (two cm for adolescents or four cm for adults) and tied in place with sutures. During recovery, adolescent and adult rats received 0.15 or 0.2 ml, respectively, of the antibiotic Timentin (Ticarcillin Disodium and Clavulanate Potassium; 100 mg/ml, i.v.) twice daily for two days post-surgery, then once daily for the remainder of the experiment. Catheters were also flushed daily with 0.15–0.3 ml heparinized saline (30 USP units/ml) to promote catheter patency. Catheter patency was tested one day before the start of experimentation and once per week on a day of recess from drug self-administration, by injecting 0.1–0.4 ml of a short-acting barbiturate anesthetic, Brevital, through the catheter. If muscle tone was not lost within 3 sec, the catheter was presumed defective and the subject was not included in the analysis.
Morphine Self-Administration
The drug dose was titrated daily based on individual body weight to administer 0.375 mg/kg/infusion morphine, and infusion volume was varied accordingly based on a 0.625 ml infusion over 5 sec for an adult rat weighing 350 g. This dose was mid-range+ based on pilot experiments (Doherty et al., 2006; Ogbonmwan et al., 2007). After each drug infusion, a 20-sec time out (TO) period was signaled by switching on a cue light above the lever and switching off a house light and white noise. Responses during TO were recorded but had no scheduled consequences. During the extinction phase only, a second lever was extended to record “non-specific” motor effects; presses on this inactive lever were recorded but had no scheduled consequences.
The four phases of behavioral testing appear in the experimental timeline (Table 1): pre-escalation for 6 daily sessions, escalation for 18 sessions, 15-day recess, extinction for 18 sessions and reinstatement for 1 session. Sessions were conducted six consecutive days per week, except for extinction sessions conducted five days per week. Adolescent and adult rats were counterbalanced across 14 test chambers. Following 5–7 days of post-surgical recovery, adolescents (P35 at start; n=10) and adults (P91 at start; n=16) were allowed to acquire lever pressing maintained by morphine on an FR1 schedule of reinforcement during six 1-hr sessions (pre-escalation). Subsequently, adolescent and adult rats were divided into four groups for the escalation phase, counterbalanced by body weight and rates of morphine self-administration averaged over the last three pre-escalation sessions. Two groups remained under conditions of 1-hr daily access to morphine (short access, or ShAcc; 5 adolescents and 7 adults), while two groups transitioned to extended access conditions of 8-hr per day (long access, or LgAcc; 5 adolescents and 9 adults). In two adolescent and two adult subjects in LgAcc conditions, self-mutilation was observed briefly during self-administration, but was immediately ameliorated by “chew toys” placed in the operant chambers.
Table 1.
Postnatal age in days (d) at start of each experimental phase.
| Arrive in lab | Catheter surgery | Pre-Escal (6 sessions) | Escalation (18 sessions) | Recess (15 days) | Extinction (18 sessions) | Cue Reinstate (1 session) | |
|---|---|---|---|---|---|---|---|
| Adolescent- onset | 22d | 28–29d | 35d | 42d | 63d | 78d | 101d |
| Adult- onset | 78d | 84–86d | 91d | 98d | 119d | 134d | 157d |
Recess, Extinction and Reinstatement
After the escalation phase, all rats received a 15 day recess from testing. They were not exposed to test chambers or drugs, although they were handled and weighed periodically. Daily catheter flushing ceased at the beginning of the recess. Extinction testing was conducted for 1-hr per day, 5 days per week over 3.5 weeks (total 18 sessions). Two levers were extended into the chamber (one previously active, one new but inactive), but presses produced no scheduled consequences (Ahmed et al., 2000). The second lever was added during extinction to record “non-specific” motor effects. Drug-associated cues were not presented, i.e. house light remained on, white noise and cue light remained off for the duration of each extinction session.
Cue-induced reinstatement of drug-seeking was tested in a single 1-hr session that began with non-contingent presentation of drug-associated cues, i.e. cue light above the active lever turned on and house light and white noise turned off for 20 sec. Subsequently, the previously active lever was extended into the chamber and each appropriate response resulted in cue presentation. Only one lever was present during reinstatement testing in order to mimic the drug-taking environment during self-administration (Ahmed et al., 2000).
Data Analysis
To assess possible effects of daily morphine intake on growth during adolescent development, body weights were analyzed during morphine self-administration, the first day of extinction testing, and one day after reinstatement. During self-administration, body weights were analyzed separately for each age group using a two-way between-within mixed measures analysis of variance (ANOVA), with access condition and sessions (repeated) as factors. Body weights before the first extinction session and the day after reinstatement sessions were compared across access conditions using Student’s t-tests for independent samples. In addition at P101, the only age at which weight was directly comparable between the younger and older age groups, body weights were analyzed using a two-way between subjects ANOVA, with age at onset and access condition as factors. Finally, as a measure of morphine dependence (Gellert and Holtzman, 1978), the percent body weight lost during weekly abstinence from morphine self-administration (weekend recess of approximately 48 hrs) was analyzed using a three-way mixed measures ANOVA with age, access condition, and time (repeated measure) as factors.
For pre-escalation (sessions 1–6), the number of morphine infusions per session was compared using a two-way mixed measures ANOVA, with age and sessions (repeated) as factors. Total morphine intake (mg/kg) summed over all pre-escalation sessions was also compared across ages using an independent samples t-test. During the escalation phase (sessions 7–24), the number of infusions per session was compared within access conditions using two-way mixed measures ANOVAs, with age and sessions (repeated) as factors. A planned comparison between the first and last escalation sessions (session 7 vs. 24) was also conducted separately for all four age and access conditions using paired samples t-tests. Total morphine intake (mg/kg) summed over the entire escalation phase was compared across ages using independent samples t-tests. To compare outcomes from ShAcc vs. LgAcc conditions, the number of infusions taken during only the first 15 min “loading phase” of each session was analyzed using a three-way mixed measures ANOVA, with age, access condition, and sessions (repeated) as factors. To analyze control of behavior exerted by discriminative cues, the percentage of “inappropriate” lever presses was calculated as the sum of presses during drug infusion and TO, divided by the total number of presses on the active lever. Percent inappropriate responding was analyzed using a three-way mixed measures ANOVA, with age and access condition as between subjects factors, and sessions as a repeated measure.
