Abstract
Purpose
Intraperitoneal (IP) chemotherapy prolongs survival in optimally reduced ovarian cancer patients. For patients in whom optimal debulking cannot be achieved, one could incorporate IP therapy post-operatively if the cancer was optimally debulked following neoadjuvant chemotherapy. We sought to evaluate overall survival (OS), progression-free survival (PFS), percent of patients optimally debulked and toxicity in patients treated with this strategy.
Methods
Women with adenocarcinoma by biopsy or cytology with stage III/IV (pleural effusions only) epithelial ovarian, fallopian tube or primary peritoneal carcinoma that presented with bulky disease were treated with neoadjuvant intravenous (IV) paclitaxel 175 mg/m2 and carboplatin AUC 6 q 21 days x 3 cycles followed by surgery (if ≥ 50% decrease in CA125). If optimally debulked they received IV paclitaxel 175 mg/m2 and IP carboplatin AUC 5 (day 1) and IP paclitaxel 60 mg/m2 (day 8) q 28 days x 6 cycles.
Results
Sixty-two patients were registered. Four were ineligible. Fifty-six were evaluated for neoadjuvant chemotherapy toxicities. One patient died of pneumonia. Five patients had grade 4 toxicity, including neutropenia (3), anemia, leukopenia, anorexia, fatigue, muscle weakness, respiratory infection, and cardiac ischemia. Thirty-six patients had debulking surgery. Two had grade 4 hemorrhage. Twenty-six patients received post-cytoreduction chemotherapy. Four had grade 4 neutropenia. At a median follow-up of 21 months, median PFS is 21 months and median OS is 32 months for all 58 patients. PFS and OS for the 26 patients who received IV/IP chemotherapy is 29 and 34 months respectively.
Conclusions
These results compare favorably with other studies of sub-optimally debulked patients.
Introduction
Standard therapy of advanced ovarian cancer involves initial surgical management to perform staging and maximal cytoreduction whenever possible. The importance of volume of residual disease as it relates to prognosis has been defined by Gynecologic Oncology Group (GOG) studies. (1,2)
Debate exists as to whether the observed survival benefits for cytoreduced patients are a function of tumor biology or surgical effort. Heintz et al. observed that cytoreduction was easier to achieve in young patients who had low grade tumors, small metastases and no ascites. (3) Burghardt showed that women in whom optimal debulking was not possible had a higher number of positive pelvic and para-aortic lymph node metastases. (4) Friedlander reported that the size of the largest residual tumor mass was not an independent prognostic factor when newer variables such as DNA ploidy were included in the multivariate analyses. (5) Hacker et al., reported that patients with extensive metastatic disease prior to cytoreduction had a poor prognosis even if disease was cytoreduced to an optimal status. (6)
Recent studies have shown conflicting results with regard to whether interval cytoreductive surgery can improve outcome in patients with advanced, bulky disease. An EORTC study randomized 319 patients who had residual lesions measuring more than 1 cm after primary surgery and received three cycles of cyclophosphamide and cisplatin to undergo either debulking surgery or no surgery, followed by further cycles of cyclophosphamide and cisplatin. (7) Progression-free (PFS) and overall survival (OS) were both significantly longer in the group that underwent interval debulking surgery. A subsequent GOG study examined the same general approach but with cisplatin and paclitaxel and found no survival benefit for patients undergoing interval debulking (8).
An alternative to conventional surgery followed by chemotherapy is to deliver neoadjuvant chemotherapy followed by surgery. Several small studies of patients with findings compatible with advanced ovarian cancer treated with neoadjuvant chemotherapy have demonstrated that neoadjuvant chemotherapy is associated with the same PFS and OS as that of patients treated conventionally. (9–11) The cost of caring for patients who have extensive but suboptimal cytoreductive surgery may be greater than for those treated with neoadjuvant chemotherapy. (9) Patients may tolerate chemotherapy better when aggressive surgery has not been done prior to the initiation of chemotherapy. (10) These studies have also suggested less surgical morbidity (less blood loss, less time in surgical ICU, shorter operative times and fewer post-operative hospital days) when neoadjuvant therapy is used. (9–15) Kumar et al. recently reported a randomized trial of 90 patients comparing upfront chemotherapy in advanced ovarian cancer vs upfront surgery. They reported a higher rate of optimal debulking in the patients receiving upfront chemotherapy with reduced surgical morbidity and mortality and better quality of life. (16). Kuhn et al reported in a non-randomized phase II study a higher resection rate and longer median survival for patients treated with a neoadjuvant approach as compared to a group of patients prospectively treated during the same period with a conventional approach (17).
