Abstract
Purpose
Strict adherence to HIV medications is critical to ensure long-term disease control, and adherence interventions that are possible in a clinic setting with limited resources are needed.
Data sources
This randomized controlled pilot study tested an adherence intervention guided by the Information-Motivation-Behavioral Skills (IMB) model. The intervention included HIV education, a peer video, motivational interviewing, and attention to behavioral skills including communication with providers and adherence-enhancing devices. Dependent variables included 3–4 week adherence recall, medication refill rate, changes in IMB subscale scores, appointment attendance, and HIV-associated laboratory findings. Seventy-three individuals starting or restarting antiretroviral therapy were enrolled and 56 were randomized.
Conclusions
Improvements were seen in most outcomes, with small to moderate effect sizes, but the study was not powered to show statistical significance. Threats to power included a 51% attrition rate, resulting mostly from loss to clinical care or prolonged gaps in care.
Implications for practice
A telephone-based intervention to improve HIV medication adherence shows promise. Further study is needed with greater attention to retention in care.
Keywords: Medication adherence, HIV, intervention, Information-Motivation-Behavioral Skills Model
In the current age of fully suppressive antiretroviral therapy (ART) for human immunodeficiency virus (HIV) disease, effective chronic management of the disease is theoretically possible. Despite this fact, actual clinical practice shows that only approximately 50% of those on ART reach this benefit. This has often been found because of incomplete adherence to ART. Poor adherence diminishes suppression of viral replication and allows the HIV virus to become resistant to the medicines. Resistant mutations naturally occur in the replication process and replicate freely in the presence of medications that apply selective pressure to susceptible virus (Bangsberg, 2008; Harman, Amico, & Johnson, 2005; Haynes, Yao, Degani, Kripalani, Garg, & McDonald, 2006).
Background and significance
Adherence in HIV disease
Adherence to medications is a complex issue that involves multiple factors. Examples of these factors include
Patient-related factors: literacy, substance abuse, health beliefs, depression.
Regimen-related factors: side effects, treatment complexity, number of pills.
Environment-related factors: beliefs of significant others, transportation, finances.
Provider-related factors: provider communication, the patient’s ability to ask questions, clinic hours, accessibility (Ickovics & Meade, 2002).
In addition to these factors related to medication adherence in general are particular issues related to adherence to HIV medications, such as perceived stigma, not wanting others to see them take medicines, nondisclosure of HIV, and difficulty in accepting this diagnosis (Konkle-Parker, Erlen, & Dubbert, 2008).
Because of the complex nature of adherence, research supports the need for multifaceted approaches that employ behavioral theory to maximize efficacy (Haynes, Yao, Degani, Kripalani, Garg, & McDonald, 2006). Motivational interviewing (MI) has emerged as an effective approach in multiple behavior change interventions, and can be combined with other modalities to provide the multifaceted approach needed (Bisono, Manuel, & Forcehimes, 2006; Cooperman & Arnsten, 2005; Lundahl, Kunz, Brownell, Tollefson, & Burke, 2010; Rubak, Sandbaek, Lauritzen, & Christensen, 2005).
Information–motivation–behavioral skills model
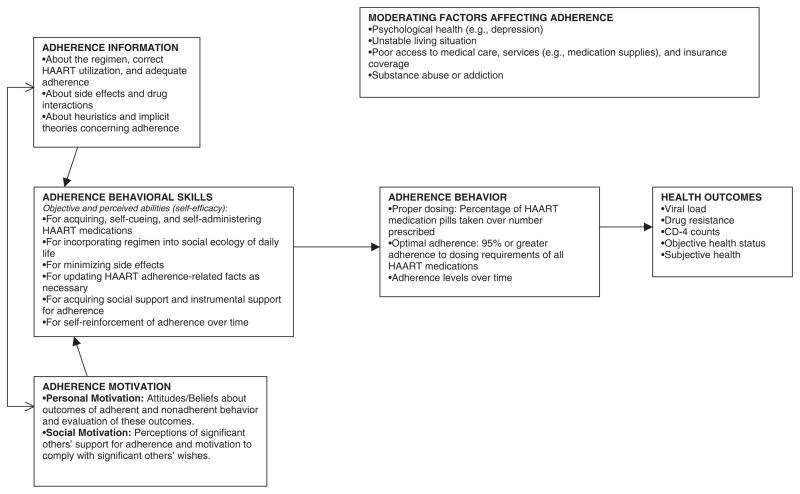
The information–motivation–behavioral skills model (IMB) has been proposed as a model applicable to a number of health promotion behaviors including adherence behavior in the setting of HIV disease (Anderson, Wagstaff, Heckman, Winett, Roffman, Solomon, 2006; Fisher, Amico, Fisher, & Harman, 2008; Fisher, Fisher, Amico, & Harman, 2006; Fisher, Fisher, & Harman, 2003; Kalichman, Picciano, & Roffman, 2008). The IMB model of ART adherence proposes that adherence-related information, motivation, and behavioral skills directly affect adherence behavior (see Figure 1; Amico, Barta, Konkle-Parker, Fisher, Cornman, Shuper, 2009; Amico, Toro-Alfonso, & Fisher, 2005; Fisher, Amico, Fisher, & Harman, 2008; Fisher, Fisher, Amico, & Harman, 2006; Starace, Massa, Amico, & Fisher, 2006). Specifically the model suggests that adherence-related information about HIV and ART, as well as personal and social motivation regarding adherence, affect the performance of adherence-related behavioral skills, which in turn affects adherence behavior. A change in adherence behavior subsequently affects health outcomes by affecting control of HIV disease and the development of resistance to ART.