During extinction the number of lever presses per session was subjected to a four-way mixed measures ANOVA with age, access condition, lever (active vs. inactive), and sessions (repeated) as factors. For the cue-induced reinstatement test, the number of presses per session was analyzed in a two-way between subjects ANOVA with age and access condition as factors. To determine whether morphine intake during self-administration influenced lever pressing during reinstatement, a Pearson’s correlation analysis was conducted on total morphine intake over 18 sessions and lever presses during reinstatement. In all cases, follow-up ANOVAs and post-hoc tests were conducted as appropriate. P ≤ 0.05 was considered significant.
Results
Body Weight
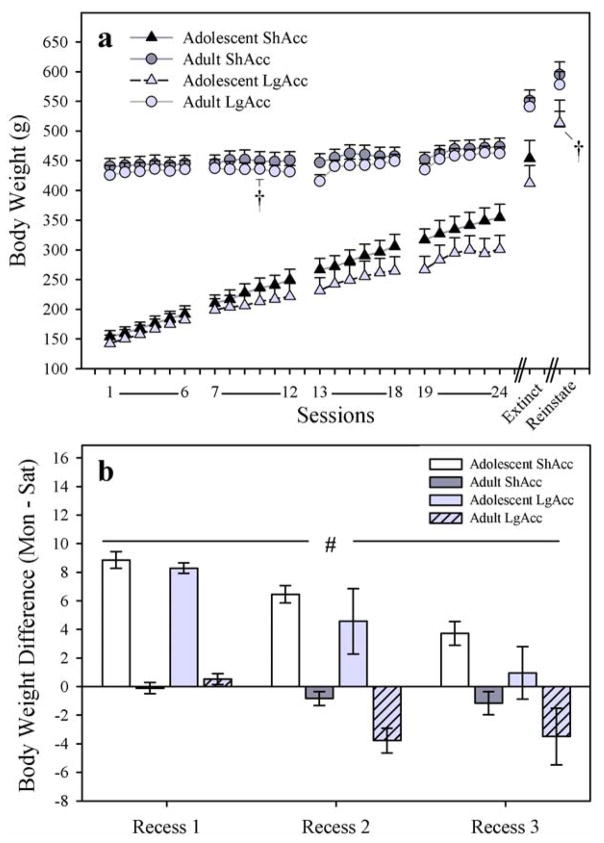
Rats in both age groups gained weight throughout morphine self-administration (Fig. 1; panel a). However, adolescent-onset rats in the ShAcc condition gained more than adolescent-onset rats in the LgAcc condition, as suggested by a significant access X session interaction (F17,136=5.73; p < 0.001), although the effect was not robust enough to reveal a significant effect of access condition on the last day of self-administration (t8=1.66; p = 0.14). Also, there was only one age (P101) at which body weights of the adolescent- vs. adult-onset groups could be compared directly, i.e. the day of reinstatement testing for the adolescent-onset group vs. the fourth day of the escalation phase for the adult-onset group, as marked with a dagger symbol on Fig. 1a. At P101, a significant main effect of age at onset revealed that the adolescent-onset group weighed more than the adult-onset group (F1,25=13.32; p < 0.001), regardless of access condition (F < 1.0; N.S.).
Fig. 1.
(a) Body Weight (g) in all subjects. Rats in both age groups gained weight over days, but ShAcc adolescents gained more weight than their LgAcc counterparts. “Extinct” indicates weight before the first session of extinction. “Reinstate” indicates weight one day after the reinstatement session. Dagger symbols indicate days on which the adolescent-onset and adult-onset groups were 101 days of age; see text for body weight comparisons. (b) Percent Body Weight Change over Weekend Recess from Morphine Self-Administration (change between sessions 6–7 for Recess 1, 12–13 for Recess 2, 18–19 for Recess 3). Over each weekend recess, adolescents continued to gain body weight, while adults lost weight (main effect of age; # p < 0.001). Subjects in the LgAcc condition tended to loose more weight than subjects in the ShAcc condition. The change in body weight tended to decrease over time. All points or bars represent mean +/− SEM (n= 5 ShAcc adolescents; n=7 ShAcc adults; n=5 LgAcc adolescents; n=9 LgAcc adults).
Loss of body weight, a classic sign of opiate withdrawal (Gellert and Holtzman, 1978), was analyzed during each weekend recess period from self-administration (post-session Saturday to pre-session Monday; Fig. 1; panel b). Adolescent body weight gains declined across successive recess periods, while adult body weight losses increased over successive recess periods, as suggested by a trend toward a significant age X time interaction (F2,44=3.19; p=0.051) in a mixed measures three-way age X access condition X time (repeated) ANOVA. A separate two-way ANOVA on body weight in only the adolescent-onset group confirmed that body weight gains declined across recess periods via a main effect of time (F2,16=18.09; p<0.001), but no main effect of access condition (F1,8=1.55; p=0.25) nor access condition X time interaction (F < 1.0; N.S.). Similarly a two-way ANOVA on body weight in only the adult-onset group confirmed that body weight losses increased across recess periods via a main effect of time (F2,28=3.76; p<0.05), but no main effect of access condition (F1,14=2.78; p=0.12) nor access condition X time interaction (F2,28=1.61; p=0.22) was recorded. Subjects in the LgAcc condition tended to either gain less (adolescents) or lose more (adults) body weight than subjects in the ShAcc condition, as revealed by a trend toward a main effect of access condition (F1,22=4.2; p = 0.052).
