Abstract
The pollen hoarding syndrome consists of a large suite of correlated traits in honey bees that may have played an important role in colony organization and consequently the social evolution of honey bees. The syndrome was first discovered in two strains that have been artificially selected for high and low pollen hoarding. These selected strains are used here to further investigate the phenotypic and genetic links between two central aspects of the pollen hoarding syndrome, sucrose responsiveness and pollen hoarding. Sons of hybrid queen offspring of these two strains were tested for sucrose responsiveness and used to produce colonies with either a highly responsive or an unresponsive father. These two colony groups differed significantly in the amount of pollen stored on brood combs and with regards to their relationship between brood and pollen amounts. Additionally, four quantitative trait loci (QTL) for pollen hoarding behavior were assessed for their effect on sucrose responsiveness. Drone offspring of two hybrid queens were phenotyped for responsiveness and genotyped at marker loci for these QTL, identifying some pleiotropic effects of the QTL with significant QTL interactions. Both experiments thus provided corroborating evidence that the distinct traits of the pollen hoarding syndrome are mechanistically and genetically linked, and that these links are complex and dependent on background genotype. The study demonstrates genetic worker-drone correlations within the context of the pollen hoarding syndrome and establishes that an indirect selection response connects pollen hoarding and sucrose responsiveness, regardless of which trait is directly selected.
Keywords: Reproductive groundplan hypothesis, Social evolution, Complex trait genetics, Division of labor, Proboscis extension reflex, Pleiotropy, Genetic background
Introduction
Social evolution requires the cooperation and coordination of individuals to form reproductively successful social groups. The group organization of social insects relies on interactions between the individual colony members and the dynamic regulation of processes at the colony level (Beshers and Fewell 2001; Gadau and Fewell 2009). The regulation of the foraging behavior of honey bees (Apis mellifera L.) has been studied in great detail and serves as a model system for social regulation and individual behavior. Honey bees mainly forage for nectar and pollen, their principal food sources. Nectar is collected and processed into honey as a long-term food reserve to provide energy-rich mono- and disaccharides during food-shortages, such as the winter season in temperate climates. Pollen, the main source of protein and lipids, is used to produce larvae and usually is stored in the immediate vicinity of the brood - the eggs, larvae, and pupae (Winston 1987; Schmickl and Crailsheim 2007). Its storage quantities are actively regulated in response to supply and demand, assessed directly (Dreller and Tarpy 2000) and via brood signals (Fewell and Winston 1992; Pankiw et al. 1998).
A social phenotype, the amount of pollen stored in the hive has been the basis for a long-term artificial selection program for bees that store pollen in large (high pollen hoarding strain) or small (low pollen hoarding strain) quantities (Page and Fondrk 1995). This divergent selection program has resulted in widely divergent pollen hoarding phenotypes at the colony level and has also changed individual foraging behavior. Other individual characteristics were also affected by the selection program including development, anatomy, physiology, and behavior (Page et al. 2012). In addition to their inclination to collect more pollen, high strain workers develop slower into adults (Linksvayer et al. 2011), have larger ovaries (Amdam et al. 2006), initiate foraging at an earlier age (Rueppell et al. 2004), learn better (Scheiner et al. 2001), and are more responsive to sucrose (Pankiw and Page 1999). Many of these phenotypic associations have been reconfirmed in unselected bees and are summarized as the pollen hoarding syndrome (Page et al. 2007; Page et al. 2012).
The pollen hoarding syndrome may be the result of different hormonal dynamics that are fundamental to social evolution in honey bees more generally (Amdam et al. 2004). This idea has been the conceptual foundation of the reproductive ground plan hypothesis of social evolution, suggesting that reproductive control modules of solitary ancestors have been coopted by social evolution to control complex social behavior (Amdam et al. 2004; Amdam et al. 2006). The hypothesis has been supported by direct genetic links between reproductive traits and social behavior in honey bees demonstrated by identifying pleiotropic effects of quantitative trait loci (QTL) for social behavior on worker ovary size (Wang et al. 2009; Graham et al. 2011). The pollen hoarding syndrome is characterized in general by a partially overlapping genetic architecture and connections at the genetic level have also been identified between pollen hoarding and the age of first foraging (Rueppell et al. 2004) and between pollen hoarding and sucrose responsiveness (Rueppell et al. 2006a).
Sucrose responsiveness has been connected to pollen hoarding at the phenotypic level in multiple studies. Workers, drones (males), and queens from the high pollen hoarding strain exhibit higher sucrose responsiveness than workers from the low strain (Pankiw and Page 1999). In unselected bees, pollen foragers are more responsive to sucrose (Scheiner et al. 2003) and the sucrose responsiveness of newly-emerged adult workers correlates with their foraging decisions later in life (Pankiw and Page 2000). The influence of sucrose responsiveness on individual foraging decisions may be due to a relationship between sucrose- and pollen-responsiveness, based on a general tuning of the sensory apparatus (Scheiner et al. 2004). In addition to pollen collection, pollen hoarding is influenced by pollen storage behavior and pollen consumption. However, these aspects have been studied less, and it is unclear how they relate to the pollen hoarding syndrome and whether they are also influenced by different sensory tuning. Pollen hoarding as the colony-level phenotype of the original selection for the high- and low pollen hoarding strains has not sufficiently been studied.