One difficulty that may be encountered is identifying which patients can be optimally surgically cytoreduced at presentation. Several authors have suggested that CT criteria may be established to identify patients who are most likely to be successfully cytoreduced. (11, 18) One study suggested that the following CT findings would predict for a low likelihood of successful cytoreduction: attachment of the omentum to the spleen; disease greater than 2 cm on the diaphragm, liver surface or parenchyma, pleura, mesentery, gallbladder fossa. (18) Successful cytoreduction was accomplished in only 6 of 18 patients with these CT findings compared with 23 of 24 patients with favorable CT findings.
Intraperitoneal (IP) chemotherapy has been shown to improve survival in optimally debulked ovarian cancer patients. IP therapy is designed to maximize drug delivery to the tumor while sparing patients many of the systemic toxicities associated with the drug. Agents that have a high level of intrinsic activity against epithelial ovarian cancer, large molecular weight, and whose plasma clearance rates substantially exceed their rates of uptake from the peritoneal cavity are especially suited for intraperitoneal administration. Carboplatin and paclitaxel are two such agents with high peritoneal-to-plasma concentration ratios following IP administration. The resulting very high IP concentrations of these drugs are maintained for a prolonged period of time which may further enhance tumor cell kill. (19)
A clinical advantage for IP therapy has been documented in three Phase III trials. In a trial from the Southwest Oncology Group (20), 544 patients with ≤ 2 cm residual disease following debulking were randomized to receive IV cisplatin 100 mg/m2 and IV cyclophosphamide 600 mg/m2, or IP cisplatin 100 mg/m2 and IV cyclophosphamide 600 mg/m2 every 3 weeks, for 6 cycles. The median survival for patients receiving IP cisplatin was significantly longer than for those receiving IV cisplatin (49 vs. 41 months, p = .03). Clinical hearing loss and neutropenia were significantly less frequent and less severe on the IP arm.
A subsequent trial (SWOG-9227/GOG 114) compared systemic carboplatin (AUC = 9) every 4 weeks for 2 cycles, followed by IV paclitaxel 135 mg/m2 over 24 hours on Days 1 – 2 and IP cisplatin 100 mg/m2 on Day 2 to standard IV paclitaxel (135 mg/m2 over 24 hours on Day 1 – 2) and IV cisplatin (75 mg/m2 on Day 2) in 523 women with optimal stage III ovarian cancer. Although toxicity was greater in the IP arm, the excessive toxicity was principally bone marrow suppression resulting from the initial two IV carboplatin cycles. In addition, 19% of patients randomized to the IP arm received two or fewer courses of IP therapy. Despite this, recurrence-free survival was 28 months in the IP arm vs. 22 months in the standard arm (p=0.01). In addition, a borderline significant difference in OS between the two regimens (median survival of 63 vs. 52 months, p=0.05) was found.(21)
Paclitaxel had been tested for IP use in a phaseI study, in which the dose-limiting toxicity was abdominal pain (22).Subsequently, an IP paclitaxel trial demonstrated improved tolerability using a low dose weekly regimen. (23). Given this information, a third randomized phase III trial was initiated by the GOG, again exploring the IP cisplatin-basedtherapy question but including the additionof IP paclitaxel (24). The experimental regimen consisted of day1 IV paclitaxel 135 mg/m2 administered during 24 hours,day 2 IP cisplatin 100 mg/m2, and day 8 IP paclitaxel 60 mg/m2.The control arm in this study was again the GOG standard IV paclitaxel and cisplatin. Although the IP program was associated with more toxicity, treatment withthis regimen resulted in a highly statistically significantimprovement in both PFS (median, 24 v 18.3 months; P = .027) and OS (median, 65.6 v 49.7 months; P = .017) (24).