Pre-Escalation Phase of Morphine Self-Administration
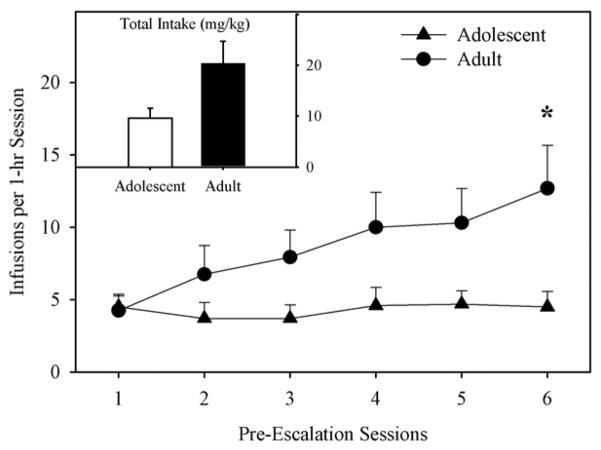
Adolescent-onset rats took fewer infusions of morphine than adults during the pre-escalation phase (Fig. 2), as confirmed by a significant age X session interaction on infusions per session (F5,120=2.43; p < 0.05), and a targeted t-test on session six (t24=−2.1; p < 0.05). The main effect of age on number of infusions (F1,24=3.75; p = 0.071), as well as the age difference in total morphine intake (t24=−1.89; p = 0.071; inset) just missed statistical significance.
Fig. 2.
Morphine Infusions during Pre-Escalation. Adolescents self-administered less morphine than adults. Post-hoc t-tests revealed a significant age effect on session 6 only (* p < 0.05). INSET. Total Morphine Intake during the Pre-Escalation Phase. All points or bars represent mean +/− SEM (n= 10 adolescents; n=16 adults).
Short Access (ShAcc) to Morphine Self-Administration (1 hr per session)
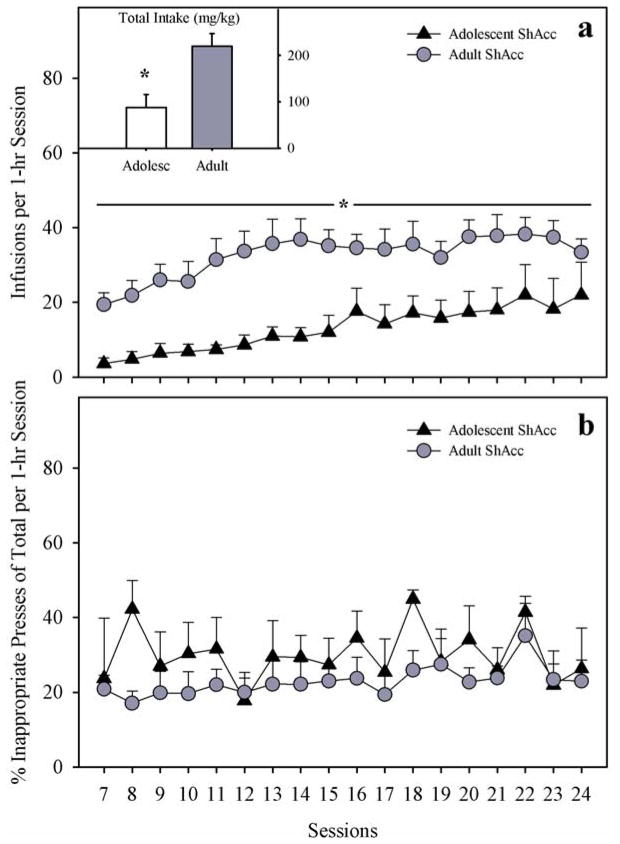
Over 18 daily 1-hr sessions (ShAcc), adolescent-onset rats continued to take fewer morphine infusions than adults (Fig. 3; panel a), although both age groups increased their morphine intake over sessions. Thus, main effects of age (F1,10=10.58; p < 0.01) and session (F17,170=7.27; p < 0.001) were significant. Specifically, adolescent-onset rats tended to increase their drug intake over all 18 sessions, whereas adult-onset rats increased their intake over approximately 7 sessions then reached a plateau. Paired t-tests comparing sessions 7 vs. 24 separately for each age group revealed only a trend toward increased infusions by adolescents (t4=−2.34; p = 0.08), but confirmed a significant increase in morphine infusions among adults (t6=−4.38; p < 0.01). Total morphine intake summed over 18 sessions was significantly lower in adolescents compared to adults (t10=−3.25; p < 0.01; inset). The percent inappropriate responding did not differ by age group (F1,10=2.31; p=0.16), session (F17,170=1.38; p=0.15), or age X session interaction (F < 1.0; N.S.) in ShAcc conditions (Fig. 3; panel b).
Fig. 3.
(a) Daily Morphine Infusions Under Short Access (ShAcc) Conditions. Adolescents took less morphine than adults over 18 sessions (main effect of age; * p<0.01). INSET. Total Morphine Intake (mg/kg) Under ShAcc Conditions. Adolescents took less morphine than adults (main effect of age; * p < 0.01). (b) Percent Inappropriate Lever Presses During Self-Administration Under ShAcc Conditions. Adolescents and adults displayed similar percent inappropriate lever responses over sessions. All points or bars represent mean +/− SEM (n= 5 adolescents; n=7 adults).