So far, most genetic tests have focused on studying individual QTL for pleiotropic effects on different aspects of the pollen hoarding syndrome (Page et al. 2012). Four QTL (pln1-4) that had been originally identified for pollen hoarding at the colony level and individual foraging behavior (Hunt et al. 1995; Page et al. 2000; Rüppell et al. 2004) were tested for their effect on sucrose responsiveness in workers and drones derived from crosses between the high and low strains. These tests revealed pleiotropic effects of all four QTL in workers, although most effects were complicated by interactions with other QTL and the genetic background (Rueppell et al. 2006a). However, the simultaneous test of a hybrid drone population demonstrated only the effect of pln1, suggesting a simpler genetic architecture in the haploid drones than in diploid workers. In addition, this result extended the pollen hoarding syndrome to males (c.f. Rueppell et al. 2006b), suggesting that male and worker evolution are genetically linked and may constrain each other’s evolution.
However, more studies are needed to understand the association between sucrose responsiveness and pollen hoarding behavior and to evaluate its robustness. This connection is particularly important because sucrose responsiveness can be evaluated in workers and drones and thus reveal cross-sexual phenotypic correlations in the pollen hoarding syndrome (Rueppell et al. 2006b). Opposite to previous studies, here we performed a single generation selection experiment based on drone sucrose responsiveness and evaluated the phenotype of resulting colonies, with special emphasis on pollen hoarding. A second study was performed to test for direct genetic linkage between pollen hoarding and sucrose sensitivity by evaluating the effects of the pollen hoarding QTL on sucrose responsiveness in drones. We demonstrate an indirect selection response in pollen hoarding when sucrose responsiveness is selected on, which substantiates the link between these two traits from an opposite perspective compared to previous studies. Furthermore, the results indicate that indirect selection responses may be more subtle than a simple change in colony mean phenotype and that the underlying genetic effects depend on the genetic background, even in haploid drones.
Methods
Colony-level selection experiment
The high- and low-pollen-hoarding strains of Page and Fondrk (1995) were used as genetic sources for the experiment. These lines were derived from commercial stocks of honey bees (Apis mellifera L.) in 1990 and have been maintained under continuous, bidirectional selection for pollen hoarding behavior. To avoid inbreeding depression the lines were bred by circular inbreeding among sub-lines and three out-crossings to commercial hives of similar (high pollen-hoarding or low-pollen hoarding) phenotype (Rueppell et al. 2004). At the beginning of the experiment, the lines were in the 18th generation of selection. Inbred lines were produced by crossing sub-lines within the high and low strain. The inbred lines provided the drones (males) and virgin queens that produced the test bees. The parental inbred high and low pollen hoarding lines differed significantly in the amount of stored pollen (812 cm2 and 97 cm2, respectively; F(1,55) = 56.1, p < 0.001).
We produced several hybrid queens by instrumentally inseminating a virgin queen from a low inbred line with semen of a single drone from one high inbred line. Female offspring of this cross were then raised to develop into new queens that produced drones with an equal mixture of high and low pollen hoarding genomes (Laidlaw and Page 1997). Newly-emerged drones produced by one hybrid queen were collected from a brood frame kept in an emergence incubator (34°C / 60% rel. hum.) and tested for their response to sucrose using the proboscis extension reflex (PER; Kuwabara 1957; Pankiw and Page 2000). The drones were tested for their responses to water and sucrose by presenting them with sucrose solutions that increased in concentration from 0.1 to 0.3, 1, 3, 10, and 30 percent (wt/vol). A droplet of water was touched to each antenna before the application of 0.1% sucrose and before each subsequent application of sucrose solution (water, 0.1%, water, 0.3%, water, 1%, water, 3%, water, 10%, water, 30%) with an inter-trial interval of about 2 minutes. Drones that responded to all concentrations of sucrose but not water were considered to be high responders, marked, and placed in cages. Drones that responded only to the highest sucrose concentration were considered low responders and were also marked and caged. Approximately 700 drones were screened resulting in about 10% high and low responders.
