Abstract
In this study, we attempted to identify threshold values for kidney function measures that maximally discriminate short-term mortality, to identify major population segments in which these thresholds apply, and to classify the hierarchical rank of the thresholds when other classic risk factors are also considered. To do this we retrospectively identified estimated glomerular filtration rate (eGFR) and urinary albumin–creatinine ratio (ACR) thresholds to maximize sensitivity and specificity predictions for death in non-institutionalized NHANES III participants, representative of the United States population from 1988 to 1994 and followed through 2000. In a classification tree excluding dichotomizing variables, age 57 years was initially selected; ACR appeared in the second round and eGFR in the third. The prognostic discrimination of optimum eGFR and ACR thresholds exceeded those of commonly advocated public health screening measures, such as LDL cholesterol and fasting blood glucose, with body mass index appearing in the third round, and smoking and LDL cholesterol in the fourth. In a tree permitting dichotomizing variables, the ACR, systolic blood pressure, and glucose first appeared in the third round, with eGFR, smoking, and LDL in the fourth. Thus, the albumin–creatinine ratio and eGFR may be at least as efficient for survival-based clinical triage as most other classic risk factors.
Keywords: albumin–creatinine ratio, estimated glomerular filtration rate, mortality, survival
Chronic kidney disease is common, and is associated with cardiovascular disease, end-stage kidney disease, and death risks in community settings.1–4 Hence, it is reasonable to question whether kidney function tests should be routinely recommended for community-dwelling adults, in much the same way that blood pressure, lipid, and blood glucose measures are recommended periodically.
Before considering screening, it is useful to evaluate how different threshold levels perform in relation to predicting major health outcomes. In the context of death within finite intervals, thresholds at which individuals classified as ‘normal’ show low mortality rates (high proportion of true negatives) and those classified as ‘abnormal’ show high mortality rates (high proportion of true positives) are attractive for selecting subgroups in which more intensive follow-up and treatment may be appropriate. As gains in sensitivity are accompanied by losses in specificity, the threshold of maximum combined sensitivity and specificity is a logical selection.
Regarding kidney function and mortality in the general population, there are many unknowns: should creatinine-based estimated glomerular filtration rate (eGFR), urinary albumin–creatinine ratio (ACR), or both be used? At what levels? Are optimal threshold values the same in all segments of the community? As declining kidney function correlates with many other classic mortality risk factors, is it more efficient to screen for factors like blood pressure, lipids, body mass index, and blood glucose? In this nationally representative study, thresholds with maximum sensitivity and specificity values (MaxSn + Sp) were identified with a receiver operating characteristic approach,5 and classification tree methodology was used to assess the performance of eGFR, ACR, and classic cardiovascular risk factors as mortality discriminators among community-dwelling adults.
RESULTS
Characteristics of the non-institutionalized US population between 1988 and 1994, based on the Third National Health and Nutrition Examination Survey (NHANES III), are shown in Table 1. Mean age was 44.9 years; 53.2% were women, 9.4% were African American, and 5.0% were Hispanic. Mean eGFR was 99.4 ml/min per 1.73 m2 and the median urinary ACR value was 5.7 mg/g. Older age was associated with lower eGFR (r = −0.76) and higher serum creatinine (r = 0.24) and ACR (r = 0.09) levels. Other positive correlations included female sex; self-reported hypertension, diabetes, and cardiovascular disease; systolic and diastolic blood pressure; body mass index; waist–hip ratio; low-density lipoprotein (LDL) cholesterol; C-reactive protein; and glucose. Negative correlations of age included African American and Hispanic race-ethnicity and smoking.
Table 1.
Characteristics of US adults, 1988–1994
| Characteristic | Mean or % (s.e.) Median (25th–75th percentiles) |
Correlation with age |
|
|---|---|---|---|
| r | P | ||
| Age (years) | 44.9 (0.6) | — | — |
| Women (%) | 53.2 (0.9) | 0.03 | 0.03 |
| Race (%) | |||
| White | 78.3 (1.4) | 0 (referent) | — |
| African American | 9.4 (0.6) | −0.06 | <0.001 |
| Hispanic | 5.0 (0.4) | −0.10 | <0.001 |
| Other | 7.4 (1.1) | −0.03 | 0.3 |
| Hypertension (%) | 23.6 (0.9) | 0.32 | <0.001 |
| Diabetes (%) | 4.2 (0.3) | 0.17 | <0.001 |
| Cardiovascular disease (%) | 5.4 (0.5) | 0.28 | <0.001 |
| Current smoker (%) | 27.1 (1.0) | −0.14 | <0.001 |
| Systolic blood pressure (mm Hg) | 121.9 (0.5) | 0.58 | <0.001 |
| Diastolic blood pressure (mm Hg) | 73.8 (0.2) | 0.16 | <0.001 |
| Body mass index (kg/m2) | 26.6 (0.2) | 0.12 | <0.001 |
| Waist-hip ratio | 0.9 (<0.1) | 0.39 | <0.001 |
| LDL cholesterol (mg/dl) | 127.9 (0.9) | 0.28 | <0.001 |
| HDL cholesterol (mg/dl) | 50.4 (0.4) | 0.02 | 0.1 |
| C-reactive protein (mg/l) | 0.4 (<0.1) | 0.12 | <0.001 |
| Glucose (mg/dl) | 98.0 (0.8) | 0.28 | <0.001 |
| Creatinine (mg/dl) | 0.8 (<0.1) | 0.24 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 99.4 (0.7) | −0.76 | <0.001 |
| ACR (mg/g) | 5.7 (3.7–10.2) | 0.09 | <0.001 |
Abbreviations: ACR, urinary albumin–creatinine ratio; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Missing data: blood pressure (n=4), body mass index (n=9), C-reactive protein (n=1), LDL cholesterol (n=168), HDL cholesterol (n=40), glucose (n=1). Reference groups for correlation–regression analysis: male, white, and absence of hypertension, diabetes, cardiovascular disease, and current smoking.
National Health and Nutrition Examination Survey-recommended analytical procedures were followed and the sampling weights implicit in this complex sample survey design were used; WTPFSD6, SDPPSU6, and SDPSTRA6 as weight, cluster, and stratum variables, respectively.
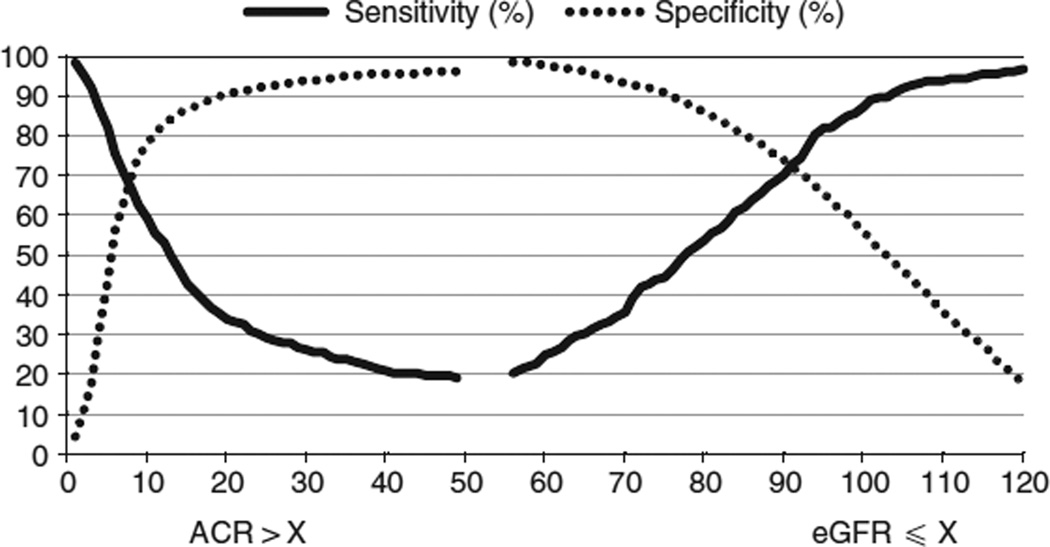
The death rate was 10.0 per 1000 person years; 8.7% of the study population died during a mean follow-up interval of 8.7 years. Sensitivity and specificity values for predicting death at different eGFR and ACR thresholds are shown in Figure 1. Sensitivity (Sn) and specificity (Sp) values for a threshold eGFR of 60 ml/min per 1.73 m2 were 0.25 and 0.98, respectively, and for an ACR threshold of 30 mg/g, 0.27 and 0.94, respectively. MaxSn + Sp thresholds were 94 ml/min per 1.73 m2 for eGFR (Sn/Sp, 0.80/0.67) and 9 mg/g (0.62/0.75) for ACR. When the study population was randomly split into five similarly sized subgroups, maximum MaxSn + Sp levels for eGFR were 85 (0.70/0.79), 94 (0.81/0.73), 95 (0.83/0.63), 93 (0.78/0.68), and 87 (0.68/0.78) ml/min per 1.73 m2. Corresponding values for ACR were 12 (0.63/0.82), 12 (0.57/0.81), 10 (0.52/0.76), 9 (0.64/0.76), and 9 (0.64/0.79) mg/g. In the subgroup with diabetes, MaxSn + Sp thresholds were 76 ml/min per 1.73 m2 for eGFR (0.49/0.82) and 12 mg/g (0.73/0.57) for ACR; corresponding values for the subgroup with hypertension were 83 ml/min per 1.73 m2 (0.69/0.69) and 12 mg/g (0.64/0.70).
Figure 1. Sensitivity (exposure among those who died) and specificity (non-exposure among those who survived) values for death on the y-axis at different estimated glomerular filtration rate (eGFR) and albumin-creatinine ratio (ACR) thresholds.
At any given threshold X, eGFR ≤ X defined exposure and eGFR > X non-exposure; corresponding ACR categories were ACR > X for exposure and ACR ≤ X for non-exposure.
MaxSn + Sp thresholds for other intrinsically continuous variables are shown in Table 2, as are mortality odds ratios. Ranked by MaxSn + Sp, age > 57 years was the best discriminator between survival and death, followed by eGFR ≤ 94 ml/min per 1.73 m2, systolic blood pressure > 127 mm Hg, ACR > 9 mg/g, waist–hip ratio > 0.91, glucose > 101 mg/dl, self-reported cardiovascular disease, self-reported hypertension, standardized serum creatinine > 0.97 mg/dl, C-reactive protein > 0.3 mg/l, LDL cholesterol > 148 mg/dl, self-reported diabetes, body mass index > 26 kg/m2, and male sex. In a similar analysis in the subgroup with self-reported diabetes, age > 62 years was the first-ranked discriminator (Sn/Sp 0.84/0.67), followed by systolic blood pressure > 136 mm Hg (0.59/0.75), eGFR ≤ 76 ml/min per 1.73 m2 (0.49/0.82), ACR > 12 mg/g (0.73/0.57), self-reported cardiovascular disease (0.42/0.88), LDL cholesterol > 165 mg/dl (0.32/0.87), HDL cholesterol ≤ 41 mg/dl (0.59/0.6), and C-reactive protein > 0.3mg/l (0.52/0.63). Among participants with self-reported hypertension, age > 65 years was the first-ranked discriminator (0.68/0.79), followed by eGFR ≤ 83 ml/min per 1.73 m2 (0.69/0.69), systolic blood pressure > 136 mm Hg (0.72/0.63), ACR > 12 mg/g (0.64/0.70), self-reported cardiovascular disease (0.33/0.92), serum creatinine > 0.97 mg/dl (0.40/0.83), glucose > 109 mg/dl (0.35/0.79), waist–hip ratio > 0.91 (0.78/0.36), C-reactive protein > 1.4 mg/l (0.14/0.95), and self-reported diabetes (0.15/0.92).
Table 2.