Long Access (LgAcc) to Morphine Self-Administration (8 hr per session)
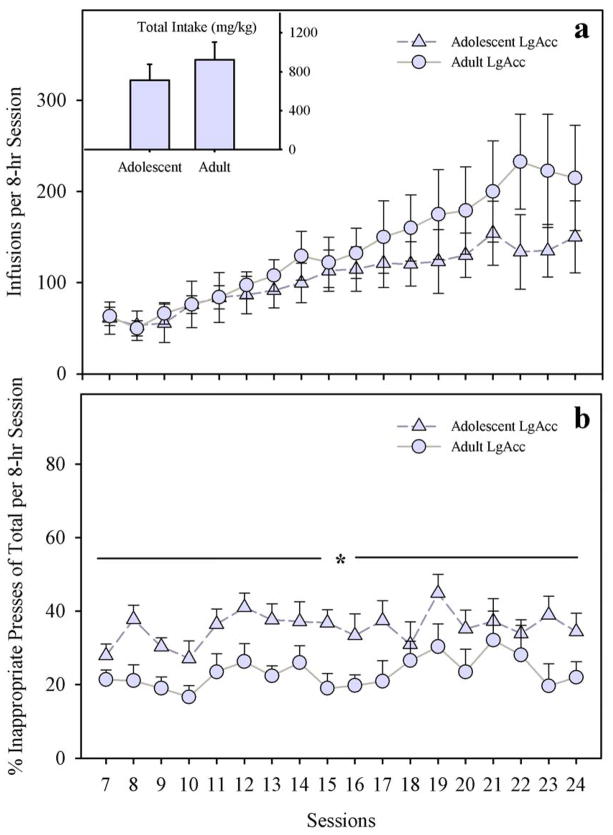
Over 18 daily 8-hr sessions (LgAcc), adolescent- and adult-onset rats escalated the number of infusions per session to similar degrees (Fig. 4; panel a). Rates of increase were similar across age groups. For example, the younger group reached an average of 61.2 ± 17.7 infusions by session 7 and 150.2 ± 39.6 by session 24, whereas adults reached 63.1 ± 9.9 infusions by session 7 and 214.9 ± 57.9 by session 24. There was no significant main effect nor any interactions with age on the number of infusions (F < 1.0; N.S.) or total morphine intake (F < 1.0; N.S.). Only the main effect of session was significant (F17,204=6.06; p < 0.001). Post-hoc tests revealed that morphine infusions increased above the level of the first LgAcc session from the fifth session onward (p < 0.025), regardless of age. However, t-tests comparing session 7 vs. 24 separately for each age group revealed only a trend toward increased infusions in adolescents (t4=−2.66; p = 0.057), but confirmed an increase in daily infusions among adults (t8=−2.77; p < 0.025). Analysis of individual subject data revealed a subset of adults (n=3) that escalated their morphine intake to a higher degree than all other animals in LgAcc conditions; dividing number of infusions data into quartiles places only this subset of adults in the upper quartile from session 12 onward (data not shown).
Fig. 4.
(a) Daily Morphine Infusions Under Long Access (LgAcc) Conditions. Adolescents and adults took similar amounts of morphine. INSET. Total Morphine Intake (mg/kg) Under LgAcc Conditions. (b) Percent Inappropriate Lever Presses During Self-Administration Under LgAcc Conditions. Adolescents exhibited more inappropriate presses compared to adults (main effect of age; * p < 0.05). All points or bars represent mean +/− SEM (n= 5 adolescents; n=9 adults).
In terms of stimulus control over lever pressing, adolescent-onset rats exhibited more inappropriate presses than adults (Fig. 4; panel b). The main effect of age was significant (F1,11=4.81; p < 0.05), but neither the main effect of sessions (F17,187=1.26; p=0.22), nor the age X sessions interaction (F < 1.0; N.S.) was significant.
Short vs. Long Access Comparisons
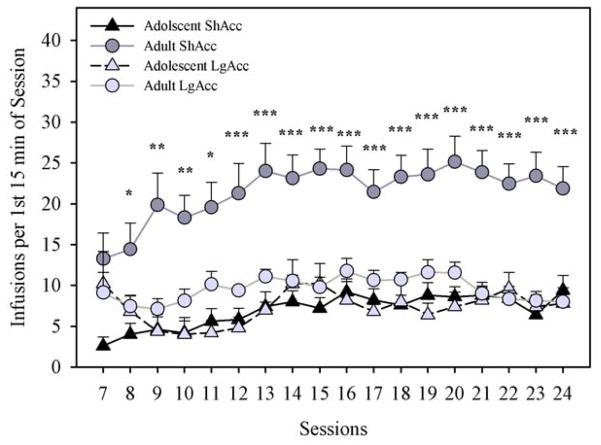
Comparisons across age and access conditions were conducted using the number of infusions during the first 15 min “loading phase” of each session (Fig. 5). Adult rats in the ShAcc condition loaded the most, compared with all other age and access groups. Thus, there were significant main effects of age (F1,22=28.29; p < 0.001), access condition (F1,22=13.31; p < 0.01), and session (F17,374=5.1; p < 0.001), as well as significant interactions of age X access condition (F1,22=15.11; p < 0.01) and session X access condition (F17,374=2.2; p < 0.01). Post-hoc one-way ANOVAs comparing all groups on each session confirmed that adults in the ShAcc condition took more morphine than any other age or access group from the eighth session onward (p < 0.05). Similar outcomes were observed when only the first hour of each session was compared across age and access conditions, per the analysis of Ahmed and colleagues (2000; data not shown).
Fig. 5.
Daily Morphine Infusions in the First 15 min per Session. Adults under ShAcc conditions took more infusions than other groups from session 8 onward (adult ShAcc different from all other groups: * p < 0.05, ** p < 0.01, *** p < 0.001). Points represent mean +/− SEM (n= 5 ShAcc adolescents; n=7 ShAcc adults; n=5 LgAcc adolescents; n=9 LgAcc adults).