Figure 1.
Information–motivation–behavioral skills model.
Note. Adapted from Fisher et al. (2006). Reprinted with permission.
Purpose
The purposes of this pilot study were to establish the feasibility of an intervention based on the IMB model in this population and begin to evaluate its effectiveness for improving medication adherence. Adherence was defined as the percentage of prescribed medications taken in the last 3–4 weeks, based on the rate of obtaining refills for the medications. We dichotomized the outcome variable as optimal adherence (at least 90% adherent) or suboptimal adherence (less than 90% adherent). Secondary outcomes included attendance at clinic appointments, HIV-associated laboratory findings, specifically CD4 counts and HIV viral load, and change in information, motivation, and behavioral skills factors related to adherence. A process evaluation of this trial is described in another paper, where the intervention is described in great detail (Konkle-Parker, Erlen, & Dubbert, 2010).
Methods
Participants and procedure
Participants were recruited from a large public HIV clinic in the southern United States that serves as a referral center for the state. Eligibility was determined by medication-start status: participants whose clinic provider reported that the patient was starting ART for the first time or was restarting ART after at least 6 months off medications were considered for inclusion. Participants were excluded if they were (1) non-English speaking, (2) dependent on others for medication administration, (3) had no access to a telephone, or (4) demonstrated impaired cognitive status indicated by the HIV Dementia Scale (Power, Selnes, Grim, & McArthur, 1995). The study was approved by the Institutional Review Board and all participants consented to participate. Data were collected on an audio-supported computer-assisted self-interview (ACASI) in conjunction with the three clinic visits after starting ART (V-1, V-2, and V-3).
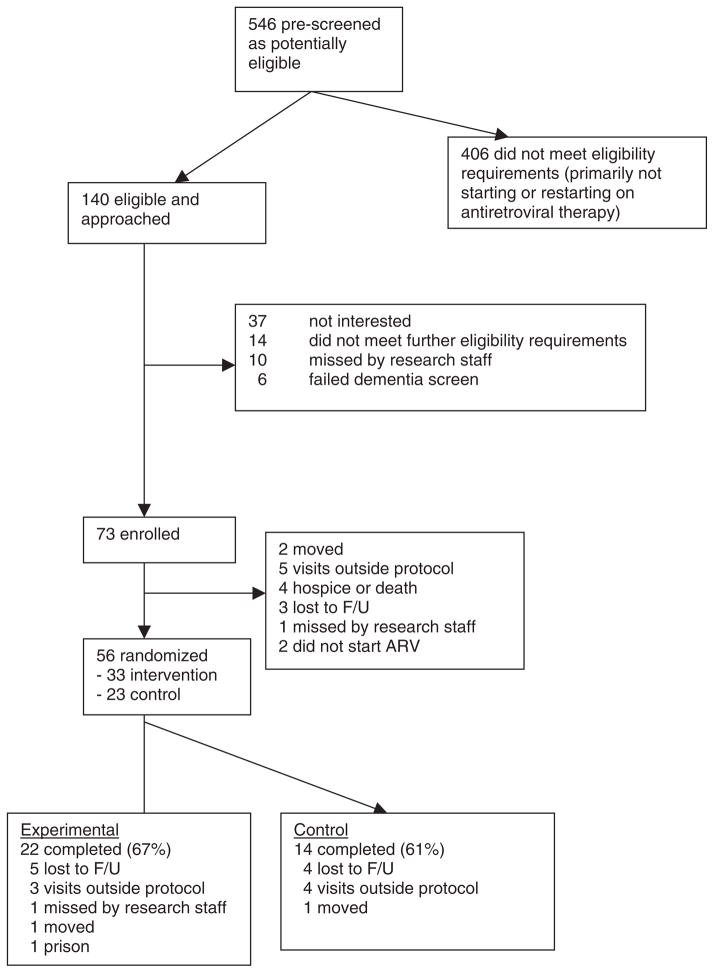
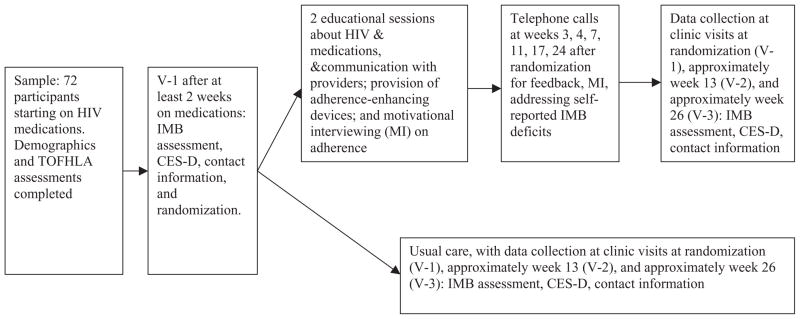
Figure 2 describes the recruitment flow. Enrollment occurred when starting medications, and randomization occurred at the next clinic visit (V-1) at least 2 weeks after enrollment, when baseline ART adherence could be assessed. After V-1 data collection, participants were assigned to one of two groups according to a pre-determined randomization schedule. The two groups were usual care or usual care plus the experimental intervention. The randomization schedule took into account gender and ART medication status (i.e., starting ART for the first time or restarting ART). See Figure 3 for a graphic illustration of data collection and intervention events of the trial.