Threshold values for mortality discrimination, ranked by maximum values of sensitivity plus specificitya
| Rank | Risk factor | Prevalence | Sensitivity/specificity | OR, death, unadjusted | P | OR, death, age-adjusted | P |
|---|---|---|---|---|---|---|---|
| Overall population (n=6165, 834 deaths) | |||||||
| 1 | Age > 57 (years) | 0.25 | 0.80/0.80 | 16.0 (12.0–21.3) | <0.001 | — | — |
| 2 | eGFR ≤ 94 (ml/min per 1.73 m2) | 0.37 | 0.80/0.67 | 8.2 (6.3–10.6) | <0.001 | 0.9 (0.7–1.3) | 0.7 |
| 3 | Systolic BP > 127 (mm Hg) | 0.30 | 0.68/0.74 | 5.8 (4.5–7.5) | <0.001 | 1.2 (0.9–1.7) | 0.2 |
| 4 | ACR > 9 (mg/l) | 0.28 | 0.62/0.75 | 4.9 (3.8–6.4) | <0.001 | 2.1 (1.6–2.8) | <0.001 |
| 5 | WHR > 0.91 | 0.49 | 0.74/0.54 | 3.3 (2.7–4.1) | <0.001 | 1.6 (1.2–2.0) | <0.001 |
| 6 | Glucose > 101 (mg/l) | 0.24 | 0.44/0.78 | 2.8 (2.3–3.4) | <0.001 | 1.2 (0.9–1.5) | 0.2 |
| 7 | CVD | 0.05 | 0.25/0.97 | 9.3 (7.1–12.1) | <0.001 | 3.0 (2.3–3.8) | <0.001 |
| 8 | Hypertension | 0.24 | 0.43/0.78 | 2.7 (2.2–3.4) | <0.001 | 1.2 (1.0–1.5) | 0.1 |
| 9 | Creatinine > 0.97 | 0.14 | 0.32/0.88 | 3.3 (2.6–4.2) | <0.001 | 1.7 (1.3–2.2 | <0.001 |
| 10 | CRP > 0.3 (mg/l) | 0.25 | 0.37/0.76 | 1.9 (1.5–2.3) | <0.001 | 1.3 (1.0–1.7) | 0.03 |
| 11 | LDL > 148 (mg/dl) | 0.27 | 0.39/0.74 | 1.8 (1.5–2.3) | <0.001 | 1.0 (0.8–1.4) | 0.7 |
| 12 | Diabetes | 0.04 | 0.12/0.97 | 3.7 (2.5–5.6) | <0.001 | 1.7 (1.1–2.6) | 0.02 |
| 13 | BMI > 26 (kg/m2) | 0.46 | 0.52/0.54 | 1.3 (1.1–1.6) | 0.009 | 1.1 (0.9–1.4) | 0.3 |
| 14 | Male | 0.47 | 0.52/0.54 | 1.3 (1.1–1.5) | 0.006 | 1.7 (1.4–2.1) | <0.001 |
| Age ≤ 57 (years), age excluded (n=4064, 128 deaths) | |||||||
| 1 | Systolic BP > 120 (mm Hg) | 0.34 | 0.66/0.67 | 3.8 (2.2–6.7) | <0.001 | 2.5 (1.3–4.5) | 0.003 |
| 2 | WHR > 0.91 | 41.5 | 0.66/0.59 | 2.8 (1.8–4.4) | <0.001 | 1.8 (1.2–2.8) | 0.009 |
| 3 | eGFR ≤ 107 (ml/min per 1.73 m2) | 0.49 | 0.70/0.52 | 2.5 (1.4–4.6) | 0.002 | 1.0 (0.6–2.0) | 0.9 |
| 4 | Hypertension | 0.17 | 0.38/0.83 | 3.1 (1.9–4.8) | <0.001 | 2.0 (1.3–3.1) | 0.002 |
| 5 | BMI > 27 (kg/m2) | 0.36 | 0.56/0.64 | 2.3 (1.3–4.1) | 0.006 | 1.7 (1.0–3.1) | 0.06 |
| 6 | Glucose > 95 (mg/dl) | 0.35 | 0.55/0.65 | 2.3 (1.4–3.7) | 0.001 | 1.4 (0.8–2.5) | 0.2 |
| 7 | ACR > 10 (mg/l) | 0.19 | 0.37/0.82 | 2.7 (1.7–4.3) | <0.001 | 2.3 (1.4–3.7) | 0.001 |
| 8 | LDL > 192 (mg/dl) | 0.04 | 0.14/0.96 | 4.2 (1.2–15) | 0.03 | 3.1 (0.8–11.5) | 0.09 |
| 9 | African American | 0.10 | 0.16/0.9 | 1.8 (1.1–2.8) | 0.01 | 1.8 (1.2–2.8) | 0.003 |
| 10 | Diabetes | 0.02 | 0.07/0.98 | 3.3 (1.2–9.2) | 0.02 | 1.9 (0.7–5.3) | 0.2 |
| 11 | CVD | 0.02 | 0.06/0.98 | 3.6 (1.4–9.1) | 0.008 | 2.1 (0.8–5.7) | 0.2 |
| Age > 57 (years), age excluded (n=2101, 706 deaths) | |||||||
| 1 | ACR > 12 (mg/l) | 0.39 | 0.58/0.68 | 3.0 (2.3–3.9) | <0.001 | 2.2 (1.7–3) | <0.001 |
| 2 | eGFR ≤ 64 (ml/min per 1.73 m2) | 0.21 | 0.36/0.85 | 3.1 (2.3–4.2) | <0.001 | 1.7 (1.2–2.3) | 0.003 |
| 3 | CVD | 0.16 | 0.30/0.89 | 3.6 (2.8–4.5) | <0.001 | 3.2 (2.4–4.3) | <0.001 |
| 4 | Creatinine > 1.07 | 0.15 | 0.25/0.89 | 2.7 (2.0–3.7) | <0.001 | 2.0 (1.5–2.8) | <0.001 |
| 5 | Systolic BP > 130 (mm Hg) | 0.60 | 0.70/0.44 | 1.8 (1.4–2.3) | <0.001 | 1.1 (0.8–1.5) | 0.4 |
| 6 | Male | 0.43 | 0.51/0.6 | 1.6 (1.2–2) | 0.001 | 1.9 (1.5–2.5) | <0.001 |
| 7 | WHR > 0.94 | 0.56 | 0.63/0.47 | 1.5 (1.2–1.9) | <0.001 | 1.6 (1.2–2.0) | <0.001 |
| 8 | CRP > 0.8 (mg/l) | 0.13 | 0.20/0.90 | 2.2 (1.6–3.0) | <0.001 | 2.4 (1.7–3.5) | 0.001 |
| 9 | Glucose > 108 (mg/l) | 0.27 | 0.31/0.74 | 1.3 (1–1.8) | 0.03 | 1.3 (0.9–1.7) | 0.1 |
| Age ≤ 57 (years), age included (n=4064, 128 deaths) | |||||||
| 1 | Age > 44 (years) | 0.25 | 0.64/0.76 | 5.6 (2.9–10.8) | <0.001 | — | — |
| 2 | Systolic BP > 120 (mm Hg) | 0.34 | 0.66/0.67 | 3.8 (2.2–6.7) | <0.001 | 2.5 (1.3–4.5) | 0.003 |
| 3 | WHR > 0.91 | 41.5 | 0.66/0.59 | 2.8 (1.8–4.4) | <0.001 | 1.8 (1.2–2.8) | 0.009 |
| 4 | eGFR ≤ 107 (ml/min per 1.73 m2) | 0.49 | 0.7/0.52 | 2.5 (1.4–4.6) | 0.002 | 1.0 (0.6–2) | 0.9 |
| 5 | Hypertension > 2 | 0.17 | 0.38/0.83 | 3.1 (1.9–4.8) | <0.001 | 2.0 (1.3–3.1) | 0.002 |
| 6 | BMI > 27 (kg/m2) | 0.36 | 0.56/0.64 | 2.3 (1.3–4.1) | 0.006 | 1.7 (1.0–3.1) | 0.06 |
| 7 | Glucose > 95 (mg/l) | 0.35 | 0.55/0.65 | 2.3 (1.4–3.7) | 0.001 | 1.4 (0.8–2.5) | 0.2 |
| 8 | ACR > 10 (mg/g) | 0.19 | 0.37/0.82 | 2.7 (1.7–4.3) | <0.001 | 2.3 (1.4–3.7) | 0.001 |
| 9 | LDL > 192 (mg/l) | 0.04 | 0.14/0.96 | 4.2 (1.2–15) | 0.03 | 3.1 (0.8–11.5) | 0.09 |
| 10 | African American | 0.10 | 0.16/0.9 | 1.8 (1.1–2.8) | 0.01 | 1.8 (1.2–2.8) | 0.003 |
| 11 | Diabetes | 0.02 | 0.07/0.98 | 3.3 (1.2–9.2) | 0.02 | 1.9 (0.7–5.3) | 0.2 |
| 12 | CVD | 0.02 | 0.06/0.98 | 3.6 (1.4–9.1) | 0.008 | 2.1 (0.8–5.7) | 0.2 |
| Age > 57 (years), age included (n=2101, 706 deaths) | |||||||
| 1 | Age > 74 (years) | 0.24 | 0.49/0.85 | 5.4 (4.2–7.1) | <0.001 | — | — |
| 2 | eGFR ≤ 64 (ml/min per 1.73 m2) | 0.21 | 0.36/0.85 | 3.1 (2.3–4.2) | <0.001 | 1.7 (1.2–2.3) | 0.003 |
| 3 | CVD | 0.16 | 0.30/0.89 | 3.6 (2.8–4.5) | <0.001 | 3.2 (2.4–4.3) | <0.001 |
| 4 | Creatinine > 1.07 | 0.15 | 0.25/0.89 | 2.7 (2.0–3.7) | <0.001 | 2.0 (1.5–2.8) | <0.001 |
| 5 | Systolic BP > 130 (mm Hg) | 0.60 | 0.70/0.44 | 1.8 (1.4–2.3) | <0.001 | 1.1 (0.8–1.5) | 0.4 |
| 6 | Male | 0.43 | 0.51/0.6 | 1.6 (1.2–2) | 0.001 | 1.9 (1.5–2.5) | <0.001 |
| 7 | WHR > 0.94 | 0.56 | 0.63/0.47 | 1.5 (1.2–1.9) | <0.001 | 1.6 (1.2–2.0) | <0.001 |
| 8 | CRP > 0.8 (mg/l) | 0.13 | 0.20/0.90 | 2.2 (1.6–3.0) | <0.001 | 2.4 (1.7–3.5) | 0.001 |
| 9 | Glucose > 108 (mg/l) | 0.27 | 0.31/0.74 | 1.3 (1–1.8) | 0.03 | 1.3 (0.9–1.7) | 0.1 |
Abbreviations: ACR, urinary albumin–creatinine ratio; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; OR, odds ratio; WHR, waist-hip ratio.
Sensitivity, the proportion of the population who died with the risk factor; specificity, the proportion of the population who survived without the risk factor. Thresholds for intrinsically continuous variables (those showing maximum combined true positive and negative predictive values for predicting death) were determined by applying the following increments: 1-unit increments to ACR (range 1–100 mg/g), age (20–90 years), BMI (18–40 kg/m2), eGFR (30–120 ml/min per 1.73m2), glucose (70–140 mg/dl), HDL cholesterol (30–80 mg/dl), LDL cholesterol (70–200 mg/dl), and systolic blood pressure (90–150mmHg); 0.1-unit increments to CRP (0.3–2.5 mg/l); and 0.01-unit increments to creatinine (0.50–2.00 mg/dl) and WHR (0.70–1.40). ORs (with 95% confidence intervals in parentheses) were calculated from logistic regression models with death (no/yes) as outcome variable. The comparator for all variables was absence of the indicated condition. National Health and Nutrition Examination Survey-recommended analytical procedures were followed and the sampling weights implicit in this complex sample survey design were used; WTPFSD6, SDPPSU6, and SDPSTRA6 as weight, cluster, and stratum variables, respectively.