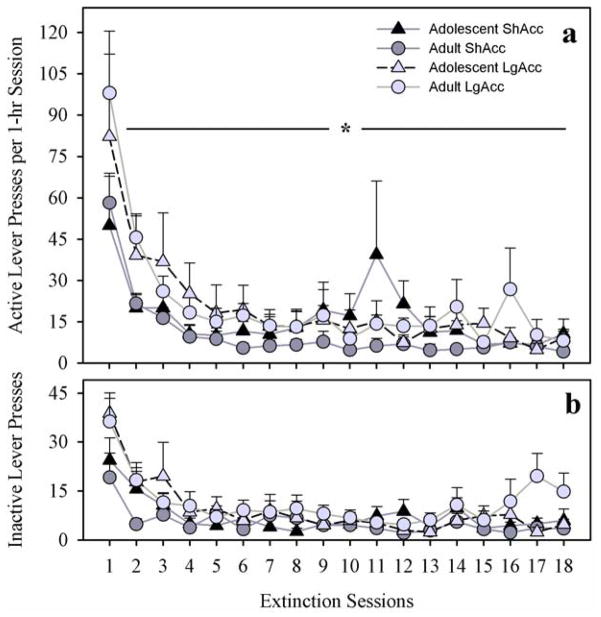
Extinction of Morphine-Seeking
In the absence of morphine, all subjects initially preferred the lever previously paired with morphine (active lever in Fig. 6; panel a) over the new (inactive; panel b) lever, but gradually decreased all lever pressing to low levels. A four-way mixed measures ANOVA [age X access condition X lever (active vs. inactive) X sessions (repeated)] produced no significant main effect of age (F < 1.0; N.S.), nor any interactions with age (F < 1.0; N.S.). However, a main effect of sessions confirmed the gradual extinction, regardless of age or access condition (F17,408=18.45; p < 0.001). Also, a main effect of access condition (F1,24=4.2; p < 0.05) showed that subjects in LgAcc conditions exhibited more extinction responding on the active lever than subjects in ShAcc conditions.
Fig. 6.
(a) Extinction of Active Lever Pressing in 1-hr Sessions. No significant age effects were observed, but LgAcc subjects pressed more than ShAcc subjects (main effect of access condition; * p < 0.05). (b) No Differences in Inactive Lever Pressing During 1-hr Extinction Sessions. All points represent mean +/− SEM (n= 5 ShAcc adolescents; n=7 ShAcc adults; n=5 LgAcc adolescents; n=9 LgAcc adults).
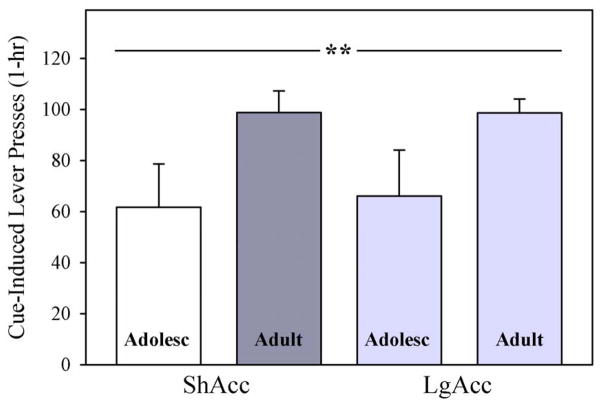
Cue-Induced Reinstatement
When drug-associated cues were reintroduced after extinction, rats that acquired morphine self-administration as adolescents reinstated lever pressing to a lesser degree than older adults, but access condition failed to influence reinstatement (Fig. 7). Thus, a two-way age X access ANOVA revealed a significant main effect of age (F1,22=9.17; p < 0.01), but neither the main effect of access (F < 1.0; N.S.) nor the age X access interaction was significant (F < 1.0; N.S.). To determine whether morphine intake during self-administration influenced lever pressing during reinstatement, a Pearson’s correlation analysis was conducted. A positive relationship was identified between total morphine intake (mg/kg) and number of lever presses during reinstatement among subjects in ShAcc, but not LgAcc conditions (ShAcc: 0.659; two-tailed p = 0.02; LgAcc: 0.188; two-tailed p = 0.52; data not shown).
Fig. 7.
Cue-Induced Reinstatement of Morphine-Seeking. Subjects that acquired morphine self-administration as adolescents reinstated lever pressing after reintroduction of drug-associated cues to a lesser degree than older adults, regardless of access conditions (main effect of age; ** p < 0.01). Bars represent mean +/− SEM (n= 5 ShAcc adolescents; n=7 ShAcc adults; n=5 LgAcc adolescents; n=9 LgAcc adults).
Discussion
This report on morphine self-administration in adolescent vs. adult rats reveals important age differences in morphine intake and reinstatement of morphine-seeking after extinction. Under conditions of short daily access (1-hr per day), rats that acquired morphine self-administration as adolescents consistently took less morphine than adults. In contrast under long access conditions (8–hr per day), rats in both age groups escalated their morphine intake similarly. Perhaps most strikingly, cue-induced reinstatement of morphine-seeking after extinction was less robust in rats that took morphine during adolescence compared to rats that self-administered as adults, regardless of daily access conditions. Together these results only partially support our hypotheses that younger rats are more sensitive than adults to the acute and long-term reinforcing effects of morphine.
Morphine self-administration and related behaviors expressed by subjects in the present study confirm prior results from this and other laboratories. First, the assertion that morphine reinforced lever pressing in our laboratory conditions is confirmed by several factors. Stable self-administration occurred in the present experiment. Lever discrimination was observed during a two-lever choice procedure in a pilot study (Doherty et al., 2006) and during extinction in the present study (Bossert et al., 2007). A burst of extinction responding occurred when saline was substituted for morphine in our pilot study (Doherty et al., 2006; Peltier et al., 2001), and reinstatement of lever pressing after reintroduction of drug-associated cues in the present study (LaLumiere and Kalivas, 2008). Second, escalation of morphine intake under long daily access conditions replicates numerous prior reports with opiate or stimulant drug reinforcers (Ahmed and Koob, 1998; Ahmed et al., 2000; Buccafusco and Bain, 2007; Chen et al., 2006; Glass et al., 2005; Glass et al., 2004; Kenny et al., 2006; Kitamura et al., 2006; Kruzich et al., 2003; Lenoir and Ahmed, 2007, 2008; Morgan et al., 2002; Paterson and Markou, 2004; Wee et al., 2007). Third, higher rates of lever pressing during extinction by subjects from LgAcc compared with ShAcc groups replicates prior work (Ahmed et al., 2000; Ferrario et al., 2005; Lenoir and Ahmed, 2007). Lastly, even the higher percentage of “inappropriate responding” exhibited by adolescent-onset rats compared to adults in the present LgAcc conditions is consistent with higher levels of impulsivity or lack of stimulus control previously noted in adolescents (Adriani and Laviola, 2003; Sagvolden and Sergeant, 1998; Shahbazi et al., 2008; Spear and Brake, 1983).