In patients for whom optimal debulking is felt to be unlikely, one strategy could be to incorporate IP therapy post-operatively in patients that are able to be optimally debulked following neoadjuvant chemotherapy. The purpose of this study was to evaluate OS, PFS and toxicity in patients treated with this strategy as well as to evaluate the percent of patients that are able to be successfully cytoreduced to optimal disease following neoadjuvant therapy.
Patients and Methods
Patients were eligible for this study if the clinical picture was consistent with stage III epithelial ovarian cancer, primary peritoneal or fallopian tube cancer with a large pelvic mass and/or bulky abdominal disease and/or malignant pleural effusion. Patients with stage IV disease with malignant pleural effusion only were allowed. Patients must have had adenocarcinoma by biopsy or cytology (nonmucinous histology) compatible with an epithelial ovarian, fallopian tube or peritoneal primary. Patients were deemed unlikely to be optimally cytoreduced by the evaluating gynecologic oncologist.
Patients must not have had prior chemotherapy, immunotherapy, or pelvic radiation for this cancer. Patients who had undergone an exploratory laparotomy prior to enrollment were eligible only if an aggressive tumor debulking procedure was not performed (e.g., BSO/TAH with omentectomy). Only minimal tumor resection for the purposes of diagnosis and palliation was allowed (e.g., salpingo oophorectomy and/or partial omentectomy).
Baseline CA-125 was required to be ≥ 70 units/ml. Patients were required to have a calculated creatinine clearance ≥ 50 ml/min, a serum bilirubin and SGOT ≤ 2 x the institutional upper limit of normal, a granulocyte count of ≥ 1,500/μl, platelets ≥ 100,000/μl and a performance status of 0 – 2. Patients with any ≥ grade 2 sensory neuropathy were not eligible. Patients were informed of the investigational nature of the study and signed and gave informed consent in accordance with institutional and federal guidelines.
Neoadjuvant chemotherapy was given for three 21 day cycles and consisted of paclitaxel 175 mg/m2 over 3 hours followed by carboplatin, target AUC 6, IV over 30–60 minutes. Patients were required to receive prophylaxis for paclitaxel-associated hypersensitivity reactions as per their institutional regimen. Carboplatin dose was determined using the Calvert formula substituting calculated creatinine clearance for the GFR (glomerular filtration rate). (25)
If following neoadjuvant chemotherapy, a patient had a ≥ 50% decrease in CA-125 from baseline, an exploratory laparotomy was performed with the goal of optimal debulking or complete resection of any residual tumor seen. These procedures were all performed by gynecologic oncologists familiar with the protocol and trained in debulking surgery techniques.
At the completion of cytoreductive surgery patients must have had optimal disease defined as no residual lesions after resection, or residual disease such that no single lesion measured ≥ 1 cm. Patients whose disease was not optimally cytoreduced were removed from protocol. Malignant pleural effusions were required to have resolved completely.
Post-operative chemotherapy consisted of paclitaxel 175 mg/m2 IV over 3 hours day 1 followed by carboplatin AUC=5 IP day 1 and paclitaxel 60 mg/m2 IP day 8 q 28 days x 6 courses. An IV Port-A-Cath™ was recommended for IP chemotherapy administration. Carboplatin was diluted in 1,000 mL of normal saline or D5W, warmed to 37°C and instilled into the peritoneal cavity as rapidly as possible immediately following the end of the IV paclitaxel infusion. On day 8, IP paclitaxel was administered in a similar fashion. Standard dose modifications were employed.
The primary goal of the study was to evaluate PFS and OS in stages III and IV, bulky ovarian, peritoneal, and fallopian tube cancer patients treated with this strategy. OS was the primary endpoint. Based on prior GOG trials in suboptimally debulked patients (26, 27), it was assumed that the study strategy would not be of further interest if the true two-year survival rate was less than 60%, while a two-year rate of 73% or higher would be of interest for further testing. This design had a power (probability of correctly declaring a regimen with a 73% true two-year survival rate to warrant further study) of 0.80.