Figure 2.
Recruitment and retention patterns of study participants (November 7, 2005, to October 3, 2006). Note. Adapted from Konkle-Parker et al. (2010, p. 93).
Figure 3.
Illustration of flow of clinical trial.
Note. TOFHLA, test of functional health literacy assessment; IMB, information–motivation–behavioral skills; CES-D, Center of Epidemiologic Studies-Depression.
Intervention
The intervention was conducted by the principal investigator (PI), a nurse practitioner with over a decade of experience treating persons with HIV. She was trained in MI and received ongoing field monitoring throughout the study on a random selection of 10% of the face-to-face intervention audiotapes for quality control from an MI expert. This expert coded the tapes using the Motivational Interviewing Treatment Integrity 2.0 Scale (Moyers, Martin, Manuel, & Miller, 2005).
The intervention consisted of (1) two one-on-one sessions with the PI at weeks 1 and 2 after randomization; and (2) six telephone calls on a tapering schedule (approximately weeks 3, 4, 6, 10, 16, 24). The one-on-one sessions included all of the following: (a) HIV education (Knobel, Escobar, Polo, Ortega, Martin-Conde, Casado, et al., 2005); (b) viewing of a video of peers describing how they overcame adherence obstacles (Fairley, Levy, Rayner, Allardice, Costello, Thomas, et al., 2003); (c) MI regarding adherence (Adamian, Golin, Shain, & DeVellis, 2004; Cooperman & Arnsten, 2005; Possidente, Bucci, & McClain, 2005; Rubak, Sandbaek, Lauritzen, & Christensen, 2005; Rutledge, Roffman, Mahoney, Picciano, Berghuis, & Kalichman, 2001; Thrasher, Golin, Earp, Tien, Porter, & Howie, 2006); (d) feedback about HIV-related laboratory studies and responses to an assessment of their IMB skills related to ART adherence administered at each data collection visit; (e) training on communication skills during a medical visit (Cegala, McClure, Marinelli, & Post, 2000); and (f) the receipt of two adherence-enhancing devices—a reminder watch and a pillbox (Fairley, Levy, Rayner, Allardice, Costello, Thomas, et al., 2003; Powell-Cope, White, Henkelman, & Turner, 2003). Telephone contacts included a continuation of MI, education, reminders of communication skills, and continued feedback about laboratory results and responses to IMB assessments (Collier, Ribaudo, Mukherjee, Feinberg, Fischl, Chesney, et al., 2005; Haynes, Yao, Degani, Kripalani, Garg, & McDonald, 2006). Table 1 displays the intervention components. One-on-one sessions required 30–60 min; telephone sessions ranged from 2 to 33 min, but averaged less than 10 min. Participants received $10 for their time and travel for the one-on-one sessions, but received no incentive for telephone intervention calls.
Table 1.
Aspects of the IMB intervention
| Element of intervention | 1 (face-to-face) | 2 (face-to-face) | 3 (phone) | 4 (phone) | 5 (phone) | 6 (phone) | 7 (phone) | 8 (phone) |
|---|---|---|---|---|---|---|---|---|
| Information | X | X | X | X | X | X | X | X |
| Social motivation—video | X | |||||||
| Personal motivation—motivational interviewing | X | X | X | X | X | X | X | X |
| Behavioral skills—adherence-enhancing devices | X | |||||||
| Behavioral skills—communication with provider | X | X | X |
Note. Adapted from Konkle-Parker et al. (2010, p. 92).
Assessment
Baseline assessment of demographics and screening for health literacy and dementia were completed. Data collection for the dependent variables (medication adherence, IMB) occurred using the ACASI at V-1 and at the next two clinic visits (V-2 and V-3) by a research assistant not blinded to group assignment; these visits were expected to be approximately 3 and 6 months after V-1. Laboratory studies were carried out at the same time as data collection as part of routine medical care. Each participant received $15 for data collection and $30 for feedback interviews after they completed the intervention.
The short test of functional health literacy in adults (STOFHLA)
The STOFHLA was used to characterize the participants’ ability to read and understand health-related information. This instrument is scored from 1 to 36, with higher scores indicating greater health literacy and scores above 22 indicating adequate health literacy (Kalichman, Cain, Fuhrel, Eaton, Di Fonzo, & Ertl, 2005; Nurss, Parker, Williams, & Baker, 2001).
The Center for Epidemiologic Studies-Depression (CES-D)
This measure has been used in multiple populations to assess depressive symptoms, including those with HIV disease (Devins & Orme, 1985), and has established reliability and validity (Radloff, 1977). Possible scores range from 0 to 60, with higher scores reflecting greater frequency of depressive symptoms. Examples of items include “I felt that I could not shake off the blues even with help from my family or friends” and “I felt that everything I did was an effort.” A score of 21 or higher indicates moderate to severe depressive symptoms (Devins & Orme, 1985). In a previous study in this clinic, population (n = 151) internal consistency reliability was high (α = 0.86; Konkle-Parker, Erlen, & Dubbert, 2007)
IMB assessment
This tool, now called the LifeWin-dows Information-Motivation-Behavioral Skills ART Adherence Questionnaire (LW-IMB-AAQ; Center for Health Intervention and Prevention, 2007), reflects the theoretical model informing this intervention by specifically measuring adequacy of information about adherence to HIV medications, the extent of personal and social motivation to adhere to HIV medicines, and the participant’s ability to use the behavioral skills deemed necessary to effectively adhere to these medications. The scale has 33 statements with a 5- or 6-point Likert scale measuring the level of agreement with the statement, or the ease by which certain behaviors are performed. Examples of statements include (1) I know what to do if I miss a dose of any of my HIV medications (e.g., whether or not to take the pill(s) later); (2) I do not like taking my HIV medications because they remind me that I am HIV-positive; and (3) How hard or easy is it for you to remember to take your HIV medication?