Among the most important of the present new findings is that adolescent-onset rats consistently took less morphine than adults under ShAcc conditions. On an FR schedule of reinforcement in short daily sessions, lower rates of intake are usually elicited by higher doses per infusion (Arnold and Roberts, 1997; Carroll and Lac, 1997; Koob et al., 1984). Therefore, the present results suggest an adolescent hypersensitivity to the reinforcing effects of morphine, consistent with age differences in morphine-stimulated motor activity and sensitization (Spear et al., 1982; White and Holtzman, 2005), as well as heightened vulnerability to opiates among human adolescents (Johnston et al., 2006; SAMHSA, 2006). On the other hand, slower rates of acquisition (Perry et al., 2007) and fewer number of infusions per session (Belluzzi et al., 2005) have been interpreted to reflect hyposensitivity. Extensive research will be necessary to confirm either interpretation and describe the neural basis for either of these age differences.
Relatively little is known about adolescent development of brain reinforcement circuitry. Very few studies on the ontogeny of opioid receptors include the adolescent phase of development, and ontological studies that do bracket adolescence do not provide a clear explanation for observed behavioral differences. For example, the density of μ-opioid receptors in the nucleus accumbens and other forebrain regions rises to adult levels already by P30 (Talbot et al., 2005) and agonist binding affinity appears similar in the forebrain of P28 and adult rats (Spain et al., 1985). However, less efficient coupling between μ-opioid receptors and G-proteins in P30 vs. adult rats fits a profile of adolescent hyposensitivity to morphine (Talbot et al., 2005). With regard to reinforcement circuits involving mesolimbic dopamine transmission, a transient overexpression of dopamine receptors in the nucleus accumbens and prefrontal cortex is observed specifically during adolescence (Andersen and Teicher, 2000; Andersen et al., 2000), along with fluctuations in basal and dopamine agonist-stimulated cAMP levels (Andersen, 2002). It is entirely possible that similar transient effects will be revealed in opioid receptor systems and/or that these known changes in dopamine signaling could contribute to the age differences in morphine self-administration presently reported.
In addition to neurochemical maturation, endocrine system changes could contribute to age differences in drug self-administration. Indeed corticosterone suppression decreases the locomotor-stimulating effects of morphine (Deroche et al., 1993); blocking glucocorticoids attenuates morphine-induced dopamine efflux in the nucleus accumbens (Marinelli et al., 1998), and gonadal steroid hormones increase the potency of morphine in a hot plate test (Stoffel et al., 2003). Unfortunately, comparisons across different self-administered drugs do not reveal the same age-dependent results as the present study [e.g. no age differences in cocaine self-administration (Frantz et al., 2007; Kerstetter and Kantak, 2007; McQuown et al., 2007) or higher intake after adolescent onset of nicotine or amphetamine self-administration (Shram et al., 2008; Belluzzi et al., 2005; Shahbazi et al., 2008)]. Thus, general conclusions about adolescent sensitivity to behavioral reinforcement by drugs of abuse are not warranted.
An unexpected observation in our experiments was the gradual increase in morphine intake under ShAcc conditions, which was significant for adults and trended toward significant for adolescents (p=0.08). This increase contrasts with numerous reports of stable intake over weeks of heroin or cocaine self-administration under similar schedules and access conditions (Ahmed and Koob, 1999; Ahmed et al., 2000; Bossert et al., 2007; Kenny et al., 2006), but corroborates a recent report of slightly increased heroin intake over 27 daily 1-hr sessions (Lenoir and Ahmed, 2008). In a manner specific to opiates, either tolerance could drive up rates of intake (Zernig et al., 2007 for review), or increasingly aversive states of withdrawal could drive up intake through negative reinforcement (Kenny et al., 2006; Koob and Le Moal, 1997; Schulteis and Koob, 1996). In either case, a lack of significant increase over sessions by adolescent-onset rats compared with adults could reflect adolescent hyposensitivity to these morphine effects. Adolescent hyposensitivity to tolerance is not supported by research on morphine analgesia (Ingram et al., 2007; Wang et al., 2005). However, adolescent hyposensitivity to aversive drug withdrawal does corroborate mounting evidence from research showing adolescent hyposensitivity to acute aversive drug effects in nicotine- or amphetamine-conditioned taste aversion (Wilmouth and Spear, 2004; Infurna and Spear, 1979), as well as physical and affective signs of nicotine withdrawal (Infurna and Spear, 1979; O’Dell et al., 2006; O’Dell et al., 2007; Shram et al., 2008; Wilmouth and Spear, 2004). Further, adolescent hyposensitivity to drug withdrawal may even be supported by the present observation that the younger cohort exhibited less “drug-loading” than adults during the first 15 min of each session, possibly reflecting less aversive interoceptive states just prior to self-administration sessions. Finally, body weight gain among rats in the younger cohort was not affected during abstinence from morphine over three intermittent periods (weekends), whereas adult rats lost weight in a classic sign of opiate withdrawal (Gellert and Holtzman, 1978). However, the interpretation of body weight change across age groups is confounded by the normal growth curve for adolescent rats during development, contrasted with the relatively flat rate of body weight gain among adults. Given that behavioral reinforcement and aversive drug withdrawal are mediated by discrete neural systems (Koob and Le Moal, 2008; Schulteis and Koob, 1996), it is possible that adult-like sensitivity to behavioral reinforcement is coupled with resistance to aversive drug withdrawal during ontological development, leading to lower and more stable rates of morphine self-administration by adolescent-onset compared with adult-onset rats under ShAcc conditions.
