Abstract
Many models from foraging theory and movement ecology assume that resources are encountered randomly. If food locations, types and values are retained in memory, however, search time could be significantly reduced, with concurrent effects on biological fitness. Despite this, little is known about what specific characteristics of foods, particularly those relevant to profitability, nonhuman animals can remember. Building upon previous observations, we hypothesized that chimpanzees (Pan troglodytes), after observing foods being hidden in a large wooded test area they could not enter, and after long delays, would direct (through gesture and vocalization) experimentally naïve humans to the reward locations in an order that could be predicted beforehand by the spatial and physical characteristics of those items. In the main experiment, various quantities of almonds, both in and out of shells and sealed in transparent bags, were hidden in the test area. The chimpanzees later directed searchers to those items in a nonrandom order related to quantity, shell presence/absence, and the distance they were hidden from the subject. The recovery sequences were closely related to the actual e/h profitability of the foods. Predicted recovery orders, based on the energetic value of almonds and independently-measured, individual-specific expected pursuit and processing times, were closely related to observed recovery orders. We argue that the information nonhuman animals possess regarding their environment can be extensive, and that further comparative study is vital for incorporating realistic cognitive variables into models of foraging and movement.
Keywords: primate cognition, evolutionary ecology, prey encounter, communication, movement ecology, handling time
In most models of diet selection, potential foods are assumed to be sequentially and/or randomly encountered, where an “encounter” occurs “when a forager, using its senses, detects an item” (Stephens & Krebs 1986, p. 13). Much research in vertebrate movement ecology has similarly emphasized random walk models as opposed to purposive, directed travel (Benhamou 2007). A complementary approach is to elucidate the knowledge animals possess regarding their environment (Collett 2002), perhaps even distant and currently imperceptible parts of their environment, and assume they can utilize this information for their selective advantage (Dall et al. 2005). Such variables can then be incorporated into predictive models of feeding and travel.
With respect to foraging, a sizable literature examines the constraints limiting animal ability to estimate food handling times (Shettleworth 1985; Rosati et al. 2006), profitability (Bélisle & Cresswell 1997), and encounter rates (Shettleworth & Plowright 1992; Berec & Křivan 2000), or considers related questions such as between-subject differences in general perceptual ability (Spencer et al. 1996) and prey discrimination (Tillett et al. 2008). Many studies of memory and foraging involve stimuli being presented, usually visually, to subjects (e.g., "Have you experienced this before?," Shettleworth 1998, p. 238). Although much is now known about nonhuman memory for locations (e.g., R. Menzel et al. 2005; Manser & Bell 2004), comparatively little work has addressed what specific characteristics of items are remembered and can be flexibly accessed when they are outside perceptual range.
The ability to remember details about distant resources (e.g., item type, value) could—by reducing search time—increase foraging efficiency considerably, and thus presents a potential target for natural selection (Kurland & Beckerman 1985; Adams-Hunt & Jacobs 2007; Barraquand et al. 2009; Janmaat & Chancellor 2010). Black-capped chickadees (Parus atricapillus), for example, search more frequently at cache sites which had contained the more favored of two seed types (Sherry 1984), and scrub jays (Aphelocoma coerulescens) will bias recovery attempts towards one of two foods based on time of caching (Clayton & Dickinson 1998) or current preference (Clayton & Dickinson 1999). Rats (Rattus norvegicus) in a radial arm task visit high reward arms earlier than low reward arms (Pratt & Mizumori 2001), and pigs (Sus scrofa) will selectively visit a test location associated with food of high quantity and low handling time compared with one providing a less profitable reward (Held et al. 2005).
Among primates, a symbol-trained chimpanzee (Pan troglodytes) can identify by lexigram which one of over 30 types of object has been hidden in a wooded test area. High accuracy on trial-unique experimental tests occurs even after delays of many hours and when subject reports item identity in a location spatially removed and not visible from where the object was seen hidden (Menzel 1999; Menzel 2005). In addition, free-ranging primates appear to possess knowledge of specific food patches—including, perhaps, information on relative value—that lie beyond direct sensory cues (Saguinus mystax and S. fuscicollis, Garber 1989; Macaca fuscata, Menzel 1991; Cebus apella, Janson 1998; Papio cynocephalus, Pochron 2001; Pithecia pithecia, Cunningham & Janson 2007; Papio ursinus, Noser & Byrne 2007; Pan troglodytes, Normand & Boesch 2009; Microcebus murinus, Joly & Zimmermann 2011).
The present study investigates what information chimpanzees possess concerning the relative profitability (energy/handling time) of foods that cannot be seen or otherwise perceived, and located at differing distances and directions from the forager. Also examined is whether multiple foods of varying values can be compared in memory, and recovered in a predictable order. Chimpanzees in previous studies have demonstrated proclivity in selecting visible or hidden food by amount (Menzel 1960; Boysen & Berntson 1995; Beran et al. 2008), remembering the location of hidden foods (Köhler 1925; Tinklepaugh 1932), recovering hidden preferred foods before those that were non-preferred (Menzel 1973), and even estimating unknown food quality, quantity, and location by observing the behaviour of conspecifics which possessed such knowledge (Menzel 1971).
Relevant to the methods employed in this study, symbol-trained chimpanzees will also direct experimentally naïve humans to unreachable locations where foods earlier had been hidden (Menzel 1999, 2005; Menzel & Menzel 2012). The general procedure is as follows: a chimpanzee observes an Experimenter hiding a food item under leaves or other cover in an outdoor area that cannot be entered by the former. The food is always hidden in a trial-unique location. This can be visualized as a chimpanzee on one side of a fence, and the Experimenter on the other side. After hiding the item, the Experimenter exits the scene. In order to secure the reward, the chimpanzee must induce another human, who is naïve to the location or type of hidden item, to retrieve the food.
In previous tests (Putney 2007; Menzel & Menzel 2012) it has been shown that chimpanzees will “recruit” an uninformed person to search for hidden items. This is accomplished by the chimpanzee first going inside the ape building and attracting the attention of a caretaker, often by vocalization. This is generally followed by the chimpanzee touching lexigrams (arbitrary symbols associated with foods, locations, actions, individuals, or events)—typically corresponding with the type of food that is hidden—and then gesturing towards the tunnel it must go through to reach the outdoor area bordering where the food is hidden. Once the chimpanzee and human searcher are outside (again, the chimpanzee on one side of the fence, and the human and hidden food on the other) direction giving by the chimpanzee will commence. The chimpanzee will orient its body and gaze towards where the target is hidden, will point manually towards the location, and will exhibit increasing signs of excitement (including arm shaking, head bobs, and vocal grunts) as the searcher reaches the correct distance and angle. Chimpanzees can use such techniques to direct uninformed humans to locations over 30 m distant, with generally few “false positives” (e.g., a human searching a location over 100 cm from where a food item was hidden) even after hours or days. For example, in 54 single-item trials, the Pearson r between the “angle of hidden object” and the “angle at which an uninformed person first searched, following the chimpanzee’s directions,” was 0.99 (Menzel 2005).
Building on these observations, we predicted that chimpanzees, after observing foods being hidden in an inaccessible 350 m2 wooded test area, and after an extended delay, would direct uninformed humans to those items in a nonrandom order that could be predicted a priori by the physical and spatial characteristics of the foods. This experimental method (Menzel 1999; Menzel et al. 2006) allows trial-specific rates of energetic return to be calculated without needing to incorporate costs of locomotion (Taylor et al. 1982; Lifjeld & Slagsvold 1988), and also reduces the information available to the subject through means other than visual perception (such as tactile cues to location, Menzel 2005).
We first gathered data allowing us to calculate expected handling time (pursuit plus processing time) for each chimpanzee. In experiment 1, assorted objects were hidden at predetermined locations in the wooded test area to construct a regression equation predicting subject-specific pursuit times—starting from when the chimpanzee commenced to direct the person and ending when the goal object was handed to the subject—using the distance items were hidden from the chimpanzee. In experiment 2, processing times for varying quantities of no-shell and shell almonds were independently measured for each subject, in conjunction with preference testing, allowing calculation of chimpanzee-specific expected processing times.
In the main foraging task, experiment 3, differing quantities of almonds, both in and out of shells and sealed in transparent bags, were shown to the subjects and hidden in the wooded test area. We predicted that the order of bag recovery would be related to almond quantity, presence/absence of shells, and distance from chimpanzee. Encouraged by foraging theory, we expected that a single measure of observed, actual profitability (energy/handling time) would correlate with recovery order. As the energetic content of almonds is known, the expected profitability for each bag could be determined before each trial by dividing the kilocalories per bag by the summed expected pursuit time (from experiment 1) and processing time (from experiment 2). A predicted order of recovery could thus be determined. Unlike most previous foraging tests, 1) the subjects were expected to solve trial-unique problems solely through the use of memory and, 2) the “ideal solution” to each problem from a theoretical perspective—the item that “should” be recovered at each decision point—was calculated using standard optimality criteria.
EXPERIMENT 1
The primary aim of this experiment was to construct a regression equation predicting the pursuit time required for an individual chimpanzee to direct an uninformed person to a hidden object, based on the distance the object was from the chimpanzee.
Methods
Ethical note
The subjects in this study were fed three meals of fruits and vegetables daily, could request specific foods via lexigrams both during and between meals, were not food deprived at any time, and had ad libitum access to water. Participation in all of the testing described below was voluntary on the part of the chimpanzees. The research methods were approved by the Institutional Animal Care and Use Committee of Georgia State University (IACUC Protocol Number A07008).
Subjects and environment
Two common chimpanzees (Pan troglodytes) at the Language Research Center, Panzee (female, 23 years old) and Sherman (male, 35 years old), were the subjects in this study. Both have a long history of cognitive testing and a background involving the use of lexigrams (Rumbaugh & Washburn 2003). Although both subjects had previous experience in the outdoor testing procedure outlined below, neither were specifically trained for any of the tasks reported here. Their rearing histories, however, undoubtedly predisposed them favorably towards the type of communication activities, including pointing, necessary for them (Menzel 1999; Menzel et al. 2006).