In this study, the LW-IMB-AAQ showed acceptable psychometric properties at baseline (n = 56), as seen by α = .68 for the Information Subscale; α = .68 for the Motivation Subscale; and α = .94 for the Behavioral Skills Subscale. The Information Subscale was significantly associated with Motivation (r = .41, p = .00), and the Information and Motivation Subscales were both associated with Behavioral Skills (Information r = .40, p = .00; Motivation r = .71, p = .00). By linear regression analysis, Motivation and Information predicted Behavioral Skills (F (2.51) = 27.78, p = .00). The behavioral skills score suggested an association with 3- to 4-week adherence recall (r = .22, p = .05). These are the hypothesized relationships according to the IMB model (Figure 1).
Medication adherence
Two measures of medication adherence were used: 3- to 4-week adherence recall by visual analog scale, and refill rate. Linear visual analog scale (VAS) measured recall of the percent of prescribed doses taken “in the previous 3–4 weeks,” ranging from 0 to 100 (Amico, Fisher, Cornman, Shuper, Redding, Konkle-Parker, et al., 2006). Refill rate was determined by contacting pharmacies reported by the participants in monthly contacts, starting at enrollment and ending at V-3 or when the participant dropped from the study. The number of refills was divided by the number of months from enrollment to end date, given that all prescriptions were written on a monthly basis.
Appointment attendance
The number of missed medical visits was obtained from the medical record during the period of enrollment to V-3, or until the time that the participant dropped from the study. The number of missed medical visits only included “no-show” visits and did not include visits that were rescheduled by the patient or canceled by the clinic.
HIV-associated laboratory studies
Laboratory findings of interest were CD4 cell count and HIV RNA viral load by polymerase chain reaction (PCR). These tests were ordered as part of routine medical care and obtained from the medical record by research staff. Viral load values below the level of detection (less than 400) were recorded as zero and values were transformed to log10 for reporting.
Statistical analysis
To determine the effectiveness of randomization, we compared the demographic characteristics based on the random group assignments by t-test or chi-square.
The primary outcomes were change in self-reported adherence by 3–4 weeks on the VAS and by refill rate. Secondary outcomes were number of missed visits, change in HIV viral load and CD4 count, and change in IMB scale scores, comparing for the effect of group assignment. Outcome analyses were conducted using SPSS 15.0 (SPSS Inc., Armonk, NY), using analysis of covariance for continuous variables (VAS adherence, IMB scores, CD4, and HIV viral load) at the last visit of record (at exit), as well as at V-3 for those who completed the study by attending all three data collection visits (completers); adjustments were made for baseline values. For dichotomous variables (>90% adherence, undetectable viral load), logistic regression was used to control for baseline and to compare the effects of group assignment (Munro, 2005).
Because of the variability of intervention dose exposure within group assignments, outcomes are also reported comparing those who received no intervention contacts (n = 28) with those who received at least one (n = 28). Through this method, those assigned to the treatment group who actually received no intervention could be compared with those assigned to that group who received intervention.
A test of adherence measures’ reliability was conducted by correlating both adherence measures (self-reported 3-to 4-week adherence and refill rate) with clinical outcomes (CD4 and HIV viral load) to compare the clinical utility of these measures. The correlations were carried out by Spearman’s rho because of the skewness evident in the clinical and adherence data using a .05 level of significance.
Results
Sample
From July 2005 to March 2006, a total of 73 participants were enrolled in the study. Because 17 participants did not return for V-1 (Figure 2), only 56 were randomized. Enrollment was expected for 75 participants for a power of 0.56 for this pilot study, but two enrolled participants were not ultimately eligible because their blood work indicated that they did not need to start ART. Study participants reflected the demographics of the clinic population: 38% female, primarily African American, low-income, and half with high school education or below (Table 2). All data collection visits were completed by January 1, 2007. Attrition was roughly equal at all stages, with 23% of those enrolled dropping out between enrollment and V-1 (17 out of 73), 20% of the remainder between V-1 and V-2 (11 out of 56), and 20% of the remainder between V-2 and v-3 (9 out of 45).
Table 2.