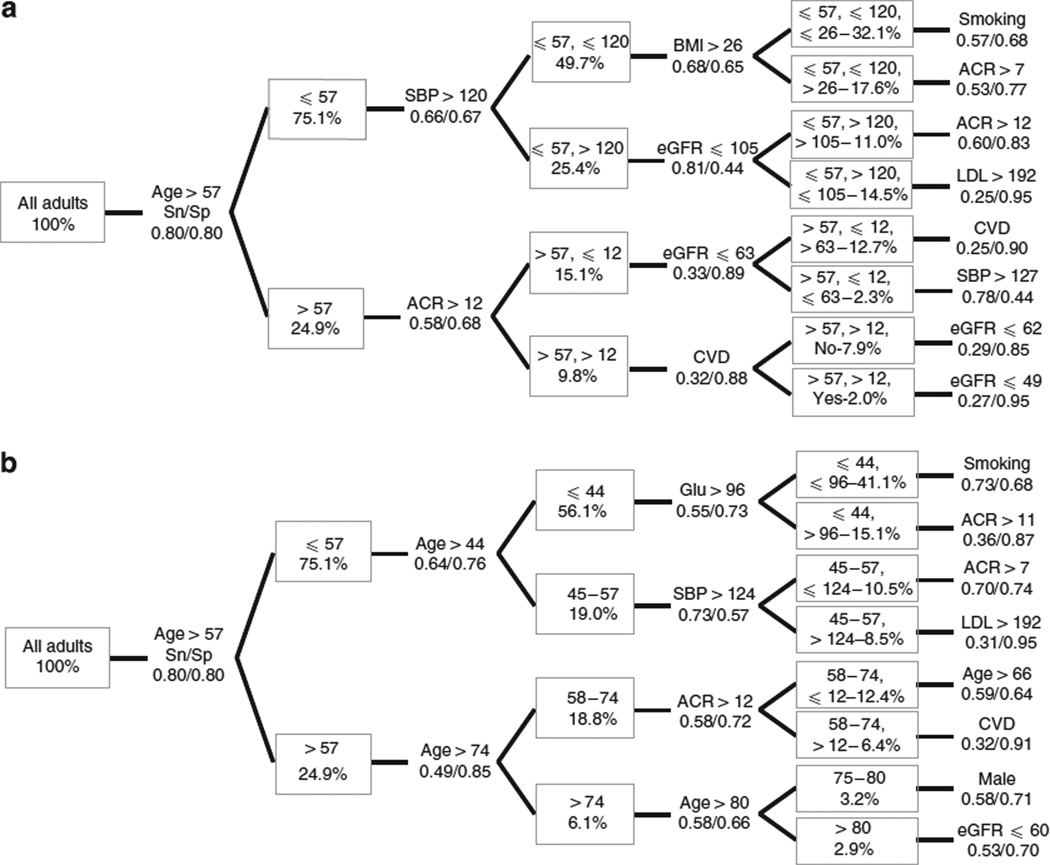
Because its MaxSp + Sn for predicting death or survival was highest, age > 57 years was the first item chosen in the classification tree, and it formed the basis for the first two subgroups chosen, ages ≤ 57 and > 57 years. When an otherwise identical approach was used in the subgroup aged ≤ 57 years and age was not considered, systolic blood pressure > 120 mm Hg was the best discriminator; ACR > 12 mg/g was selected for the subgroup aged > 57 years (Table 2, Figure 2a). eGFR first appeared in the third round in two branches of the classification tree: age ≤ 57 years and systolic blood pressure > 120 mm Hg, eGFR ≤ 105 ml/min per 1.73 m2; age > 57 years and ACR ≤ 12 mg/g, eGFR ≤ 63 ml/min per 1.73 m2. ACR also appeared in the fourth round: age ≤ 57 years, systolic blood pressure > 120 mm Hg and body mass index > 26 kg/m2, ACR > 7 mg/g. eGFR also appeared in the fourth round: age > 57 years, ACR > 12 mg/g and no cardiovascular disease, eGFR ≤ 62 ml/min per 1.73 m2; age > 57 years, ACR > 12 mg/g and cardiovascular disease, eGFR ≤ 49 ml/min per 1.73 m2. Regarding other classic risk factors, body mass index first appeared in the third round of the classification tree, and smoking and LDL cholesterol in the fourth round.
Figure 2. Classification tree analyses.
Successive subgroups defined by threshold values showing maximum sensitivity and specificity values for predicting death with (a) exclusion of variables used at parent nodes in subsequent child nodes, and (b) without exclusion of variables used at parent nodes in subsequent child nodes. Percentages are referenced to the overall population. Age in years. Complete rankings at each node are shown in Appendix 1 and Appendix 2. ACR, albumin-creatinine ratio (mg/g); BMI, body mass index (kg/m2); CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate (ml/min per 1.73 m2); glu, glucose (mg/dl); LDL, low-density lipoprotein cholesterol (mg/dl); SBP, systolic blood pressure, mm Hg; Sn/Sp, sensitivity/specificity.
When age was not excluded as a candidate variable, age > 44 years and age > 74 years, respectively, were the best mortality discriminators in the subgroups aged ≤ 57 and > 57 years (Table 2). Figure 2b shows the corresponding four-level mortality classification tree, in which dichotomizing variables were not excluded. ACR first appeared in the third round (ACR > 12 mg/g, in the subset aged 58–74 years); ACR also appeared in the fourth round, in two subsets (ACR > 11 mg/g in the subset aged ≤ 44 years with glucose > 96 mg/dl; ACR > 7 mg/g in the subset aged 45–57 years with systolic blood pressure ≤ 124 mm Hg. eGFR first appeared in the fourth round (eGFR ≤ 60 ml/min per 1.73 m2 in the subset aged > 80 years). Regarding other classic risk factors, glucose and systolic blood pressure first appeared in the third round of the classification tree, and smoking and LDL cholesterol in the fourth round.
Table 3 shows mean ages, death rates, and mortality odds ratios when the terminal nodes of the two classification trees were used to classify the study population. Overall, both classification systems appeared to exhibit satisfactory mortality discrimination characteristics, with or without adjustment for age, as C-statistics were ≥ 0.85 in each model.
Table 3.
Mortality risk estimates from categories derived from classification tree analysisa
| Category | Mean age (years) | Death rate | OR, death, unadjusted | OR, death, age adjusted |
|---|---|---|---|---|
| Dichotomization variables excluded, classification based on terminal nodes in Figure 2a | ||||
| Age ≤ 57, SBP ≤ 120, BMI ≤ 26, non-smoker | 33.6 | 0.4 | 1 (referent) | 1 (referent) |
| Age ≤ 57, SBP ≤ 120, BMI ≤ 26, smoker | 33.4 | 1.2 | 2.8 (1.1–7.3) | 2.9 (1.1–7.4) |
| Age ≤ 57, SBP ≤ 120, BMI > 26, glucose ≤ 107 | 36.1 | 1.7 | 4 (1.6–9.8) | 3.4 (1.4–8.5) |
| Age ≤ 57, SBP ≤ 120, BMI > 26, glucose > 107 | 42.2 | 11.2 | 26.4 (9.5–73.1) | 15.7 (4.9–50.2) |
| Age ≤ 57, SBP > 120, eGFR > 105, ACR ≤ 12 | 33.8 | 1.0 | 2.5 (1.2–5.5) | 2.6 (1.2–5.6) |
| Age ≤ 57, SBP > 120, eGFR > 105, ACR > 12 | 39.4 | 7.4 | 18.2 (5.5–60) | 12.1 (3.4–42.3) |
| Age ≤ 57, SBP > 120, eGFR ≤ 105, LDL ≤ 192 | 45.6 | 6.4 | 14.8 (7.3–30.1) | 6.7 (3.1–14.3) |
| Age ≤ 57, SBP > 120, eGFR ≤ 105, LDL > 192 | 48.3 | 27.3 | 82.8 (19.9–344.5) | 34.1 (7–166.2) |
| Age > 57, ACR ≤ 12, eGFR > 63, no CVD | 66.4 | 14.8 | 37.8 (21.8–65.6) | 4 (1.9–8.6) |
| Age > 57, ACR ≤ 12, eGFR > 63, CVD | 67.8 | 41.4 | 120.8 (53.4–273.2) | 12.1 (4.4–33.3) |
| Age > 57, ACR ≤ 12, eGFR ≤ 63, SBP ≤ 127 | 70.9 | 33.3 | 89.2 (39.4–201.6) | 6.9 (2.4–19.9) |
| Age > 57, ACR ≤ 12, eGFR ≤ 63, SBP > 127 | 75.1 | 73.7 | 246.5 (114.4–531.1) | 15 (5.7–39.1) |
| Age > 57, ACR > 12, no CVD, eGFR > 62 | 69.7 | 39.6 | 117.6 (64.3–215) | 10.1 (4.6–22.6) |
| Age > 57, ACR > 12, no CVD, eGFR ≤ 62 | 76.5 | 78.3 | 262.3 (128.8–534.4) | 14.5 (5.6–37.4) |
| Age > 57, ACR > 12, CVD, eGFR > 49 | 72.2 | 93.3 | 366.6 (189.4–709.6) | 28.7 (11.4–72.7) |
| Age > 57, ACR > 12, CVD, eGFR ≤ 49 | 79.2 | 199.8 | 2372.1 (634.1–8873.4) | 118.3 (27–517.6) |
| C-statistic 0.85 | C-statistic 0.88 | |||
| Model P <0.001 | Model P <0.001 | |||
| Dichotomization variables included, classification based on terminal nodes in Figure 2b | ||||
| Age ≤ 44, glucose ≤96, non-smoker | 31.2 | 0.3 | 1 (referent) | 1 (referent) |
| Age ≤ 44, glucose ≤ 96, smoker | 30.6 | 1.7 | 5.7 (2.0–16.1) | 5.8 (2.0–16.5) |
| Age ≤ 44, glucose > 96, ACR ≤ 11 | 35.3 | 2.0 | 6.3 (2.0–19.2) | 5.6 (1.7–18.1) |
| Age ≤ 44, glucose > 96, ACR > 11 | 35.2 | 7.2 | 24.5 (6.1–98.9) | 22.1 (5.2–94.6) |
| Age 45–57, SBP ≤ 124, ACR ≤ 7 | 50.1 | 1.4 | 4.5 (1.4–14.8) | 2.7 (0.8–9.5) |
| Age 45–57, SBP ≤ 124, ACR > 7 | 49.8 | 8.8 | 30.2 (10.2–89.8) | 18.5 (5.5–61.8) |
| Age 45–57, SBP > 124, LDL ≤ 192 | 51.3 | 9.0 | 30.4 (12.3–74.7) | 17.9 (5.7–56.2) |
| Age 45–57, SBP > 124, LDL > 192 | 50.2 | 46.4 | 230.9 (50.9–1046.9) | 140.3 (21.3–925.5) |
| Age 58–66, ACR ≤ 12 | 61.8 | 9.1 | 31.8 (13.6–74.2) | 14.1 (3.7–54) |
| Age 67–74, ACR ≤ 12 | 70.2 | 22.1 | 81.5 (35.3–188) | 28.7 (6.3–130.7) |
| Age 58–74, ACR > 12, no CVD | 66.6 | 32.0 | 127.6 (53.8–302.5) | 49.4 (11.8–206.4) |
| Age 58–74, ACR > 12, CVD | 66.7 | 99.1 | 614.1 (243.2–1550.6) | 239.2 (54.3–1053.8) |
| Age 75–80, female | 77.2 | 43.3 | 175.1 (73.4–417.8) | 51.1 (8.3–314.2) |
| Age 75–80, male | 77.1 | 98.5 | 575 (237.2–1393.5) | 168.2 (26.1–1085.9) |
| Age > 80, eGFR > 60 | 83.8 | 91.4 | 533 (217.7–1304.8) | 130.3 (18.6–911) |
| Age > 80, eGFR ≤60 | 84.6 | 152.8 | 1357.6 (539.3–3417.2) | 324.4 (42.8–2458.1) |
| C-statistic 0.88 | C-statistic 0.88 | |||
| Model P <0.001 | Model P <0.001 | |||
Abbreviations: ACR, urinary albumin-creatinine ratio; BMI, body mass index; eGFR, estimated glomerular filtration rates, LDL, low-density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure.