Fifty-five patients were deemed sufficient to estimate the optimal debulking rate or the probability of a particular neoadjuvant treatment toxicity to within ± 13%. Assuming a baseline 20% optimal debulking rate for this group of patients, the power to detect an improvement in this rate would be higher than 0.90 if the neoadjuvant treatment increased it to at least 40%. Any toxicity with a true incidence rate of 5% or higher would be likely (94% chance) to be seen at least once.
All patients entering the study underwent pathology review for both the initial diagnostic procedure and for the interval cytoreduction procedure. A review of the surgical procedure was carried out prior to the second registration by gynecologic oncologists familiar with the techniques used, to confirm that optimal debulking was performed.
Results
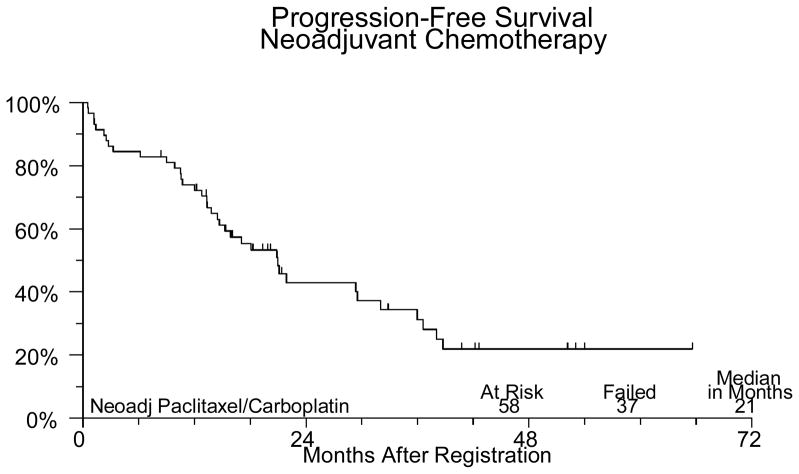
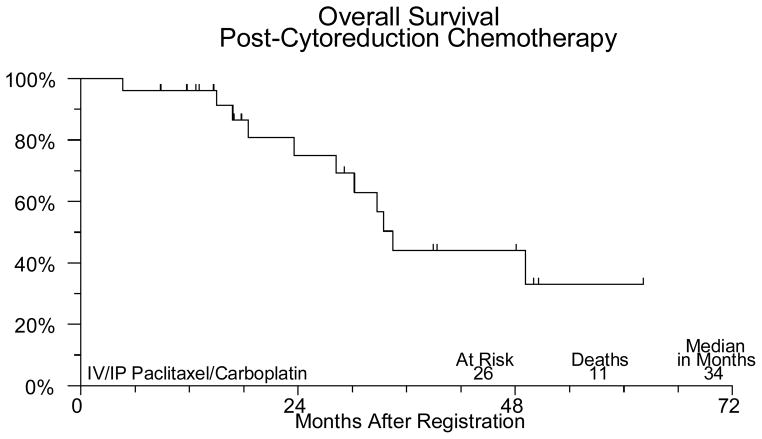
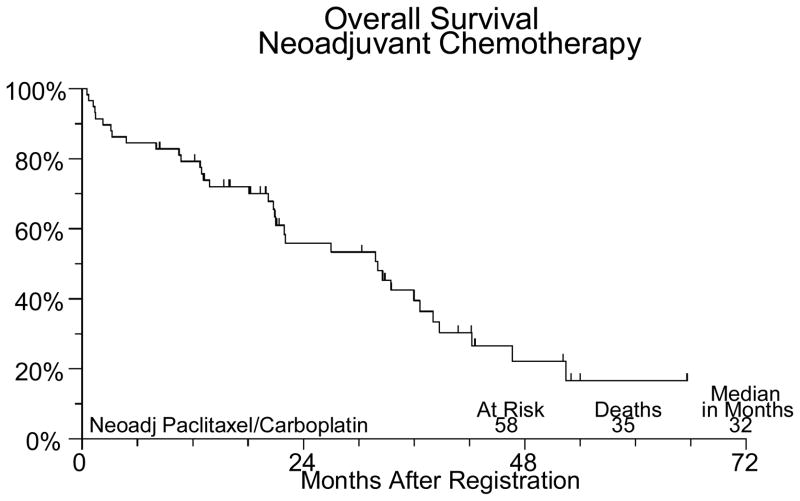
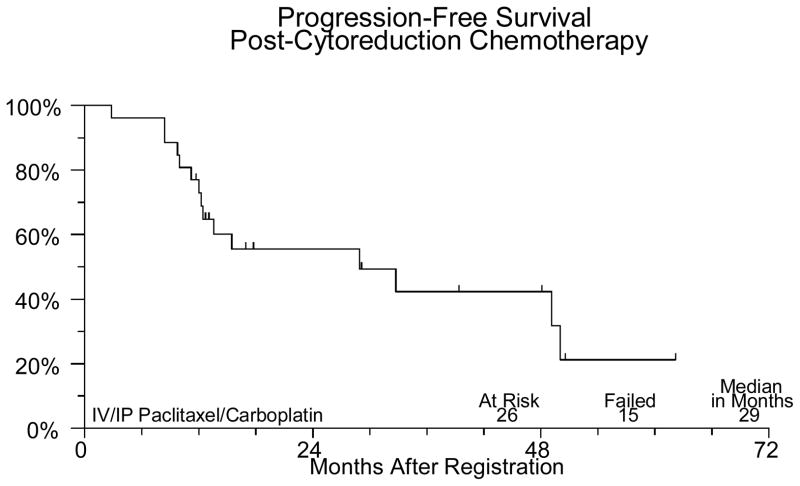
Sixty-two patients were registered between March 2001 and February 2006. Four patients were ineligible (1 had extensive surgery, 2 distant metastases, 1 CA125 < 70). The median age for the patients on study was 62.6 (31.5–79.8). Forty-three (74%) patients had ovarian cancer, 14 (24%) had primary peritoneal cancer and in one patient (2%) the primary site was not reported. Forty-three (74%) patients had stage III disease and 15 (26%) had stage IV. Of the 58 eligible patients undergoing neoadjuvant chemotherapy, 45 completed the planned treatment. Two patients were discontinued for adverse events, 3 discontinued for refusal unrelated to adverse events. Three patients were discontinued for disease progression and four died (3 from disease, 1 from toxicity). One patient was found to have a second primary cancer and was removed from study. There were no major protocol deviations. Fifty-six of the 58 eligible patients have been evaluated for neoadjuvant chemotherapy adverse events (2 institutions did not submit toxicity data). One patient died of pneumonia and sepsis probably due to treatment. Five other patients had grade 4 adverse events, including neutropenia (3), anemia, leukopenia, anorexia, fatigue/malaise/lethargy, muscle weakness, respiratory infection, and cardiac ischemia/infarction. Thirty-six (62%) eligible patients had interval debulking surgery. Two patients experienced grade 4 hemorrhage due to surgery. No other significant surgical complications were noted. Thirty one patients were registered for post-cytoreduction chemotherapy. Five were ineligible due to suboptimal cytoreduction. The median age for this group of patients was 62.3 (45.2–80.1). Of the 26 optimally debulked eligible patients (72% of the group undergoing surgery), 18 (69%) completed the planned treatment. Six patients were discontinued secondary to toxicity, 1 for progression and 1 was discontinued for personal reasons. The only grade 4 toxicity seen among the 26 eligible patients who received post-cytoreduction chemotherapy was neutropenia in 4 patients. Thirteen patients had grade 3 adverse events including neutropenia in 7 patients, 1 episode of febrile neutropenia, anemia in 3 patients, thrombocytopenia in 2 patients, abdominal pain in 3 patients, anorexia in 1 patient, IP catheter infection in 1 patient, fatigue in 1 patient and vomiting in 3 patients (Table 1). At a median follow-up of 21 months for those still alive, median PFS is 21 months and median OS is 32 months for all 58 eligible patients. PFS and OS (from the time of starting post-operative chemotherapy) for the 26 patients who received IV/IP chemotherapy are 29 and 34 months respectively (Figures 1–IV).
Table 1.
Comparison of IP Toxicity With Other IP Studies
| IP 114 | IP 172 | S0009 | |
|---|---|---|---|
| Neuro | 12% | 40% | 0 |
| GI | 37% | 50% | 25% |
| Platelets | 50% | 12% | 12% |
| Neutrophils | 80% | 76% | 50% |
| Completion | 71% | 42% | 69% |
| PFS | 28 | 24 | 29 |
| OS | 63 | 66 | 34 |
Figure I.
Figure IV.
Discussion
Previous small studies have suggested similar PFS and OS with the use of neoadjuvant chemotherapy as compared with initial surgery followed by chemotherapy with reduced surgical morbidity, better optimal debulking rates, less cost and better chemotherapy tolerability (12–17). The EORTC randomized neoadjuvant chemotherapy study recently closed to accrual should shed further light on the value of the neoadjuvant approach.
Neoadjuvant therapy should be distinguished from interval debulking. Neoadjuvant therapy, as performed in this study, is defined as a chemical cytoreduction occurring prior to any significant attempt at surgical resection of the tumor. In contrast, interval debulking implies that an attempt at optimal debulking has already been made prior to the patients’ receiving any chemotherapy. This approach has not consistently been shown to improve survival (7,8). Studies of interval debulking therefore address a different patient population from the group described by this report. The impact of the neoadjuvant approach in a select group of patients who receive chemotherapy prior to any or little debulking effort is not known. In our study 21 patients had –pre-registration “surgery” of any kind, meaning that 37 patients had biopsy or cytology only. Among the 58 patients, reasons given for these patients to be “unlikely to be debulked” were that 27 were unresectable by CT scan criteria, 9 by physical examination findings, 11 by findings at surgery and 11 unknown.
There are conflicting data regarding whether various criteria used correlate well with unresectability. (28, 29) It is important to identify the patients who are truly unlikely to be optimally debulked so as not to potentially “deprive” a patient of the possibility of being optimally debulked. A recent meta-analysis (30) summarizes the need for better universal criteria for unresectability.
We estimated an approximately 20% optimal debulking for this group of patients without neoadjuvant therapy and 45% of the entire cohort and 72% of the group who underwent surgery were optimally debulked.
Although the survival results obtained using this treatment regimen compare favorably with other studies of sub-optimally debulked patients, our primary statistical endpoint was not met. In 2 GOG trials of suboptimally debulked stage III and IV ovarian cancer patients treated with cisplatin and paclitaxel combinations, the 2 year OS rates were approximately 60%. (26, 27). We assumed that a 2 year survival rate of greater than 73% would be of considerable interest for further development and our 2 year survival rate was 52%. Perhaps in this poor prognosis group of patients, our survival goal was too optimistic. It is true that because only approximately half of the patients went on to receive the IP component of the study, it is difficult in a non-randomized trial to decipher whether these patients did relatively well because of selecting out the best prognosis patients by response to neoadjvuant therapy or because of the surgery or the IP route of chemotherapy. The numbers are certainly too small to compare the median survival for the entire cohort of 32 months versus the 34 month median survival for the patients who completed surgery with <1 cm disease and then received IP chemotherapy, but it should be noted that the PFS and OS estimates reported for the latter group is calculated from the time of starting the IV/IP therapy.
Although randomized trials in ovarian cancer have established a survival advantage for IP chemotherapy for optimally debulked ovarian cancer, toxicity has been significant in the IP arms of these trials. Because IV carboplatin and cisplatinhave equivalent efficacy in ovarian cancer, andcarboplatin can be safely administered IP with a similar pharmacokineticadvantage as IP cisplatin (31, 32), and IP carboplatin has been shown to have activity (33–35), we chose to use IP carboplatin in our post-cytoreductive chemotherapy regimen and this certainly may have accounted for the comparatively good tolerability for our IP regimen. The 28 day cycle as compared to 21 day cycle may have contributed to the favorable toxicity profile as well. In addition, the participants in this study appeared to tolerate surgery with less morbidity than might have been expected from women with very advanced disease. Certainly, randomized comparisons will be needed to make conclusions about the toxicity and efficacy of this IP regimen in this group of patients.
Figure II.
Figure III.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA12213, CA46368, CA105409, CA13612, CA46441, CA35262, CA45560, CA22433, CA67575, CA12644, CA35176, CA86780, CA46368, CA58861, CA58723, CA52654, CA35281, CA35261 and supported in part by Oncotech
Footnotes
This study was presented in part at the 2008 meeting of the Society of Gynecologic Oncologists
References
- 1.Hoskins WJ, Bundy BN, Thigpen JT, et al. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume Stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47:159–166. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 2.Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974–980. doi: 10.1016/s0002-9378(94)70090-7. [DOI] [PubMed] [Google Scholar]
- 3.Heintz APM, Van Oosterom AT, Trimbos JBMC, et al. The treatment of advanced ovarian carcinoma (I): clinical variables associated with prognosis. Gynecol Oncol. 1988;30:347–358. doi: 10.1016/0090-8258(88)90249-1. [DOI] [PubMed] [Google Scholar]
- 4.Burghardt E, Girardi F, Lahousen M, et al. Patterns of pelvic and para-aortic lymph node involvement in ovarian cancer. Gynecol Oncol. 1991;40:103–106. doi: 10.1016/0090-8258(91)90099-q. [DOI] [PubMed] [Google Scholar]
- 5.Friedlander ML, Hedley DW, Swanson C, et al. Prediction of long-term survival by flow cytometric analysis of cellular DNA content in patients with advanced ovarian cancer. J Clin Oncol. 1988;6:282–290. doi: 10.1200/JCO.1988.6.2.282. [DOI] [PubMed] [Google Scholar]
- 6.Hacker NF, Berek JS, Lagasse LD, et al. Primary cytoreductive surgery for epithelial ovarian cancer. Obstet Gynecol. 1983;61:413–420. [PubMed] [Google Scholar]
- 7.Van der Burg ME, van Lent M, Buyse M, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecologic Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. NEJM. 1995;332:629–634. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 8.Rose PG, Nerenstone S, Brady MF, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. NEJM. 2004;351(24):2489–97. doi: 10.1056/NEJMoa041125. [DOI] [PubMed] [Google Scholar]
- 9.Chambers JT, Chambers SK, Voynick IM, et al. Neoadjuvant chemotherapy in stage X ovarian carcinoma. Gynecol Oncol. 1990;37:327–331. doi: 10.1016/0090-8258(90)90361-n. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PE, Chambers JT, Makuch R. Neoadjuvant chemotherapy for advanced ovarian cancer. Gynecol Oncol. 1994;53:33–37. doi: 10.1006/gyno.1994.1083. [DOI] [PubMed] [Google Scholar]
- 11.Surwit E, Childers J, Atlas I, et al. Neoadjuvant chemotherapy for advanced ovarian cancer. Int J Gynecol Cancer. 1996;6:356–361. doi: 10.1016/s1074-3804(96)80131-9. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz PE, Rutherford TJ, Chambers JT, et al. Neoadjuvant chemotherapy for advanced ovarian cancer: long-term survival. Gynecol Oncol. 1999;72:93–99. doi: 10.1006/gyno.1998.5236. [DOI] [PubMed] [Google Scholar]
- 13.Jacob JH, Gershenson DM, Morris M, et al. Neoadjuvant chemotherapy and interval debulking for advanced epithelial ovarian cancer. Gynecol Oncol. 1991;42:146–50. doi: 10.1016/0090-8258(91)90335-3. [DOI] [PubMed] [Google Scholar]
- 14.Lim JTW, Green JA. Neoadjuvant carboplatin and ifosfamide chemotherapy for inoperable FIGO stage III and IV ovarian carcinoma. Clin Oncol R Coll Radiol. 1993;5:198–202. doi: 10.1016/s0936-6555(05)80227-4. [DOI] [PubMed] [Google Scholar]
- 15.Vergote I, De Wever I, Tjalma W, et al. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol. 1998;71:431–6. doi: 10.1006/gyno.1998.5213. [DOI] [PubMed] [Google Scholar]
- 16.Kumar L, Hariprasad R, Kumar S, et al. Neoadjuvant chemotherapy (NACT) followed by interval debulking surgery versus upfront surgery followed by chemotherapy (CT) in advanced epithelial ovarian carcinoma (EOC): A prospective randomized study--Interim results. J Clin Oncol. 2007;25(18s) (suppl; abstr 5531) [Google Scholar]