Sample characteristics
| Total | Intervention
|
Control
|
p | |||
|---|---|---|---|---|---|---|
| N = 56 N (%) or mean (SD) | N | Percent or mean (SD) | N | Percent or mean (SD) | ||
| Age | 34.6(8.4) | 33 | 34.9 (7.4) | 23 | 33.2 (8.6) | 0.63 |
| Gender (%) | ||||||
| Male | 35(62%) | 21 | 64% | 14 | 61% | 0.83 |
| Female | 21(38%) | 12 | 36% | 9 | 39% | |
| Race (%) | ||||||
| Black | 50(89%) | 27 | 82% | 23 | 100% | 0.03* |
| White | 6(11%) | 6 | 18% | 0 | 0% | |
| Income | ||||||
| <$10K | 36(64%) | 21 | 63% | 15 | 65% | 0.69 |
| >$10K | 20(34%) | 12 | 33% | 8 | 34% | |
| Education (%) | ||||||
| Less than HS | 16(29%) | 10 | 30% | 6 | 26% | 0.81 |
| HS grad or GED | 15(27%) | 8 | 24% | 7 | 30% | |
| More than HS | 25(44%) | 15 | 45% | 10 | 44% | |
| Naïve vs. experienced | ||||||
| Naïve to ART | 32(57%) | 18 | 55% | 14 | 61% | 0.64a |
| ART Experienced | 24(43%) | 15 | 45% | 9 | 39% | |
| Log baseline HIV viral load | 4.7(.70) | 33 | 4.7 (0.8) | 22 | 4.6 (0.6) | 0.55 |
| Baseline CD4 | 131.2(110.0) | 33 | 129.9 (113.5) | 23 | 137.5 (107.1) | 0.80 |
| Health literacy (STOFHLA) | 31.0(5.4) | 32 | 30.6 (5.6) | 23 | 31.5 (5.4) | 0.52 |
| Depressive symptoms (CES-D) | ||||||
| No or mild symptoms | 37(67.3%) | 33 | 21 (63.6%) | 22 | 16 (72.7%) | 0.68a |
| Moderate or severe | 18(32.7%) | 33 | 12 (36.4%) | 22 | 6 (27.3%) | |
| VAS 3–4 month adherence | .82(.28) | 33 | .78 (.33) | 23 | .88 (.18) | 0.21 |
Chi-square.
p < .05.
Visit-1 with randomization occurred at an average of 2.06 (SD = 1.15) months after enrollment (range: 0.2–5.59 months). At baseline, the control group and the intervention group were comparable in all demographic characteristics and baseline laboratory values except for race; all Caucasians (n = 6) were randomly assigned to the intervention group. Analysis of variance (ANOVA) showed there were no relationships between race and adherence at baseline or at V-3, or between race and study completion. Attrition was the same in both treatment and control groups.
Twenty-two of the 33 (67%) participants in the experimental and 14 of the 23 (61%) participants in the control groups completed the study by attending V-1, V-2, and V-3. There were no statistically significant differences in demographic characteristics or group assignment by completion status except for a trend for depressive symptoms, where those individuals with more depressive symptoms were less likely to complete the study (p = .06). Of those randomized to the intervention group, half (18; 55%) completed seven to eight of the eight planned intervention contacts, 30% (n = 10) completed one to six contacts, and five (15%) did not complete any intervention contacts.
Self-reported adherence outcomes
There were small nonstatistically significant improvements in most primary and secondary outcome measures in the intervention group compared to the control group, except for a significantly greater decrease in log viral load for the intervention group in those who completed the study (t = 2.416; n = 31, p < .022, data not shown). Effect sizes were small to moderate, both at exit and for those who completed the study.
Because of the small sample size of this pilot study and 15% of the intervention group actually received no intervention, sensitivity analyses were conducted, comparing those with no intervention contacts with those who received at least one (Table 3). There were nonstatistically significant improvements for most primary outcomes in those who received intervention contacts over those who did not, with small to moderate effect sizes, except for self-reported adherence at exit.
Table 3.
Outcomes at last visit by number of intervention contacts, adjusted for baseline
| Difference related to intervention contacts at exit
|
Difference related to intervention contacts for completers
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable, adjusted for baseline values | N | ≥1 intervention contact mean (SD) or n (%) | N | No intervention contact mean (SD) or n (%) | Effect size | N | ≥1 intervention contact mean (SD) or n (%) | N | No intervention contact mean (SD) or n (%) | Effect size |
| Primary outcomes | ||||||||||
| Mean change in adherence by VAS | 28 | 7.36(16.51) | 28 | 9.00(16.51) | d = −0.10 | 21 | 13.45 (9.99) | 15 | 11.69(9.99) | d = 0.22 |
| 90% adherent by VAS | 28 | 22(78%) | 28 | 22(78%) | OR = 1.0 | 21 | 18 (86%) | 15 | 13(87%) | OR = 1.08 |
| Refill/month | 28 | .85(.28) | 28 | .74(.32) | d = .37 | 21 | .93 (.23) | 15 | .92(.27) | d = .04 |
| Secondary outcomes | ||||||||||
| Mean change in information scale | 27 | 2.62(3.95) | 28 | 0.58(3.97) | d = 0.52 | 20 | 3.08 (4.61) | 15 | 0.43(4.57) | d = 0.58 |
| Mean change in motivation scale | 27 | 3.50(3.95) | 28 | 1.01(6.19) | d = 0.48 | 21 | 3.43 (6.78) | 14 | 2.22(7.05) | d = 0.18 |
| Mean change in behavioral skills scale | 28 | 2.62(4.02) | 28 | 0.58(3.97) | d = 0.51 | 21 | 5.22 (6.96) | 15 | 3.23(6.97) | d = 0.28 |
| Mean change in CD4 | 28 | 112(122) | 27 | 70(125) | d = 0.34 | 20 | 110 (139) | 15 | 100(139) | d = 0.07 |
| Mean log change HIV viral load | 28 | −3.69(1.75) | 27 | −3.55(1.77) | d = 0.08 | 20 | −3.86 (2.15) | 15 | −3.31(1.82) | d = 0.27 |
| Number of missed visits | 28 | .79(1.00) | 28 | 1.11(1.00) | d = 0.32 | 21 | .33 (.48) | 15 | .40(.51) | d = 0.14 |
| Undetectable viral load (<400) | 28 | 21(75%) | 28 | 22(78%) | OR = .82 | 20 | 16 (80%) | 15 | 11(73%) | OR = 1.45 |
Note. Mean change scores are adjusted for baseline values.