Long access self-administration conditions are thought to model the transition from recreational drug use to compulsive drug addiction (Ahmed and Koob, 1998). In contrast to the clear age differences in the present ShAcc conditions, no significant age differences in long access testing were observed, although the three highest “escalators” were all adult rats and they comprised the upper quartile in the data range from session 12 onward. The gradual escalation of drug intake exhibited by both age groups extends numerous reports on heroin, fentanyl, cocaine, and methamphetamine (Ahmed and Koob, 1998; Ahmed et al., 2000; Chen et al., 2006; Kenny et al., 2006; Kitamura et al., 2006; Lenoir and Ahmed, 2007, 2008; Morgan et al., 2002; Wee et al., 2007) to include morphine and adolescent male rats. However, insofar as a faster rate and greater degree of escalation reflect increased vulnerability to the transition from periodic drug use to compulsive drug abuse (Ahmed and Koob, 1999; Ahmed et al., 2000; Chen et al., 2006; Mantsch et al., 2003; O’Brien et al., 1986; Walker et al., 2003), our data do not support the contention that adolescent onset of drug-taking heightens vulnerability to transition from periodic to compulsive drug-seeking or addiction (Anthony and Petronis, 1995; Clark et al., 1998; Kandel et al., 1992; Laviola et al., 1999; SAMHSA, 2006; Smith, 2003).
Lastly, we considered the long-term effects of morphine self-administration using an animal model of drug craving and relapse following abstinence and extinction. Unlike prior reports (Ahmed and Koob, 1998; Ahmed et al., 2000; Lenoir and Ahmed, 2007), rats that took morphine under LgAcc conditions in our study failed to exhibit more robust reinstatement of drug-seeking after extinction, relative to rats in ShAcc conditions. Two factors could explain this contradiction: 1) drug-associated cues rather than acute drug administration or stressors were used to trigger reinstatement, and 2) although escalation was greater in LgAcc conditions, rats in both access groups actually increased intake over sessions. Despite these factors, rats that acquired morphine self-administration during adolescence showed less robust cue-induced reinstatement of lever pressing than older adults. Whereas total drug intake during self-administration has correlated with lever pressing during reinstatement previously (Liu et al., 2008) as well as in the present ShAcc cohorts, no such correlation was observed among the present LgAcc cohorts, suggesting that prior drug intake does not entirely explain the age differences in reinstatement. Alternatively, the amount of cue-induced reinstatement may reflect the prior strength of the reinforcing stimulus (Kenny, 2007; Shaham et al., 2003; Zhou et al., 2007), or the negative affect triggered by cue presentation during withdrawal (Kenny et al., 2006; Kenny and Markou, 2005). In either case, our results suggest that younger rats are less sensitive than older adults to these enduring effects of drug self-administration. Similarly, rats that self-administered cocaine as adolescents showed lower rates of cue-induced reinstatement of cocaine-seeking than adults (Li and Frantz, manuscript submitted). Also, rats that took cocaine as adolescents showed less long-term cognitive impairment than adults (Kerstetter and Kantak, 2007); and rats given MDMA during adolescence failed to cross-sensitize to cocaine reinforcement when their adult counterparts did (Frantz and Parsons, 2001). Together these results suggest that adolescence could be a period during which neuroprotective factors dampen some long-term drug effects.
In all, the present results extend research on age differences in vulnerability to both acute and long-term reinforcing effects of morphine, using the i.v. self-administration model which has strong face and predictive validity (Ator and Griffiths, 2003). Although hypersensitivity of adolescent rats to the acute reinforcing effects of morphine could explain some of the present results, hyposensitivity of adolescent rats to morphine-associated tolerance, withdrawal, and/or cue-induced reinstatement may reflect developmental protection from some acute and long-term detrimental effects of morphine. If verified in future studies, including clinical investigations, these findings might suggest a better prognosis for adolescent compared with adult drug abusers in behavioral or pharmacological therapy for drug dependence.
Acknowledgments
The authors would like to thank Chen Li and Nathan Waldron for technical assistance, as well as Dr. S. Ahmed for helpful comments on this manuscript. This research was supported in part by a National Institute on Drug Abuse B/START grant to KJF (1 RO3 DA020110-01) and the Center for Behavioral Neuroscience, an NSF Science & Technology Center (IBN-9876754).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behavioral neuroscience. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behavioural pharmacology. 1995;6:229–237. [PubMed] [Google Scholar]
- Andersen SL. Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD) Behavioural Brain Research. 2002;130:197–201. doi: 10.1016/s0166-4328(01)00417-x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neuroscience and biobehavioral reviews. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug and Alcohol Dependence. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacology, biochemistry and behavior. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug and Alcohol Dependence. 2003;70:S55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. The journal of neuroscience. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. At the threshold: the developing adolescent. In: Feldman S, Elliott G, editors. Peer Groups. Cambridge, MA: Harvard University Press; 1990. pp. 171–196. [Google Scholar]
- Buccafusco JJ, Bain JN. A 24-h access I.V. self-administration schedule of morphine reinforcement and the estimation of recidivism: Pharmacological modification by arecoline. Neuroscience. 2007;149:487–498. doi: 10.1016/j.neuroscience.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology. 2004;47:47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology. 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug and Alcohol Dependence. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, biochemistry and behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Piazza PV, Le Moal M, Simon H. Individual differences in the psychomotor effects of morphine are predicted by reactivity to novelty and influenced by corticosterone secretion. Brain Res. 1993;623:341–4. doi: 10.1016/0006-8993(93)91451-w. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Li C, Moffett AE, Ogbonmwan Y, Whyte AC, Williams BF, Frantz KJ. Society for Neuroscience abstract. 2006. Morphine self-administration in adolescent vs. adult male rats. [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology & behavior. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Duedahl TH, Hansen EH. A qualitative systematic review of morphine treatment in children with postoperative pain. Paediatr Anaesth. 2007;17:756–774. doi: 10.1111/j.1460-9592.2007.02213.x. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biological psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, Parsons LH. Society for Neuroscience abstract. 2001. Effects of MDMA on acquisition of cocaine self-administration in periadolescent and adult rats. [Google Scholar]