The chimpanzees were housed in a building with 4 indoor cages, the site of indoor testing, and 3 outdoor enclosures. Three of 4 indoor cages, and all 3 of the outdoor enclosures, contained lexigram boards with 256 unique geometrical symbols. Each of the outdoor enclosures bordered unfenced woodland, and approximately 350 m2 of this woodland served as an outdoor testing area. The woodland could not be entered by the chimpanzees, but was visible from a fenced tower where the subjects would sit during outdoor testing. The chimpanzees were given daily access to the outdoor enclosures, and indoor cages connected to them by sliding doors that they could operate.
Design and procedure
Each trial consisted of a cue-giving phase, a delay phase, and a response phase.
The cue-giving phase commenced with the chimpanzee in the fenced tower of an outdoor enclosure. The Experimenter stood in the wooded test area facing the chimpanzee. The test area could not be entered by the chimpanzee, as fencing separated the ape from it, as well as the experimenter (and later the uninformed searcher, see below). The Experimenter carried an opaque box containing 10 transparent, numbered plastic bags, each with an equivalent quantity of food. The type of food varied between trials but was identical within any given trial. One of the bags was removed from the box, held in the air and shown to the subject for 5 seconds, and then hidden in a unique location, ≤ 17 m from the outdoor enclosure, in the available cover of the test area. Care was taken to ensure that the chimpanzee attended visually to the hiding of each bag. The hiding place was generally within a small hole covered by broad-leaves or pine needles, with care taken to ensure that, after hiding, the reward was completely hidden and the ground surface looked undisturbed. The preparation of individual holes (which occurred during and not before cue-giving), in addition to the placement and covering of the associated individual bag, generally was completed in two minutes. This protocol was then repeated with the remaining bags. Note that no two bags were ever shown to the subject simultaneously for direct comparison.
The order in which bags were shown to the subjects, and the location in which each was hidden, was determined randomly by computer before each trial with the restriction that the exact location had never been used before. The location of each hidden bag was plotted to the nearest 30 cm or less on a detailed, scaled map of the test area, and subsequently transcribed to an identical map in QuickCad ©. The distance between the center of the observation tower (location of chimpanzee) and each bag was calculated using xy coordinates.
The delay between the hiding of the last bag and the time in which the subject could interact with an uninformed person was ≥ 15 minutes.
In the response phase, the chimpanzee could interact with a familiar caregiver indoors and was given the opportunity to “recruit” this person to go outside and locate, under subject direction, the hidden bags. Although aware that a trial was about to be run, this person was naïve as to the food types, quantities, or locations, and was not available for recruitment by the chimpanzee until the end of the delay phase. As noted above and reported previously (Menzel 1999; Menzel 2005), recruitment generally consists of, on the part of the chimpanzee, vocalizations to attract the attention of the person, lexigram use, and gestures towards the tunnel leading to the outdoor enclosure adjacent to the test area.
If directed outside by the chimpanzee, the uninformed person overturned leaves or other cover at a specific location (individual searches uncovered approximately 30 cm diameter of debris) to which the chimpanzee directed them. When such an inspection revealed a bag it was given to the chimpanzee through the fencing of the outdoor enclosure and the ape was able to consume its contents. The uninformed person, during this time, stood next to the outdoor enclosure, on the opposite side of the fencing from the chimpanzee, and would not take further direction until the bag contents were consumed and the empty bag itself handed through the fence to the human. The subject was then free to continue directing to other locations, and could terminate the trial at any time by leaving the tower. Chimpanzees, again, were unable to enter the test area, or to touch the bags until they were handed to them. After trial termination, unrecovered bags were removed by the Experimenter.
For the outdoor portion of the response phase, the experimentally naïve person announced the following into a radio: when the chimpanzee began directing, where (using compass direction and landmarks) the chimpanzee was directing him/her to, when a bag and its contents were recovered, when a bag was handed to the subject, and when contents of a bag were masticated and the bag itself handed back to the person. These radio announcements were digitally recorded, transcribed, and used to calculate handling times for recovered bags. Handling time includes pursuit time (time from beginning of direction to when the bag was handed to the subject) and processing time (time from receiving bag to when the empty bag was handed back to the human). A maximum of one test trial was conducted per day per animal. Ten (10) trials were conducted for each chimpanzee, with inter-trial intervals ≥ 16 hours per subject.
Results and discussion
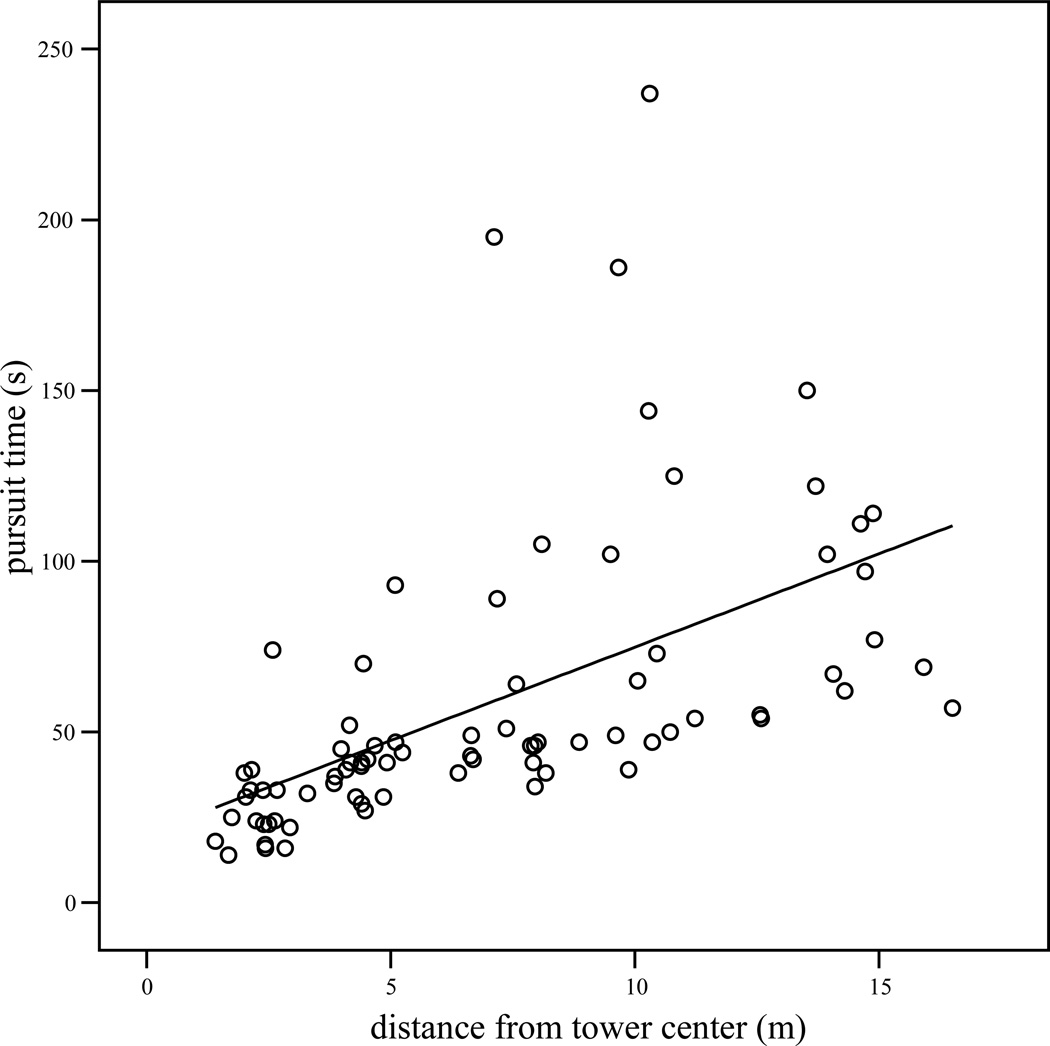
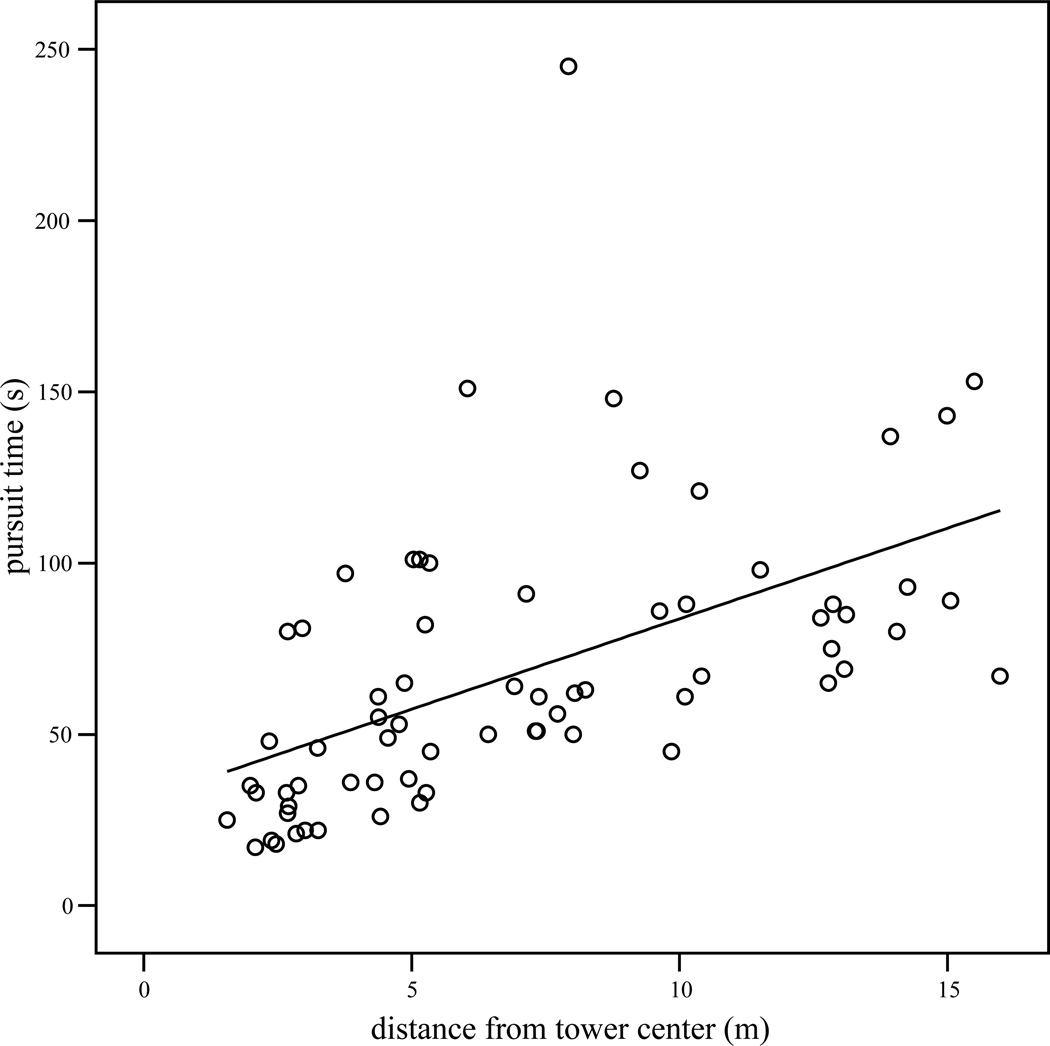
Only data from bags actually recovered were considered for those calculations involving pursuit time. For both subjects, bags hidden closer to the observation tower were recovered earlier in the sequence than more distant items (order recovered and distance; Panzee: Pearson’s r = 0.79, n = 100, p < 0.001; Sherman: r = 0.66, n = 100, p < 0.001). There was no significant relationship between the order in which bags were shown to the chimpanzee and the order in which they were recovered (Panzee: r = −0.01, n = 100, p = 0.93; Sherman: r = −0.09, n = 100, p = 0.36). Pursuit time was positively related to the distance bags were hidden from the chimpanzee (Figures 1 and 2, Panzee: r = 0.55, n = 79, p < 0.001; Sherman: r = 0.54, n = 68, p < 0.001).