- 17.Kuhn W, Rutke S, Späthe K, et al. Neoadjuvant chemotherapy followed by tumor debulking prolongs survival for patients with poor prognosis in International Federation of Gynecology and Obstetrics Stage IIIC ovarian carcinoma. Cancer. 2001;92(10):2585–91. doi: 10.1002/1097-0142(20011115)92:10<2585::aid-cncr1611>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Nelson BE, Rosenfield AT, Schwartz PE. Preoperative abdominopelvic computed tomographic prediction of optimal cytoreduction in epithelial ovarian carcinoma. J Clin Oncol. 1993;11:166–72. doi: 10.1200/JCO.1993.11.1.166. [DOI] [PubMed] [Google Scholar]
- 19.Schneider JG. Intraperitoneal chemotherapy. Obstet Gynecol Clin North Am. 1994;21:195–212. [PubMed] [Google Scholar]
- 20.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. NEJM. 1996;335:1950–1956. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 21.Markman M, Bundy B, Alberts D, et al. Phase III Trial of Standard-Dose Intravenous Cisplatin Plus Paclitaxel Versus Moderately High-Dose Carboplatin Followed by Intravenous Paclitaxel and Intraperitoneal Cisplatin in Small-Volume Stage III Ovarian Carcinoma: An Intergroup Study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 22.Markman M, Rowinsky E, Hakes T, et al. Phase I trial of intraperitoneal Taxol: A Gynecologic Oncology Group study. J Clin Oncol. 1992;10:1485–1491. doi: 10.1200/JCO.1992.10.9.1485. [DOI] [PubMed] [Google Scholar]
- 23.Francis P, Rowinsky E, Schneider J, et al. Phase I feasibility and pharmacologic study of weekly intraperitoneal paclitaxel: A Gynecologic Oncology Group pilot study. J Clin Oncol. 1995;13:2961–2967. doi: 10.1200/JCO.1995.13.12.2961. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 25.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Onc. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 26.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with Stage III and Stage IV ovarian cancer. NEJM. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 27.Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2000;18:106–115. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 28.Risum S, Høgdall C, Loft A, et al. Prediction of suboptimal primary cytoreduction in primary ovarian cancer with combined positron emission tomography/computed tomography—A prospective study. Gynecol Oncol. 2008;108:265–270. doi: 10.1016/j.ygyno.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Salani R, Axtell A, Gerardi M, et al. Limited utility of conventional criteria for predicting unresectable disease in patients with advanced stage epithelial ovarian cancer. Gynecol Oncol. 2008;108:271–275. doi: 10.1016/j.ygyno.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Bristow R, Eisenhauer E, Santillan A, et al. Delaying the primary surgical effort for advanced ovarian cancer: A systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecol Oncol. 2007;104:480–490. doi: 10.1016/j.ygyno.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.DeGregorio MW, Lum BL, Holleran WM, et al. Preliminary observations of intraperitoneal carboplatin pharmacokinetics during a phase I study of the Northern California Oncology Group. Cancer Chemother Pharmacol. 1986;18:235–238. doi: 10.1007/BF00273393. [DOI] [PubMed] [Google Scholar]
- 32.Elferink F, van der Vijgh WJF, Klein I, et al. Pharmacokinetics of carboplatin after intraperitoneal administration. Cancer Chemother Pharmacol. 1988;21:57–60. doi: 10.1007/BF00262740. [DOI] [PubMed] [Google Scholar]
- 33.Speyer JL, Beller U, Colombo N, et al. Intraperitoneal carboplatin: Favorable results in women with minimal residual ovarian cancer after cisplatin therapy. J Clin Oncol. 1990;8:1335–1341. doi: 10.1200/JCO.1990.8.8.1335. [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer P, Bennedbaek O, Bertelsen K. Intraperitoneal carboplatin in the treatment of minimal residual ovarian cancer. Gynecol Oncol. 1990;36:306–311. doi: 10.1016/0090-8258(90)90131-4. [DOI] [PubMed] [Google Scholar]
- 35.Fujiwara K, Markman M, Morgan M, et al. Intraperitoneal carboplatin-based chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2005;97:10–15. doi: 10.1016/j.ygyno.2004.12.005. [DOI] [PubMed] [Google Scholar]