OR, odds ratio.
All individuals who participated in the study, regardless of group assignment or intervention contacts, improved in all outcome variables. These differences did attain statistical significance.
Secondary outcomes
Secondary outcomes were the number of missed medical visits, change in CD4 and log HIV RNA viral load, the proportion of those with undetectable viral loads, and change in the IMB skills subscales. Effect sizes were small to moderate in all secondary outcomes, with no statistically significant differences discernable.
When comparing those who received at least one intervention contact with those who received none, differences in all secondary outcome variables were not statistically significant, but were favorable to those who received the intervention, with small to moderate effect sizes (Table 3). The only exception to this was the proportion of those with an undetectable viral load, which showed a slightly greater percent of those in the control group who had undetectable viral loads (viral load < 400). In addition, there was a significantly greater decrease in depressive symptoms in those who received intervention contacts (F = 5.65, p = .008) as compared to those who did not.
Reliability of assessments
The validity of adherence measures was determined by assessing the correlation with HIV-associated laboratory values. Self-report 3- to 4-week adherence showed a moderate nonsignificant correlation with change in CD4 (rs = .31, p = .07), and the correlation with log HIV viral load was minimal (rs = −.12, p = .48). Of the adherence variables, refill rate demonstrated a moderate significant correlation with all laboratory values. In particular, refill rate at V-3 had statistically significant correlations with change in CD4 (rs = .39, p = .02), change in viral load (rs = −.34, p = .04), and undetectable viral load (rs = .55, p = .00) for those who completed the study.
Discussion
We evaluated the feasibility and effectiveness of a clinic-based MI adherence intervention using the IMB model for improving medication adherence and related variables. A clinic-based intervention using few additional resources was tested specifically because an intervention that is cost-effective and implementable in poorly resourced clinic settings could improve outcomes for many currently underserved patients.
The fact that there were small to moderate effect sizes in favor of the intervention in most of the selected outcome measures indicates that this intervention may be promising (see Table 3). This small feasibility pilot study was not expected to show significance because of low power.
In published literature, average adherence is generally 70%–75% for individuals on ART, so a baseline adherence rate of 82% was unexpectedly high. Possible explanations include the fact that all eligible participants were newly started on ART, thus in a “honeymoon period” of greater adherence. An additional explanation is that those who were not prepared or committed to take ART in an adherent fashion dropped out of the study, based on the finding that 23% of those who enrolled, all newly starting or restarting ART, did not return for clinical care. In any case, this high level of baseline adherence makes it more difficult to show differential improvement in the experimental group. An additional finding was that all participants in this study significantly improved in most outcome variables, regardless of group assignment, caused perhaps by exposure to the study and greater attention to adherence through the data collection instruments.
Thirty-eight percent of those who dropped out of the study dropped out of care completely, and another 38% experienced a gap in care of greater than 4–6 months, which put them outside the research protocol. This diminished the power of the study, but also underscored the fact that retaining patients in care is a significant challenge in this underserved clinic population. This study was designed to explore an intervention that could reasonably be performed in a clinic setting with limited resources. The only retention strategy that was used was to call each participant once a month to update contact information, and give them a reminder call 1 week before their clinic appointment, which is when they completed the data collection instruments.
The percentage of participants who had extended gaps in care is consistent with overall clinic statistics. Multiple studies on this “no show” phenomenon are currently being conducted. The factors related to this phenomenon appear to be a combination of structural, personal, and contextual (Konkle-Parker, Amico, & Henderson, 2011; Mugavero, 2008; Mugavero, Lin, Allison, Willig, Chang, Marler, et al., 2007; Williams, Amico, & Konkle-Parker, 2010). In fact, these factors may be as complex as those that influence adherence to medications, and therefore warrant additional study. The complexity of adherence and the importance of the factors related to the patient, medications, environment, and patient-provider relationship make adherence a phenomenon that is challenging to address (Ickovics & Meade, 2002; Konkle-Parker, Erlen, & Dubbert, 2008). For this reason, an intervention that primarily focuses on patient-related factors may be inadequate, and may need to be extended to address other factors as well, including adherence to care.
Limitations of this study include the small sample and the high attrition. In addition, this study was conducted in only one public clinic in the southeastern United States, and cannot be generalized to other settings. Despite these limitations, further research in a larger population may better determine the effect of this clinic-based intervention based on the IMB model. Making modifications to the intervention may improve retention of participants in clinical care and increase the value of the intervention.
In future research, barriers to staying in care, especially immediately after starting or restarting ART, need to be assessed and interventions included that are designed to remove these barriers. This may involve information about why adherence to care is important, MI specifically related to adherence to care, and appointment reminder devices. Individuals who did not complete the study were possibly different from completers in relation to depressive symptoms (p = .06). This finding needs further exploration; it is particularly interesting in light of the well-established association between depression and medication adherence (Reynolds, Testa, Marc, Chesney, Neidig, Smith, 2004).