Units: ACR, mg/g; age, years; BMI, kg/m2; eGFR, ml/min per 1.73m2; glucose, mg/dl; LDL, mg/dl; SBP, mmHg.
National Health and Nutrition Examination Survey-recommended analytical procedures were followed and the sampling weights implicit in this complex sample survey design were used; WTPFSD6, SDPPSU6, and SDPSTRA6 as weight, cluster, and stratum variables, respectively.
Death rates are per 1000 subject years. Logistic regression was used to calculate ORs for death. Values in parentheses are 95% confidence intervals.
DISCUSSION
We attempted to identify threshold values for kidney function measures that maximally discriminate short-term mortality, to identify major population segments in which these thresholds apply, and to identify the hierarchical rank of the thresholds when other classic risk factors are also considered. We found that optimal eGFR and ACR thresholds showed prognostic discrimination close to optimal age thresholds, and, except for systolic blood pressure, higher discrimination than exhibited by optimal values of commonly advocated public health screening measures such as LDL cholesterol and fasting blood glucose. For urinary ACR, the maximally discriminatory threshold value was considerably lower than the values usually used to define microalbuminuria. Finally, classification tree analysis suggested that measures of kidney function were most discriminatory in older segments of the population, possibly because of their strong positive correlation with age.
From a public health perspective, discrete threshold values of intrinsically continuous risk factors are often used identify individuals at higher risk who might benefit from more extensive follow-up and treatment with established therapies. For adverse health outcomes like death, one common approach involves examination of risk ratios across several risk factor levels, with risk estimates in the exposed population appearing in the numerator and risk estimates in the non-exposed population appearing in the denominator of the risk ratio. However, this approach ignores the prevalence of risk factor levels, and it can easily be shown that, depending on prevalence, thresholds defined by maximum risk ratio gradients may not maximize the combined proportions of true positive and true negative predictions. If reduced mortality is the major goal, it is difficult to argue against using a threshold that maximizes the chances of ‘good’ levels of the risk factor predicting survival and ‘bad’ levels predicting death.
Many studies have examined associations between kidney function and death/survival. For example, a PubMed search of human studies in April 2010 with the search terms (mortality or survival) (glomerular filtration rate or albuminuria or chronic kidney disease) and (community or general population) yielded 1230 citations. Adding the terms ‘sensitivity’ and ‘specificity’ reduced this number to 72 citations, and adding the term ‘threshold’ resulted in zero citations. Thus, although many studies have examined associations between levels of kidney function and mortality, few if any have attempted to establish threshold values of maximum combined sensitivity and specificity. A notable recent community-based study from Norway used diagnostic test methodology to evaluate the ability of eGFR and albuminuria, as continuous variables, to predict end-stage renal disease and found that both variables provided complementary information.6 Another community-based study from Sweden attempted to identify optimal creatinine clearance thresholds for the outcomes fatal or nonfatal myocardial infarction and cardiovascular death in 2176 50-year-old men without diabetes or cardiovascular disease.7 Follow-up extended to age 70 years. Optimal eGFR thresholds were 98 ml/min per 1.73 m2 for myocardial infarction and 92 ml/min per 1.73 m2 for cardiovascular death. Some-what paralleling the observations in this study, the authors concluded that optimal eGFR thresholds for discriminating cardiovascular risk in the general population may be higher than generally appreciated.
When using intrinsically continuous variables to define a ‘disease,’ using the same criteria for clinical triage in a public health setting may not be optimal. For example, eGFR and ACR thresholds of 60 ml/min per 1.73 m2 and 30 mg/g seem to be very far from optimal in this study; only a small fraction of individuals who died during the study were identified with these criteria. In addition, this study does not support the primacy of eGFR over ACR, or vice versa, for clinical triage; both measures were discriminatory, albeit in different segments of the population. These findings mirror findings of other recent community-based studies with death and end-stage renal disease as outcomes.8–10
The limitations of this study deserve scrutiny. Gold standard techniques, such as inulin or radioisotope clearance to measure GFR and accurately timed urine collection to measure urinary albumin excretion, were not used. As eGFR is calculated with age, and age is a potent predictor of mortality, the prognostic discrimination of eGFR may be inflated.11 As serum creatinine and urinary ACR were measured only once, identification of participants with progressive loss of kidney function was not feasible. The accuracy of cause-specific mortality determination has not been assessed in NHANES III and we did not attempt to identify eGFR and ACR thresholds for renal and cardiovascular death. This study examined the US population between 1988 and 1994, and generalizability of our findings to other countries and other times cannot be guaranteed. Similarly, these findings may not be generalizable to hospital and out patient settings. No attempts were made to weigh sensitivity differently from specificity. For public health screening, confidence that a test has a high true negative rate might be attractive from a service provision perspective; in contrast, missing certain conditions might be so catastrophic that high false positive test rates could be justified. This being said, applying relative weights to true-negative and true-positive test results requires value judgments, whether by individuals, the caring professions, or those who decide public health policy. Thus, we made no attempt to differentially weigh sensitivity and specificity in this study. Finally, we only examined short-term mortality, which is strongly associated with age or with variables highly correlated with age, such as kidney function. Whether reduced kidney function is causally related to the increased short-term mortality in older adults is not known.
Despite these limitations, we believe this study has useful features. The study population is large, and, by design, representative of the US population from 1988 to 1994. Several commonly measured risk-stratification measures, such as blood pressure, glucose, lipids, and body mass index were carefully measured, and study participants were examined in a fasting state. Defining discrete threshold values that discriminate between death and survival may help current efforts to establish clinical guidelines for chronic kidney disease,12,13 but future studies that identify risk factors for premature mortality instead of short-term mortality are needed.
MATERIALS AND METHODS
Objectives
Among adult participants in NHANES III (1988 and 1994), the objectives of this study were:
To identify eGFR and ACR thresholds with maximal sensitivity and specificity (MaxSn + Sp) for death through 31 December 2000 (8.7 years of follow-up);
To use classification tree analysis (based on MaxSn + Sp) to rank eGFR and ACR thresholds in a framework considering other major mortality risk factors, especially those commonly recommended for screening in community-dwelling adults.
Study population and measurements
NHANES samples are multistage, cross-sectional, stratified, clustered probability samples of the non-institutionalized US civilian population. NHANES III was performed in two phases (1988–1991 and 1991–199414); as recommended by the National Center for Health Statistics,15,16 data from the 1988–1991 and 1991–1994 sub-populations were combined in this study. Population subgroups of elderly, Mexican American, and non-Hispanic African American participants were systematically oversampled. Participants were interviewed at home, and physical examinations and blood and urine collections were performed at mobile examination centers. For this study, we limited the study population to participants examined in a mobile examination center after 12 h fasting, aged ≥ 20 years, with serum creatinine and urinary albumin–creatinine measurements.
Serum creatinine was measured by the kinetic alkaline picrate method at the White Sands Research Center (Coulston Foundation) laboratory (Alamogordo, NM, USA) with a Roche/Hitachi 737 analyzer (Roche Diagnostics, Indianapolis, IN, USA). Serum creatinine measurements were aligned to standardized creatinine measured at the Cleveland Clinic Research Laboratory (Cleveland, OH, USA).17 In summary, a random sample of 200 stored specimens was obtained from participants aged X60 years and creatinine was measured with a coupled enzymatic assay (creatininase, creatinase, sarcosine oxidase; kits no. 1775677 and 1775766) on a Roche P Module instrument. College of American Pathologists Creatinine Accuracy Calibration Verification/Linearity Survey LN24 samples were used to confirm traceability to methods based on isotope dilution gas chromatography mass spectrometry. Ultimately, standardized creatinine values (in mg/dl) were calculated from actual creatinine, as follows: standardized creatinine = 0.960 × actual creatinine −0.184.
For GFR estimates, the body-surface-area-adjusted Cockcroft–Gault equation,18–20 the abbreviated Modification of Diet in Renal Disease equation,21,22 the Mayo Clinic equation,23 and the Chronic Kidney Disease Epidemiology Collaboration formula24 were all assessed. As findings were similar with all four equations, only findings using the Chronic Kidney Disease Epidemiology Collaboration equation are reported, calculated from the following functions of age and serum creatinine (Scr, mg/dl):
African American, female, Scr ≤ 0.7: eGFR = 166 × (Scr/0.7)−0.329 × (0.993)age
African American, female, Scr > 0.7: eGFR = 166 × (Scr/0.7)−1.209 × (0.993)age
African American, male, Scr ≤ 0.9: eGFR = 163 × (Scr/0.9)−0.411 × (0.993)age
African American, male, Scr > 0.9: eGFR = 163 × (Scr/0.9)−1.209 × (0.993)age
White or other race, female, Scr ≤ 0.7: eGFR = 144 × (Scr/0.7)−0.329 × (0.993)age
White or other race, female, Scr > 0.7: eGFR = 144 × (Scr/0.7)−1.209 × (0.993)age
White or other race, male, Scr ≤ 0.9: eGFR = 141 × (Scr/0.9)−0.411 × (0.993)age
White or other race, male, Scr > 0.9: eGFR = 141 × (Scr/0.9)−1.209 × (0.993)age
Urinary albumin and creatinine concentrations were measured at the University of Minnesota, (Minneapolis, MN, USA) from random urine spot samples by the modified kinetic Jaffe method with a Synchron AS/Astra Analyzer (Beckman Coulter, Fullerton, CA, USA). High-sensitivity C-reactive protein concentrations were measured by nephelometric immunoassay (Department of Laboratory Medicine, Immunology Division, University of Washington, Seattle, Washington, DC, USA). Current smokers were defined by affirmative answers to the questions ‘Do you now smoke cigarettes?’ and ‘Have you smoked at least 100 cigarettes in your life?’ Diabetes, hypertension, and cardiovascular disease (previous history of myocardial infarction, congestive heart failure, or stroke) were defined by self-report.
Outcomes
Vital status for NHANES III participants was ascertained through 31 December 2000, through linkage with death certificate data in the National Death Index. To protect confidentiality, the public use file was subjected to data perturbation techniques that introduce statistical noise into the dataset to reduce the risk of identification. Synthetic dates were substituted for actual dates of death for selected deceased participants, whereas information regarding vital status was not perturbed. A validation study has shown that mortality hazards ratios from the public access, perturbed dataset closely correspond with those from the restricted access, unperturbed dataset.25
To identify mortality MaxSn + Sp levels for eGFR, sensitivity (exposure among those who died) and specificity (non-exposure among those who survived) were computed separately for whole-number eGFR thresholds between 30 and 120 ml/min per 1.73 m2 and for ACR thresholds between 1 and 100 mg/g. To assess the reproducibility of eGFR and ACR thresholds, we randomly split the dataset into five similarly sized subgroups and calculated MaxSn + Sp levels. As the discriminatory power of many variables might reflect correlations with other variables, most notably age, we constructed classification trees for death based on the highest MaxSn + Sp across all variables, provided P-values for mortality association were < 0.05 with logistic regression. At any given node, subsequent branches were defined by MaxSn + Sp, a process repeated within subgroups until four orders of dichotomization had been completed. Two four-level classification trees were constructed, based on exclusion or inclusion of dichotomizing variables from downstream analyses. Thereafter, terminal branches of the classification trees were used to classify the study population. Logistic regression was used to compute mortality odds ratios and overall model C-statistics.