Figure 1.
Scatterplot of the time taken to uncover hidden objects (pursuit time) by the distance they were hidden from Panzee (tower center) in experiment 1, with least-squares regression line.
Figure 2.
Scatterplot of the time taken to uncover hidden objects (pursuit time) by the distance they were hidden from Sherman (tower center) in experiment 1, with least-squares regression line.
Chimpanzee-specific linear regressions were also constructed with pursuit time (seconds) as dependent variable and distance from tower center (meters) as the sole independent variable. The models (Figures 1 and 2) were as follows: for Panzee, pursuit time (seconds) = 20.17 + (5.47 * distance from chimpanzee in meters) (R Square = 0.30; Beta (distance) = 0.55, t = 5.76, p < 0.001). For Sherman, pursuit time (s) = 30.95 + (5.28 * distance [m]) (R Square = 0.29; Beta = 0.54, t = 5.15, p < 0.001). Thus, Panzee generally required less time to direct uninformed persons to food items (i.e., exhibited shorter pursuit times) than Sherman.
EXPERIMENT 2
The aims of this experiment were to determine subject-specific preference for, and processing time associated with, no-shell and shell almonds.
Methods
Subjects
The subjects were the same as in experiment 1.
Apparatus
Experiment 2 was conducted using an apparatus containing a horizontal test board that could be pushed forward towards a subject or pulled backwards away. Identical plastic bowls were placed on the test board approximately 45 cm apart, and the subject could choose a single bowl from a two-bowl array by touching it through the wire mesh of its enclosure.
Design and procedure
Each chimpanzee was tested while in one of the four indoor cages, facing the Experimenter and testing apparatus. Outside the cage, the testing apparatus with test board was located between the Experimenter and subject.
Prior to each trial, the Experimenter baited two plastic bowls with one or more almonds. In one bowl was (were) 1,3,5,7, or 9 almond(s) without shells, and in the other bowl an equivalent number of almonds within shells. The two bowls were then placed on the test board, with left-right placement determined randomly. The test board was pushed forward towards the chimpanzee, who indicated its choice by touching one bowl, which was given to the subject. Baiting was then conducted in preparation for the next trial.
A split-time stopwatch was utilized to record elapsed time from when the bowl or food reward was first touched by the subject to when the chimpanzee had stopped chewing or swallowing the almond kernel(s). This was considered “processing time.”
Inter-trial intervals were approximately 30 seconds. Each subject underwent two sessions per testing day, with five trials per session. Each quantity (1,3,5,7, and 9) appeared once per session, and the order in which the quantities were presented was varied randomly between sessions. The experiment ended when the subject had completed 20 trials per quantity (100 trials total), or displayed a consistent (100%) choice of shell or no shell after 12 trials per quantity (60 trials total). After this testing had been completed, forced trials, where only one option was present, were performed to reach adequate sample sizes (n ≥ 15) to obtain a reliable measure of processing time for all quantities and shell states.
Results and discussion
As expected, in dichotomous choice trials, the chimpanzees selected almonds without shells significantly more often than almonds with shells (Panzee: binomial test, proportion no shell = 1.00, n = 60, p < 0.001; Sherman: proportion no shell = 0.67, n = 100, p = 0.001).
Processing times for both no-shell and shell almonds increased as quantity increased (Table 1). Almonds with shells were associated with longer processing times than those without them (paired t-tests, paired by ascending trial number, Panzee, all quantities p < 0.001; Sherman, all quantities p < 0.001). Mean processing times for Panzee were longer than those for Sherman under all quantities and shell states (independent sample t-tests for unequal variances, all comparisons, p < 0.005).
Table 1.
Mean processing times (seconds) for almonds by quantity and shell state
| Panzee | Sherman | ||||||
|---|---|---|---|---|---|---|---|
| object | n | mean | s.d. | object | n | mean | s.d. |
| 9N | 23 | 132.35 | 36.44 | 9N | 32 | 58.47 | 12.37 |
| 7N | 20 | 99.60 | 23.90 | 7N | 32 | 49.19 | 11.51 |
| 5N | 22 | 78.45 | 20.02 | 5N | 32 | 43.66 | 10.46 |
| 3N | 19 | 49.84 | 11.89 | 3N | 33 | 30.94 | 5.00 |
| 1N | 21 | 25.86 | 8.71 | 1N | 28 | 19.43 | 3.35 |
| 9S | 16 | 231.69 | 39.80 | 9S | 24 | 125.88 | 26.91 |
| 7S | 16 | 172.81 | 41.42 | 7S | 23 | 99.30 | 20.08 |
| 5S | 16 | 142.94 | 35.37 | 5S | 23 | 84.65 | 28.51 |
| 3S | 18 | 105.17 | 45.16 | 3S | 23 | 60.70 | 18.68 |
| 1S | 15 | 41.27 | 9.48 | 1S | 28 | 30.86 | 10.89 |
N = no shell, S = shell, s.d. = standard deviation
EXPERIMENT 3
The purpose of this experiment was to determine whether multiple hidden foods would be recovered by chimpanzees in a nonrandom order, and whether the recovery sequence could be predicted beforehand based on the known energetic value of the items, as well as the expected pursuit time (estimated from distance, experiment 1) and mean processing time (from experiment 2) associated with them.
Methods
Subjects
The subjects were the same as in experiments 1 and 2.
Design and procedure
The procedure in this experiment was generally the same as in experiment 1, but with food rewards corresponding in type and quantity to those used in experiment 2: 1, 3, 5, 7, or 9 almonds in or out of shell (10 bags total per trial). The no-shell almonds from each bag were weighed prior to each trial, with the edible portion of in-shell almonds assigned the same weight as the no-shell almonds. The value of 6 kcal per gram was utilized as the energetic yield for almonds; this represents energy available after subtraction of urinary/digestive losses in human models (United States Department of Agriculture 2010). As only one food type was utilized in this study, gross food weight could have as easily been used as currency, and any differences concerning available energy in chimpanzees from that of humans (Sayers et al. 2010) could safely be ignored. Unrecovered bags were left in place 16–48 hours after initial recruitment to allow for possible re-recruitment and bag recovery.
The x-y coordinates of each unsuccessful search, where cover was removed by the uninformed person but no bag was found, were plotted on a map of the test area. A bag was scored as “de facto recovered” if: 1) a search was conducted within 100 cm of where the bag was hidden, and 2) it was the bag closest to the search in absolute distance. Actual and de facto recovered bags were rank-ordered by sequence of recovery, with unrecovered bags assigned a tied score for last place. For each trial, sum of ranks totaled 55.
The size of the test area, the density of its vegetation, and the conditions of testing rendered it improbable that any given bag would be recovered by chance alone. To establish this, two control bags, each containing 9 almonds, were placed by the Experimenter in the test area prior to most trials, without being shown to the chimpanzee or the uninformed person. Twenty (20) trials were conducted for each chimpanzee, with inter-trial intervals ≥ 16 hours per subject.