The cages of selected high and low responding drones were placed into a nursery colony that had been prepared to care for the drones while they matured for 14 days. Concomitantly, daughter virgin queens were raised from the inbred high line queen (Laidlaw and Page 1997). Each virgin queen was instrumentally inseminated with the semen of a caged high or low responding drone and placed into a nucleus colony to initiate egg laying. Colonies were allowed to grow into full-scale hives for at least 59 days before being evaluated. After 6 weeks, more than 90% of the former worker bees within the hive should be replaced by the offspring of the test queen (Rueppell et al. 2007; Rueppell et al. 2009). The phenotype of these colonies was evaluated using established methods (Page and Fondrk 1995). A total of 13 queens mated to low responding drones (LRD) and 18 mated to high responding drones (HRD) were evaluated. We measured the viability of the brood produced, the amount of brood produced, the estimated number of bees, and the area of stored honey and pollen in the colony. The quantity of adult bees, honey, and brood was measured in reference to full Langstroth frames, while pollen was quantified in cm2. Pollen quantity was further divided into pollen stored on brood combs, because this quantity is inspected by returning pollen foragers (Dreller and Tarpy 2000), and the total amount of pollen stored in the colony. The simultaneous influences of brood quantity (Pankiw et al. 1998) and the selective breeding on the latter quantity were evaluated with a generalized linear model including an interaction term and both main effects. Bonferroni corrections for multiple tests of the same hypothesis were applied but uncorrected p-values are reported.
Genetic Test of Pleiotropy
Two hybrid sister queens were produced as described above and selected to produce drone offspring for testing the genetic effect of the pln QTL on sucrose responsiveness of drones. One of these queens was the hybrid queen used in the first experiment (Q1), but a second queen (Q2) was used as an independent test of the genetic effects in a related, but differing genetic background. Drones emerged and were tested for sucrose responsiveness as described above. However, the drone responsiveness for this experiment was recorded in more detail, resulting in numerical scores of total responsiveness (all positive PER responses to water and sucrose) and sucrose-specific responsiveness (all positive PER responses to sucrose corrected by PER responses to water). These scores reflected drone behavior better than response thresholds (Pankiw and Page 2000) because they are more robust against single random responses. They also allowed the distinction between a general and a sucrose-specific response. Drones that did not respond at all (n = 18, corresponding to 9.4%) were eliminated from the analysis.
Directly after the PER assay, drones were frozen at −80°C until genomic DNA was extracted following a modified CTAB protocol and diluted to 100 ng/ul as a template for the subsequent PCR reactions. PCR was performed using previously developed sequence tagged site (sts) markers and protocols (Rueppell et al. 2004) to assess the genotype at pln1-4. Two flanking markers were used for pln1 and pln2 but only one marker was used for the smaller QTL pln3 and pln4 (Table 3). The two alternative genotypes were scored at each locus by agarose gel-electrophoresis of the PCR products with a prior restriction enzyme digestion if necessary (Rueppell et al. 2004). The effects of marker genotypes on total response score and adjusted PER score were evaluated by multi-way ANOVAs, applying Bonferroni corrections to determine significance but reporting uncorrected values.
