Abstract
Psychological stress is a major risk factor for the development and progression of a number of diseases, including cardiovascular disease, cancer, arthritis, and major depression. A growing body of research suggests that long-term, stress-induced activation of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis may lead to increases in inflammation, which is known to play a key role in the pathophysiology of a variety of diseases. Furthermore, the burgeoning fields of social neuroscience and health neuroscience have begun to identify the neurocognitive mechanisms by which stress may lead to these physiological changes. Here we review the literature examining the neurocognitive correlates of stress-induced SNS, HPA, and inflammatory responses. Specifically, we summarize the results of neuroimaging studies that have examined the neural correlates of stress-related increases in SNS, HPA, and inflammatory activity. A set of neural systems involved in threat processing, safety processing, and social cognition are suggested as key contributors to stress-related changes in physiology. We conclude by offering suggestions for future research in the exciting new field of health neuroscience.
Keywords: neuroimaging, social neuroscience, stress, health, inflammation
“Of course it is happening inside your head, Harry, but why on earth should that mean that it is not real?” Harry Potter and the Deathly Hallows
by J. K. Rowling
Psychological stress is commonplace in modern life. Within a day, a person is likely to endure many types of stressful experiences: From minor stressful events, such as giving a presentation at work or taking a midterm exam, to major life events, such as a serious argument with a partner or the loss of a job. All of these stressors can take place against a backdrop of chronic stress, such as serious financial debt or ongoing relationship difficulties. Indeed, stressful experiences are the norm, not the exception, in the lives of most individuals.
Far from being a mere nuisance that may impact our feelings of happiness, stress contributes to disease risk and early mortality. Indeed, stress has been linked with the development or progression of a number of major diseases, including cardiovascular disease, cancer, arthritis, and major depression (Cohen et al., 2007; Juster et al., 2010; Slavich et al., 2010a). The past thirty years of research have seen vital developments in our understanding of the physiological mechanisms that may explain how stress influences health. These studies generally point to pathways that include stressed-related activation of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis, which, over time, can alter inflammatory processes—a correlate of many chronic diseases of aging (Gruenewald et al., 2006; Miller et al., 2009). Although there are many remaining questions about the precise physiological mechanisms by which stress affects health, we have now made significant progress in understanding the physiological mediators of the stress-health link.
Less is known, however, about the central neurobiological mechanisms that may lead to stress-related physiological responses in humans. Understanding how stress is represented in the brain and how neural activity during stress relates to physiology is important, as the brain is the key organ of stress perception, appraisal, and coping processes (e.g., McEwen & Gianaros, 2010), and thus any complete model of stress-health mediators must include the brain. The availability of neuroimaging tools together with a growing understanding of the neural systems involved in a variety of social, affective, and cognitive processes (Dalgleish et al., 2009; Lieberman, 2010; Singer, 2012) have coalesced to provide an opportunity to investigate the neural correlates of physiological stress responses.
Capitalizing on this opportunity, recent studies in “health neuroscience” have been conducted attempting to link physiological indicators with measures of neural activity during stress (Critchley, 2005; Gianaros & Sheu, 2009). In the present paper, we review the literature that has examined the neural correlates of stress-related changes in physiology. First, we identify and review the target physiological systems that have been linked with stress and health as well as the neural systems that may be related to physiological stress responding. Then, we review and synthesize the results from neuroimaging studies that have examined stressor-evoked physiological changes. Finally, we offer suggestions for future research in the exciting new field of health neuroscience.
Overview of Physiological Stress Systems
The human body contains a number of physiological systems that have been implicated in stress and disease. One of the most critical systems is the inflammatory component of the immune system. Inflammation is the body’s primary response to illness and infection, and can be local (such as when one has a cut) or systemic (such as when the body attempts to fight off infections). The primary mediators of the inflammatory response are proteins called pro-inflammatory cytokines, which facilitate cell-to-cell communication during times of injury or infection. The inflammatory response is critical for wound healing and preventing the spread of infection. However, exaggerated, repeated, or prolonged inflammatory activation can, over time, increase risk for disease and early mortality (McEwen, 1998; Finch, 2010). Indeed, inflammatory processes have been shown to contribute to the development of cardiovascular disease, rheumatoid arthritis, and asthma (Fahy, 2009; Libby, 2008; Taube et al., in press). Importantly, inflammatory responses are regulated by the SNS and the HPA axis, which activate in response to stressors.
The SNS is one branch of the autonomic nervous system, and, generally speaking, markers of SNS activity increase in response to psychological stress (Bradley et al., 2008; Nater & Rohleder, 2009; Uchino et al., 1995). Other correlates of SNS activity, including heart rate and blood pressure, are also often measured in the context of stress (though increases in heart rate and blood pressure reflect some combination of sympathetic increases and parasympathetic decreases and are thus not solely driven by SNS activity). Numerous studies have shown that psychological stressors such as public speaking or interpersonal conflict evoke increases in blood pressure and heart rate (Kamarck & Lovallo, 2003; Smith et al., 2009). Furthermore, activation of the SNS has been linked to increased inflammation (Grebe et al., 2010; Irwin & Cole, 2011; Levick et al., 2010), suggesting that one mechanism by which SNS activity affects health is via increasing levels of inflammation.