- Frantz KJ, Van Hartesveldt C. The locomotor effects of quinpirole in rats depend on age and gender. Pharmacol Biochem Behav. 1999;64:821–826. doi: 10.1016/s0091-3057(99)00162-8. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. The Journal of pharmacology and experimental therapeutics. 1978;205:536–546. [PubMed] [Google Scholar]
- Glass MJ, Kruzich PJ, Colago EE, Kreek MJ, Pickel VM. Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse. 2005;58:1–12. doi: 10.1002/syn.20176. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Kruzich PJ, Kreek MJ, Pickel VM. Decreased plasma membrane targeting of NMDA-NR1 receptor subunit in dendrites of medial nucleus tractus solitarius neurons in rats self-administering morphine. Synapse. 2004;53:191–201. doi: 10.1002/syn.20049. [DOI] [PubMed] [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacology, biochemistry and behavior. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology. 2007;32:600–606. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No. 06-5883. 2006. Monitoring the Future national survey results on drug use 1975–2005:Volume I, Secondary school students. [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Brain reward systems and compulsive drug use. Trends in Pharmacological Sciences. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. The journal of neuroscience. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology. 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Pettit HO, Ettenberg A, Bloom FE. Effects of opiate antagonists and their quaternary derivatives on heroin self-administration in the rat. The Journal of pharmacology and experimental therapeutics. 1984;229:481–486. [PubMed] [Google Scholar]
- Kruzich PJ, Chen AC, Unterwald EM, Kreek MJ. Subject-regulated dosing alters morphine self-administration behavior and morphine-stimulated [35S]GTPgammaS binding. Synapse. 2003;47:243–249. doi: 10.1002/syn.10173. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. The journal of neuroscience. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology. 2008;33:2272–2282. doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology. 2008;196:365–375. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high-dose, extended-access rat self-administration model of escalating cocaine use. Psychoneuroendocrinology. 2003;28:836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Aouizerate B, Barrot M, Le Moal M, Piazza PV. Dopamine-dependent responses to morphine depend on glucocorticoid receptors. Proc Natl Acad Sci U S A. 1998;95:7742–7. doi: 10.1073/pnas.95.13.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AD, Campbell UC, Fons RD, Carroll ME. Effects of agmatine on the escalation of intravenous cocaine and fentanyl self-administration in rats. Pharmacol Biochem Behav. 2002;72:873–880. doi: 10.1016/s0091-3057(02)00774-8. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology. 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Ehrman R, Ternes JW. Classical conditioning in human opioid dependence. In: Goldberg SR, Stolerman IP, editors. Behavioral analysis of Drug Dependence. Orlando, FL: Academic Press; 1986. pp. 329–356. [Google Scholar]
- Ogbonmwan Y, Doherty JM, Williams BF, Waldron N, Frantz KJ. Society for Neuroscience abstract. 2007. Escalation and reinstatement of morphine self-administration in periadolescent vs. adult male rats. [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology. 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Peltier RL, Guerin GF, Dorairaj N, Goeders NE. Effects of saline substitution on responding and plasma corticosterone in rats trained to self-administer different doses of cocaine. The Journal of pharmacology and experimental therapeutics. 2001;299:114–120. [PubMed] [Google Scholar]
- Perry JL, Anderson MM, Nelson SE, Carroll ME. Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiology & behavior. 2007;91:126–133. doi: 10.1016/j.physbeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder--from brain dysfunctions to behaviour. Behavioural Brain Research. 1998;94:1–10. [PubMed] [Google Scholar]
- SAMHSA; Depart. of Health and Human Services, OoAS. Substance Abuse and Mental Health Services Administration, results from the 2005 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2006. [Google Scholar]
- Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochemical research. 1996;21:1437–1454. doi: 10.1007/BF02532385. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Siu ECK, Li Z, Tyndale RF, Lê AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology. 2008;198:181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- Smith RF. Animal models of periadolescent substance abuse. Neurotoxicol Teratol. 2003;25:291–301. doi: 10.1016/s0892-0362(02)00349-5. [DOI] [PubMed] [Google Scholar]
- Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa) J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4:279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Dev Psychobiol. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JN, Happe HK, Murrin LC. Mu opioid receptor coupling to Gi/o proteins increases during postnatal development in rat brain. J Pharmacol Exp Ther. 2005;314:596–602. doi: 10.1124/jpet.104.082156. [DOI] [PubMed] [Google Scholar]
- Walker JR, Chen SA, Moffitt H, Inturrisi CE, Koob GF. Chronic opioid exposure produces increased heroin self-administration in rats. Pharmacology, biochemistry and behavior. 2003;75:349–354. doi: 10.1016/s0091-3057(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mitchell J, Moriyama K, Kim KJ, Sharma M, Xie GX, Palmer PP. Age-dependent morphine tolerance development in the rat. Anesth Analg. 2005;100:1733–1739. doi: 10.1213/01.ANE.0000152192.23851.40. [DOI] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. The Journal of pharmacology and experimental therapeutics. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stöckl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liu H, Zhang F, Tang S, Zhu H, Lai M, Kalivas PW. Role of acetylcholine transmission in nucleus accumbens and ventral tegmental area in heroin-seeking induced by conditioned cues. Neuroscience. 2007;144:1209–1218. doi: 10.1016/j.neuroscience.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation-seeking: the balance between risk and reward. In: Lipsitt LP, Mitnick LL, editors. Self-regulatory behavior and risk taking. Norwood, NJ: Ablex Publishing; 1992. [Google Scholar]