Table 3.
Markers used to assess genetic effects of the pln QTL on sucrose response
| Marker name |
QTL target |
Genomic location |
Primer sequences or fragment sequence |
|---|---|---|---|
| stsD8-.3 | pln1 | Chr.13 (3.4Mb) |
Forward: ACAACCAGAGAGCAAACGCC Reverse: CGGTGCAACGGTATATTATTACC |
| E15-.43 | pln1 | Chr.13 (7.1Mb) |
ACGCACAACCACGGGAAGAAAGAACGAGCACAACCGGAACCACGA TTTCTCCCTACGATCCTTTTACGAGCGCGGGATAAACGAGCCGGA TGAATCGAGATAACGAAGTGGGTGAAACAAAGGATTTGAAATTCG CGCCATTTCGTTTGCTCGATACGCCTTTCGGATACGGCGAACGTTT CGTGGAAGATTCGT |
| W5-1.36 | pln2 | Chr.1 (16.9Mb) |
Forward: CTAATTTCCGGTCGTAGAGATACGTG Reverse: CGGTACTAGTATTTATCGAAAAATTGTGTC |
| F8-1.04 | pln2 | Chr.1 (19.7Mb) |
Forward: GGGATATCGG Reverse: GGGATATCGG |
| stsQ4-LF | pln3 | Chr.1 (9.2Mb) |
Forward: GCGCTGTTCAAAAATTCCACATATCTG Reverse: GTATTTATTACCACGTTGTGACAAATCG |
| sts-AmFOR | pln4 | Chr.13 (9.0Mb) |
Forward: CCAAGACGTTCTGCTGGGTTGTC Reverse: TATACACGGCCATAATCGCGATCG |
Results
Colony-level selection experiment
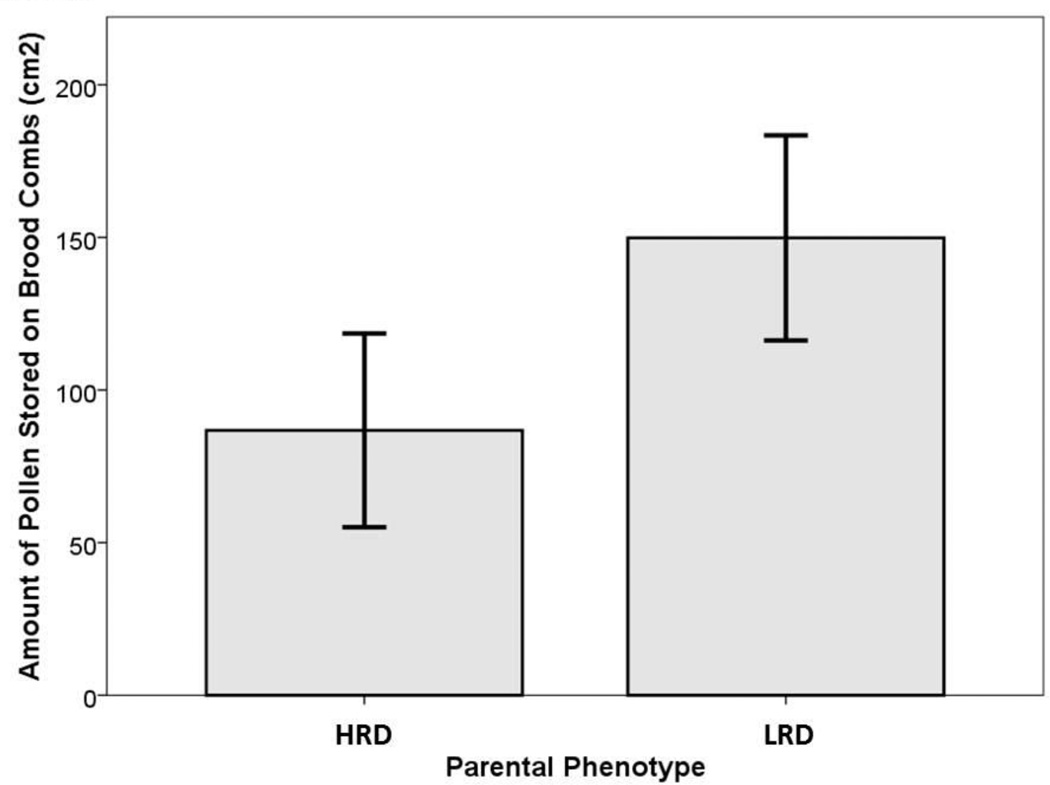
Across all 31 evaluated colonies, the amount of brood was significantly correlated with the number of bees (colony size). The only other significant association among the evaluated variables after Bonferroni correction was a positive correlation between the total quantity of pollen stored in the colony and the quantity of pollen stored on brood combs (Table 1). The comparison of colony phenotype between HRD and LRD colonies revealed a significant difference in the amount of pollen stored on brood combs (Figure 1) but no other evaluated variable showed a significant difference between the two groups (Table 2). The simultaneous assessment of the effects of brood amount and experimental selection on the total amount of stored pollen revealed a strong effect of selection (F(1,27) = 11.3, p = 0.002), no significant effect on brood amount (F(1,27) = 3.1, p = 0.087), and a significant interaction between both factors (F(1,27) = 9.3, p = 0.005). The interaction was a result of a non-significant increase in stored pollen with an increase in brood area for colonies derived from high responding drones (post-hoc test: r = 0.31, n = 18, p = 0.209), while colonies with low responding drone fathers produced colonies with a steep decrease in pollen area with increasing brood area (r = −0.70, n = 13, p = 0.008; Figure 2).
Table 1.
Correlations among phenotypic variables evaluated in the selectively crossed colonies*
| Pollen near brood |
Stored pollen |
Stored honey |
Number of bees |
Brood amount |
|
|---|---|---|---|---|---|
| Brood viability | r = 0.23, p = 0.214 |
r = 0.20, p = 0.292 |
r = 0.05, p = 0.794 |
r = 0.31, p = 0.089 |
r = 0.38, p = 0.037 |
| Brood amount | r = 0.39, p = 0.032 |
r = 0.04, p = 0.843 |
r = 0.34, p = 0.065 |
r = 0.76, p < 0.001 |
|
| Number of bees | r = 0.30, p = 0.106 |
r = 0.30, p = 0.103 |
r = 0.33, p = 0.069 |
||
| Stored honey | r = 0.07, p = 0.727 |
r = 0.17, p = 0.365 |
|||
| Stored pollen |
r = 0.64, p < 0.001 |
||||
uncorrected, two-tailed significance is given, significant results after Bonferroni correction are printed in bold.
Figure 1.
Difference (p = 0.008) in the two experimental colonies types, fathered by drones with either low or high sucrose responsiveness (LRD and HRD, respectively) in the amount of pollen stored on frames that contained brood. Averages are given with ±95% confidence intervals.
Table 2.