Results and discussion
The mean delay length between the completion of cue-giving and first recruitment of a person for searching was 25.80 minutes for Panzee (n = 20, range: 16–131 min, standard deviation = 24.97) and 20.80 min for Sherman (n = 20, range: 15–30 min, s.d. = 4.42). In addition, Panzee re-recruited on three occasions and directed to items that had not been recovered on previous days (delays between cue-giving and re-recruitment: 1421, 2900, and 1384 min). As in experiment 1, there was no significant relationship between the order in which bags were presented to the chimpanzee and the order in which they were recovered (Panzee: Pearson’s r = −0.01, n = 200, p = 0.88; Sherman: Pearson’s r = −0.01, n = 200, p = 0.94). Panzee recovered 154/200 experimental bags, with 10/200 de facto recovered and 36/200 unrecovered. Sherman recovered 105/200 experimental bags, with 12/200 de facto recovered and 83/200 unrecovered. Panzee recovered 1/40 control bags, while Sherman recovered 0/38. No control bags for either subject were de facto recovered.
The hidden bags were recovered in a highly nonrandom, orderly fashion (Figure 3, Table 2). Panzee first directed searchers to larger quantities of no-shell almonds, next to smaller quantities of no-shell almonds, and lastly to almonds-in-shell. Sherman also directed to larger quantities of no-shell almonds at the outset, but followed this by recovering the larger quantities of almonds-in-shell. Small quantities of almonds were taken last in the sequence, regardless of shell presence or absence. There were significant interactions between the fixed factors quantity and shell state in relation to recovery order for both Panzee (GLM ANOVA, F = 2.60, p < 0.05) and Sherman (F = 3.31, p < 0.05).
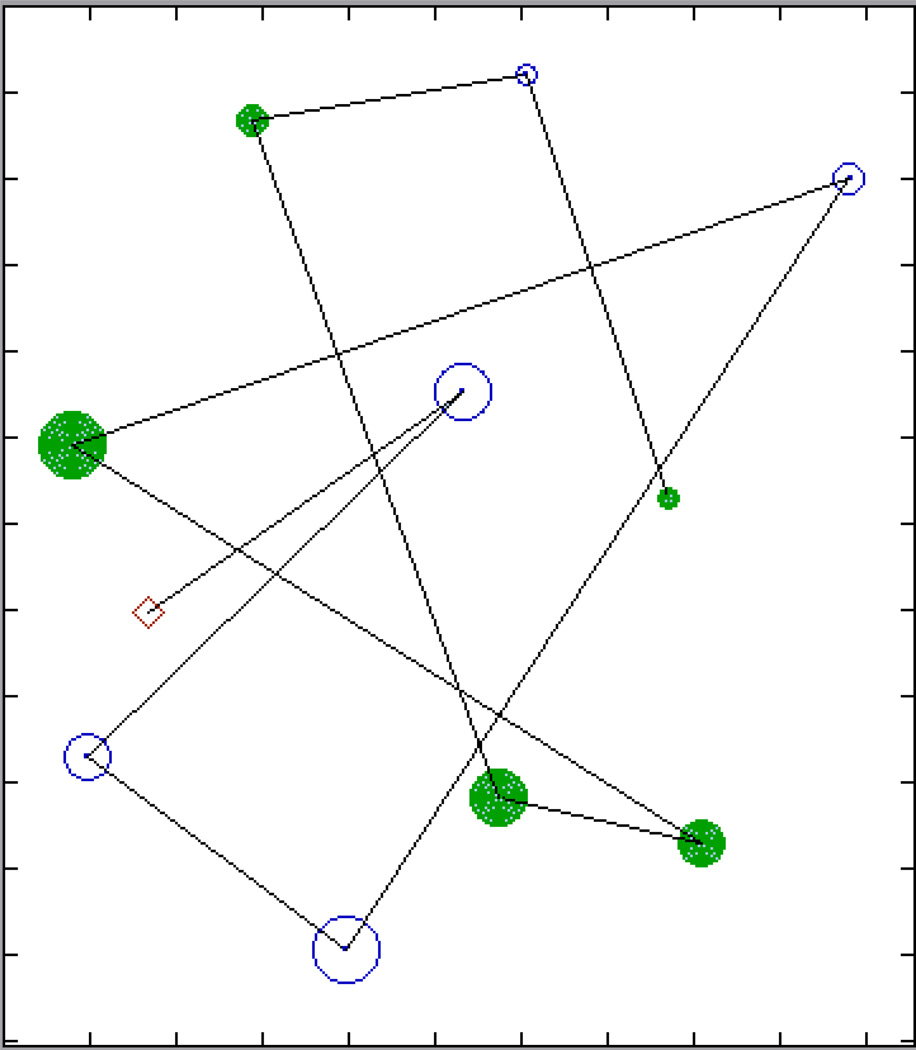
Figure 3.
Panzee’s recovery order for trial 6 in experiment 3. Diamond denotes subject’s position in tower; the line emanating from this point traces through hidden items in the order Panzee directed an uninformed person to them. Size of circle denotes almond quantity, open circles no-shell almonds, closed circles shell almonds. Hatches on the perimeter represent 1-meter increments. On this particular trial, all 10 hidden items were recovered and there were no unsuccessful searches.
Table 2.
Experiment 3: mean order of bag recovery by quantity and shell state
| Panzee LSD = 1.20 |
Sherman LSD = 1.30 |
||||||
|---|---|---|---|---|---|---|---|
| object | mean | s.d. | objects recovered significantly earlier |
object | mean | s.d. | objects recovered significantly earlier |
| 9N | 2.70 | 1.49 | none | 9N | 2.35 | 1.57 | None |
| 7N | 2.75 | 1.48 | none | 7N | 3.58 | 2.63 | none |
| 5N | 2.30 | 2.00 | none | 5N | 4.50 | 2.73 | 9N |
| 3N | 4.23 | 2.44 | 9N, 7N, 5N | 3N | 7.25 | 1.92 | 9N, 7N, 5N, 9S, 7S |
| 1N | 5.28 | 2.35 | 9N, 7N, 5N | 1N | 7.98 | 1.12 | 9N, 7N, 5N, 9S, 7S, 5S, 3S |
| 9S | 7.10 | 1.74 | all N | 9S | 4.18 | 2.21 | 9N |
| 7S | 7.30 | 1.84 | all N | 7S | 4.15 | 2.33 | 9N |
| 5S | 7.53 | 1.51 | all N | 5S | 6.48 | 1.86 | 9N, 7N, 5N, 9S, 7S |
| 3S | 7.68 | 1.62 | all N | 3S | 6.68 | 1.45 | 9N, 7N, 5N, 9S, 7S |
| 1S | 8.15 | 1.51 | all N | 1S | 7.88 | 1.18 | 9N, 7N, 5N, 9S, 7S, 5S |
LSD = least significant difference statistic (Conover 1999, p. 371), N = no shell, S =shell, s.d. = standard deviation. The differences in the mean ranks must exceed the LSD value to be considered statistically significant at the 0.05 level. For example, Panzee took 9N significantly earlier in the recovery sequence than 3N, 1N, and all five S bags.
In a forward selection linear regression model for Panzee (R2 = 0.57), the observed 1-2-3…10 recovery order was positively related to shell presence (Beta = 0.72, t = 15.46, p < 0.001) and negatively related to quantity (Beta = −0.23, t = −4.85, p < 0.001). Quantity added only 0.05 to the R2 value from a model containing shell absence/presence only. The variable “distance from chimpanzee” was positively related to recovery order but excluded (Beta In = 0.08, t = 1.66, p = 0.10). For Sherman (R2 = 0.46), quantity (Beta = −0.66, t = −12.48, p < 0.001) was a significant negative predictor of recovery order, with shell presence (Beta = 0.13, t = 2.37, p < 0.05) and distance (Beta = 0.11, t = 2.01, p < 0.05) being positively related to recovery order. Shell absence/presence added only 0.02, and distance only an additional 0.01, to the R2 value of the model containing quantity alone.