In addition to the IMB variables influencing adherence, a recent addition to the model is the recognition that contextual factors may moderate IMB variables to influence adherence (Fisher, Amico, Fisher, & Harman, 2006). Severe contextual difficulties may act as moderators to make the IMB variables extraneous (see Figure 1) as they may suppress the influence of knowledge, motivation, and ability to act. Examples of these factors are mental illness, substance abuse, homelessness, and poor access to medications. Further research needs to include a focus on these contextual factors and the intervention needs to be broadened to address these factors in order to improve adherence and decrease attrition. Attrition may both decrease study power and HIV treatment efficacy as continuation in clinical care is essential to maintain medication supply and management.
Acknowledgments
This study was supported by the National Institute of Nursing Research, through grant K23NR09186.
References
- Adamian MS, Golin CE, Shain LS, DeVellis B. Brief motivational interviewing to improve adherence to antiretroviral therapy: Development and qualitative pilot assessment of an intervention. AIDS Patient Care and STDs. 2004;18(4):229–238. doi: 10.1089/108729104323038900. [DOI] [PubMed] [Google Scholar]
- Amico KR, Barta W, Konkle-Parker DJ, Fisher JD, Cornman DH, Shuper PA, Fisher WA. The Information-Motivation-Behavioral Skills Model of ART adherence in a Deep South HIV+ clinic sample. AIDS & Behavior. 2009;13:66–75. doi: 10.1007/s10461-007-9311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico KR, Fisher WA, Cornman DH, Shuper PA, Redding CG, Konkle-Parker DJ, Fisher WA. Visual analog scale of ART adherence: Association with 3-day self-report and adherence barriers. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2006;42(4):455–459. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the information, motivation and behavioral skills model of antiretroviral therapy adherence. AIDS Care. 2005;17(6):661–673. doi: 10.1080/09540120500038058. [DOI] [PubMed] [Google Scholar]
- Anderson ES, Wagstaff DA, Heckman TG, Winett RA, Roffman RA, Solomon LJ, Sikkema KJ. Information-Motivation-Behavioral Skills (IMB) Model: Testing direct and mediated treatment effects on condom use among women in low-income housing. Annals of Behavioral Medicine. 2006;31(1):70–79. doi: 10.1207/s15324796abm3101_11. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. Journal of Infectious Diseases. 2008;197(Suppl 3):S272–S278. doi: 10.1086/533415. [DOI] [PubMed] [Google Scholar]
- Bisono AM, Manuel JK, Forcehimes AA. Promoting treatment adherence: A practical handbook for health care providers. Thousand Oaks, CA: Sage Publications, Inc; 2006. Promoting treatment adherence through motivational interviewing; pp. 71–84. [Google Scholar]
- Cegala DJ, McClure L, Marinelli TM, Post DM. The effects of communication skills training on patients’ participation during medical interviews. Patient Education & Counseling. 2000;41(2):209–222. doi: 10.1016/s0738-3991(00)00093-8. [DOI] [PubMed] [Google Scholar]
- Center for Health Intervention and Prevention. The LifeWindows Information-Motivation-Behavioral Skills ART Adherence Questionnaire (LW-IMB-AAQ) 2007 Retrieved from http://www.chip.uconn.edu/chipweb/documents/Research/FLWIMBARTQuestionnaire.pdf.
- Collier AC, Ribaudo H, Mukherjee AL, Feinberg J, Fischl MA, Chesney M Adult AIDS Clinical Trials Group 746 Substudy Team. A randomized study of serial telephone call support to increase adherence and thereby improve virologic outcome in persons initiating antiretroviral therapy. Journal of Infectious Diseases. 2005;192(8):1398–1406. doi: 10.1086/466526. [DOI] [PubMed] [Google Scholar]
- Cooperman NA, Arnsten JH. Motivational interviewing for improving adherence to antiretroviral medications. Current HIV/AIDS Reports. 2005;2(4):159–164. doi: 10.1007/s11904-005-0010-x. [DOI] [PubMed] [Google Scholar]
- Devins GM, Orme CM. Center for Epidemiologic Studies Depression Scale. In: Keyser DJ, Sweetland RC, editors. Test critiques. Vol. 2. Kansas City, MO: Test Corporation of America; 1985. pp. 144–159. [Google Scholar]
- Fairley CK, Levy R, Rayner CR, Allardice K, Costello K, Thomas C Melbourne Adherence Group. Randomized trial of an adherence programme for clients with HIV. International Journal of STD & AIDS. 2003;14(12):805–809. doi: 10.1258/095646203322556129. [DOI] [PubMed] [Google Scholar]
- Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Current HIV/AIDS Reports. 2008;5(4):193–203. doi: 10.1007/s11904-008-0028-y. [DOI] [PubMed] [Google Scholar]
- Fisher JD, Fisher W, Amico K, Harman J. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology. 2006;25(4):462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- Fisher W, Fisher JD, Harman J. The information-motivation-behavioral skills model: A general social psychological approach to understanding and promoting health behavior. In: Suls J, Wallston KA, editors. Social psychological foundations of health and illness. Malden, MA: Blackwell Publishing; 2003. pp. 82–106. [Google Scholar]
- Harman JJ, Amico KR, Johnson BT. Standard of care: Promoting antiretroviral adherence in clinical care. AIDS Care. 2005;17(2):237–251. doi: 10.1080/09540120512331325707. [DOI] [PubMed] [Google Scholar]
- Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews. 2005;4:CD000011. doi: 10.1002/14651858.CD000011.pub2. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Meade CS. Adherence to HAART among patients with HIV: Breakthroughs and barriers. AIDS Care. 2002;14(3):309–318. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Cain D, Fuhrel A, Eaton L, Di Fonzo K, Ertl T. Assessing medication adherence self-efficacy among low-literacy patients: Development of a pictographic visual analogue scale. Health Education Research. 2005;20(1):24–35. doi: 10.1093/her/cyg106. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Picciano JF, Roffman RA. Motivation to reduce HIV risk behaviors in the context of the information, motivation and behavioral skills (IMB) model of HIV prevention. Journal of Health Psychology. 2008;13(5):680–689. doi: 10.1177/1359105307082456. [DOI] [PubMed] [Google Scholar]
- Knobel H, Escobar I, Polo R, Ortega L, Martin-Conde MT, Casado JL, Chamorro L. Recommendations from GESIDA/SEFH/PNS to improve adherence to antiviral treatment (2004) Enfermedades Infecciosas y Microbiologia Clinica. 2005;23(4):221–231. doi: 10.1157/13073149. [DOI] [PubMed] [Google Scholar]
- Konkle-Parker D, Amico K, Henderson HM. Barriers and facilitators for initiation and continuity of HIV clinical care in a Southern minority population. Journal of the Association of Nurses in AIDS care. 2011;22(2):90–96. doi: 10.1016/j.jana.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle-Parker D, Erlen JA, Dubbert PM. Deficits in information, motivation and behavioral skills associated with HIV medication adherence identified in a southern population. Paper presented at the Southern Nursing Research Society Annual Conference; Galveston, TX.. 2007. [Google Scholar]
- Konkle-Parker D, Erlen JA, Dubbert PM. Barriers and facilitators to medication adherence in a southern minority population with HIV disease. Journal of the Association of Nurses in AIDS Care. 2008;19(2):98–104. doi: 10.1016/j.jana.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle-Parker D, Erlen JA, Dubbert PM. Lessons learned from an HIV adherence pilot study in the Deep South. Patient Education and Counseling. 2010;78(1):91–96. doi: 10.1016/j.pec.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of motivational interviewing: Twenty-Five years of empirical studies. Research on Social Work Practice. 2010;20(2):137–160. [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Miller WR. The Motivational Interviewing Treatment Integrity (MITI) Code: Version 2.0. Albuquerque, NM: University of New Mexico, Center on Alcoholism, Substance Abuse and Addictions (CASAA); 2005. [Google Scholar]
- Mugavero MJ. Improving engagement in HIV care: What can we do? Topics in HIV Medicine. 2008;16(5):156–161. [PubMed] [Google Scholar]
- Mugavero MJ, Lin HY, Allison JJ, Willig JH, Chang PW, Marler M, Saag MS. Failure to establish HIV care: Characterizing the “no show” phenomenon. Clinical Infectious Diseases. 2007;45(1):127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- Munro BH. Statistical methods for health care research. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- Nurss JR, Parker RM, Williams MV, Baker DW. TOFHLA: Test of functional health literacy in adults. 2. Snow Camp, NC: Peppercorn Books & Press; 2001. [Google Scholar]
- Possidente CJ, Bucci KK, McClain WJ. Motivational interviewing: A tool to improve medication adherence? American Journal of Health-System Pharmacy. 2005;62(12):1311–1314. doi: 10.1093/ajhp/62.12.1311. [DOI] [PubMed] [Google Scholar]
- Powell-Cope GM, White J, Henkelman EJ, Turner BJ. Qualitative and quantitative assessments of HAART adherence of substance-abusing women. AIDS Care. 2003;15(2):239–249. doi: 10.1080/0954012031000068380. [DOI] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: A rapid screening test. Journal of Acquired Immune Deficiency Syndromes & Human Retrovirology. 1995;8(3):273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Reynolds NR, Testa MA, Marc LG, Chesney MA, Neidig JL, Smith SR, Robbins GK. Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: A multicenter, cross-sectional study. AIDS and Behavior. 2004;8(2):141–150. doi: 10.1023/B:AIBE.0000030245.52406.bb. [DOI] [PubMed] [Google Scholar]
- Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: A systematic review and meta-analysis. British Journal of General Practice. 2005;55(513):305–312. [PMC free article] [PubMed] [Google Scholar]
- Rutledge SE, Roffman RA, Mahoney C, Picciano JF, Berghuis JP, Kalichman SC. Motivational enhancement counseling strategies in delivering a telephone-based brief HIV prevention intervention. Clinical Social Work Journal. 2001;29(3):291–306. [Google Scholar]
- SPSS Inc. Statistical package for social sciences (version 15.0) Chicago, IL: 2006. [Google Scholar]
- Starace F, Massa A, Amico KR, Fisher JD. Adherence to antiretroviral therapy: An empirical test of the information-motivation-behavioral skills model. Health Psychology. 2006;25(2):153–162. doi: 10.1037/0278-6133.25.2.153. [DOI] [PubMed] [Google Scholar]
- Thrasher AD, Golin CE, Earp JAL, Tien H, Porter C, Howie L. Motivational interviewing to support antiretroviral therapy adherence: The role of quality counseling. Patient Education & Counseling. 2006;62(1):64–71. doi: 10.1016/j.pec.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Williams B, Amico KR, Konkle-Parker DJ. Barriers to HIV care: Patients’ perspectives. Journal of the Association of Nurses in AIDS Care. 2010;22(4):307–312. doi: 10.1016/j.jana.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]