Several sensitivity analyses were performed. Because abnormal kidney function could reflect ongoing serious illness, analyses were repeated in which we excluded participants who died within the first year of follow-up. As baseline assessments were performed over a 6-year period, potential duration of follow-up was shorter for later participants; hence, analyses were repeated that truncated follow-up at the shortest follow-up time among survivors. Findings were similar with all strategies and are not tabulated in detail. Finally, both logistic regression and proportional hazards regression were used to assess mortality risk ratios, and as the findings were similar only odds ratios with the logistic regression model were reported. NHANES-recommended analytical procedures were followed and the sampling weights implicit in this complex sample survey design were used;15,26 specifically, we used WTPFSD6, SDPPSU6, and SDPSTRA6 as weight, cluster, and stratum variables, respectively. An alpha level < 0.05 was considered statistically significant. SUDAAN, v10 (Research Triangle Institute, Research Triangle Park, NC, USA) and SAS, v9.1.3 (Cary, NC, USA) were used for data analysis.
ACKNOWLEDGMENTS
The authors thank the United States Renal Data System colleagues Beth Forrest for regulatory assistance, Shane Nygaard for manuscript preparation, and Nan Booth, MSW, MPH, for paper editing. This study was performed as a deliverable under Contract No. HHSN267200715002C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland).
Appendix 1
Detailed enumeration of threshold values for mortality discrimination, ranked by maximum true positive and true negative values at each node of the classification tree in Figure 2a, wherein first-ranked discriminators in any analysis are removed from subsequent analysisa
| Rank | Risk factor | Prevalence | Sensitivity/ specificity |
OR, Death, Unadjusted |
P | OR, death, age-adjusted |
P |
|---|---|---|---|---|---|---|---|
| Overall population | |||||||
| 1 | Age >57 (years) | 0.25 | 0.80/0.80 | 16.0 (12.0–21.3) | <0.001 | — | |
| 2 | eGFR ≤94 (ml/min per 1.73m2) | 0.37 | 0.80/0.67 | 8.2 (6.3–10.6) | <0.001 | 0.9 (0.7–1.3) | 0.7 |
| 3 | Systolic BP >127 (mm Hg) | 0.30 | 0.68/0.74 | 5.8 (4.5–7.5) | <0.001 | 1.2 (0.9–1.7) | 0.2 |
| 4 | ACR >9 (mg/g) | 0.28 | 0.62/0.75 | 4.9 (3.8–6.4) | <0.001 | 2.1 (1.6–2.8) | <0.001 |
| 5 | WHR >0.91 | 0.49 | 0.74/0.54 | 3.3 (2.7–4.1) | <0.001 | 1.6 (1.2–2.0) | <0.001 |
| 6 | Glucose >101 (mg/dl) | 0.24 | 0.44/0.78 | 2.8 (2.3–3.4) | <0.001 | 1.2 (0.9–1.5) | 0.2 |
| 7 | CVD | 0.05 | 0.25/0.97 | 9.3 (7.1–12.1) | <0.001 | 3.0 (2.3–3.8) | <0.001 |
| 8 | Hypertension | 0.24 | 0.43/0.78 | 2.7 (2.2–3.4) | <0.001 | 1.2 (1.0–1.5) | 0.1 |
| 9 | Creatinine >0.97 | 0.14 | 0.32/0.88 | 3.3 (2.6–4.2) | <0.001 | 1.7 (1.3–2.2) | <0.001 |
| 10 | CRP >0.3 (mg/l) | 0.25 | 0.37/0.76 | 1.9 (1.5–2.3) | <0.001 | 1.3 (1.0–1.7) | 0.03 |
| 11 | LDL >148 (mg/dl) | 0.27 | 0.39/0.74 | 1.8 (1.5–2.3) | <0.001 | 1.0 (0.8–1.4) | 0.7 |
| 12 | Diabetes | 0.04 | 0.12/0.97 | 3.7 (2.5–5.6) | <0.001 | 1.7 (1.1–2.6) | 0.02 |
| 13 | BMI >26 (kg/m2) | 0.46 | 0.52/0.54 | 1.3 (1.1–1.6) | 0.009 | 1.1 (0.9–1.4) | 0.3 |
| 14 | Male | 0.47 | 0.52/0.54 | 1.3 (1.1–1.5) | 0.006 | 1.7 (1.4–2.1) | <0.001 |
| Age ≤57 (years) | |||||||
| 1 | Systolic BP >120 (mm Hg) | 0.34 | 0.66/0.67 | 3.8 (2.2–6.7) | <0.001 | 2.5 (1.3–4.5) | 0.003 |
| 2 | WHR >0.91 | 0.42 | 0.66/0.59 | 2.8 (1.8–4.4) | <0.001 | 1.8 (1.2–2.8) | 0.009 |
| 3 | GFR ≤107 (ml/min per 1.73m2) | 0.49 | 0.70/0.52 | 2.5 (1.4–4.6) | 0.002 | 1.0 (0.6–2.0) | 0.9 |
| 4 | Hypertension | 0.17 | 0.38/0.83 | 3.1 (1.9–4.8) | <0.001 | 2 (1.3–3.1) | 0.002 |
| 5 | BMI >27 (kg/m2) | 0.36 | 0.56/0.64 | 2.3 (1.3–4.1) | 0.006 | 1.7 (1–3.1) | 0.06 |
| 6 | Glucose >95 (mg/dl) | 0.35 | 0.55/0.65 | 2.3 (1.4–3.7) | 0.001 | 1.4 (0.8–2.5) | 0.2 |
| 7 | ACR >10 (mg/l) | 0.19 | 0.37/0.82 | 2.7 (1.7–4.3) | <0.001 | 2.3 (1.4–3.7) | 0.001 |
| 8 | CRP >0.3 (mg/l) | 0.22 | 0.34/0.78 | 1.9 (1.2–2.9) | 0.008 | 1.4 (0.9–2.3) | 0.2 |
| 9 | LDL >192 (mg/dl) | 0.04 | 0.14/0.96 | 4.2 (1.2–15) | 0.03 | 3.1 (0.8–11.5) | 0.09 |
| 10 | African American | 0.10 | 0.16/0.9 | 1.8 (1.1–2.8) | 0.01 | 1.8 (1.2–2.8) | 0.003 |
| 11 | Diabetes | 0.02 | 0.07/0.98 | 3.3 (1.2–9.2) | 0.02 | 1.9 (0.7–5.3) | 0.2 |
| 12 | CVD | 0.02 | 0.06/0.98 | 3.6 (1.4–9.1) | 0.008 | 2.1 (0.8–5.7) | 0.2 |
| Age >57 (years) | |||||||
| 1 | ACR >12 (mg/l) | 0.39 | 0.58/0.68 | 3 (2.3–3.9) | <0.001 | 2.2 (1.7–3) | <0.001 |
| 2 | GFR ≤64 (ml/min per 1.73m2) | 0.21 | 0.36/0.85 | 3.1 (2.3–4.2) | <0.001 | 1.7 (1.2–2.3) | 0.003 |
| 3 | CVD | 0.16 | 0.3/0.89 | 3.6 (2.8–4.5) | <0.001 | 3.2 (2.4–4.3) | <0.001 |
| 4 | Creatinine >1.07 | 0.15 | 0.25/0.89 | 2.7 (2.0–3.7) | <0.001 | 2.0 (1.5–2.8) | <0.001 |
| 5 | Systolic BP >130 (mm Hg) | 0.60 | 0.7/0.44 | 1.8 (1.4–2.3) | <0.001 | 1.1 (0.8–1.5) | 0.4 |
| 6 | Male | 0.43 | 0.51/0.6 | 1.6 (1.2–2) | 0.001 | 1.9 (1.5–2.5) | <0.001 |
| 7 | WHR >0.94 | 0.56 | 0.63/0.47 | 1.5 (1.2–1.9) | <0.001 | 1.6 (1.2–2) | 0.001 |
| Age ≤57 (years), SBP ≤120 (mm Hg) | |||||||
| 1 | BMI >26 (kg/m2) | 0.35 | 0.68/0.65 | 3.9 (2–7.7) | <0.001 | 3.4 (1.8–6.6) | <0.001 |
| 2 | WHR >0.87 | 0.47 | 0.71/0.54 | 2.8 (1.3–5.9) | 0.009 | 2.2 (1.0–4.9) | 0.04 |
| 3 | Glucose >106 (mg/dl) | 0.07 | 0.30/0.93 | 6.2 (2.5–15.0) | <0.001 | 4.8 (1.6–14.4) | 0.05 |
| 4 | CRP >1.1 (mg/l) | 0.04 | 0.17/0.96 | 5.7 (2.3–14.2) | <0.001 | 5.8 (2.3–14.8) | <0.001 |
| 5 | African American | 0.09 | 0.19/0.91 | 2.3 (1.2–4.5) | 0.02 | 2.0 (1.0–3.9) | 0.04 |
| Age ≤57 (years), SBP >120 (mmHg) | |||||||
| 1 | eGFR ≤105 (ml/min per 1.73m2) | 0.57 | 0.81/0.44 | 3.4 (1.4–8.3) | 0.006 | 1.7 (0.6–5.1) | 0.3 |
| 2 | ACR >12 (mg/l) | 0.19 | 0.37/0.82 | 2.7 (1.3–5.7) | 0.01 | 1.9 (0.9–4.4) | 0.1 |
| 3 | LDL >192 (mg/dl) | 0.04 | 0.21/0.96 | 6.8 (1.8–26.2) | 0.005 | 5.5 (1.4–22.5) | 0.02 |
| 4 | Hypertension | 0.32 | 0.48/0.69 | 2.0 (1.1–3.8) | 0.03 | 1.5 (0.8–2.8) | 0.2 |
| Age >57 (years), ACR ≤12 (mg/l) | |||||||
| 1 | eGFR ≤63 (ml/min per 1.73m2) | 0.16 | 0.33/0.89 | 3.9 (2.4–6.1) | <0.001 | 1.9 (1.2–3.2) | 0.007 |
| 2 | CVD | 0.13 | 0.27/0.90 | 3.3 (2.3–4.8) | <0.001 | 3.1 (2.0–4.8) | <0.001 |
| 3 | Creatinine >0.88 | 0.40 | 0.52/0.63 | 1.8 (1.3–2.6) | 0.001 | 1.5 (1.1–2.2) | 0.01 |
| 4 | WHR >0.95 | 0.51 | 0.60/0.52 | 1.6 (1.1–2.3) | 0.007 | 1.6 (1.1–2.3) | 0.01 |
| 5 | CRP >0.8 (mg/l) | 0.10 | 0.18/0.92 | 2.4 (1.6–3.7) | <0.001 | 2.6 (1.5–4.4) | 0.001 |
| 5 | Male | 0.45 | 0.55/0.57 | 1.6 (1.1–2.2) | 0.006 | 1.9 (1.4–2.8) | <0.001 |
| 6 | Systolic BP >130 (mm Hg) | 0.51 | 0.58/0.51 | 1.4 (1–2) | 0.03 | 0.9 (0.6–1.3) | 0.5 |
| 7 | LDL >146 (mg/dl) | 0.42 | 0.49/0.59 | 1.4 (1–1.9) | 0.03 | 1.6 (1.1–2.3) | 0.007 |
| Age >57 (years), ACR >12 (mg/l) | |||||||
| 1 | CVD | 0.21 | 0.32/0.88 | 3.4 (2.1–5.3) | <0.001 | 3.1 (2.0–5.0) | <0.001 |
| 2 | Creatinine >0.97 | 0.28 | 0.38/0.79 | 2.4 (1.7–3.3) | <0.001 | 2.0 (1.4–3.0) | <0.001 |
| 3 | GFR ≤62 (ml/min per 1.73m2) | 0.25 | 0.34/0.82 | 2.5 (1.8–3.4) | <0.001 | 1.6 (1.1–2.4) | 0.02 |