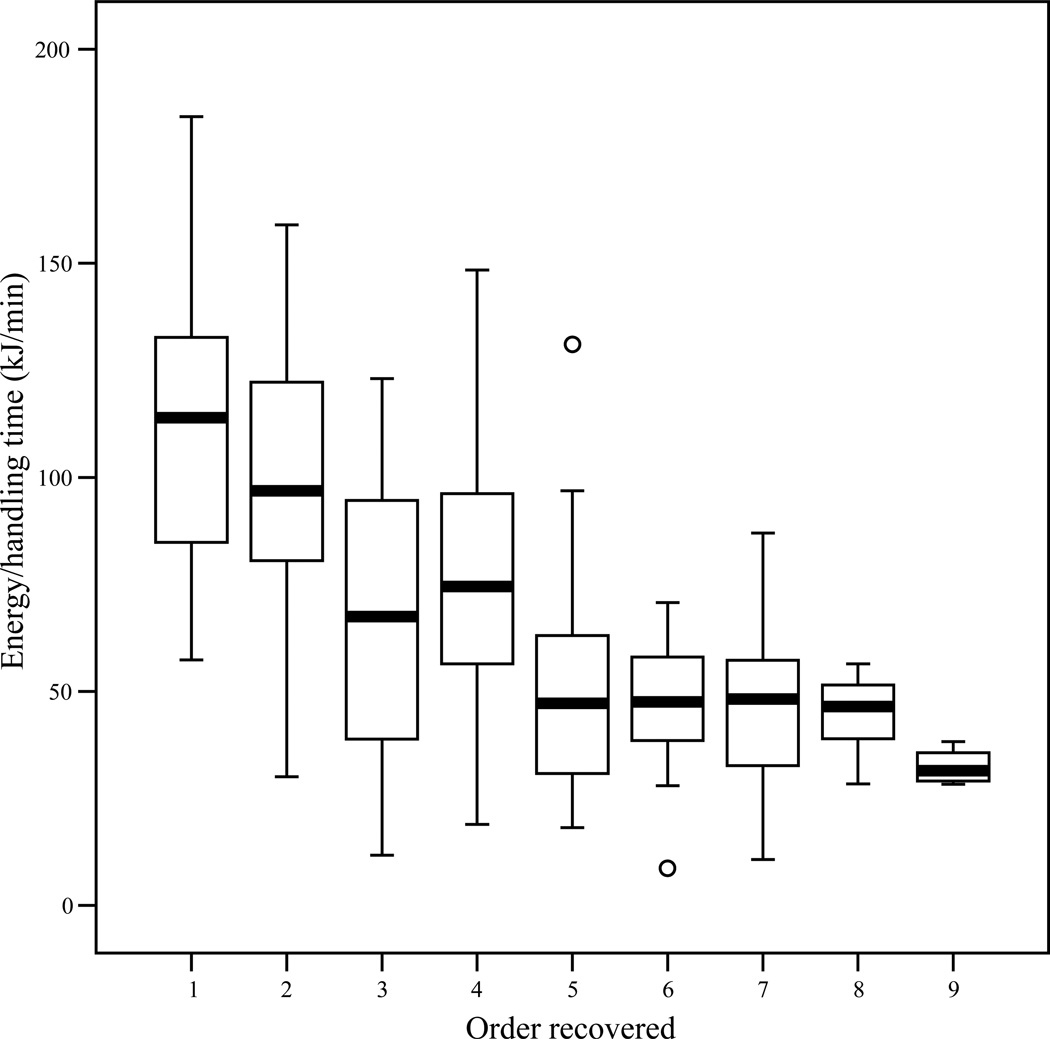
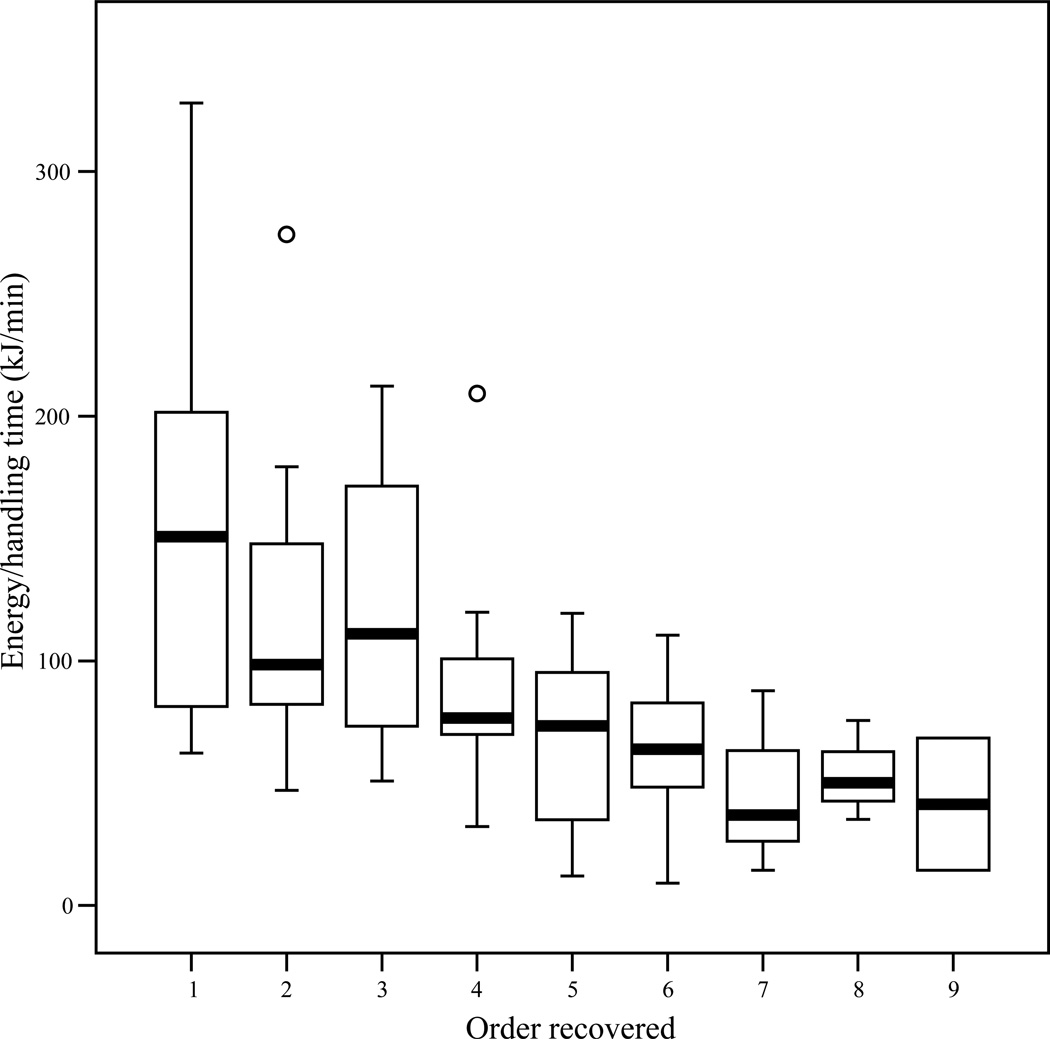
Based on the known energetic values of the almonds, and the actual pursuit and processing times, both subjects recovered items in close accordance to the actual e/h profitability of the items (Figures 4 and 5). Note that actual profitability (which requires data on processing time) was not calculated for unrecovered and “de facto recovered” bags. Across all trials, there was a significant negative correlation between the 1-2-3…10 order of recovery and the kilocalories per minute handling time for recovered bags (Panzee, Pearson’s r = −0.62, n = 133, p < 0.001; Sherman, Pearson’s r = −0.56, n = 103, p < 0.001). For specific trials, the Pearson correlation coefficient between order recovered and kcal/minute was negative in 20/20 trials for Panzee, with a statistically significant negative correlation in eight. For Sherman, 16/20 correlation coefficients were negative (four statistically significant) and 3/20 correlation coefficients were positive (none significant). Pearson’s r could not be calculated for the remaining trial (Trial 6; 1 bag recovered, 3 additional bags “de facto recovered”).
Figure 4.
Boxplot of actual profitability (observed kcal/min) versus order recovered for Panzee in experiment 3. Line denotes median, boxes extend to the 25th and 75th percentiles, and whiskers extend to observed values within 1.5 box lengths. Circles are outliers. In trials where Panzee recovered the 10th and final bag, she left the tower before eating the almonds. Therefore, actual profitability is not available for any bag recovered in this position due to lack of processing time data.
Figure 5.
Boxplot of actual profitability (observed kcal/min) versus order recovered for Sherman in experiment 3. Notation as in Figure 6. Actual profitability was not calculated for the tenth position in the one trial (Trial 10) where Sherman directed searchers to the locations of all ten bags, as this last item was de facto recovered.
The final question addressed in Experiment 3 was how well the order of recovery of 10 bags on each trial-unique foraging problem could be predicted in advance by an independent measurement of the expected e/h value for each bag. This includes all bags, whether unrecovered, recovered, or “de facto recovered.” Using the known energetic contents per bag, and the expected pursuit and processing times from experiments 1 and 2, predicted and observed recovery orders were closely related (Table 3, Panzee: Pearson’s r = 0.61, n = 200, p < 0.001; Sherman: Pearson’s r = 0.66, n = 200, p < 0.001). For specific trials, the Pearson correlation coefficient between predicted and observed recovery order was positive in 20/20 trials for both Panzee (10/20 significant) and Sherman (11/20 significant).
Table 3.
Experiment 3: trial-specific observed versus predicted order of recovery
| Observed (Predicted) Order of Recovery |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Trial | Delay (min) | 9N | 7N | 5N | 3N | 1N | 9S | 7S | 5S | 3S | 1S | r |
| Panzee | 1 | 24 | 1(1) | 2(2) | 9d(3) | 3(6) | 8d(10) | 6(5) | 4d(4) | 5(7) | 7d(8) | 10(9) | 0.66* |
| 2 | 21 | 1(1) | 3(2) | 2(3) | 8(6) | 7(10) | 4(5) | 5(4) | 9.5♦(7) | 6d(8) | 9.5♦(9) | 0.83** | |
| 3 | 131 | 2(1) | 3(3) | 1(2) | 9♦(4) | 4(9) | 6(6) | 5(5) | 9♦(7) | 7(8) | 9♦(10) | 0.64* | |
| 4 | 17 | 3(2) | 2(3) | 1d(1) | 8.5♦(4) | 8.5♦(9) | 6(6) | 5d(5) | 8.5♦(7) | 4(8) | 8.5♦10) | 0.73* | |
| 5 | 17 | 1(1) | 2(3) | 5(2) | 3(5) | 10♦(9) | 8(4) | 7(6) | 4(7) | 9(8) | 6(10) | 0.65* | |
| 6 | 17 | 3(1) | 1(2) | 2(3) | 4(6) | 9(9) | 5(4) | 7(5) | 6(7) | 8(8) | 10(10) | 0.90** | |
| 7 | 27; 1421 | 4(2) | 7(1) | 1(3) | 3(6) | 2(7) | 6(5) | 10♦(4) | 8(8) | 9(9) | 5(10) | 0.15 | |
| 8 | 19; 2900 | 1(2) | 4(1) | 5(3) | 2(4) | 3(8) | 9♦(5) | 7(6) | 9♦(7) | 6(9) | 9♦(10) | 0.55 | |
| 9 | 18 | 4(2) | 2(1) | 1(3) | 9♦(4) | 3(10) | 5(5) | 7(6) | 6(7) | 9♦(8) | 9♦(9) | 0.47 | |
| 10 | 22; 1384 | 4(2) | 1(1) | 3(3) | 2(4) | 8♦(9) | 8♦(5) | 5(6) | 8♦(7) | 8♦(8) | 8♦(10) | 0.85** | |
| 11 | 25 | 1(2) | 4(1) | 2(3) | 5d(6) | 3(8) | 8(4) | 9(5) | 7(7) | 10♦(9) | 6(10) | 0.48 | |
| 12 | 16 | 3(1) | 1(2) | 2(3) | 4(4) | 5(8) | 7(5) | 9d(6) | 6(7) | 10(10) | 8(9) | 0.82** | |
| 13 | 17 | 5(1) | 2(2) | 1(3) | 3(4) | 4(10) | 10(5) | 6(6) | 7(7) | 8(8) | 9(9) | 0.50 | |
| 14 | 23 | 5(2) | 1(1) | 2(3) | 4(7) | 3(10) | 8♦(5) | 8♦(4) | 8♦(6) | 8♦(8) | 8♦(9) | 0.37 | |
| 15 | 21 | 1(1) | 3(2) | 2(3) | 4(4) | 5(9) | 7(5) | 9(6) | 8(7) | 6(8) | 10♦(10) | 0.78** | |
| 16 | 18 | 2(1) | 4(2) | 1(3) | 3(4) | 5(9) | 6(5) | 7(6) | 9♦(7) | 9♦(8) | 9♦(10) | 0.79** | |
| 17 | 24 | 4(2) | 2(1) | 1(3) | 3(6) | 5(9) | 10♦(4) | 9(5) | 7(7) | 8(8) | 6(10) | 0.38 | |
| 18 | 17 | 2(1) | 4(3) | 3(2) | 1(4) | 5(9) | 10d(5) | 9(6) | 8(7) | 6(8) | 7(10) | 0.54 | |
| 19 | 20 | 5(1) | 4(2) | 1(3) | 2(5) | 3(9) | 6(4) | 8(6) | 9.5♦(7) | 9.5♦(8) | 7(10) | 0.43 | |
| 20 | 22 | 2(1) | 3(2) | 1(3) | 4(5) | 5(9) | 7(6) | 10♦(4) | 8(7) | 6(8) | 9(10) | 0.60 | |
| Subject | Trial | Delay (min) | 9N | 7N | 5N | 3N | 1N | 9S | 7S | 5S | 3S | 1S | r |
| Sherman | 1 | 20 | 3(1) | 1(2) | 8.5♦(3) | 8.5♦(6) | 8.5♦(10) | 5(4) | 2(5) | 4(7) | 8.5♦(8) | 6(9) | 0.55 |
| 2 | 17 | 2(1) | 1(2) | 3(4) | 9♦(7) | 9♦(9) | 9♦(3) | 4(5) | 6(6) | 5(8) | 7(10) | 0.62 | |
| 3 | 20 | 1(1) | 5(3) | 8♦(2) | 8♦(5) | 8♦(9) | 3(4) | 4(6) | 2(7) | 8♦(8) | 8♦(10) | 0.46 | |
| 4 | 16 | 4d(2) | 1(1) | 3(3) | 9♦(6) | 9♦(9) | 5(5) | 2(4) | 7(7) | 6(8) | 9♦(10) | 0.87** | |
| 5 | 19 | 3(2) | 2(1) | 7♦(3) | 7♦(6) | 7♦(10) | 1(4) | 7♦(5) | 7♦(7) | 7♦(8) | 7♦(9) | 0.68* | |
| 6 | 21 | 4d(1) | 7.5♦(2) | 7.5♦(3) | 1d(6) | 7.5♦(9) | 2d(4) | 3(5) | 7.5♦(7) | 7.5♦(8) | 7.5♦(10) | 0.31 | |
| 7 | 30 | 3(2) | 2(1) | 1(3) | 7(7) | 9.5♦(9) | 4d(4) | 6(5) | 8(6) | 5(8) | 9.5♦(10) | 0.88** | |
| 8 | 15 | 1(1) | 8.5♦(2) | 4(3) | 8.5♦(6) | 8.5♦(10) | 2(4) | 3d(5) | 6(7) | 5d(8) | 8.5♦(9) | 0.56 | |
| 9 | 19 | 1(1) | 8♦(2) | 2(3) | 8♦(6) | 8♦(9) | 3d(4) | 4(5) | 5(7) | 8♦(8) | 8♦(10) | 0.67* | |
| 10 | 23 | 2(1) | 3(2) | 10d(4) | 8(7) | 9(9) | 4(3) | 1(5) | 5(6) | 6(8) | 7(10) | 0.58 | |
| 11 | 26 | 4(1) | 1(2) | 2(3) | 7.5♦(7) | 7.5♦(10) | 3(4) | 7.5♦(5) | 7.5♦(6) | 7.5♦(8) | 7.5♦(9) | 0.81** | |
| 12 | 16 | 2(1) | 5(2) | 3(4) | 6(7) | 7(9) | 1(3) | 9(5) | 8(6) | 4(8) | 10♦(10) | 0.66* | |
| 13 | 23 | 1(1) | 2(2) | 5(4) | 9♦(7) | 9♦(9) | 3(3) | 6(5) | 4(6) | 7(8) | 9♦(10) | 0.93** | |
| 14 | 17 | 1(2) | 7.5♦(1) | 7.5♦(3) | 4(6) | 7.5♦(10) | 3(5) | 2(4) | 7.5♦(7) | 7.5♦(8) | 7.5♦(9) | 0.43 | |
| 15 | 25 | 2(1) | 1(2) | 3(3) | 7(7) | 9♦(9) | 4(4) | 5(5) | 9♦(6) | 9♦(8) | 6(10) | 0.83** | |
| 16 | 24 | 3(1) | 6(2) | 2(3) | 7d(6) | 5d(9) | 8d(4) | 1(5) | 9.5♦(7) | 4(8) | 9.5♦(10) | 0.44 | |
| 17 | 29 | 7♦(1) | 3(2) | 2(5) | 7♦(6) | 7♦(9) | 7♦(4) | 1(3) | 7♦(7) | 7♦(8) | 7♦(10) | 0.47 | |
| 18 | 15 | 1(1) | 2(2) | 3(3) | 8♦(6) | 8♦(10) | 4(4) | 5(5) | 8♦(7) | 8♦(8) | 8♦(9) | 0.94** | |
| 19 | 22 | 1(1) | 2(2) | 6.5♦(4) | 6.5♦(6) | 6.5♦(9) | 6.5♦(3) | 6.5♦(5) | 6.5♦(7) | 6.5♦(8) | 6.5♦(10) | 0.70* | |
| 20 | 19 | 1(1) | 3(2) | 2(4) | 9♦(7) | 9♦(10) | 6(3) | 4(5) | 5(6) | 7(8) | 9♦(9) | 0.87** | |
See text for methods regarding the calculation of predicted order of recovery (given in parentheses). Secondary recruitments to recover items remaining hidden after initial recruitment are denoted by italicized delay lengths; any bags recovered after secondary recruitment also denoted by italics. N = no shell, S = shell,
de facto recovered bag,
bag left unrecovered,
r = Pearson rank order correlation coefficient between predicted and observed recovery order,
p < 0.05,
p < 0.01.
GENERAL DISCUSSION
Chimpanzees, by memory, directed experimentally naïve persons to hidden almonds in approximate order of the food’s e/h profitability, with multiple reward characteristics being utilized concurrently to select a recovery sequence. Bags with larger almond quantities, almonds without shells, and almonds hidden closer to the subject were generally recovered before those with smaller quantities, those with shells, and those located at greater distances. It is probable that visual perception of these physical and spatial food characteristics during the cue-giving phase of each trial provided information that was stored, weighted, and later retrieved to guide recovery. This differs from traditional “rules of thumb” (Janetos & Cole 1981), some of which are elegantly simple (e.g., patch departure rules in crab spiders (Misumena vatia), Kareiva et al. 1989), in that the present performance requires the differential and simultaneous consideration of several criteria relevant to profitability (see Ydenberg et al. 2007) without the benefit of being able to perceive any of the items at the time of decision-making. In addition, the chimpanzees were required to access their memory of items while at the same time manually directing a human to their locations, adding an interactive, social dimension to the task (Kahneman 1973).
Independently-constructed, chimpanzee-specific regressions for predicted pursuit time, and individual mean almond processing times, provided predicted recovery orders that were closely related to observed recovery orders. Unrecovered bags were generally those with low expected profitability. Iteratively selecting the “best remaining item” at each decision point, as observed here and variably called instantaneous or short-term rate maximization, is considered most likely to occur in social foraging contexts or situations where feeding bouts may terminate unexpectedly (Holt & Kotler 1987; Mitchell 1990) and is likely especially common whenever multiple foods of differing value are presented to a forager in a restricted space.
In foraging theory, decisions are regarded to be functions of individuals, and its predictions are based on factors at the level of the organism and not simply data averaged across multiple subjects (Krebs & McCleery 1984). From this standpoint, it is especially interesting that the apes in this study exhibited individual differences in recovery order in relation to quantity, shell state and distance, and that these were consistent with their individual abilities in pursuit and processing (see also Sullivan 1988). The female Panzee, who exhibited long almond processing times but relatively short pursuit times, placed most importance on shell state, with quantity secondary and distance only weakly related to recovery order. In fact, in trials 11–20 of experiment 3, Panzee actually devalued shell almonds to a greater degree than would be expected by the profitability criteria utilized here. The male Sherman, who processed almonds more quickly but required longer pursuit times, recovered bags largely in relation to quantity, with shell state and distance as secondary factors. Note that the preference for no-shell almonds (as in kangaroo rats (Dipodomys merriami) with millet, Brown & Mitchell 1989) is predicted by foraging theory, but is against the predilection that some animals (but not all, Shettleworth 1985) show in selectively feeding on the item with largest physical appearance (e.g., rats, Yoshioka 1930).
Although the chimpanzees performed very well under the present e/h model, in no trials were recovery order and actual or expected profitability perfectly correlated, and some causes, or potential causes, for such deviation can be identified or hypothesized. The first is that expected food energy to handling time, as utilized here, is unlikely the final word as a measure of optimal performance. Food items vary in relation to handling costs, such as the energy required to process and consume a shell or no-shell almond, and further differ as to the wear they pose to the dentition. These aspects could certainly be related to Panzee’s “overestimating” the importance of the shell factor. A second reason for deviation is that the chimpanzees only rarely (Panzee: 5/20 trials; Sherman: 1/20) recovered or made attempts on all ten bags. As unrecovered items were almost always of low expected profitability (see Table 3 and earlier discussion), it is possible these were considered unworthy of effort rather than forgotten. Although difficult to determine on a case-by-case basis, a third cause of deviation was almost certainly true perceptual and/or memory errors. Given that bags were visible for only seconds before being hidden, for example, some quantity discriminations (e.g., between 7 and 9 almonds) may have been difficult, and such fine distinctions may have been taken into consideration only variably during the response phase.
Unlike many investigations of food selection, however, the chimpanzees in this study recovered items in a systematic and ordered fashion even though the items were presented in trial-unique locations and were not visible, and had not been seen for delays ≥ 15 minutes. In several cases, locations were remembered and items recovered after 1–2 days. Although it is possible that memory errors would significantly increase at delays of days rather than minutes or hours, the comparatively small number of items recovered after such time frames in the present experiments render any such conclusions equivocal. In this respect, it is important to note that while the delays utilized here (generally 15–30 min) could be deemed long by some laboratory standards, they are miniscule compared to a free-ranging animal that may be outside of perceptual contact with a given immobile food patch for days, weeks or months. It is likely that myriad factors are associated with how well individual features of the environment are remembered by animals in the wild. How often a resource (such as a tree) has been encountered in the past, the delay since it has last been encountered, the preference value of the food in it, and its size could all potentially contribute to the existence and/or strength of the memory trace (e.g., Lynch 2004).
Another difference between the experiments presented and animal foraging in the wild is that, in the former, a chimpanzee must direct a human to recover hidden objects, adding additional complexity to the task. As noted, however, one benefit of this technique from an experimental perspective is that, by removing locomotion costs and potential detection cues, examinations of trade-offs between factors such as food quantity and expected pursuit time, in relation to memory, are relatively straightforward. If the chimpanzees were given a complementary task where they themselves recovered the items, the “optimal solution” would possibly differ. Locomotion costs, for example, might shift emphasis towards minimizing distance (Menzel 1973, as in the mathematical traveling salesman problem. In addition, the experiments presented here, while over a larger spatial scale than most other laboratory experiments, were over a much smaller spatial scale than that occupied by free-ranging animals. In such situations, increased distance could reduce the relative importance of food type or quantity as it pertains to goal-oriented movement (e.g., Janson 1998, 2007).
Much work on ecological cognition has focused on whether or not food patches are encountered non-randomly; for example, whether the locations of particular trees are stored in memory (e.g., primates, Menzel 1997; Janson & Byrne 2007). While even sequential and random encounter models of diet selection have proven useful (e.g., primates, Winterhalder & Smith 1981; Hawkes et al. 1982; Sayers et al. 2010), much remains to be learned regarding the influence of memory on foraging efficiency. When an animal is presented with two or more food items concurrently, it is termed simultaneous encounter, and a number of foraging models have been developed to address this scenario (e.g., Waddington & Holden 1979; Engen & Stenseth 1984). When details of immobile foods, such as identity and relative preference value, can be accessed via long-term memory, the diet selection process could be viewed as a series of simultaneous encounters, where potential food items or patches within perceptual range may be compared or contrasted with key resources located outside of it, and foraging path determined by profitability criteria. Thus, resources may be “encountered” in memory. In spider monkeys (Ateles geoffroyi), for example, feeding movements have been found to be consistent with a Lévy random walk model (Ramos-Fernandez et al. 2004) and also an optimal foraging, directed-search model that presumes partial knowledge of resource location and value (Boyer et al. 2006). Although both types of model are constructive, and indeed can and should be investigated concurrently (Bartumeus & Catalan 2009), present interest is tilted heavily in favor of the former. Given our results and those of previous primate spatial cognition studies (Menzel 1991), 1996; Janson 1998), we consider the directed-search paradigm in particular need of further theoretical, experimental, and observational investigation (see also Benhamou 2007).
Chimpanzees, given trial-unique problems, are able to remember multiple dimensions of foods that are outside the range of their perception and utilize this information to select an efficient recovery order. Much remains to be learned concerning the extent of such abilities in other nonhumans, and thus future comparative work is vital (Collett 2002; Sayers & Lovejoy 2008). The species-specific elucidation of what food characteristics are remembered, how accurately and how long, will be of primary importance for the incorporation of realistic cognitive variables into models of diet selection and movement.
-
➢
Chimpanzees recovered hidden foods in order of their e/h (energy/handling time) profitability.
-
➢
Chimpanzees remembered multiple details of foods.
-
➢
Researchers, using foraging theory variables, could predict food recovery order.
ACKNOWLEDGMENTS
We would like to thank John Kelley, Betty Chan, and Sarah Hunsberger for their assistance during the data collection phase of this project. David Washburn, Mike Beran, Michael Owren, R. Thompson Putney, and Duane Rumbaugh engaged in helpful discussions with the authors. Dorothy Cheney, Emilie Menzel, and two anonymous reviewers provided useful comments. We also thank Emil Menzel for offering a critical analysis of an earlier version of this paper, as well as for performing much of the research that serves as its inspiration. The project was supported by grants HD-056352, HD-38051, HD-060563, and 1F32HD061177 from the National Institutes of Health (NIH). The contents of this article do not necessarily represent the official views of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams-Hunt MM, Jacobs LF. Cognition for foraging. In: Stephens DW, Brown JL, Ydenberg RC, editors. Foraging: Behavior and Ecology. Chicago: The University of Chicago Press; 2007. pp. 105–138. [Google Scholar]
- Barraquand F, Inchausti P, Bretagnolle V. Cognitive abilities of a central place forager interact with prey spatial aggregation in their effect on intake rate. Animal Behaviour. 2009;78:505–514. [Google Scholar]
- Bartumeus F, Catalan J. Optimal search behavior and classic foraging theory. Journal of Physics A: Mathematical and Theoretical. 2009;42:434002. [Google Scholar]
- Bélisle C, Cresswell J. The effects of a limited memory capacity on foraging behavior. Theoretical Population Biology. 1997;52:78–90. doi: 10.1006/tpbi.1997.1319. [DOI] [PubMed] [Google Scholar]
- Benhamou S. How many animals really do the Lévy walk? Ecology. 2007;88:1962–1969. doi: 10.1890/06-1769.1. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Harris EH. Perception of food amounts by chimpanzees based on the number, size, contour length and visibility of items. Animal Behaviour. 2008;75:1793–1802. doi: 10.1016/j.anbehav.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berec L, Křivan V. A Mechanistic Model for Partial Preferences. Theoretical Population Biology. 2000;58:279–289. doi: 10.1006/tpbi.2000.1491. [DOI] [PubMed] [Google Scholar]
- Boyer D, Ramos-Fernández G, Miramontes O, Mateos JL, Cocho G, Larralde H, Ramos H, Rojas F. Scale-free foraging by primates emerges from their interaction with a complex environment. Proceedings of the Royal Society B: Biological Sciences. 2006;273:1743–1750. doi: 10.1098/rspb.2005.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boysen ST, Berntson GG. Responses to quantity: perceptual 518 versus cognitive mechanisms in chimpanzees (Pan troglodytes) Journal of Experimental Psychology. Animal Behavior Processes. 1995;21:82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- Brown JS, Mitchell WA. Diet selection on depletable resources. Oikos. 1989;54:33–43. [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Memory for the content of caches by scrub jays (Aphelocoma coerulescens) Journal of Experimental Psychology Animal Behavior Processes. 1999;25:82–91. [PubMed] [Google Scholar]
- Collett TS. Spatial learning. In: Gallistel E, Pashler H, editors. Steven's Handbook of Experimental Psychology, Volume 3, Learning, motivation, and emotion. 3rd edition. New York: John Wiley & Sons; 2002. pp. 301–364. [Google Scholar]
- Conover WJ. Practical Nonparametric Statistics. New York: John Wiley & Sons; 1999. [Google Scholar]
- Cunningham E, Janson C. Integrating information about location and value of resources by white-faced saki monkeys (Pithecia pithecia) Animal Cognition. 2007;10:293–304. doi: 10.1007/s10071-007-0077-4. [DOI] [PubMed] [Google Scholar]
- Dall SRX, Giraldeau LA, Olsson O, McNamara JM, Stephens DW. Information and its use by animals in evolutionary ecology. Trends in Ecology & Evolution. 2005;20:187–193. doi: 10.1016/j.tree.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Engen S, Stenseth NC. A general version of optimal foraging theory: The effect of simultaneous encounters. Theoretical Population Biology. 1984;26:192–204. [Google Scholar]
- Garber PA. Role of spatial memory in primate foraging patterns: Saguinus mystax and Saguinus fuscicollis. American Journal of Primatology. 1989;19:203–216. doi: 10.1002/ajp.1350190403. [DOI] [PubMed] [Google Scholar]
- Hawkes K, Hill K, O'Connell JF. Why hunters gather: optimal foraging and the Ache of eastern Paraguay. American Ethnologist. 1982;9:379–398. [Google Scholar]
- Held S, Baumgartner J, KilBride A, Byrne RW, Mendl M. Foraging behaviour in domestic pigs (Sus scrofa): remembering and prioritizing food sites of different value. Animal Cognition. 2005;8:114–121. doi: 10.1007/s10071-004-0242-y. [DOI] [PubMed] [Google Scholar]
- Holt RD, Kotler BP. Short-Term Apparent Competition. American Naturalist. 1987;130:412–430. [Google Scholar]
- Janetos AC, Cole BJ. Imperfectly optimal animals. Behavioral Ecology and Sociobiology. 1981;9:203–209. [Google Scholar]
- Janmaat KRL, Chancellor RL. Exploring new areas: How important is long551 term spatial memory for mangabey (Lophocebus albigena johnstonii) foraging efficiency? International Journal of Primatology. 2010;31:863–886. [Google Scholar]
- Janson CH. Experimental evidence for spatial memory in foraging wild capuchin monkeys, Cebus apella. Animal Behaviour. 1998;55:1229–1243. doi: 10.1006/anbe.1997.0688. [DOI] [PubMed] [Google Scholar]
- Janson CH. Experimental evidence for route integration and strategic planning in wild capuchin monkeys. Animal Cognition. 2007;10:341–356. doi: 10.1007/s10071-007-0079-2. [DOI] [PubMed] [Google Scholar]
- Janson CH, Byrne R. What wild primates know about resources: opening up the black box. Animal Cognition. 2007;10:357–367. doi: 10.1007/s10071-007-0080-9. [DOI] [PubMed] [Google Scholar]
- Joly M, Zimmermann E. Do solitary foraging nocturnal mammals plan their routes? Biology Letters. 2011;7:638–640. doi: 10.1098/rsbl.2011.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Attention and Effort. Englewood Cliffs: Prentice Hall; 1973. [Google Scholar]
- Kareiva P, Morse DH, Eccleston J. Stochastic prey arrivals 562 and crab spider giving-up times: simulations of spider performance using two simple"rules of thumb”. Oecologia. 1989;78:542–549. doi: 10.1007/BF00378746. [DOI] [PubMed] [Google Scholar]
- Köhler W. The Mentality of Apes. London: Routledge and Kegan Paul; 1925. [Google Scholar]
- Krebs JR, McCleery RH. Optimization in behavioural ecology. In: Krebs JR, Davies NB, editors. Behavioural Ecology: An Evolutionary Approach. Second Edition. Sutherland: Sinauer; 1984. pp. 91–121. [Google Scholar]
- Kurland JA, Beckerman SJ. Optimal foraging and hominid evolution: labor and reciprocity. American Anthropologist. 1985;87:73–93. [Google Scholar]
- Lifjeld JT, Slagsvold T. Effects of energy costs on the optimal diet: An experiment with pied flycatchers Ficedula hypoleuca feeding nestlings. Ornis Scandinavica. 1988;19:111–118. [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiological Reviews. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Manser MB, Bell MB. Spatial representation of shelter locations in meerkats, Suricata suricatta. Animal Behaviour. 2004;68:151–157. [Google Scholar]
- Menzel CR. Cognitive aspects of foraging in Japanese monkeys. Animal Behaviour. 1991;41:397–402. [Google Scholar]
- Menzel CR. Spontaneous use of matching visual cues during foraging by long-tailed macaques (Macaca fascicularis) Journal of Comparative Psychology. 1996;110:370–376. doi: 10.1037/0735-7036.110.4.370. [DOI] [PubMed] [Google Scholar]
- Menzel CR. Primates' knowledge of their natural habitat: As indicated in foraging. In: Whiten A, Byrne R, editors. Machiavellian Intelligence II: Extensions and Evaluations. Cambridge: Cambridge University Press; 1997. pp. 207–239. [Google Scholar]
- Menzel CR. Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. Journal of Comparative Psychology. 1999;113:426–434. doi: 10.1037/0735-7036.113.4.426. [DOI] [PubMed] [Google Scholar]
- Menzel CR. Progress in the study of chimpanzee recall and episodic memory. In: Terrace HS, Metcalfe J, editors. The Missing Link in Cognition: Origins of Self-Reflective Consciousness. Oxford: Oxford University Press; 2005. pp. 188–224. [Google Scholar]
- Menzel CR, Kelley JW, Hunsberger SA, Chan B, Menzel EW. A chimpanzee as a traveling salesman's director. American Journal of Primatology. 2006;68(Supplement 1):55. [Google Scholar]
- Menzel CR, Menzel EW. Enquiries concerning chimpanzee understanding. In: de Waal FBM, Ferrari PF, editors. The Primate Mind. Cambridge: Harvard; 2012. pp. 265–287. [Google Scholar]
- Menzel EW. Selection of food by size in the chimpanzee, and comparison with human judgments. Science. 1960;131:1527–1528. doi: 10.1126/science.131.3412.1527. [DOI] [PubMed] [Google Scholar]
- Menzel EW. Communication about the environment in a group of young chimpanzees. Folia Primatologica. 1971;15:220–232. doi: 10.1159/000155381. [DOI] [PubMed] [Google Scholar]
- Menzel EW. Chimpanzee spatial memory organization. Science. 1973;182:943–945. doi: 10.1126/science.182.4115.943. [DOI] [PubMed] [Google Scholar]
- Menzel R, Greggers U, Smith A, Berger S, Brandt R, Brunke S, Bundrock G, Hulse S, Plumpe T, Schaupp F, Schuttler E, Stach S, Stindt J, Stollhoff N, Watzl S. Honey bees navigate according to a map-like spatial memory. Proceedings of the National Academy of Sciences. 2005;102:3040–3045. doi: 10.1073/pnas.0408550102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell WA. An optimal control theory of diet selection: The effects of resource depletion and exploitative competition. Oikos. 1990;58:16–24. [Google Scholar]
- Normand E, Boesch C. Sophisticated Euclidean maps in forest chimpanzees. Animal Behaviour. 2009;77:1195–1201. [Google Scholar]
- Noser R, Byrne RW. Travel routes and planning of visits to out-607 of-sight resources in wild chacma baboons, Papio ursinus. Animal Behaviour. 2007;73:257–266. [Google Scholar]
- Pochron ST. Can concurrent speed and directness of travel indicate purposeful encounter in the yellow baboons (Papio hamadryas cynocephalus) of Ruaha National Park, Tanzania? International Journal of Primatology. 2001;22:773–785. [Google Scholar]
- Pratt WE, Mizumori SJY. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behavioural Brain Research. 2001;123:165–183. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- Putney RT. Willful apes revisited: The concept of prospective control. In: Washburn DA, editor. Primate Perspectives on Behavior and Cognition. Washington, D.C.: American Psychological Association; 2007. pp. 207–219. [Google Scholar]
- Ramos-Fernandez G, Mateos JL, Miramontes O, Cocho G, Larralde H, Ayala-Orozco B. Lévy walk patterns in the foraging movements of spider monkeys (Ateles geoffroyi) Behavioral Ecology and Sociobiology. 2004;55:223–230. [Google Scholar]
- Rosati AG, Stevens JR, Hauser MD. The effect of handling time on temporal discounting in two New World primates. Animal Behaviour. 2006;71:1379–1387. [Google Scholar]
- Rumbaugh DM, Washburn DA. Intelligence of Apes and Other Rational Beings. New Haven: Yale University Press; 2003. [Google Scholar]
- Sayers K, Lovejoy CO. The chimpanzee has no clothes: a critical examination of Pan troglodytes in models of human evolution (with comments and reply) Current Anthropology. 2008;49:87–114. [Google Scholar]
- Sayers K, Norconk MA, Conklin-Brittain NL. Optimal foraging on the roof of the world: Himalayan langurs and the classical prey model. American Journal of Physical Anthropology. 2010;141:337–357. doi: 10.1002/ajpa.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DF. Food storage by black-capped chickadees: Memory for the location and contents of caches. Animal Behaviour. 1984;32:451–464. [Google Scholar]
- Shettleworth SJ. Handling time and choice in pigeons. Journal of the Experimental Analysis of Behavior. 1985;44:139. doi: 10.1901/jeab.1985.44-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shettleworth SJ. Cognition, Evolution, and Behavior. Oxford: Oxford University Press; 1998. [Google Scholar]
- Shettleworth SJ, Plowright CMS. How pigeons estimate rates of prey encounter. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18:219–235. [PubMed] [Google Scholar]
- Spencer HG, Kennedy M, Gray RD. Perceptual constraints on optimal foraging: the effects of variation among foragers. Evolutionary Ecology. 1996;10:331–339. [Google Scholar]
- Stephens DW, Krebs JR. Foraging Theory. Princeton: Princeton University Press; 1986. [Google Scholar]
- Sullivan KA. Age-specific profitability and prey choice. Animal Behaviour. 1988;36:613–615. [Google Scholar]
- Taylor CR, Heglund NC, Maloiy GM. Energetics and mechanics of terrestrial locomotionIMetabolic energy consumption as a function of speed and body size in birds and mammals. Journal of Experimental Biology. 1982;97:1–21. doi: 10.1242/jeb.97.1.1. [DOI] [PubMed] [Google Scholar]
- Tillett BJ, Tibbetts IR, Whithead DL. Foraging behaviour and prey discrimination in the bluespotted maskray Dasyatis kuhlii. Journal of Fish Biology. 2008;73:1554–1561. [Google Scholar]
- Tinklepaugh OL. Multiple delayed reaction with chimpanzees and monkeys. Journal of Comparative Psychology. 1932;13:207–243. [Google Scholar]
- United States Department of Agriculture, A. R. S. USDA National 651 Nutrient Database for Standard Reference, Release 23. Nutrient Data Laboratory Home Page. 2010 http://www.ars.usda.gov/nutrientdata.
- Waddington KD, Holden LR. Optimal foraging: on flower selection by bees. The American Naturalist. 1979;114:179–196. [Google Scholar]
- Winterhalder B, Smith EA. Hunter-Gatherer Foraging Strategies: Ethnographic and Archeological Analyses. Chicago: The University of Chicago Press; 1981. [Google Scholar]
- Ydenberg RC, Brown JS, Stephens DW. Foraging: An overview. In: Stephens DW, Brown JS, Ydenberg RC, editors. Foraging: Behavior and Ecology. Chicago: The University of Chicago Press; 2007. pp. 1–28. [Google Scholar]
- Yoshioka JG. Size preference of albino rats. Journal of Genetic Psychology. 1930;37:427–430. [Google Scholar]