Another regulator of inflammatory processes is the HPA axis. The hormone cortisol is the end-product of activation of the HPA axis, and acute stressors are likely to elicit increases in cortisol. Interestingly, a meta-analysis that investigated cortisol responses to stress found that two features of stressors--uncontrollability and social-evaluation--were especially likely to be associated with elevations in cortisol (Dickerson & Kemeny, 2004). Stressor-evoked elevations in cortisol are important for energy mobilization to deal with impending threats; however, elevated levels of cortisol may also lead to deleterious health outcomes via associated increases in inflammation. Although cortisol is typically considered to be anti-inflammatory, chronic activation of the HPA axis can lead to a condition called glucocorticoid resistance where immune cells no longer “hear” a cortisol signal (Miller et al., 2008), thus leading to increases in both cortisol and inflammation. As with SNS activation, HPA activation may ultimately affect health via its influence on inflammation.
Overview of stress-related neural systems
Here, we review several neural “systems” that may be relevant for stress-related physiological responding, including those involved in threat-processing, safety processing, and self- and social cognitive-processing.
Neural circuitry involved in threat-processing
In order for environmental circumstances or cues to be considered “stressful”, a potential threat must be detected by and represented in the brain. A number of neural regions including (but not limited to) the amygdala, the dorsal anterior cingulate cortex (dACC), and the anterior insula (AI) are thought to make up a basic “neural alarm system” that detects potential threats in the environment and coordinates responses to such threats (Eisenberger, in press; Eisenberger & Cole, 2012; Eisenberger & Lieberman, 2004; see Figure 1). Activity in threat-related neural regions may be related to physiological responses to stress, especially insofar as appraisals of threat are linked to increased physiological reactivity (Blascovich & Mendes, 2010; O’Donovan et al., 2012).
Figure 1.
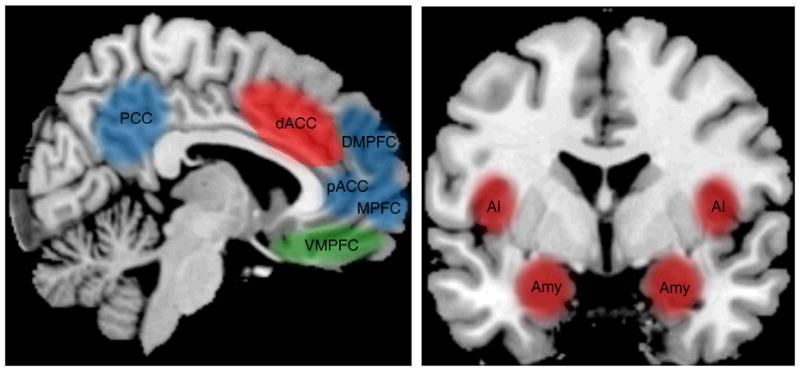
Schematic of neural regions implicated in regulating physiological responses to stress. Three primary neural systems have been shown to play a role in stressor-evoked physiological responses: a threat-related neural system, made up of the dACC, AI, and amygdala (displayed in red), a safety-related neural system, made up of the VMPFC (displayed in green), and a self/social cognition-related neural system, made up of the MPFC, pACC, DMPFC, and PCC (displayed in blue).
Arguably the most well known structure within the neural threat-processing system is the amygdala. The amygdala is thought to respond to stimuli that are especially relevant for the goals and motivations of the perceiver (Cunningham & Brosch, 2012). Thus, the amygdala is often active in studies involving threat and fear (Phelps, 2006; Whalen, 2007) given that these types of stimuli are expected to be especially salient. The amygdala likely leads to increases in SNS activity via its connections with brainstem regions, such as the locus coeruleus (LC; Herman et al., 2003; Ulrich-Lai & Herman, 2009), a key region for norepinephrine production. Amygdala activation may trigger HPA responses via projections to the bed nucleus of the striaterminalus, which provides a link between the amygdala and the paraventricular nucleus (PVN) of the hypothalamus (LeDoux, 2000; Herman & Cullinan, 1997). PVN neurons secrete corticotrophin releasing hormone, which begins the cascade eventually leading to cortisol production.
A second important brain region in the threat-processing system is the dACC. Activity in the dACC is correlated with the distressing feelings experienced during threat or pain, including physical pain (experiencing painful electric shocks; Apkarian et al., 2005), and social pain (being excluded from a group; Eisenberger, 2012). At the broadest level, the dACC is also involved in detecting the need for cognitive control (conflict monitoring, error processing; Egner, 2011; Schulz et al., 2011), such as when relevant goals are not being met. Acute stressors, which demand effortful, goal-directed behavior, may require these types of processes, and may thus involve dACC activity. The dACC is hypothesized to play a role in generating SNS responses via its connections with the amygdala (LeDoux et al., 1988), which projects to the LC and initiates production of norepinephrine. Furthermore, the dACC may lead to HPA activity through its projections to the hypothalamus, which initiates CRH production and starts the HPA axis cascade (Burgos-Robles et al., 2009; Gabbott et al., 2005).
Finally, the anterior insula (AI) is also considered a component of the threat-processing neural system. The insula is thought of as a key region for interoceptive awareness, or the conscious representation of the physiological condition of the body (Craig, 2009). In the context of stress research, activation of the AI is hypothesized to represent efferent outputs from physiological systems activated by stress or arousal (Critchley, 2005). Thus, activity in the AI is thought to reflect awareness of physiological changes that have taken place in the body.
Neural circuitry involved in safety-processing
While perceptions of threat relate to greater physiological stress responses, perceptions of safety and security may be related to lesser physiological stress responses. Evidence from behavioral research suggests that the presence of social support during a stressor, which may signal safety and security, is related to less physiological reactivity (for a meta-analysis, see Thorsteinsson & James, 1999). Thus, neural regions involved in the processing of safety and security may also be related to reduced physiological stress responses.
Prior neuroimaging research suggests that the ventromedial prefrontal cortex (VMPFC) is involved in processing safety and inhibiting threat-related responding (see Figure 1). Activity in VMPFC is observed during fear extinction when a previously feared cue is subsequently associated with safety (Schiller & Delgado, 2010). Inhibitory connections between VMPFC and the amygdala may be one mechanism by which VMPFC activity leads to decreased threat perception and lower physiological stress responses (Delgado et al., 2006; Eisenberger & Cole, 2012). Furthermore, studies of psychological stress show that low-stress (vs. high stress) conditions lead to greater VMPFC activity, (e.g., Preussner et al., 2008), and studies of physical pain show that low pain trials (vs. high pain trials; e.g., Atlas et al., 2010) lead to greater VMPFC activity. Moreover, a recent study showed that VMPFC was active during the viewing of a social support figure while experiencing pain (Eisenberger et al., 2011a) and that greater VMPFC activity was associated with greater reductions in threat-related neural activity (in dACC), again suggesting that VMPFC inhibit threat-related responding in the context of safety. Thus, in studies linking neural activity to physiology, activity in safety-related neural regions such as VMPFC is expected to be negatively related to physiological responses.
Neural circuitry involved in self and social cognitive processing
A final neural system likely related to physiological responses to stress is the self- and social cognitive-processing system. Self-related processing refers to circumstances in which individuals reflect upon their traits, thoughts, or feelings, whereas social cognitive-processing occurs when individuals think about the traits, thoughts, or feelings of others. Self-related processing is likely to be related to physiological stress responses, as increases in self-conscious emotions (e.g., shame) and decreases in self-esteem, both of which involve reflecting upon the self, are associated with heightened stress responses (Dickerson et al., 2004a; Gruenewald et al., 2004). Furthermore, stressors that involve social evaluation and the presence of other people are among the most potent activators of physiological stress response systems (Bosch et al., 2009; Bowers et al., 2008; Dickerson & Kemeny, 2004; Dickerson et al., 2004a). Individuals undergoing these stressors are likely to be thinking about the thoughts and feelings of the evaluators (e.g., “Why did she make that face?”), suggesting that social cognitive processes may relate to increased stressor-evoked physiological responsivity. As such, neural regions involved in thinking about the self and others, including the medial prefrontal cortex (MPFC) and nearby pregenual anterior cingulate cortex (pACC), the dorsomedial prefrontal cortex (DMPFC), and the posterior cingulate cortex (PCC) may be associated with increased physiological responses to stress, in part via their projections to regions important for generating SNS and HPA responses, including the amygdala and its projections to other brainstem regions as well as the hypothalamus (Ongur & Price, 2000; see Figure 1).
Across a variety of tasks and paradigms, activity in the MPFC (Broadmann Area 10), pACC, and PCC is associated with thinking about the self (Krienen et al., 2010; Lieberman, 2007; Murray et al., 2012). In the context of stress, activity in these regions may reflect the experience of self-conscious emotions (e.g., shame), which have previously been associated with physiological stress responses (Dickerson et al., 2004a), or the interpretation of negative, stressful information as more self-relevant (Eisenberger et al., 2011b).
MPFC, pACC, DMPFC and PCC are also often active in tasks that involve thinking about the thoughts and feelings of others (Frith & Frith, 2006; Krienen et al., 2010; Lieberman, 2010; Mitchell, 2009). Thus, these regions may be especially likely to activate during social stressors, as individuals try to understand the thoughts, feelings, and behaviors of others.
In sum, activity in neural regions that make up systems involved in processing threat (amygdala, dACC, AI), safety (VMPFC), and the self and others (MPFC, pACC, DMPFC, PCC) are expected to be associated with stressor-evoked changes in SNS and HPA activity, which may lead to increases in inflammatory responding. With these systems in mind, we now review evidence from neuroimaging studies that have examined the neural correlates of physiological responses to stress. We focus specifically on published reports that: 1) employed a task designed to elicit a stress response from participants; 2) measured neural activity using functional MRI (fMRI), or positron emission tomography (PET); 3) included a measure of SNS, HPA, or inflammatory output; and 4) used non-patient populations.
Neural correlates of SNS-related activity during stress
To date, only one known study has investigated how neural activity during a stress task is related to physiological responses in a purely sympathetic measure. This study examined how neural activity during a demanding Stroop task related to pupil diameter, a pure measure of SNS responding (Critchley et al., 2005). Results indicated that neural activity in the dACC, a core region of the threat-processing system, during incorrect (relative to correct) trials of a Stroop task was related to greater pupil dilation, suggesting that the dACC may be important for stress-induced SNS activity.
The majority of research in this area has focused on the neural systems related to changes in physiological measures that are correlates of SNS-related activity—reflecting some combination of sympathetic increases and parasympathetic decreases (e.g., blood pressure, heart rate, baroreflex sensitivity). In an early study by Critchley and colleagues (2000) participants completed challenging mental arithmetic and a difficult hand-grip task, and neural activity associated with both stressful tasks was related to changes in mean arterial pressure (MAP; a weighted averaging of changes in systolic and diastolic blood pressure [SDP/DBP]) and heart rate. Neural activity in threat-related neural circuitry was related to higher blood pressure (dACC) and higher heart rate (AI) during stress, whereas activity in a safety-related neural region (VMPFC) was related to lower blood pressure.
Another study on the neural correlates of heart rate responses to stress examined how neural activity during a demanding working memory task was related to the low-frequency band of heart rate variability (LF-HRV; Critchley et al., 2003), which is thought to represent a combination of SNS activation and PNS withdrawal. Once again, neural activity in threat-related neural regions (e.g., dACC and AI) was positively related to increases in LF-HRV.
More recently, a number of studies by Gianaros and colleagues (2005a; 2005b; 2007; 2008) have examined how neural activity during stress relates to blood pressure reactivity (for a quantitative review, see Gianaros & Sheu, 2009). These studies all employed a modified Stroop color-word interference task as a form of psychological stress. Importantly, performance on incongruent trials of the Stroop was fixed so that correct responses were given on only 60% of trials, perhaps contributing to the already stressful nature of having to inhibit a pre-potent response. In addition to measuring neural activation to the stressful task, measures of blood pressure (SBP or MAP) were taken. In all four studies, neural activity in a region of the self/social cognition network (PCC) was related to greater stressor-evoked increases in blood pressure. Neural activity in other regions of the self and social cognitive processing system (pACC, MPFC) and in threat-related neural regions (dACC, AI, amygdala) was associated with greater increases in blood pressure in some, but not all, studies (Gianaros et al., 2005a, 2007, 2008). Thus, activation in neural regions involved with thinking about the self and others, and in regions involved with processing threat, was related to increases in blood pressure.
In another set of recent studies, (Wager et al., 2009a, 2009b), participants performed a speech preparation task in which they were told they would have to mentally prepare to give a speech that they would subsequently deliver to a panel of experts. Subjects were scanned using fMRI while they prepared for the speech as well as during a baseline period before and after the speech prep. Measurements of heart rate were taken throughout the scan. The authors examined which neural regions mediated the relation between speech preparation stress and changes in heart rate. In one study, neural activity in a threat-related neural region (dACC) mediated the relationship between social threat and heart rate increases (Wager et al., 2009a), while activity in a region implicated in thinking about the self and others (pACC) was a mediator of the stress-heart rate increase in another study (Wager et al., 2009b). Finally, in both studies, the extent of activity in VMPFC, a safety-related neural region, was negatively related to heart rate, suggesting that participants who showed greater VMPFC activity during stress actually showed less of an increase in heart rate.
To date, only one study has examined the neural correlates of baroreflex suppression1, another index of increased cardiovascular arousal (Gianaros et al., 2011). In this study, participants completed a task similar to the Stroop task, and accuracy was fixed so that 40% of the time, participants received negative feedback indicating they had answered incorrectly. The task was completed twice: once during an fMRI scan, and once in a separate session during which beat-to-beat blood pressure and interbeat intervals were measured to form an index of baroreflex sensitivity. Greater suppression of the baroreflex (indicative of greater sympathetic arousal) was associated with heightened neural activation in a number of brain regions, including threat-processing related regions (dACC, AI, amygdala), and regions involved in thinking about the self and others (MPFC, pACC, PCC). Thus, activity in threat-processing and self/other-processing neural systems during stress was related to greater suppression of the baroreflex and thus greater sympathetic activity.
In sum, increases in neural activity in threat-related brain regions (dACC, AI, amygdala) in response to stress are associated with subsequent increases in SNS-related activity. This pattern of activation makes sense given that these regions are part of a threat-processing neural system, and increased perception of threat is associated with increased physiological responses. On the flip side, relatively greater activity in a safety-related neural region (e.g., VMPFC) is associated with relatively less of an increase in SNS activity, suggesting that greater perceptions of safety during stress may be associated with lower SNS responses. A number of studies also find neural activity in MPFC/pACC, and PCC associated with increases in SNS reactivity. These brain regions are part of a neural system involved in thinking about the self and others, suggesting that to the extent that individuals are thinking about themselves or others during a stressor (e.g., worrying about how they are perceived, or what others are thinking), they may show greater increases in SNS responses. Given that many of the SNS measures used in neuroimaging studies are not “pure” measures of sympathetic activity, future research should incorporate more direct measures of SNS activation to specify the relation between neural activity and purely sympathetic measures.
Neural correlates of cortisol responses to stress
A handful of studies have examined how cortisol responses are related to neural activity during stress (Dedovic et al., 2009; Eisenberger et al., 2007; Kern et al., 2008; Preussner et al., 2008; Wang et al., 2005, 2007). The first set of studies to examine this issue had participants perform difficult math problems in an MRI scanner while being prompted to go faster and to start over if an error occurred (Wang et al., 2005, 2007). Results from this study indicated that greater neural activity in a region involved in self-processing and thinking about others (MPFC) was associated with greater cortisol production across the session. Interestingly, results also indicated that during a rest period following the stress task, greater activity in dACC, a threat-related neural region, was associated with higher cortisol, suggesting the possibility that individuals who continue to experience some degree of threat following a stressor show a greater increase in cortisol. In a companion paper that examined gender differences in these responses, analyses revealed that, particularly for women, dACC activity following the stressor was associated with greater cortisol responses (Wang et al., 2007).
Another pair of studies compared the neural activity of individuals who showed a cortisol increase to a stressor (i.e., “responders”) to neural activity of individuals who did not show a cortisol increase (i.e., “non-responders”; Dedovic et al., 2009; Preussner et al., 2008). In these studies, in addition to performing difficult mental arithmetic, participants were given multiple forms of negative social feedback: they were told they were performing worse than the average participant, and the experimentertold participants they needed to perform better or their data would be useless. Results from one study using these procedures (Dedovic et al., 2009) indicated that the neural activity that distinguished cortisol responders from non-responders was in the DMPFC, a region often associated with thinking about the thoughts and feelings of others. In other words, individuals who showed a cortisol response to the stress task showed greater activity in this core region for understanding the behavior of others. A second study revealed that cortisol responders showed less activity in a safety-related neural region (VMPFC), and less activity in regions involved in thinking about the self (pACC/MPFC), compared with non-responders (Pruessner et al., 2008).
Another study on this topic had participants complete two versions of a speech and math task. In the stress condition, participants gave a speech and did mental arithmetic in front of a panel of evaluators, while in the no-stress condition, they completed these tasks alone (Kern et al., 2008). Neural activity was measured using PET following both conditions, and correlated with the extent of cortisol response during the stress condition. Results indicated that greater activity in self- and social-cognitive processing brain regions (MPFC, DMPFC) was related to less of a cortisol response to the stressor, while activity in a different sub-region of MPFC/pACC was positively related to cortisol responses. Thus, findings were mixed regarding the precise role of the self- and social-cognition neural systems in cortisol responses to stress, with distinct regions within this system correlating in opposite directions.
In a final study to investigate the neural correlates of cortisol responses to stress, participants underwent an fMRI scan while they experienced an episode of social rejection likely involving elements of social evaluation, which was elicited using a virtual ball-tossing game (i.e., Cyberball; Eisenberger et al., 2007). In a separate session, participants completed the TSST and cortisol responses to this social evaluative stressor were taken throughout the session. Greater cortisol responses to the TSST were associated with greater neural activity in dACC, dMPFC, and PCC during social rejection. These findings suggest that, to the extent that individuals engaged neural circuitry associated with threat-processing and thinking about others during an episode of social rejection, they also showed greater cortisol responses to a social-evaluative stressor.
In sum, only a handful of studies to date have investigated the neural correlates of cortisol responses to stress. In reviewing this literature, we found that data linking neural activity in brain regions often associated with self- and social-cognitive-processing and cortisol are mixed, with some studies finding activity in these regions positively correlated with cortisol production, and other studies finding negative correlations. We also found evidence that greater neural activity in the dACC, a threat-related neural region, is associated with heightened cortisol responses. There is also initial evidence linking activity in a safety-related neural region (i.e., VMPFC) with decreased cortisol responses.
Neural correlates of inflammatory responses to stress
Only one study to date has investigated the neural correlates of inflammatory responses to stress (Slavich et al., 2010b). In this study, participants were scanned using fMRI while they experienced an episode of social rejection. In a separate session, participants underwent a social evaluative stressor while levels of pro-inflammatory cytokines were measured (soluble receptor for TNF-alpha, IL-6). Greater neural activity in threat-related neural regions (dACC, AI) and in one region of the self/social cognition system (PCC) during social rejection was associated with greater inflammatory responses to the social stressor. Although suggestive, more research is needed before we are able to fully understand the neural systems involved in inflammatory responses to stress.
Conclusions
Understanding the brain regions that are related to physiological responses to stress is an important undertaking, and a growing body of literature is now beginning to link neural activity during a stressor with stressor-evoked changes in physiology. In numerous studies, neural activity in threat-related neural regions, including the dACC, AI, and amygdala is linked with physiological responses in SNS, HPA, and inflammatory systems. Given that the identification and experience of threat is a key feature of psychological stressors, it makes sense that activity in these regions would be linked with heightened physiological responses. However, many interesting questions regarding the role of threat-related neural processing and physiological outcomes remain to be answered.
One particularly intriguing question is the specificity of the relationship between dACC activity and physiological stress responses. Many different types of stress tasks, including those that are primarily cognitive (e.g., performing a difficult working memory task) and social in nature (e.g., being rejected) elicit dACC activity that is related to physiological activation. Thus, at this point it appears that many different types of stressors lead to dACC-physiological correlations. One plausible interpretation of this lack of specificity is that many tasks that we typically think of as purely “cognitive” may invoke more feelings of performance-anxiety and/or implicit social evaluation than we might expect. Another possible interpretation is that the dACC responds to tasks in which relevant goals are being challenged, regardless of whether those goals are cognitive or social. It will be interesting for future research to investigate how the brain and body respond to stressors with different psychological characteristics and to begin to disentangle the distinct neurocognitive processes engaged during different types of stressors.
In addition to threat-related neural activity, physiological responses to stress have also been linked with activity in safety-related neural regions, especially the VMPFC. In this case, more VMPFC activity is typically associated with blunted physiological responses, suggesting the possibility that greater perceptions of safety or security during stress are associated with less physiological reactivity. Once again, there are exciting avenues for future research investigating the role of VMPFC in physiological stress responses: For example, how do individual differences influence the likelihood that people will show VMPFC activity during a stressor? Behavioral research suggests that a variety of social and personality factors, including trait and state experiences of social support (Uchino, 2006), optimism (Brydon et al., 2009), feelings of personal control or mastery (Roepke & Grant, 2011), and self-esteem (Taylor et al., 2003) may buffer physiological stress responses, perhaps via increasing perceptions of safety and security during stress. Thus, a novel hypothesis would be that these and other psychosocial resources are related to greater VMPFC activity during stress (e.g., Eisenberger et al., 2011), which may be involved in inhibiting physiological stress responses. Much more research is needed on how individual differences moderate the relation between neural activity and physiological stress responses in an effort to understand who may be most at risk for the health-damaging effects of stress.
Finally, neural activity in brain regions often active in tasks that involve self-reflection (MPFC, pACC, PCC) and thinking about the thoughts and feelings of others (MPFC, DMPFC, PCC) are linked with heightened physiological stress responses, though the data here are more mixed. There are exciting opportunities for future research in this area. For example, one reason why MPFC may be linked with physiological responses to stress is because MPFC activity may be associated with increased self-conscious emotions. Future studies could aim to address this hypothesis directly by explicitly eliciting self-conscious emotions in the scanner and measuring changes in SNS, HPA, and inflammatory measures. Future research could also test whether activity in social cognitive-related brain regions (MPFC, DMPFC, PCC) during stress is related to greater feelings of evaluation, and attention to the thoughts of the evaluators by manipulating whether participants are attending to the evaluators and examining both neural and physiological responses. Although the precise role of self-related and social-cognitive related neural activity in predicting physiological stress responses is still unclear, this is an exciting avenue for future research.
As reflected in the wizardly wisdom at the beginning of this paper, though psychological stress may often “all be in our heads”, it can still have very real effects on physiology, and ultimately, health. Initial evidence suggests that neural regions implicated in processing threat, safety, and the self and others are related to physiological responses to stress, but much more research is needed to fully understand these relationships. It is an exciting time, as the “black box” of the brain can now be opened and we can begin to identify the neurocognitive processes that translate stressful situations into physiological changes that are relevant for health.
Table 1.
Neural regions associated with physiological stress responses.
| Citation | Physiological Measure | dACC | AI | Amygdala | MPFC (BA 10) | DMPFC | pACC | PCC | VMPFC |
|---|---|---|---|---|---|---|---|---|---|
| Sympathetic-related responses: | |||||||||
| Critchley 2005 | Pupil dilation | + | |||||||
| Critchely 2003* | LF- HRV | + | + | ||||||
| Critchley 2000* | MAP | + | − | − | |||||
| Critchley 2000* | Heart rate | + | − | − | |||||
| Wager 2009a | Heart rate | + | − | ||||||
| Wager 2009b | Heart rate | + | − | ||||||
| Gianaros 2004 | Cardio acceleration | + | − | + | − | − | |||
| Gianaros 2005a* | MAP | + | + | + | + | + | |||
| Gianaros 2005b* | SBP | + | |||||||
| Gianaros 2007 | SBP | + | + | + | + | ||||
| Gianaros 2008 | MAP | + | + | + | + | + | |||
| Gianaros 2011 | Baroreflex suppression | + | + | + | + | + | + | ||
| Cortisol responses: | |||||||||
| Wang 2005 | Cortisol | + | + | ||||||
| Eisenberger 2007 | Cortisol | + | + | + | − | ||||
| Pruessner 2008 | Cortisol | − | − | − | |||||
| Kern 2008 | Cortisol | +/− | − | + | |||||
| Dedovic 2009 | Cortisol | + | |||||||
| Inflammatory responses: | |||||||||
| Slavich 2010 | TNF-alpha | + | + | + | |||||
Note. +, regions that were positive correlated with stressor-evoked physiology; −, regions that were negative correlated with stressor-evoked physiology;
, data are from the same subjects using the same task, but are analyzed differently or examine two different physiological measures;
MAP = mean arterial blood pressure; SBP = systolic blood pressure; dACC = dorsal anterior cingulate cortex; AI = anterior insula; MPFC = medial prefrontal cortex; DMPFC = dorsomedial prefrontal cortex; pACC = perigenual anterior cingulate cortex; PCC = posterior cingulate cortex; VMPFC = ventromedial prefrontal cortex
Acknowledgments
The authors would like to thank George Slavich, Tristen Inagaki, Alexandra Dupont, and members of the UCLA Social & Affective Neuroscience Lab for their helpful comments on previous drafts of this paper. Preparation of this article was supported by an NSF Graduate Research Fellowship (to KAM), a pre-doctoral NRSA training fellowship (T32 MH015750-31A1 to KAM), and a National Institutes of Mental Health grant (R01MH091352 to NIE).
Footnotes
Under non-stressful conditions, increases in blood pressure are typically associated with decreases in heart rate, and this inverse relationship is mediated by a set of specialized neurons called baroreceptors. Baroreceptors, which respond to increases in blood pressure, increase their firing rate to initiate decelerations in heart rate, a phenomenon known as the baroreflex (Dampney, 1994). Under conditions of stress, the baroreflex is suppressed, thus unlinking the inverse relationship between blood pressure and heart rate and allowing for simultaneous rises in both (Gianaros et al., 2011).
References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9:463–464. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. Journal of Neuroscience. 2010;29:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blascovich K, Mendes WB. Social psychophysiology and embodiement. In: Fiske S, Gilbert DT, editors. The Handbook of Social Psychology. New York: Wiley; 2010. [Google Scholar]
- Bosch JA, de Geus EJC, Carroll D, Goedhart AD, Anane LA, Veldhuizen van Zanten JJ, et al. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosomatic Medicine. 2009;71:877–885. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain, Behavior, and Immunity. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. A secure base: Parent-child attachment and healthy human development. Basic Books; USA: 1988. [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiologyc. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak AJ, Chart H, Steptoe A. Dispositional optimism and stress-induced changes in immunity and negative mood. Brain, Behavior, and Immunity. 2009;23:810–816. doi: 10.1016/j.bbi.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic neurons are correlated with fear expression and extinction failure. The Journal of Neuroscience. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. Journal of Physiology. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–156. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchely HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Brosch T. Motivational salience: Amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science. 2012;21:54–59. [Google Scholar]
- Dalgleish T, Dunn BD, Mobbs D. Affective neuroscience: Past, present, and future. Emotion Review. 2009;1:355–368. [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, et al. Neural correlates of processing stressful information: An event-related fMRI study. Brain Research. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biological Psychology. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stresors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: Shame, physiology, and health. Journal of Personality. 2004a;72:1191–1216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosomatic Medicine. 2004b;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Egner T. Surprise! A unifying model of dorsal anterior cingulate function? Nature Neuroscience. 2011;14:1219–1220. doi: 10.1038/nn.2932. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: The shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience. doi: 10.1038/nrn3231. (in press) [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. Broken hearts and broken bones: A neural perspective on the similarities between social pain and physical pain. Current Directions in Psychological Science. 2012;21:41–47. [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: Neurophysiological mechanisms linking social ties with physical health. Nature Neuroscience. 2012;15:1–6. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Psychology. 2004;8:264–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Master SL, Inagaki TI, Taylor SE, Shirinyan D, Lieberman MD, et al. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences, USA. 2011a;1081:11721–11726. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Muscatell KA, Haltom KEB, Leary MR. The neural sociometer: A mechanism for translating interpersonal appraisals into state self-esteem. Journal of Cognitive Neuroscience. 2011b;23:3448–3455. doi: 10.1162/jocn_a_00027. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. NeuroImage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: Insights from clinical studies. Proceedings of the American Thoracic Society. 