| 4 | ACR >47 (mg/l) | 0.28 | 0.37/0.78 | 2.1 (1.4–3.2) | 0.001 | 1.9 (1.2–3.1) | 0.005 |
| 5 | Male | 0.41 | 0.49/0.65 | 1.8 (1.2–2.6) | 0.002 | 2.1 (1.4–3.0) | <0.001 |
| 6 | WHR >0.91 | 0.72 | 0.78/0.31 | 1.6 (1.1–2.3) | 0.02 | 1.7 (1.1–2.5) | 0.02 |
| 7 | Systolic BP >132 (mm Hg) | 0.69 | 0.74/0.34 | 1.5 (1.1–2.1) | 0.02 | 1.1 (0.8–1.6) | 0.6 |
| 8 | CRP >0.8 (mg/l) | 0.16 | 0.21/0.87 | 1.7 (1.1–2.6) | |||
| Age ≤57 (years), SBP ≤120 (mm Hg), BMI ≤26 (kg/m2) | |||||||
| 1 | Smoking | 0.32 | 0.57/0.68 | 2.8 (1.1–7.2) | 0.03 | 2.9 (1.1–7.3) | 0.03 |
| 2 | African American | 0.08 | 0.31/0.93 | 5.5 (1.7–17.6) | 0.004 | 5.0 (1.6–15) | 0.005 |
| 3 | CRP >1.1 (mg/l) | 0.02 | 0.14/0.98 | 7.5 (1.5–37.1) | 0.01 | 7.6 (1.5–38.3) | 0.01 |
| Age ≤57 (years), SBP ≤120 (mm Hg), BMI >26 (kg/m2) | |||||||
| 1 | Glucose >107 (mg/dl) | 0.11 | 0.42/0.90 | 6.6 (2.1–20.6) | 0.001 | 5.1 (1.4–19.0) | 0.02 |
| 2 | ACR >7 (mg/l) | 0.24 | 0.53/0.77 | 3.7 (1.3–10.7) | 0.01 | 3.7 (1.3–10.4) | 0.01 |
| 3 | CRP >1.1 (mg/l) | 0.06 | 0.19/0.94 | 3.6 (1.1–12.0) | 0.04 | 3.9 (1.1–13.5) | 0.04 |
| Age ≤57 (years), SBP >120 (mmHg), GFR >105 (ml/min per 1.73m2) | |||||||
| 1 | ACR >12 (mg/l) | 0.18 | 0.6/0.83 | 7.2 (2.6–20) | <0.001 | 5.2 (1.1–25.1) | 0.04 |
| 2 | Smoking | 0.35 | 0.7/0.66 | 4.5 (1.3–16) | 0.02 | 4.8 (1.3–17.8) | 0.02 |
| 3 | African American | 0.17 | 0.46/0.84 | 4.4 (1.5–13) | 0.007 | 4.4 (1.9–10.3) | 0.001 |
| 4 | LDL >232 (mg/dl) | <0.01 | 0.06/1.00 | 55.3 (4.6–667.6) | 0.002 | 47.4 (7.8–287.6) | <0.001 |
| 5 | WHR >0.74 | 0.99 | 1.00/0.01 | >100 | <0.001 | >100 | <0.001 |
| 6 | Male | 0.68 | 0.32/0.31 | 0.2 (0.1–0.5) | <0.001 | 0.3 (0.1–1.4) | 0.1 |
| 7 | CVD | 0.03 | <0.01/0.97 | <0.1 | <0.001 | <0.01 | <0.001 |
| Age ≤57 (years), SBP >120 (mmHg), GFR ≤105 (ml/min per 1.73m2) | |||||||
| 1 | LDL >192 (mg/dl) | 0.06 | 0.25/0.95 | 6.6 (1.5–28.5) | 0.01 | 6.2 (1.4–27.5) | 0.02 |
| 2 | Hypertension | 0.36 | 0.54/0.66 | 2.3 (1.1–4.7) | 0.03 | 2 (0.9–4) | 0.07 |
| Age >57 (years), ACR ≤12 (mg/l), GFR >63 (ml/min per 1.73m2) | |||||||
| 1 | CVD | 0.12 | 0.25/0.9 | 3.2 (1.9–5.4) | <0.001 | 3 (1.6–5.6) | <0.001 |
| 2 | Male | 0.45 | 0.58/0.57 | 1.9 (1.2–2.8) | 0.003 | 2.1 (1.4–3.3) | 0.001 |
| 3 | WHR >0.95 | 0.50 | 0.60/0.52 | 1.6 (1.1–2.4) | 0.02 | 1.6 (1.0–2.5) | 0.03 |
| 4 | Smoking | 0.17 | 0.26/0.85 | 2 (1.1–3.6) | 0.02 | 2.9 (1.6–5.3) | 0.001 |
| 5 | LDL >146 (mg/dl) | 0.42 | 0.51/0.59 | 1.5 (1.1–2.2) | 0.02 | 1.8 (1.2–2.7) | 0.004 |
| 6 | CRP >0.7 (mg/l) | 0.12 | 0.19/0.89 | 1.9 (1.0–3.6) | 0.05 | 1.9 (0.9–3.8) | 0.08 |
| 7 | Systolic BP >110 (mm Hg) | 0.93 | 0.98/0.08 | 3.5 (1.3–9.7) | 0.01 | 2.4 (0.8–6.8) | 0.1 |
| 8 | BMI >40 (kg/m2) | <0.01 | <0.01/0.99 | <0.1 | 0.009 | 0.1 (0–0.6) | 0.02 |
| Age >57 (years), ACR ≤12 (mg/l), GFR ≤63 (ml/min per 1.73m2) | |||||||
| 1 | Systolic BP >127 (mm Hg) | 0.65 | 0.78/0.44 | 2.8 (1.5–5.2) | 0.002 | 2.0 (1.0–3.8) | 0.04 |
| 2 | Creatinine >1.27 | 0.22 | 0.32/0.85 | 2.6 (1.2–5.7) | 0.02 | 3.7 (1.4–9.4) | 0.006 |
| 3 | CVD | 0.20 | 0.3/0.86 | 2.7 (1.3–5.8) | 0.009 | 2.8 (1.1–7.1) | 0.03 |
| 4 | WHR >0.87 | 0.84 | 0.93/0.22 | 4.0 (1.4–11.4) | 0.01 | 3.2 (1.2–8.2) | 0.02 |
| 5 | CRP >0.8 (mg/l) | 0.12 | 0.21/0.94 | 4.4 (1.4–13.8) | 0.01 | 5.6 (1.6–20.3) | 0.008 |
| Age >57 (years), ACR >12 (mg/l), No CVD | |||||||
| 1 | GFR ≤62 (ml/min per 1.73m2) | 0.20 | 0.29/0.85 | 2.2 (1.5–3.4) | <0.001 | 1.4 (0.9–2.3) | 0.1 |
| 2 | Creatinine >0.97 | 0.23 | 0.31/0.82 | 2 (1.2–3.3) | 0.006 | 1.8 (1–3.2) | 0.06 |
| 3 | WHR >0.91 | 0.70 | 0.76/0.33 | 1.6 (1–2.4) | 0.036 | 1.6 (1–2.5) | 0.04 |
| 4 | HDL ≤41 (mg/dl) | 0.26 | 0.31/0.77 | 1.5 (1.1–2.1) | 0.02 | 1.7 (1.1–2.6) | 0.02 |
| 5 | Hypertension | 0.51 | 0.46/0.46 | 0.7 (0.5–1) | 0.03 | 0.8 (0.6–1.1) | 0.1 |
| Age >57 (years), ACR >12 (mg/l), CVD | |||||||
| 1 | GFR ≤49 (ml/min per 1.73m2) | 0.19 | 0.27/0.95 | 6.5 (2–21) | 0.002 | 5 (1.5–16.8) | 0.008 |
Abbreviations: ACR, urinary albumin creatinine ratio; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; OR, odds ratio; WHR, waist-hip ratio.
Sensitivity, the proportion of the population who died with the risk factor; specificity, the proportion of the population who survived without the risk factor. Logistic regression was used to calculated ORs (with 95% confidence intervals in parentheses) for death; reference groups were those without each risk factor.
National Health and Nutrition Examination Survey-recommended analytical procedures were followed and the sampling weights implicit in this complex sample survey design were used; WTPFSD6, SDPPSU6, and SDPSTRA6 as weight, cluster, and stratum variables, respectively.
True positive is defined by death when the risk factor is present; true negative is defined by survival when the risk factor is absent. Logistic regression was used to calculated ORs (with 95% confidence intervals in parentheses) for death; reference groups were those without each risk factor.
Appendix 2
Detailed enumeration of threshold values for mortality discrimination, ranked by maximum true positive and true negative values at each node of the classification tree in Figure 2b, wherein first-ranked discriminators in any analysis can be considered in subsequent analysisa
| Rank | Risk factor | Prevalence | Sensitivity/ specificity |
OR, Death, Unadjusted |
P | OR, death, age-adjusted |
P |
|---|---|---|---|---|---|---|---|
| Overall population | |||||||
| 1 | Age > 57 (years) | 0.25 | 0.80/0.80 | 16.0 (12.0–21.3) | <0.001 | — | — |
| 2 | GFR ≤94 (ml/min per 1.73 m2) | 0.37 | 0.80/0.67 | 8.2 (6.3–10.6) | <0.001 | 0.9 (0.7–1.3) | 0.7 |
| 3 | Systolic BP > 127 (mmHg) | 0.30 | 0.68/0.74 | 5.8 (4.5–7.5) | <0.001 | 11.2 (0.9–1.7) | 0.2 |
| 4 | ACR > 9 (mg/g) | 0.28 | 0.62/0.75 | 4.9 (3.8–6.4) | <0.001 | 2.1 (1.6–2.8) | <0.001 |
| 5 | WHR > 0.91 | 0.49 | 0.74/0.54 | 3.3 (2.7–4.1) | <0.001 | 1.6 (1.2–2.0) | <0.001 |
| 6 | Glucose > 101 (mg/dl) | 0.24 | 0.44/0.78 | 2.8 (2.3–3.4) | <0.001 | 1.2 (0.9–1.5) | 0.2 |
| 7 | CVD | 0.05 | 0.25/0.97 | 9.3 (7.1–12.1) | <0.001 | 3.0 (2.3–3.8) | <0.001 |
| 8 | Hypertension | 0.24 | 0.43/0.78 | 2.7 (2.2–3.4) | <0.001 | 1.2 (1.0–1.5) | 0.1 |
| 9 | Creatinine > 0.97 | 0.14 | 0.32/0.88 | 3.3 (2.6–4.2) | <0.001 | 1.7 (1.3–2.2) | <0.001 |
| 10 | CRP > 0.3 (mg/l) | 0.25 | 0.37/0.76 | 1.9 (1.5–2.3) | <0.001 | 11.3 (1.0–1.7) | 0.03 |
| 11 | LDL > 148 (mg/dl) | 0.27 | 0.39/0.74 | 1.8 (1.5–2.3) | <0.001 | 1.0 (0.8–1.4) | 0.7 |
| 12 | Diabetes | 0.04 | 0.12/0.97 | 3.7 (2.5–5.6) | <0.001 | 1.7 (1.1–2.6) | 0.02 |
| 13 | BMI > 26 (kg/m2) | 0.46 | 0.52/0.54 | 1.3 (1.1–1.6) | 0.009 | 1.1 (0.9–1.4) | 0.3 |
| 14 | Male | 0.47 | 0.52/0.54 | 1.3 (1.1–1.5) | 0.006 | 1.7 (1.4–2.1) | <0.001 |
| Age ≤57 (years) | |||||||
| 1 | Age > 44 (years) | 0.25 | 0.64/0.76 | 5.6 (2.9–10.8) | <0.001 | — | — |
| 2 | Systolic BP > 120 (mmHg) | 0.34 | 0.66/0.67 | 3.8 (2.2–6.7) | <0.001 | 2.5 (1.3–4.5) | 0.003 |
| 3 | WHR > 0.91 | 0.42 | 0.66/0.59 | 2.8 (1.8–4.4) | <0.001 | 1.8 (1.2–2.8) | 0.009 |
| 4 | eGFR ≤107 (ml/min per 1.73 m2) | 0.49 | 0.7/0.52 | 2.5 (1.4–4.6) | 0.002 | 1 (0.6–2) | 0.9 |