Phenotypic comparison of colonies fathered by a drone with high (HRD) and low (LRD) responsiveness to sucrose*
| Variable | HRD (mean ± S.E.) | LRD (mean ± S.E.) | ANOVA |
|---|---|---|---|
| Brood viability | 76.9 ± 7.2 % | 69.4% ± 5.9 | F(1,30) = 0.7, p = 0.426 |
| Brood amount | 1.65 ± 0.11 frames | 1.57 ± 0.15 frames | F(1,30) = 0.1, p = 0.719 |
| Number of bees | 2.81 ± 0.23 frames | 2.73 ± 0.30 frames | F(1,30) = 0.0, p = 0.845 |
| Stored honey | 2.00 ± 0.18 frames | 1.93 ± 0.22 frames | F(1,30) = 0.1, p = 0.807 |
| Stored pollen | 227.5 ± 20.2 cm2 | 188.1 ± 17.5 cm2 | F(1,30) = 2.2, p = 0.151 |
| Pollen near brood | 149.8 ± 15.4 cm2 | 86.8 ± 15.0 cm2 | F(1,30) = 8.2, p = 0.008 |
uncorrected, two-tailed significance is given, significant results after Bonferroni correction are printed in bold.
Figure 2.
Paternal sucrose responsiveness interacted with the effect of the amount of brood on colony pollen storage. While colonies with a sucrose responsive father showed a slightly positive relationship (r2 = 0.10), the colonies with unresponsive fathers showed a strong, negative relationship between brood and pollen amounts (r2 = 0.49).
Genetic Test of Pleiotropy
General responsiveness and sucrose-specific responsiveness were significantly different between the offspring of the two queens (respectively: F(1,172) = 6.7, p = 0.010; F(1,172) = 6.3, p = 0.013). The correlation between these two scores also differed significantly between the offspring of the two queens (Q1: r = −0.28, n = 121, p = 0.002; Q2: r = 0.42, n = 53, p = 0.002). Marker effects were therefore analyzed for each queen separately. Single marker analyses revealed a significant effect of F8 (pln2) on general responsiveness (F(1,119) = 10.8, p = 0.001) and sucrose response (F(1,119) = 8.4, p = 0.004) for the Q1 queen (Figure 3) but no significant effects in the offspring of her Q2 sister. The results of the 4-way ANOVAs to assess the effect of all pln QTL simultaneously depended on which of the specific markers for pln1 and pln2 were used but differed between queens in all instances. Table 4 lists these results for all possible marker combinations for both queens and both traits separately. Regardless of the marker selection, some genetic effects of the pln QTL are detected and the sister queens differ in these results.
Figure 3.
Significant single marker effects of the pln2-related F8 marker on sucrose response (p = 0.004) and general responsiveness (p = 0.001) in drone offspring of the hybrid Q1 queen, showing opposite effects on the two measures.
Table 4.
Four-way ANOVA results# to assess all pln QTL effects on drone responsiveness simultaneously
| ANOVA Model (using different genetic markers for the 4 pln QTL) |
Total Response Score | Sucrose-specific Response Score | ||
|---|---|---|---|---|
| Q1-queen offspring |
Q2-queen offspring |
Q1-queen offspring |
Q2-queen offspring |
|
| D8 × F8 × Q4 × AmFOR |
F8* | D8* | F8*** | Q4* D8 × Q4* Q4 × AmFOR*** |
| D8 × W5 × Q4 × AmFOR |
--- | W5* D8×W5×Q4×AmFOR* |
--- |
Q4*** W5 × AmFOR* Q4 × AmFOR**** |
| E15 × F8 × Q4 × AmFOR |
E15 × F8 × Q4** | --- | F8* |
Q4 × AmFOR*** E15×F8×Q4×AmFOR* |
| E15 × W5 × Q4 × AmFOR |
--- | E15* W5*** AmFOR** W5 × AmFOR*** E15 × W5 × Q4* |
--- | E15* W5* Q4*** E15 × AmFOR* W5 × Q4* W5 × AmFOR*** Q4 × AmFOR**** E15 × W5 × Q4*** W5 × Q4 × AmFOR*** |
For clarity, only terms with an uncorrected significance value below 0.05 are listed (* < 0.05, ** < 0.01, *** < 0.005, **** < 0.001). Significant results after Bonferroni correction are printedin bold.
Discussion
The investigated connection between sucrose responsiveness and pollen hoarding in honey bee workers is central to the pollen hoarding syndrome (Page et al. 2012) and our results at the phenotypic and genetic levels confirm the relationship between the two traits but indicate that it may not be as simple as previously implied. The results suggest that individual sucrose responsiveness affects the colony-level regulation of pollen hoarding relative to the amount of brood (Pankiw et al. 1998) and that pollen hoarding QTL interact in a complex manner with each other and with the genetic background of an individual to influence gustatory responsiveness.
The single generation selection based on sucrose responsiveness of individual males resulted in colonies of significantly different pollen hoarding phenotypes. Colonies derived from drone fathers that were relatively unresponsive to sucrose solutions stored relatively high amounts of pollen on brood combs and showed a negative relationship between the amount of stored pollen and the amount of brood in the colony. In contrast, colonies with highly responsive fathers stored significantly less pollen on brood combs and showed no significant relationship between the overall amount of pollen and brood in the colonies. Single-generation selection on different aspects of the pollen hoarding syndrome has previously resulted in profound selection responses (Page and Fondrk 1995; Linksvayer et al. 2009). In contrast to those direct selection experiments, this short-term selection relied on an individual trait of drones, which do not contribute to colony function, to elicit a social phenotypic response at the colony level. These findings also demonstrate that the indirect selection response connecting pollen hoarding and sucrose responsiveness acts in both directions. The initial selection on pollen hoarding caused changes in individual sucrose responsiveness (Pankiw and Page 1999) and the selection on sucrose responsiveness in this experiment changed pollen hoarding behavior. Combined, these results argue strongly for a direct connection between the two phenotypes.
Contrary to our expectations based on previous results (Pankiw and Page 2000; Scheiner et al. 2004), no difference in the overall amount of stored pollen between the two types of colonies was detected. However, the pollen areas directly on the brood combs that are actively inspected (Dreller and Tarpy 2000) differed between colonies that were fathered by high and low responding drones. These areas are also closest to the brood (Winston 1987), which suggests that the difference could indicate a difference between the experimental groups either in directly evaluating the stored pollen quantity (Dreller and Tarpy 2000) or in responsiveness to brood signals. In support of the latter explanation, workers from the high pollen hoarding strain are more responsive to sucrose and stimuli that regulate pollen hoarding, as shown by changes in foraging choice and the age of foraging onset (Pankiw and Page 2001). Differences in sensitivity to brood may cause returning pollen foragers to unload close to the brood or further away. The more responsive workers might unload further away from the brood because they are already sensing the brood while less responsive workers might only unload close to the brood when their response threshold for unloading is exceeded.
In general, the amount of young brood is positively correlated to pollen hoarding because brood pheromone elicits pollen foraging behavior (Pankiw et al. 1998) but our results show that the details of this relationship depend on genotype. The negative relation between brood and pollen in colonies of low responding males suggests that pollen hoarding by these workers is not stimulated but inhibited by brood. The artificial selection imposed on the parental high and low pollen hoarding strain may have generated more extreme genotypic variation for pollen hoarding behavior than normally observed in unselected honey bee populations. However, the reversal of the relation between pollen and brood by selection on sucrose responsiveness suggest a fundamental connection to the general pollen hoarding syndrome that is also observed in unselected honey bees (Pankiw 2003; Page et al. 2007). Potential space constraints forcing a negative correlation between brood and pollen quantity in colonies (Page and Fondrk 1995) cannot explain the difference between the experimental groups because suitable open cells were observed during colony inspections throughout the experiment and the experimental groups did not differ in brood quantity or overall pollen amount.
Although the reproductive ground-plan hypothesis has been proposed based on female physiology and life-history regulation (Amdam et al. 2004; Amdam et al. 2006) the pollen hoarding syndrome extends to drone phenotypes, such as locomotor activity when newly emerged, the age of flight onset, and sucrose responsiveness (Pankiw and Page 1999; Rueppell et al. 2006b). The results of our selection experiment further strengthen the argument that genetic worker-drone correlations exist for multiple traits of the pollen hoarding syndrome, representing a constraint on the adaptive evolution of workers and drones. More cross-sexual studies are needed on additional phenotypes and in unselected populations to empirically assess the importance of “genetic release followed by diversifying evolution” (Gadagkar 1997) in honey bee social evolution.
Cross-sexual correlations for sucrose responsiveness have already been shown at the QTL level: pln1 influences sucrose responsiveness in workers and drones (Rueppell et al. 2006a). However, the same study also demonstrated a more complex genetic architecture of sucrose responsiveness in workers than in drones, implicating further loci and epistatic interactions in workers but not in drones (Rueppell et al. 2006a). The same QTL also influence the foraging behavior of workers in a highly epistatic fashion (Rüppell et al. 2004) but drones could not be evaluated in this regard because they do not forage. Our presented analyses also suggest epistasis among the pln QTL for their influence on the responsiveness of drones. When the same marker loci are considered as in our previous study (D8, W5, Q4, and AmFOR), the drone offspring of the Q2 queen mirrored the consistent pln3 × pln4 interaction for worker sucrose response (Rueppell et al. 2006a) but the genetic effects reporte here are more complicated than the simple pln1 effect reported earlier (Rueppell et al. 2006a).
In contrast to previous studies, two different marker loci were used here for each of the larger two pln QTL. In previous mapping studies these markers were found at variable recombinational distances from the mapped QTL (Hunt et al. 1995; Page et al. 2000; Rueppell et al. 2004; Rüppell et al. 2004; Rueppell et al. 2006a; Hunt et al. 2007). Therefore, we cannot determine a priori which of these markers represented the QTL effects most accurately. The four different marker combinations (models) suggested significantly different effects of the pln QTL on sucrose responsiveness. In the Q1 queen, only the F8 marker when used in place of pln2 showed a singular effect. In the Q2 queen, pln1 and pln2 showed each a direct effect in one of the four possible models and interaction effects in two additional models. The effects of the other two QTL were also influenced by the marker selection for pln1 and pln2 but they were more consistent, with pln3 exhibiting a direct effect in three models and 2-way interaction effects in all four models and pln4 showing interaction effects in all four models. The divergent results exemplify the conservative nature of testing QTL by genotyping markers that may only be loosely linked to the QTL (Rueppell 2009; Graham et al. 2011). However, all models consistently suggested a complex genetic architecture with epistatic interactions for the Q2 queen offspring and a simpler architecture for the Q1 queen offspring. Thus, our main conclusions are not influenced by the choice of particular markers.
The two sister queens also differed in the relationship between total responsiveness and sucrose responsiveness. The two variables were negatively correlated in the offspring of the Q1 queen but showed a positive correlation in the offspring of the Q2 queen. Surprisingly, these different relationships did not predict the similarity of the genetic architectures of total responsiveness and sucrose responsiveness. The two traits seem to share more marker effects in the Q1 offspring than in Q2 offspring, although this impression could not be evaluated statistically. In the latter, more effects were found in all models for sucrose responsiveness and the two measures also differed in the identity of the significant effects. For example, pln3 did not have a direct effect in any model on total responsiveness. In general, correlated sensitivity to different stimuli was observed for the pollen hoarding syndrome (Page and Erber 2002; Scheiner et al. 2004; Page et al. 2007). However, our QTL data suggest that these correlations may not be as straightforward at the genetic level as previously thought. Moreover, the differences between offspring of the two queens suggest that significant genetic variation remains in the high and low pollen hoarding strains that can lead to variable results in their genetic study (c.f. Rueppell et al. 2011). These considerations may also apply to the sensitivity to brood pheromone which plays presumably a key role in the regulation of pollen hoarding behavior.
Acknowledgements
Tanya Pankiw conducted the PER analyses for the drones used in this study. Financial support for this project was provided by the National Science Foundation (grants: # 0090482 and # 0076811 to REP and #0615502 and #0926288 to OR), the Agriculture and Food Research Initiative of the USDA National Institute of Food and Agriculture (#2010-65104-20533 to OR), and the National Institute on Aging (NIA P01 AG22500 to REP).
References
- Amdam GV, Csondes A, Fondrk MK, Page RE. Complex social behaviour derived from maternal reproductive traits. Nature. 2006;439:76–78. doi: 10.1038/nature04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE., Jr Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc Nat Acad Sci USA. 2004;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshers SN, Fewell JH. Models of division of labor in social insects. Annu Rev Entomol. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- Dreller C, Tarpy DR. Perception of the pollen need by foragers in a honeybee colony. Anim Behav. 2000;59:91–96. doi: 10.1006/anbe.1999.1303. [DOI] [PubMed] [Google Scholar]
- Fewell JH, Winston ML. Colony state and regulation of pollen foraging in the honey-bee, Apis mellifera L. Behav Ecol Sociobiol. 1992;30:387–393. [Google Scholar]
- Gadagkar R. The evolution of caste polymorphism in social insects: genetic release followed by diversifying evolution. J Genet. 1997;76:167–179. [Google Scholar]
- Gadau J, Fewell J. Organization of Insect Societies - From Genome to Sociocomplexity. Cambridge, MA: Harvard University Press; 2009. p. 617. [Google Scholar]
- Graham AM, Munday MD, Kaftanoglu O, Page RE, Jr, Amdam GV, Rueppell O. Support for the reproductive ground plan hypothesis of social evolution and major QTL for ovary traits of Africanized worker honey bees (Apis mellifera L.) BMC Evol Biol. 2011;11:95. doi: 10.1186/1471-2148-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GJ, Amdam GV, Schlipalius D, Emore C, Sardesai N, Williams CE, Rueppell O, Guzman-Novoa E, Arechavaleta-Velasco M, Chandra S, Fondrk MK, Beye M, Page RE. Behavioral genomics of honeybee foraging and nest defense. Naturwissenschaften. 2007;94:247–267. doi: 10.1007/s00114-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GJ, Page RE, Jr, Fondrk MK, Dullum CJ. Major quantitative trait loci affecting honey bee foraging behavior. Genetics. 1995;141:1537–1545. doi: 10.1093/genetics/141.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M. Bildung des bedingten Reflexes vom Pavlov Typus bei der Honigbiene (Apis mellifica) J Fac Sci Hokkaido Univ Zool. 1957;13:458–464. [Google Scholar]
- Laidlaw HH, Page RE. Queen Rearing and Bee Breeding. Cheshire, CT: Wicwas Press; 1997. [Google Scholar]
- Linksvayer TA, Kaftanoglu O, Akyol E, Blatch S, Amdam GV, Page RE. Larval and nurse worker control of developmental plasticity and the evolution of honey bee queen-worker dimorphism. J Evol Biol. 2011;24:1939–1948. doi: 10.1111/j.1420-9101.2011.02331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linksvayer TA, Rueppell O, Siegel A, Kaftanoglu O, Page RE, Amdam GV. The genetic basis of transgressive ovary size in honey bee workers. Genetics. 2009;183:693–707. doi: 10.1534/genetics.109.105452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Erber J. Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften. 2002;89:91–106. doi: 10.1007/s00114-002-0299-x. [DOI] [PubMed] [Google Scholar]
- Page RE, Fondrk MK. The effects of colony level selection on the social organization of honey bee (Apis mellifera L) colonies - colony level components of pollen hoarding. Behav Ecol Sociobiol. 1995;36:135–144. [Google Scholar]
- Page RE, Fondrk MK, Hunt GJ, Guzman-Novoa E, Humphries MA, Nguyen K, Greene AS. Genetic dissection of honeybee (Apis mellifera L.) foraging behavior. J Hered. 2000;91:474–479. doi: 10.1093/jhered/91.6.474. [DOI] [PubMed] [Google Scholar]
- Page RE, Rueppell O, Amdam GV. A review of the connections between reproductive and social behavioral traits with special emphasis on the honey bee. Annu Rev of Genet. 2012;46 in press. [Google Scholar]
- Page RE, Scheiner R, Erber J, Amdam GV. The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.) Curr Top Dev Biol. 2007;74:253–286. doi: 10.1016/S0070-2153(06)74008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiw T. Directional change in a suite of foraging behaviors in tropical and temperate evolved honey bees (Apis mellifera L.) Behav Ecol Sociobiol. 2003;54:458–464. [Google Scholar]
- Pankiw T, Page RE. The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.) J Comp Physiol A. 1999;185:207–213. doi: 10.1007/s003590050379. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE. Response thresholds to sucrose predict foraging division of labor in honeybees. Behav Ecol Sociobiol. 2000;47:265–267. [Google Scholar]
- Pankiw T, Page RE. Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behav Ecol Sociobiol. 2001;51:87–94. [Google Scholar]
- Pankiw T, Page RE, Fondrk MK. Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera) Behav Ecol Sociobiol. 1998;44:193–198. [Google Scholar]
- Rueppell O. Characterization of quantitative trait loci for the age of first foraging in honey bee workers. Behav Genet. 2009;39:541–553. doi: 10.1007/s10519-009-9278-8. [DOI] [PubMed] [Google Scholar]
- Rueppell O, Bachelier C, Fondrk MK, Page RE., Jr Regulation of life history determines lifespan of worker honey bees (Apis mellifera L.) Exp Gerontol. 2007;42:1020–1032. doi: 10.1016/j.exger.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Chandra SBC, Pankiw T, Fondrk MK, Beye M, Hunt GJ, Page RE. The genetic architecture of sucrose responsiveness in the honey bee (Apis mellifera L.) Genetics. 2006a;172:243–251. doi: 10.1534/genetics.105.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Fondrk MK, Page RE. Male maturation response to selection of the pollen-hoarding syndrome in honey bees (Apis mellifera L.) Anim Behav. 2006b;71:227–234. doi: 10.1016/j.anbehav.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Kaftanouglu O, Page RE. Honey bee (Apis mellifera) workers live longer in small than in large colonies. Exp Gerontol. 2009;44:447–452. doi: 10.1016/j.exger.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Metheny JD, Linksvayer TA, Fondrk MK, Page RE, Jr, Amdam GV. Genetic architecture of ovary size and asymmetry in European honeybee workers. Heredity. 2011;106:894–903. doi: 10.1038/hdy.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueppell O, Pankiw T, Nielson DI, Fondrk MK, Beye M, Page RE., Jr The genetic architecture of the behavioral ontogeny of foraging in honey bee workers. Genetics. 2004;167:1767–1779. doi: 10.1534/genetics.103.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüppell O, Pankiw T, Page RE., Jr Pleiotropy, epistasis and new QTL: the genetic architecture of honey bee foraging behavior. J Hered. 2004;95:481–491. doi: 10.1093/jhered/esh072. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Barnert M, Erber J. Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie. 2003;34:67–72. [Google Scholar]
- Scheiner R, Page RE, Erber J. Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav Brain Res. 2001;120:67–73. doi: 10.1016/s0166-4328(00)00359-4. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera) Apidologie. 2004;35:133–142. [Google Scholar]
- Schmickl T, Crailsheim K. HoPoMo: A model of honeybee intracolonial population dynamics and resource management. Ecol Model. 2007;204:219–245. [Google Scholar]
- Wang Y, Amdam GV, Rueppell O, Wallrichs MA, Fondrk MK, Kaftanoglu O, Page RE., Jr PDK1 and HR46 gene homologs tie social behavior to ovary signals. PLoS ONE. 2009;4:e4899. doi: 10.1371/journal.pone.0004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Cambridge, Massachusetts: Harvard University Press; 1987. [Google Scholar]