2009;6:256–259. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- Finch CE. Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proceedings of the National Academy of Sciences, USA. 2010;107:1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, van der Veen FM, Jennings RJ. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–530. doi: 10.1111/1469-8986.2004.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Derbyshire SWG, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005a;42:627–635. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, May JC, Siegle GJ, Jennings JR. Is there a functional neural correlate of individual differences in cardiovascular reactivity? Psychosomatic Medicine. 2005b;67:31–39. doi: 10.1097/01.psy.0000151487.05506.dc. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SWG, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. The Journal of Neuroscience. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease. NeuroImage. 2009;47:922–936. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflext suppression during stress in humans. Human Brain Mapping. 2011 doi: 10.1002/hbm.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe KM, Takeda K, Hickman HD, Bailey AM, Embry AC, Bennik JR, et al. Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbate influenza A virus pathogenesis. Journal of Immunology. 2010;184:540–544. doi: 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: Shame, social self-esteem, and cortisol activity. Psychosomatic Medicine. 2004;66:915–924. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proceedings of the National Academy of Sciences, USA. 2006;103:14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuity of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: Conceptual and measurement considerations. Psychosomatic Medicine. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Tu PC, Buckner RL. Clan mentality: Evidence that the medial prefrontal cortex responds to close others. Journal of Neuroscience. 2010;13:13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neurosceince. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick SP, Murray DB, Janicki JS, Brower GL. Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension. 2010;55:270–276. doi: 10.1161/HYPERTENSIONAHA.109.142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. American Journal of Medicine. 2008;121:S21–S31. doi: 10.1016/j.amjmed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: A review of core processes. Annual Review of Psychology. 2007;59:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 5. New York, NY: McGraw-Hill; 2010. pp. 143–193. [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JK, Doll R, et al. A genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biological Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Inferences about mental states. Philosophical Transactions of the Royal Society B. 2009;364:1309–1316. doi: 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbane M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neuroscience and Biobehavioral Reviews. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, et al. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain, Behavior, and Immunity. 2012;26:573–579. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. Deactivation of the limbic system during acute psychological stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biological Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Roepke SK, Grant I. Toward a more complete understanding of the effect of personal mastery on cardiometabolic health. Health Psychology. 2011;30:615–632. doi: 10.1037/a0023480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal, and regulation of fear. Trends in Cognitive Sciences. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Bedard ACV, Czarnecki R, Fan J. Preparatory activity and connectivity in dorsal anterior cingulate cortex for cognitive control. NeuroImage. 2011;57:242–250. doi: 10.1016/j.neuroimage.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. The past, present and future of social neuroscience: A European perspective. NeuroImage. 2012;61:437–449. doi: 10.1016/j.neuroimage.2012.01.109. [DOI] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience and Biobehavioral Reviews. 2010a;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proceedings of the National Academy of Sciences, USA. 2010b;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Uchino BN, Beg CA, Florsheim P, Pearce G, Hawkins M, et al. Conflict and collaboration in middle-aged and older couples: II. Cardiovascular reactivity during marital interaction. Psychology and Aging. 2009;24:274–286. doi: 10.1037/a0016067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sherman DK, Sage RM, McDowell NK. Are self-enhancing cognitions associated with healthy or unhealthy biological profiles? Journal of Personality and Social Psychology. 2003;85:605–615. doi: 10.1037/0022-3514.85.4.605. [DOI] [PubMed] [Google Scholar]
- Taube A, Schlick R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: Links to cardiovascular disease. American Journal of Physiology: Heart and Circulatory Physiology. doi: 10.1152/ajpheart.00907.2011. (in press) [DOI] [PubMed] [Google Scholar]
- Thorsteinsson EB, James JE. A meta-analysis of the effects of experimental manipulations of social support during laboratory stress. Psychology and Health. 1999;14:869–886. [Google Scholar]
- Uchino BN. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo JT, Malarkey W, Glaser R. Individual differences in cardiac sympathetic control predict endocrine and immune responses to acute psychological stress. Journal of Personality and Social Psychology. 1995;69:736–743. doi: 10.1037//0022-3514.69.4.736. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular response to social threat, Part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. NeuroImage. 2009a;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social stress, Part II: Prefrontal-subcorticol pathways and relationship with anxiety. NeuroImage. 2009b;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, et al. Gender difference in neural response to psychological stress. Social Cognitive and Affective Neuroscience. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Sciences. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]