| 5 | Hypertension | 0.17 | 0.38/0.83 | 3.1 (1.9–4.8) | <0.001 | 2(1.3–3.1) | 0.002 |
| 6 | BMI > 27 (kg/m2) | 0.36 | 0.56/0.64 | 2.3 (1.3–4.1) | 0.006 | 1.7(1–3.1) | 0.06 |
| 7 | Glucose > 95 (mg/dl) | 0.35 | 0.55/0.65 | 2.3 (1.4–3.7) | 0.001 | 1.4 (0.8–2.5) | 0.2 |
| 8 | ACR > 10 (mg/l) | 0.19 | 0.37/0.82 | 2.7 (1.7–4.3) | <0.001 | 2.3 (1.4–3.7) | 0.001 |
| 9 | CRP > 0.3 (mg/l) | 0.22 | 0.34/0.78 | 1.9 (1.2–2.9) | 0.008 | 1.4 (0.9–2.3) | 0.2 |
| 10 | LDL > 192 (mg/dl) | 0.04 | 0.14/0.96 | 4.2 (1.2–15) | 0.03 | 3.1 (0.8–11.5) | 0.09 |
| 11 | African American | 0.10 | 0.16/0.9 | 1.8 (1.1–2.8) | 0.01 | 1.8 (1.2–2.8) | 0.003 |
| 12 | Diabetes | 0.02 | 0.07/0.98 | 3.3 (1.2–9.2) | 0.02 | 1.9 (0.7–5.3) | 0.2 |
| 13 | CVD | 0.02 | 0.06/0.98 | 3.6 (1.4–9.1) | 0.008 | 2.1 (0.8–5.7) | 0.2 |
| Age >57 (years) | |||||||
| 1 | Age > 74 (years) | 0.24 | 0.49/0.85 | 5.4 (4.2–7.1) | <0.001 | — | — |
| 2 | ACR > 12 (mg/l) | 0.39 | 0.58/0.68 | 3.0 (2.3–3.9) | <0.001 | 2.2 (1.7–3.0) | <0.001 |
| 3 | eGFR ≤64 (ml/min per 1.73 m2) | 0.21 | 0.36/0.85 | 3.1 (2.3–4.2) | <0.001 | 1.7 (1.2–2.3) | 0.003 |
| 4 | CVD | 0.16 | 0.3/0.89 | 3.6 (2.8–4.5) | <0.001 | 3.2 (2.4–4.3) | <0.001 |
| 5 | Creatinine > 1.07 | 0.15 | 0.25/0.89 | 2.7 (2.0–3.7) | <0.001 | 2.0 (1.5–2.8) | <0.001 |
| 6 | Systolic BP > 130 (mmHg) | 0.60 | 0.7/0.44 | 1.8 (1.4–2.3) | <0.001 | 1.1 (0.8–1.5) | 0.4 |
| 7 | Male | 0.43 | 0.51/0.6 | 1.6 (1.2–2.0) | 0.001 | 1.9 (1.5–2.5) | <0.001 |
| 8 | WHR > 0.94 | 0.56 | 0.63/0.47 | 1.5 (1.2–1.9) | <0.001 | 1.6 (1.2–2.0) | 0.001 |
| 9 | CRP > 0.8 (mg/l) | 0.13 | 0.20/0.90 | 2.2 (1.6–3.0) | <0.001 | 2.4 (1.2–3.5) | <0.001 |
| 10 | Glucose > 108 (mg/dl) | 0.27 | 0.31/0.74 | 1.3 (1.0–1.8) | 0.03 | 1.3 (0.9–1.7) | 0.1 |
| Age ≤44 (years) | |||||||
| 1 | Glucose > 96 (mg/dl) | 0.27 | 0.55/0.73 | 3.4 (1.4–8.1) | 0.005 | 3.3 (1.2–9.4) | 0.03 |
| 2 | Systolic BP > 110 (mmHg) | 0.60 | 0.85/0.41 | 3.8 (1.8–7.9) | 0.001 | 3.6 (1.7–7.7) | 0.001 |
| 3 | Smoking | 0.31 | 0.49/0.69 | 2.1 (1.1–4.1) | 0.02 | 2.2 (1.2–4.2) | 0.02 |
| 4 | ACR > 11 (mg/l) | 0.14 | 0.29/0.86 | 2.5 (1.1–5.9) | 0.03 | 2.6 (1.1–6) | 0.03 |
| 5 | African American | 0.11 | 0.21/0.89 | 2.1 (1.0–4.4) | 0.04 | 1.9 (0.9–3.8) | 0.08 |
| 6 | Hypertension | 0.13 | 0.04/0.87 | 0.3 (0.1–0.9) | 0.04 | 0.3 (0.1–0.8) | 0.02 |
| Age 45–57 (years) | |||||||
| 1 | Systolic BP > 124 (mmHg) | 0.45 | 0.73/0.57 | 3.5 (1.7–7.1) | <0.001 | 3.4 (1.6–7.2) | 0.002 |
| 2 | Hypertension | 0.30 | 0.56/0.72 | 3.3 (1.7–6.4) | 0.001 | 3.2 (1.7–6.1) | <0.001 |
| 3 | BMI > 29 (kg/m2) | 0.32 | 0.53/0.7 | 2.6 (1.3–5.3) | 0.007 | 2.6 (1.3–5.1) | 0.007 |
| 4 | ACR > 5 (mg/l) | 0.57 | 0.77/0.45 | 2.6 (1.2–6) | 0.02 | 2.5 (1.1–5.9) | 0.03 |
| 5 | WHR > 0.91 | 0.61 | 0.79/0.4 | 2.5 (1.3–5.0) | 0.009 | 2.5 (1.2–5.0) | 0.01 |
| 6 | LDL > 192 (mg/dl) | 0.06 | 0.22/0.94 | 5.0 (1.4–17.6) | 0.01 | 4.9 (1.3–18.4) | 0.02 |
| 7 | eGFR ≤77 (ml/min per 1.73 m2) | 0.10 | 0.23/0.91 | 2.8 (1.2–6.8) | 0.02 | 2.7 (1.2–6.1) | 0.02 |
| 8 | Creatinine > 1.07 | 0.04 | 0.13/0.97 | 4.3 (1.5–12.4) | 0.007 | 4.4 (1.5–12.6) | 0.006 |
| 9 | African American | 0.07 | 0.14/0.93 | 2.3 (1.2–4.3) | 0.01 | 3.1 (1.7–5.8) | <0.001 |
| Age 58–74 (years) | |||||||
| 1 | ACR > 12 (mg/l) | 0.34 | 0.58/0.72 | 3.5 (2.3–5.2) | <0.001 | 3.2 (2.1–4.8) | <0.001 |
| 2 | Age > 66 (years) | 0.43 | 0.61/0.61 | 2.5 (1.7–3.5) | <0.001 | — | — |
| 3 | CVD | 0.13 | 0.29/0.91 | 4.0 (2.8–5.6) | <0.001 | 4.0 (2.8–5.7) | <0.001 |
| 4 | WHR > 0.99 | 0.33 | 0.45/0.7 | 1.9 (1.3–2.7) | <0.001 | 11.9 (1.3–2.7) | 0.001 |
| 5 | Male | 0.45 | 0.57/0.58 | 1.8 (1.3–2.5) | <0.001 | 1.8 (1.3–2.5) | <0.001 |
| 6 | CRP > 0.8 (mg/l) | 0.13 | 0.23/0.9 | 2.5 (1.6–3.9) | <0.001 | 2.5 (1.6–3.8) | 0.000 |
| 7 | Smoking | 0.20 | 0.29/0.83 | 2.0 (1.3–3.1) | 0.002 | 2.2 (1.4–3.5) | 0.001 |
| 8 | Systolic BP > 130 (mmHg) | 0.54 | 0.63/0.48 | 1.5 (1.1–2.1) | 0.005 | 1.3 (1.0–1.8) | 0.1 |
| 9 | eGFR ≤63 (ml/min per 1.73 m2) | 0.12 | 0.21/0.89 | 2.2 (1.3–3.7) | 0.002 | 1.9 (1.1–3.3) | 0.01 |
| 10 | Creatinine > 1.17 | 0.07 | 0.15/0.95 | 3.5 (2–6.1) | <0.001 | 3.2 (1.8–5.6) | <0.001 |
| 11 | Glucose > 114 (mg/dl) | 0.20 | 0.28/0.82 | 1.8 (1.1–2.9) | 0.02 | 1.7 (1.1–2.8) | 0.02 |
| Age >74 (years) | |||||||
| 1 | Age > 80 (years) | 0.47 | 0.58/0.66 | 2.7 (1.8^) | <0.001 | — | — |
| 2 | Creatinine > 0.88 | 0.51 | 0.59/0.59 | 2.1 (1.2–3.5) | 0.005 | 2.0 (1.2–3.4) | 0.005 |
| 3 | eGFR > 56 (ml/min per 1.73 m2) | 0.29 | 0.37/0.81 | 2.5 (1.3^.8) | 0.005 | 2.1 (1.1–4.0) | 0.02 |
| 4 | ACR > 32 (mg/l) | 0.24 | 0.32/0.85 | 2.7 (1.7^.2) | <0.001 | 2.3 (1.4–3.7) | 0.001 |
| 5 | Male | 0.38 | 0.46/0.71 | 2.1 (1.3–3.2) | 0.001 | 2.4 (1.6–3.6) | <0.001 |
| 6 | WHR > 0.86 | 0.87 | 0.93/0.2 | 3.2 (1.8–5.6) | <0.001 | 3.2 (1.9–5.6) | <0.001 |
| 7 | CVD | 0.26 | 0.31/0.81 | 1.9 (1.1–3.4) | 0.03 | 2.1 (1.2–3.8) | 0.01 |
| Age ≤44 (years), glucose ≤96 (mg/dl) | |||||||
| 1 | Smoking | 0.32 | 0.73/0.68 | 5.7 (2–16.1) | 0.001 | 6.0 (2.0–18.5) | 0.002 |
| 2 | Age > 39 (years) | 0.15 | 0.45/0.85 | 4.8 (1.7–13.2) | 0.002 | — | — |
| 3 | Systolic BP > 110 (mmHg) | 0.54 | 0.79/0.46 | 3.3 (1.3–8.2) | 0.01 | 3.1 (1.3–7.4) | 0.01 |
| 4 | BMI > 30 (kg/m2) | 0.14 | 0.37/0.86 | 3.7 (1.2–11.4) | 0.02 | 3.4 (1.2–9.2) | 0.02 |
| 5 | African American | 0.11 | 0.28/0.89 | 3.2 (1.4–7.3) | 0.007 | 2.9 (1.2–7) | 0.01 |
| 6 | CVD | 0.01 | 0.13/0.99 | 14.2 (2.1–94.9) | 0.006 | 11.5 (1.9–70.5) | 0.009 |
| Age ≤44 (years), glucose > 96 (mg/dl) | |||||||
| 1 | ACR > 11 (mg/l) | 0.13 | 0.36/0.87 | 3.9 (1.4–11.1) | 0.01 | 3.9 (1.4–10.9) | 0.009 |
| 2 | WHR > 0.74 | 0.99 | 1.00/0.01 | >100 | <0.001 | >100 | <0.001 |
| 3 | Hypertension | 0.17 | 0.03/0.82 | 0.1 (0.0–0.6) | 0.01 | 0.2 (0.0–0.7) | 0.01 |
| 4 | CVD | 0.01 | <0.01./0.99 | <0.01 | <0.001 | <0.01 | <0.001 |
| Age 45–57 (years), systolic BP ≤124 (mm Hg) | |||||||
| 1 | ACR > 7 (mg/l) | 0.27 | 0.7/0.74 | 6.7 (2.2–20.5) | 0.001 | 7.0 (2.3–21.2) | 0.001 |
| 2 | BMI > 29 (kg/m2) | 0.22 | 0.56/0.79 | 4.7 (1.4–15.6) | 0.01 | 4.6 (1.4–15.1) | 0.01 |
| 3 | Hypertension | 0.16 | 0.44/0.85 | 4.5 (1.6–12.5) | 0.004 | 4.5 (1.6–12.7) | 0.005 |
| 4 | Age > 50 (years) | 0.43 | 0.71/0.58 | 3.3 (1.2–9.4) | 0.02 | — | — |
| 5 | Smoking | 0.28 | 0.55/0.73 | 3.3 (1.5–7.3) | 0.003 | 3.4 (1.6–7.5) | 0.002 |
| 6 | Systolic BP > 112 (mmHg) | 0.62 | 0.88/0.39 | 4.5 (1.6–12.8) | 0.005 | 4.1 (1.5–11.5) | 0.007 |
| 7 | Glucose > 107 (mg/dl) | 0.16 | 0.42/0.85 | 3.9 (1.6–9.7) | 0.003 | 3.8 (1.5–9.5) | 0.004 |
| 8 | CRP > 0.5 (mg/l) | 0.14 | 0.37/0.87 | 3.8 (1.4–10.5) | 0.01 | 3.5 (1.3–9.4) | 0.02 |
| 9 | HDL >47 (mg/dl) | 0.48 | 0.67/0.53 | 2.4 (1.1–4.9) | 0.03 | 2.4 (1.1–4.9) | 0.02 |
| 10 | eGFR ≤76 (ml/min per 1.73 m2) | 0.08 | 0.22/0.93 | 3.4 (1.1–10.3) | 0.03 | 2.9 (1.0–8) | 0.04 |
| 11 | African American | 0.07 | 0.19/0.94 | 3.6 (1.4–9.2) | 0.007 | 3.8 (1.6–9.1) | 0.002 |
| 12 | Diabetes | 0.03 | 0.15/0.98 | 7.2 (1.4–37.9) | 0.02 | 5.8 (1.0–33.1) | 0.05 |
| Age 45–57 (years), Systolic BP > 124 (mm Hg) | |||||||
| 1 | LDL > 192 (mg/dl) | 0.07 | 0.31/0.95 | 9.5 (2.5–35.8) | 0.001 | 9.1 (2.6–32.4) | 0.001 |
| 2 | CRP > 1.7 (mg/l) | 0.03 | 0.1/0.98 | 6.6 (2–22.1) | 0.002 | 6.7 (2.1–21.9) | 0.002 |
| Age 58–74 (years), ACR ≤12 (mg/l) | |||||||
| 1 | Age > 66 (years) | 0.38 | 0.59/0.64 | 2.6 (1.6–4.2) | <0.001 | — | — |
| 2 | CRP > 0.7 (mg/l) | 0.13 | 0.27/0.89 | 3.0 (1.7–5.3) | <0.001 | 2.8 (1.6–5.0) | <0.001 |
| 3 | WHR > 0.95 | 0.50 | 0.64/0.52 | 1.9 (1.2–3) | 0.006 | 1.9 (1.2–2.9) | 0.008 |
| 4 | Male | 0.46 | 0.6/0.56 | 1.9 (1.2–3.0) | 0.006 | 1.9 (1.2–3) | 0.007 |
| 5 | CVD | 0.11 | 0.25/0.91 | 3.1 (1.8–5.5) | <0.001 | 3.1 (1.8–5.4) | <0.001 |
| 6 | Smoking | 0.17 | 0.29/0.85 | 2.2 (1.1–4.4) | 0.03 | 2.3 (1.2–4.6) | 0.01 |
| 7 | eGFR ≤63 (ml/min per 1.73 m2) | 0.10 | 0.2/0.91 | 2.6 (1.3–5.3) | 0.009 | 2.3 (1.1–4.7) | 0.03 |
| Age 58–74 (years), ACR >12 (mg/l) | |||||||
| 1 | CVD | 0.16 | 0.32/0.91 | 4.8 (3.0–7.8) | <0.001 | 4.9 (3–7.9) | <0.001 |
| 2 | Male | 0.43 | 0.55/0.62 | 2.0 (1.3–3.1) | 0.002 | 2.0 (1.3–3.1) | 0.002 |
| 3 | Age > 65 (years) | 0.57 | 0.69/0.48 | 2.0 (1.2–3.4) | 0.007 | — | — |
| 4 | WHR > 0.99 | 0.36 | 0.46/0.69 | 1.8 (1.0–3.3) | 0.04 | 1.8 (1.0–3.3) | 0.04 |
| 5 | Creatinine > 0.97 | 0.24 | 0.32/0.81 | 2.1 (1.2–3.3) | 0.006 | 1.9 (1.2–3.2) | 0.01 |
| 6 | HDL > 40 (mg/dl) | 0.26 | 0.35/0.78 | 1.9 (1.2–3.1) | 0.01 | 1.9 (1.2–3.2) | 0.01 |
| Age 75–80 (years) | |||||||
| 1 | Male | 0.42 | 0.58/0.71 | 3.3 (2–5.4) | <0.001 | 3.3 (2–5.5) | <0.001 |
| 2 | WHR > 0.97 | 0.38 | 0.49/0.71 | 2.3 (1.2–4.5) | 0.02 | 2.3 (1.2–4.6) | 0.02 |
| 3 | Creatinine > 0.88 | 0.48 | 0.58/0.6 | 2.1 (1.2–3.6) | 0.009 | 2.1 (1.2–3.6) | 0.01 |
| 4 | ACR > 33 (mg/l) | 0.18 | 0.27/0.89 | 2.9 (1.3–6.7) | 0.01 | 3.0 (1.4–6.8) | 0.007 |
| 5 | CRP > 0.6 (mg/l) | 0.18 | 0.27/0.89 | 2.8 (1.4–5.4) | 0.003 | 2.8 (1.5–5.3) | 0.002 |
| 6 | HDL > 65 (mg/dl) | 0.83 | 0.9/0.23 | 2.7 (1.1–6.3) | 0.03 | 2.7 (1.1–6.3) | 0.027 |
| 7 | eGFR ≤50 (ml/min per 1.73 m2) | 0.10 | 0.17/0.95 | 4.4 (1.7–11.3) | 0.002 | 4.4 (1.7–11.1) | 0.002 |
| 8 | Smoking | 0.10 | 0.16/0.95 | 3.9 (1.4–11.2) | 0.1 | 4.0 (1.4–11.3) | 0.009 |
| 9 | Diabetes | 0.14 | 0.2/0.9 | 2.2 (1.0–4.8) | 0.05 | 2.2 (1.0–4.8) | 0.05 |
| Age >80 (years) | |||||||
| 1 | eGFR ≤60 (ml/min per 1.73 m2) | 0.46 | 0.53/0.7 | 2.5 (1.1–6.1) | 0.04 | 2.4 (1–5.7) | 0.05 |
| 2 | Age > 82 (years) | 0.63 | 0.69/0.49 | 2.1 (1.4–3.2) | <0.001 | — | — |
| 3 | Creatinine >0.88 | 0.54 | 0.6/0.58 | 2.1 (1.0–4.1) | 0.04 | 2.0 (1.0–3.9) | 0.05 |
| 4 | ACR > 32 (mg/l) | 0.29 | 0.34/0.83 | 2.5 (1.7–3.7) | <0.001 | 2.2 (1.5–3.3) | <0.001 |
| 5 | CVD | 0.25 | 0.29/0.86 | 2.5 (1.1–5.3) | 0.02 | 2.5 (1.2–5.3) | 0.02 |
| 6 | WHR > 0.91 | 0.67 | 0.72/0.41 | 1.8 (1.0–3.1) | 0.04 | 1.8 (1.1–3.1) | 0.03 |
| 7 | HDL > 54 (mg/dl) | 0.58 | 0.62/0.5 | 1.7 (1.1–2.6) | 0.02 | 1.6 (1.1–2.5) | 0.02 |
| 8 | Systolic BP > 147 (mmHg) | 0.46 | 0.5/0.62 | 1.6 (1.1–2.3) | 0.02 | 1.5 (1.0–2.3) | 0.06 |
| 9 | Glucose > 130 (mg/dl) | 0.08 | 0.1/0.97 | 3.6 (1.3–10.3) | 0.02 | 3.8 (1.4–10.3) | 0.009 |
| 10 | CRP > 2.0 (mg/l) | 0.04 | 0.06/0.99 | 11.6 (1.9–69.5) | 0.007 | 13.1 (2.2–77.6) | 0.005 |
| 11 | Hispanic | 0.01 | 0.01/0.98 | 0.3 (0.1–0.8) | 0.02 | 0.5 (0.2–1.5) | 0.2 |
Abbreviations: ACR, urinary albumin creatinine ratio; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; OR, odds ratio; WHR, waist hip ratio.
National Health and Nutrition Examination Survey-recommended analytical procedures were followed and the sampling weights implicit in this complex sample survey design were used; WTPFSD6, SDPPSU6, and SDPSTRA6 as weight, cluster, and stratum variables, respectively.
True positive is defined by death when the risk factor is present; true negative is defined by survival when the risk factor is absent. Logistic regression was used to calculated ORs (with 95% confidence intervals in parentheses) for death; reference groups were those without each risk factor.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Kidney disease outcome quality initiative. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 2.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 4.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circul. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 5.Swets JA. The Relative operating characteristic in psychology: a technique for isolating effects of response bias finds wide use in the study of perception and cognition. Science. 1973;182:990–1000. doi: 10.1126/science.182.4116.990. [DOI] [PubMed] [Google Scholar]
- 6.Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soveri I, Arnlov J, Berglund L, et al. Kidney function and discrimination of cardiovascular risk in middle-aged men. J Intern Med. 2009;266:406–413. doi: 10.1111/j.1365-2796.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 8.Hallan SI, Ritz E, Lydersen S, et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gansevoort RT, De Jong PE. Challenges for the present CKD classification system. Curr Opin Nephrol Hypertens. 2010;19:308–314. doi: 10.1097/MNH.0b013e328337bbbe. [DOI] [PubMed] [Google Scholar]
- 10.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 11.Rule AD, Bailey KR, Schwartz GL, et al. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009;75:1071–1078. doi: 10.1038/ki.2008.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe E, Halpin D, Stevens P. Early identification and management of chronic kidney disease: summary of NICE guidance. BMJ. 2008;337:a1530. doi: 10.1136/bmj.a1530. [DOI] [PubMed] [Google Scholar]
- 13.Taal MW, Brenner BM. Renal risk scores: progress and prospects. Kidney Int. 2008;73:1216–1219. doi: 10.1038/ki.2008.36. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. [Accessed 12 May 2010];National Health and Nutrition Examination Survey. Available at http://www.cdc.gov/nchs/nhanes.htm.
- 15.National Center for Health Statistics and Centers for Disease Control and Prevention. [Accessed 12 May 2010];Analytic and Reporting Guidelines: the Third National Health and Nutrition Examination Survey, NHANES III (1988–1994) Available at http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf.
- 16.National Health and Nutrition Examination Survey (NHANES) [Accessed 12 May 2010];NHANES Analytic Guidelines, June 2004 Version. Available at http://www.cdc.gov/nchs/data/nhanes/nhanes_general_guidelines_june_04.pdf.
- 17.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 19.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 20.Snyder JJ, Foley RN, Collins AJ. Prevalence of CKD in the United States: a sensitivity analysis using the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2009;53:218–228. doi: 10.1053/j.ajkd.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention and National Health and Nutrition Examination Survey (NHANES) [Accessed 12 May 2010];Comparative Analysis of the NHANES III Public-Use and Restricted-Use Linked Mortality Files. Available at http://www.cdc.gov/nchs/data/datalinkage/nh3_mort_compare_2007_final.pdf.
- 26.National Health and Nutrition Examination Survey (NHANES) [Accessed 12 May 2010];Analytic and Reporting Guidelines. Available at http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf.