Abstract
Bone marrow (BM) and lymphocyte samples from aplastic anemia patients show up-regulated Fas and Fas-ligand (FasL) expression respectively, supporting a relationship between immune-mediated BM destruction and the Fas apoptotic pathway. Mice with spontaneous lymphoproliferation (lpr) and generalized lymphoproliferative disease (gld) mutations exhibit abnormal expression of Fas and FasL; serving as potential models to elucidate underlying mechanisms of BM failure. We examined cellular and functional characteristics of lpr and gld mutants on the C57BL/6 (B6) background. Lymph node (LN) cells from lpr and gld mice produced less apoptosis when co-incubated with C.B10-H2b/LilMcd (C.B10) BM cells in vitro. This functional difference was confirmed by infusing lpr, gld, and B6 LN cells into sub-lethally irradiated CB10 mice; all donor LN cells showed significant T cell expansion and activation but only B6 LN cells caused severe BM destruction. Mice infused with gld LN cells developed mild to moderate BM failure, despite receiving FasL-deficient effectors, thus suggesting the existence of alternative pathways or incomplete penetrance of the mutation. Paradoxically, mice that received Fas-deficient lpr LN cells also had reduced BM failure, likely due to down-regulation of pro-apoptotic genes, an effect that can be overcome by higher doses of lpr LN cells. Our model demonstrates that abnormal Fas or FasL expression interferes with the development of pancytopenia and marrow hypoplasia, validating a major role for the Fas/FasL cytotoxic pathway in immune-mediated BM failure, although disruption of this pathway does not completely abolish marrow destruction.
Keywords: Autoimmunity, Hematopoiesis, Systemic Lupus Erythematosus, Cell Activation, Stem Cell
The key cellular events in the development of aplastic anemia (AA) and other bone marrow (BM) failure syndromes are the expansion and activation of self-reactive pathogenic T cells that are polarized towards a Th1-type response and hypersecrete inflammatory cytokines, such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α)1–3. The autoimmune response results in direct and bystander destruction of hematopoietic stem and progenitor cells in the BM by cytotoxic lymphocytes, as well as stem cell suppression by inhibitory cytokines4–6. The clinical outcome is peripheral pancytopenia and severe marrow aplasia that, untreated, is fatal due to bleeding and infection. Immune-mediated BM failure has been modeled in the mouse by infusion of allogeneic donor lymph node (LN) cells into major- or minor-histocompatibility antigen-mismatched recipients, for the purposes of understanding the underlying disease mechanisms as well as to test novel treatments7–10. The murine models mimic many features of human disease, including significantly up-regulated Fas expression on residual BM cells11 as well as modulation of lymphocyte expansion and clinical symptoms with immunosuppressive agents such as cyclosporine, anti-thymocyte globulin (ATG), anti-gamma-interferon (IFN-γ), and anti-tumor necrosis factor-alpha (TNF-α)9,11. While the inciting auto-antigen(s) remains unknown, considerable research into the underlying pathophysiology has implicated the Fas system as the predominant pathway in immune-mediated BM failure12–14.
Engagement of Fas, a 48-kDa type 1 transmembrane protein, by its natural ligand Fas-Ligand (FasL), results in a signal transduction cascade that culminates in apoptosis15. Mice with autosomal recessive spontaneous mutations at the lymphoproliferation (lpr) or generalized lymphoproliferative disease (gld) locus, abnormally express Fas and FasL, respectively, and similarly accelerate systemic autoimmunity16–18. One characteristic feature of these animals is massive lymphadenopathy, associated with the proliferation of aberrant T cells that are composed predominantly of a unique population of TCRαβ+CD4−CD8−B220+ T cells (DN T cells). DN T cells are presumed to be resting19,20, although recent studies suggest they may be capable of suppressing proliferation of autologus and syngeneic T cells through a Fas-independent mechanism21. In addition, a small proportion of another unique Ly-5(B220)+ T cell population is present and expresses low levels of CD4 resulting in increased total cell numbers of CD4+ and CD8+ T cells because of the extent of lymphadenopathy22. Historically, lpr and gld mice have been useful models for studying systemic lupus erythematosus (SLE) and autoimmune lymphoproliferative syndrome (ALPS), although only ALPS patients can have homozygous mutations within the Fas gene23,24. Hematopoietic development has also been probed in these mice, with the observation of increased peripheral clonogenic progenitors secondary to extramedullary hematopoiesis from splenomegaly25. The utility of lpr and gld mice in testing the contribution of the Fas/FasL pathway in immune-mediated BM failure has not been explored. Lymphocytes isolated from AA patients, although expressing low-levels of surface FasL, demonstrate very high levels of intracellular FasL that are released upon stimulation26,27. T cells12 and BM CD34+ cells from patients with AA show upregulated Fas expression13,28,29 that is inducible in the presence of IFN-γ and TNF-α and modulated by immunosuppressive therapies28–31, reinforcing the requirement of FasL-bearing effectors and Fas-bearing targets in the promotion of marrow cell death. Since Fas-mediated cell apoptosis is not always up-regulated in AA32–34, alternative pathways such as granzyme and perforin may also be involved.
The aim of our study is to define the effectiveness of effector T cells lacking expression of Fas or FasL in the induction of BM failure in a minor-H mismatched model, and thus assess the contribution of Fas/FasL-mediated apoptosis in the development of immune-mediated BM failure. We analyzed hematopoietic tissues of C57BL/6 (B6), B6.MRL-Faslpr (lpr) and B6Smn.C3-Faslgld (gld) mice to establish whether at baseline these animals show evidence of marrow dysfunction. Furthermore, unfractionated LN cells from lpr and gld donors were compared with those from normal B6 donors in the induction of BM failure after infusion into congenic C.B10-H2b/LilMcd (C.B10) recipients. Data from this study indicate that LN cells deficient in Fas or FasL mitigate immune-mediated attack against allogeneic BM cells.
Materials and Methods
Mice and cell extraction
Inbred C57BL/6 (B6) and congenic B6.MRL-Faslpr (lpr), B6Smn.C3-Faslgld (gld) and C.B10-H2b/LilMcd (C.B10) mice were originally obtained from the Jackson Laboratory (Bar Harbor, Maine USA), and were bred and maintained in the NIH animal facility under standard care and nutrition conditions. The lpr and gld mice were backcrossed to B6 for 11 and 10 generations respectively while C.B10 mice were backcrossed to BALB for 13 generations. Mice were used at 2–6 months of age and were gender-matched between donors and recipients. All animal studies were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee.
Cells from BM, peripheral blood (PB), lymph nodes (LN), spleen (SP), and thymus (TH) were obtained from normal B6, lpr and gld mice or C.B10 recipients. BM cells were extracted from bilateral femurs and tibiae; PB was obtained by retro-orbital sinus bleeding; LN cells were obtained from bilateral inguinal, axillary and cervical regions. TH, SP and LN tissues were processed in a tissue grinder and were filtered through 90 μM nylon mesh to obtain single cell suspensions. Cells were counted using a ViCell counter (Beckman Coulter Inc., Miami, FL) and complete blood counts (CBC) were obtained using a Hemavet 950 analyzer (Drew Scientific Inc., Oxford, CT).
Flow cytometry cell staining and cell sorting
Monoclonal antibodies for mouse CD3 (clone 145-2C11), CD4 (clone GK 1.5), CD8 (clone 53-6.72), CD11a (clone 2D7), CD11b (clone M1/70), CD25 (clone 3C7), CD45R (B220, clone RA3-6B2), CD95 (Fas, clone Jo2), CD178 (FasL, clone Kay-10), CD117 (c-Kit, clone 2B8), erythroid cells (clone Ter119), granulocytes (Gr1/Ly6-G, clone RB6-8C5), and stem cell antigen 1 (Sca1, clone E13-161) were obtained from BD Biosciences (San Diego, CA) while anti-Fox-P3 (clone FJK-16s) was obtained from eBioscience (San Diego, CA). Antibodies were conjugated to either fluorescein isothyocyanate (FITC), phycoerythrin (PE), CyChrome, PE-Cyanin 5 (PE-Cy5) or allophycocyanin (APC).
Cells were first incubated with Gey’s solution (130.68 mM NH4Cl, 4.96 mM KCl, 0.82 mM Na2HPO4, 0.16 mM KH2PO4, 5.55 mM Dextrose, 1.03 mM MgCl2, 0.28 mM MgSO4, 1.53 mM CaCl2 and 13.39 mM NaHCO3) for ten minutes on ice to lyse red blood cells, and were then washed and stained with various antibody mixtures in a flow buffer (2.68 mM KCl, 1.62 mM Na2HPO4, 1.47 mM KH2PO4, 137 mM NaCl, 7.69 mM NaN3, and 1% BSA) at 4°C or on ice. For the measurement of regulatory T cells (Treg cells), samples were first stained with cell surface antigens CD4 and CD25 and then fixed, permeabilized and stained for intracellular FoxP3. Stained cells were analyzed on a BD LSR II flow cytometer equipped with the FACSDiva software (Becton Dickinson, San Jose, CA).
Lymphocytes and splenocytes from lpr mice were stained with the marker combination B220+CD4−CD8−TCRαβ+ to sort double negative (DN) T cells using a FACSVantage cell sorter from Becton Dickinson. We also sorted CD3−CD4−CD8−CD45R− residual BM (RBM) cells from C.B10 recipients infused with either B6 or lpr LN cells to elucidate molecular differences using a PCR-based array analysis.
Lymphocyte cytotoxicity in vitro
BM cell targets from C.B10 mice were co-incubated with effector LN cells from lpr, gld and B6 donors, or with sorted B220+CD4−CD8−TCRαβ+ DN T cells from lpr mice, in a 96-well flat bottom culture plate. Cytotoxicity was assayed using a CyToxiLux Plus kit (OncoImmunin Inc., Gaithersburg, MD) according to the manufacturer’s instructions. Briefly, C.B10 BM targets were first labeled with a red fluorescence dye at 37°C for 30 minutes and then dispensed into each well. Each well contained a mixture of 2 ×104 labeled C.B10 BM cells (targets) and 40 × 104 B6 LN cells (effectors), or mixtures of 5 × 104 C.B10 BM cells and 100 × 104 lpr or gld LN cells (Effector:Target ratio = 20:1). The cell mixtures were incubated at 37°C for 60 minutes, after which a fluorescent green caspase substrate was added for the detection of cell apoptosis by LSR-II flow cytometry. Targets only and effectors only were used as controls.
Induction of BM failure
Procedures for BM failure induction have been described previously. In brief, C.B10 mice, irradiated four to six hours earlier with 5 Gy total body irradiation (TBI) from a Shepherd Mark 1 137cesium gamma source (J. L. Shepherd & Associates, Glendale CA), were infused with LN cells from B6, lpr and gld donors at varying doses of 5–30 × 106 cells per mouse as specified in each experiment. Mice that received 5 Gy TBI without LN cell infusion were controls. Recipient mice were bled at two and three weeks to measure CBC, and euthanized at three weeks post LN-cell infusion for pathological, cellular and molecular analyses.
Pathology
C.B10 mice that received TBI with or without infusion of LN cells were euthanized on day 21. Sternebrae, stomach, intestines, colon, liver, SP and skin were collected, fixed, embodied, sectioned, stained with hematoxylin and eosin, and examined using an Olympus IX50 microscope (Optical Elements). Photographic images of BM morphology were captured at 20× magnification using a SPOT INSIGHT camera with the SPOT version 4.0.8 software.
Enzyme-linked immunosorbent assay (ELISA)
Orbital sinus blood from C.B10 mice infused with B6 or lpr LN cells were centrifuged at 1000g for 10 minutes. Serum was removed and stored at −20°C and analyzed for soluble FasL concentration using the Quantikine mouse FasL ELISA kit (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. Light absorbance was measured using the Wallace 1420 Victor 3 reader (Perkin Elmer, Wellesley, MA, USA), at 450 nm with a correction wavelength set at 570 nm.
Gene expression analysis
BM cells from C.B10 mice that received 5 Gy TBI and 5 × 106 B6 or lpr LN cells three weeks earlier were stained with an antibody cocktail containing CD3-APC-Cy7, CD4-APC, CD8-PeCy5 and CD45R-FITC and were then sorted to obtain CD3-, CD4-, CD8-, CD45R- residual BM cells (RBM). Total mRNA (100–200 ng/assay) was extracted from these samples using RT2 qPCR-Grade RNA isolation kit (SABiosciences, Frederick, MD, USA), omitting the enrichment step, and quantified by spectrophotometry (NanoDrop Technologies, Wilmington, DE, USA). Samples were treated with DNAse using the Turbo DNA-free kit (Ambion, Austin, TX, USA) and then used as templates for cDNA synthesis (RT2 First Strand Kit, SABiosciences, Frederick, MD), according to the manufacturer’s instructions. The synthesized cDNA were applied to cataloged PCR-based SuperArray (SABiosciences, Frederick, MD) plates to detect expression of genes related to apoptosis, chemokines, toll-like receptors and stem cell function using the ABI 7900 HT sequence detector (Applied Biosystems, USA). The relative expression level of each gene was normalized to six murine housekeeping genes.
Data analyses
Cellular composition data were analyzed by JMP Statistical Discovery Software (SAS Institute Inc., NC) using different one-way analysis of variance platforms. Results are shown as means with standard errors. Statistical significance was declared at p <0.05 and p <0.01 levels. Data from the PCR array were exported to Microsoft Excel spreadsheets and analyzed with PCR Array Data Analysis Web Portal software provided by SABiosciences. Genes with more than 2-fold changes in expression between recipients of lpr and B6 LN cells were considered significant.
Results
Cellular composition
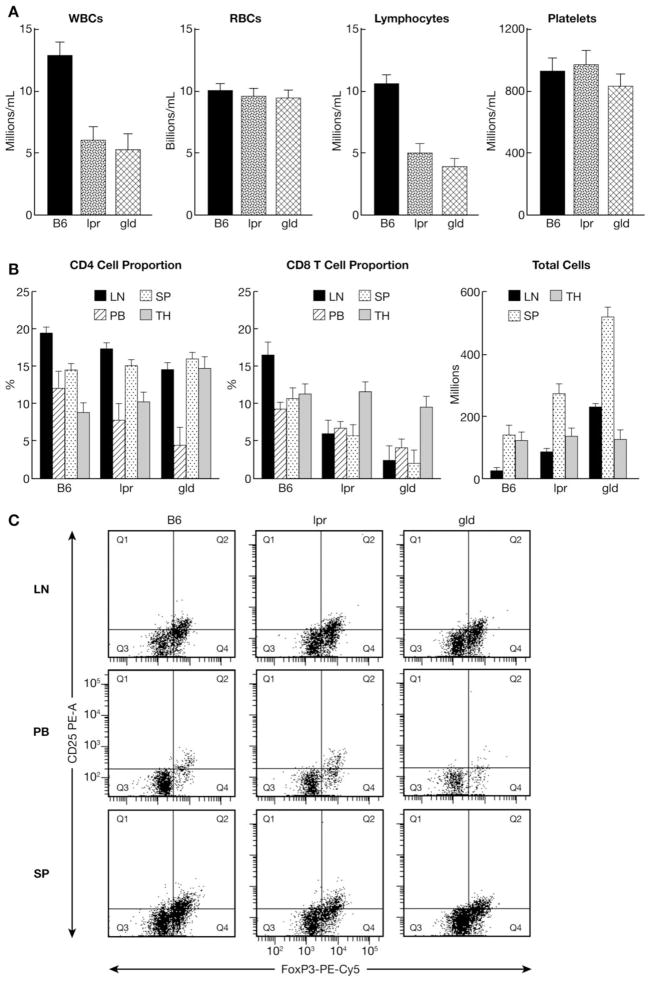
We first examined the cellular composition of various tissues in order to compare lpr and gld mice with normal B6 controls. In the PB, the white blood cell concentration, especially the concentration of lymphocytes, was significantly lower in lpr and gld mice (p<0.0005) than in normal B6 (Figure 1A). There was no difference among the three genotypes in the concentrations of red blood cells or platelets (Fig. 1A). Further characterization of the lymphocyte subsets revealed reduced proportions of CD8+ T cells in lpr and gld mice, not exclusive to peripheral blood but also apparent in the SP and LN (Fig. 1B). Although the total number of thymocytes per mouse was similar among the three genotypes, lpr and gld mice had many more LN cells (P<0.01) and splenocytes (P<0.05) than did normal B6 controls, consistent with their known phenotype (Fig. 1B). We further examined the presence of immunosuppressive Treg cells and found that the proportion of CD4+CD25+FoxP3+ Treg cells was similar among the three genotypes in LN and SP but was reduced in lpr and gld mice (P<0.05) in the PB (Fig. 1C).
FIGURE 1.
Reduced lymphocyte proportion in lpr and gld mice. Complete blood counts were performed on B6, lpr and gld mice using Hemavet 950 (A). Proportions of CD4+ and CD8+ T cells in LN, PB, SP and TH were analyzed by FACS after staining with a CD8-PeCY5 + CD4-APC antibody mixture, while total cells in LN, SP and TH were counted by a Vicell Counter (B). The presence of CD4+CD25+FoxP3+ Treg cells in LN, PB and SP were also analyzed by FACS (C). Data shown are representative FACS dot plots or means with standard errors from six B6, six lpr mice and five gld mice used for each group.
In the BM, the proportion of CD4+ T cells was similar among the three genotypes, whereas the proportion of CD8+ T cells was slightly lower in lpr and gld mice, but these differences were not statistically significant (Fig. 2A). There was no difference in the total BM cells per mouse or the proportion and total number of hematopoietic stem cells (HSCs) and progenitor cells as defined by the Lin−Sca1+Kit+ phenotype (Fig. 2B). In addition, no genotype effect was observed in BM Treg cell proportion (Fig. 2C).
FIGURE 2.
Normal lymphocyte composition in the BM of lpr and gld mice. The same animals described in Figure 1 were also analyzed for total BM cells per mouse (Vicell counter, Beckman Coulter, FL) and proportions of BM CD4+ and CD8+ T cells by FACS analysis (A). BM cells were stained with the antibody mixture Lin (CD3, CD4, CD8, CD11b CD45R, Gr1, Ter119)-PE + CD117-APC, + CD34-FITC for the proportion of Lin−Sca1+Kit+ (LSK) cells (B), and with CD25-PE + FoxP3-PE-Cy5 + CD4-APC for the proportion of Treg cells (C).
Cytotoxicity in vitro
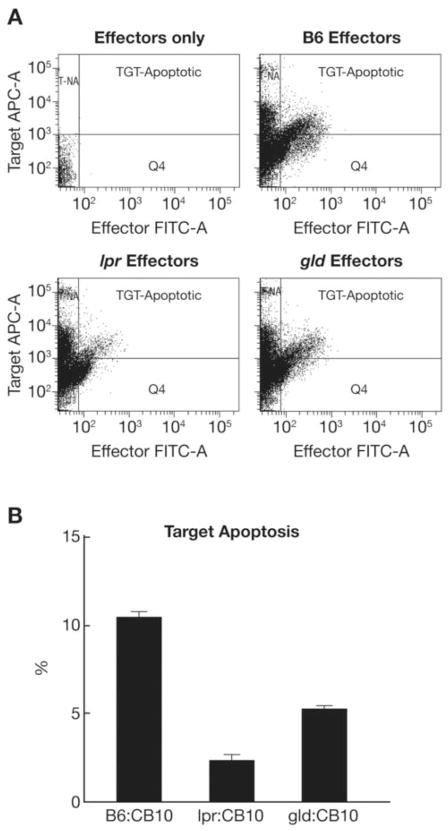
We examined LN cell cytotoxicity in vitro by incubating effector LN cells from lpr, gld and B6 mice with target BM cells from C.B10 mice. The lpr, gld and B6 mice are matched at the H2 locus with C.B10 mice, all bearing the H2b/b allele, but mismatched at multiple minor histocompatibility antigen loci, thereby permitting B6, lpr, or gld LN cells to recognize C.B10 BM cells as foreign targets. In this assay, lpr and gld LN effectors were 60–80% less efficient than B6 LN effectors in inducing apoptosis of C.B10 BM cell targets (Fig. 3A, B).
FIGURE 3.
Reduced lpr and gld LN cell cytotoxicity in vitro. LN cell-induced apoptosis of C.B10 BM cell targets is shown in the representative FACS dot plots after effectors and targets were mixed 20:1 and incubated at 37°C for 60 minutes using a CyToxilux kit from Onco-Immunin (A). In comparison to B6 LN cells, effector LN cells from lpr and gld donors caused less apoptosis of C.B10 BM cell targets (B). Data shown are means with standard errors from two B6 animals, one lpr, one gld and one C.B10 animal per group each measured in triplicates.
Induction of BM failure
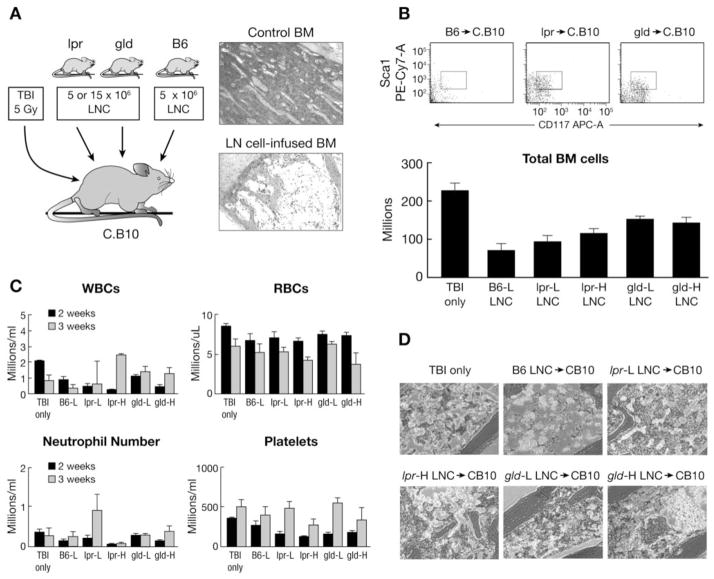
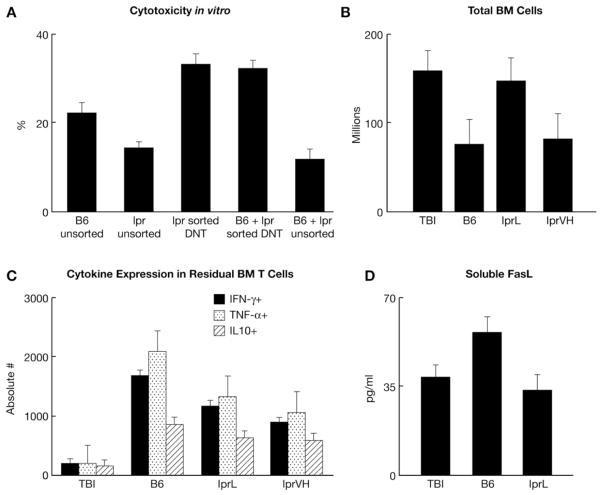
To evaluate the function of Fas- and FasL-deficient lymphocytes in vivo, we infused LN cells from lpr and gld donors into sub-lethally irradiated C.B10 mice at a high (15 × 106 cells/recipient) or low (5 × 106 cells/recipient) cell dose and compared outcomes to our standard model of BM failure, in which infusion of 5 × 106 cells/recipient of B6 LN cells produced severe BM hypoplasia within two weeks (Fig. 4A). Total BM cell counts at three weeks after cell infusion showed a significant (P<0.0005) treatment effect with 49% and 33% less residual BM cells in mice infused with lpr and gld LN cells respectively, compared to C.B10 mice that received TBI only. In contrast, mice infused with B6 LN cells had 68% less residual BM cells compared to TBI only mice (Fig. 4B). Changes in the BM were consistent with changes observed in the PB at two weeks post-induction with severe leukopenia and thrombocytopenia in mice infused with B6 LN cells, whereas C.B10 mice that received lpr and gld LN cell infusions had blood counts similar to mice that received TBI only (Fig. 4C). At three weeks post-induction, recipient mice infused with lpr and gld LN cells had a greater proportion of Lin−Sca1+Kit+ cells than recipients infused with B6 LN cells (Fig. 4B). BM histopathology was also consistent, showing that C.B10 mice infused with lpr and gld LN cells had mild to moderate reduction in marrow cellularity in comparison to mice that received B6 LN cells, which had moderate to severe atrophy and TBI only mice that had mild atrophy (Fig. 4D). Overall, mice infused with mutant LN cells had less severe clinical manifestations than mice infused with LN cells from normal B6 mice.
FIGURE 4.
Induction of BM failure using LN cells (LNC) from B6, lpr and gld donors. Pre-irradiated (5 Gy TBI) C.B10 mice were infused with 5 × 106 or 15 × 106 LN cells from lpr and gld donors, or 5 × 106 LN cells from B6 donors, to induce BM failure (A). The presence of LSK hematopoietic stem and progenitor cells in the BM are shown as representative FACS dot plots while total BM cells per mouse are shown as means with standard error bars for four or five mice of each treatment group (B). Recipient CBC was analyzed at two and three weeks respectively (C). Histological analysis revealed mild hematopoietic cell atrophy in mice treated with TBI only, mild to moderate atrophy in mice treated with TBI + lpr LN cells or TBI + gld LN cells, and moderate to severe atrophy in mice treated with TBI + B6 LN cells (D).
T-cell activation
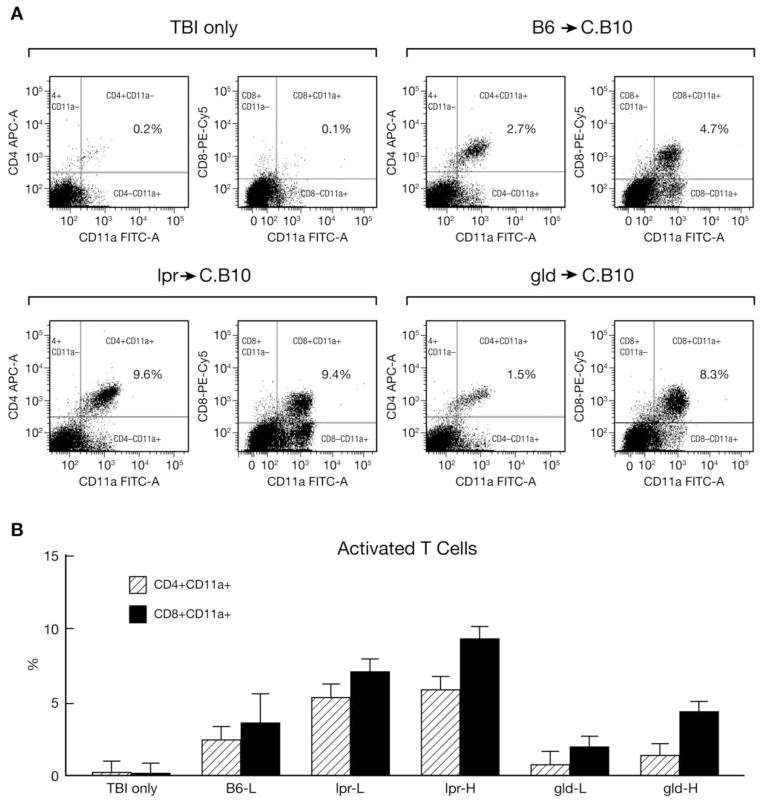
We further analyzed residual BM cells from recipient C.B10 mice for the presence of CD4+ and CD8+ T cells, as well as for T cell activation status using CD11a as a marker. Mice that received TBI only showed no T-cell expansion or activation in the BM whereas mice that received low and high doses of gld LN cells had significant expansion and activation of CD4+ and CD8+ T cells in the BM similar to the level observed in mice infused with B6 LN cells (Fig. 5A, B). Mice that received low and high doses of lpr LN cells also showed expansion and activation of CD4+ and CD8+ T cells, but the extent surpassed that of recipient mice infused with B6 LN cells (Fig. 5A, B). Although donor lpr and gld LN cells expanded and were activated in C.B10 recipient BM, they failed to destroy host hematopoietic cells or produce severe BM failure.
FIGURE 5.
Expansion and activation of T cells. Residual BM cells from mice discussed in Figure 4 were stained with an antibody mixture containing CD4-APC, CD8-PeCY5 and CD11a-FITC. The majority of CD4+ and CD8+ T cells were positively stained for the activation marker CD11a in mice that received LN cell infusions (A). CD4+ and CD8+ T cell proportions were significantly higher in mice that received lpr LN cells (B).
Role of DN T cells
LN cells from lpr mice contain a large fraction of DN T cells, which we postulated could serve as immune suppressors and mitigate BM failure. We sorted B220+CD4−CD8−TCRαβ+ DN T cells and used them alone or in combination with B6 LN cells as potential effectors/suppressors in a cytotoxicity assay in vitro. Results from this experiment showed that: 1) lpr DN T cells did not alter the ability of B6 LN cells to induce target cell apoptosis; 2) sorted lpr DN T cells alone induced the same level of target cell apoptosis as did B6 LN cells; and 3) unsorted lpr LN cells suppressed B6 LN cells in target cell apoptosis (Figure 6A). Although lpr LN cells clearly reduced target cell apoptosis, their suppressive effect was not caused by DN T cells.
FIGURE 6.
The roles of DN T cells, cell dose, class I cytokines and soluble FasL. Sorted DN T cells from lpr donors caused increased apoptosis of C.B10 BM cell targets and did not suppress the apoptotic effect of B6 LN cells. Data shown are means with standard errors from two B6 and two lpr donors each measured in triplicates (A). Pre-irradiated (5 Gy TBI) C.B10 mice were infused with 5 × 106 or 30 × 106 LN cells from lpr donors, or 5 × 106 LN cells from B6 donors, to induce BM failure. Total BM cells per mouse are shown as means with standard error bars for three mice in each treatment group (B). BM cells were stained with IFN-γ-FITC, TNFa-APC and IL10-PE; cytokine profiles are shown as means with standard error bars for three mice in each treatment group (C). Serum soluble FasL levels are shown as means with standard error bars for treatment groups TBI, B6 and lprL (D).
Cell dose effect
We further tested the effect of cell dose by injecting sub-lethally irradiated C.B10 recipients with low (5 × 106 cells/recipient) or very high (30 × 106 cells/recipient) doses of lpr LN cells and compared outcomes with mice infused with 5 × 106 cells/recipient B6 LN cells. Total BM cell counts at three weeks after cell infusion were relatively similar between recipients of B6 LN cells and lpr-VH LN cells, suggesting that the reduction in BM failure observed in lpr infused recipients could be overcome by a 6-fold increase in cell dose (Fig. 6B). Further analysis of residual BM cells showed a reduction in Th1-type cytokines in lpr infused recipients (Fig. 6C). Soluble FasL levels were also measured but showed no elevations to explain the ineffectiveness of lpr LN cells (Fig. 6D).
Gene expression
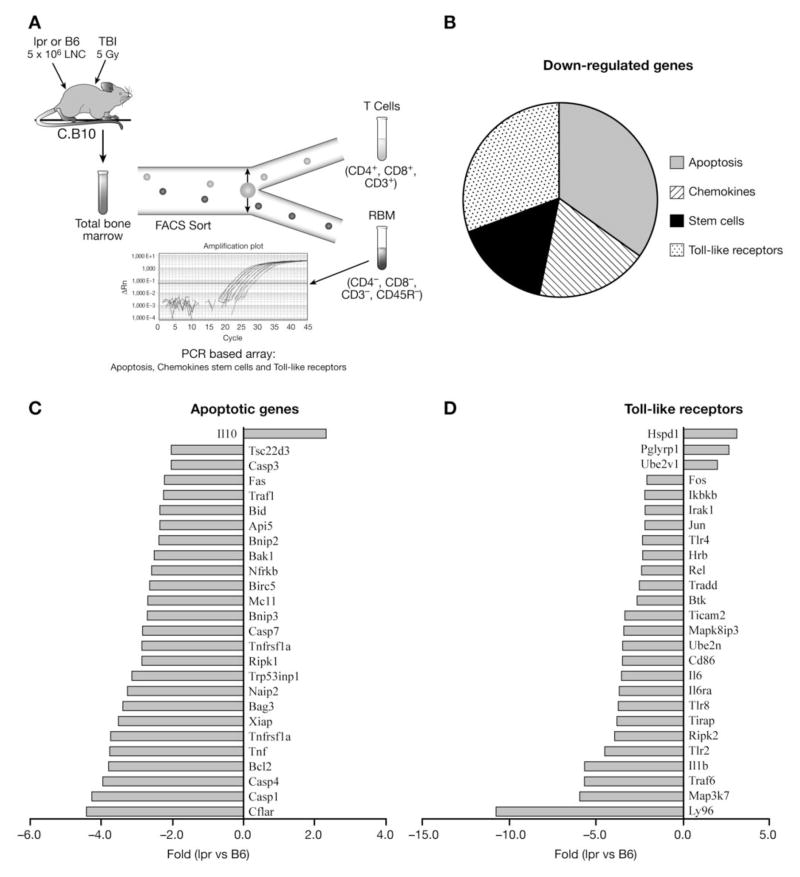
To discover potential mechanisms responsible for the reduced function of lpr LN cells, we compared gene expression in RBM from recipients of B6 and lpr LN cells using a PCR-based SuperArray method focusing on chemokines, toll-like receptors, apoptosis and stem cell function (Fig. 7A). Of the 360 genes analyzed, 75 genes showed >2 fold down-regulation and 6 genes showed >2 fold up-regulation in recipients of lpr compared to B6 LN cell recipients. The up-regulated genes showed 2–3 fold changes in all four gene categories. Of the75 down-regulated genes, 26 are related to apoptosis, 23 to the toll-like receptor family, 14 associate with chemokines, and 12 are genes related to stem cell function (Fig. 7B). Of particular interest to our study was the low expression of several genes involving apoptosis and toll-like receptor pathways. Several caspase family members were down-regulated, including caspase 1 (4.3 fold), 3 (2.1), 4 (4.0) and 7 (2.8), all of which play important roles in the apoptosis cascade mediated by Fas/FasL (Fig. 7C). There was also a 2.2-fold decline in Fas expression on RBM cells from recipients infused with lpr LN cells (Fig. 7C). Although anti-apoptotic genes, such as Bcl2 and Xiap, also were down-regulated in RBM; this effect was counter-balanced by over-expression of IL10, a potent anti-apoptosis regulator. Toll-like receptors including Tlr4 and Tlr2 were also down-regulated (2.2 and 4.5 fold), a pattern known to inhibit inflammation. Thus, the limiting factors accounting for the decline in the function of lpr LN cells in BM destruction may be decreased expression of key molecules such as Fas, caspase 3 and toll-like receptors in cell death signaling pathways.
FIGURE 7.
Down-regulation of apopotic genes in lpr LN cell-infused recipient BM. C.B10 recipients pre-irradiated with 5 Gy TBI were infused with 5 × 106 LN cells from either lpr or B6 donors. Three weeks following cell infusion, recipients were euthanized and CD3−CD4−CD8−CD45R− BM cells were sorted for gene expression analysis using a PCR-based SuperArray system (A). Of the 360 total genes analyzed in the four categories, 75 genes showed more than two-fold down-regulation in lpr LN cell-infused recipient BM when compared to B6 (B). There was significant down-regulation in the expression of genes important in the regulation of cell apoptosis, such as Fas and caspase genes (C) as well as toll-like receptor genes (D). Data shown are from three recipients analyzed in each group.
Discussion
Murine models have been used for years to explore the basic mechanisms underlying autoimmunity and to interrogate isolated gene effects, while avoiding the limitations of human studies, particularly the heterogeneity of patient populations35–43. Spontaneous mouse models and later transgenic models have provided insight into the immune system with revelations such as an early wave of pancreatic β-cell death leading to stimulation of autoreactive T cells in non-obese diabetic mice (NOD)35,38,42; induction of experimental allergic encephalomyelitis (EAE) after immunization with myelin antigens39,40,43,44; and the incidental development of spontaneous colitis in immunodeficient knockout strains such as TCRα and IL2, directing further exploration of T cell mechanisms in human inflammatory bowel disease (IBD)45.
Our study used lpr and gld mice to determine the contribution of Fas/FasL pathway as well as substantiate the presence of competing cytolytic pathways in the AA mouse model. The lpr and gld mice are good models due to their lack of Fas and FasL expression24, respectively. The gld mice are particularly relevant because they provide FasL-deficient effectors which would be expected to abolish immune-mediated BM failure if HSC destruction were solely Fas-dependent. The mechanism of Fas/FasL-mediated cell apoptosis in the development of BM failure was directly tested when we infused LN cells from lpr, gld and B6 donors into sub-lethally irradiated C.B10 recipients. The infusion of 5 × 106 LN cells from lpr and gld donors had markedly reduced ability to destroy recipient hematopoietic cells, supporting the predominance of this pathway. Initial cellular composition analysis indicated that, despite significantly enlarged LN, both lpr and gld mice had mild peripheral leukopenia with a reduction in lymphocyte concentration, particularly CD8+ T cells, prompting increased LN cell dose to a much higher level of 15–30 × 106 per recipient. The differences between mice infused with B6 and gld effectors persisted despite the increased cell dose, while lpr effectors were more similar to B6 at higher doses.
Expansion and activation of infused lymphocytes from lpr and gld donors in vivo in recipient BM was comparable or beyond the level observed in mice infused with B6 donors. Based on these observations, Fas and FasL deficiency did not alter T cell proliferation or activation and these mutations may promote these processes through abnormal activation-induced cell death46, particularly in the case of lpr (Fas negative) effectors. T cell activation evident by the level of CD11a expression was much greater in C.B10 recipients injected with mutant LN cells than in recipients infused with B6 LN cells. We speculate that T cells derived from lpr and gld donors may be pre-activated, predisposing them to heightened expansion and activation upon antigen encounter. Prior studies in C3H-lpr and C3H-gld mice indicate that CD4+ T cells have pre-activated or memory T cells which hypersecrete TH1-type cytokines, IL-2, IFN-γ and TNF-α, which would provide a favorable immunologic milieu for BM destruction22. In contrast, our study shows that expanded and activated lpr and gld LN cells do not destroy C.B10 hematopoietic cells, further supporting the hypothesis that marrow destruction is largely mediated by the Fas-Fas-ligand pathway.
The presence of BM failure, albeit a milder form, in C.B10 mice infused with LN cells from gld donors supports that the mutation is “leaky”, leading to the presence of a small amount of normal T cells expressing FasL. Davidson et al reported that 8–14% of LN T cells from lpr and gld mutants were normal T cells expressing Fas or FasL16. Another possible explanation for the BM failure observed in C.B10 mice infused with LN cells from gld mice is the existence of alternative cytolytic pathways, including perforin and granzyme, which may become more obvious in the absence of normal Fas-FasL interactions. We have recently reported perforin gene polymorphisms in a subset of AA patients47. The amelioration of inflammatory bowel disease in perforin knock-out mice48 is also supportive.
Conversely, the observation of reduced BM failure in mice infused with lpr LN cells is less well understood since FasL is the key molecule on or released by effector T cells responsible for BM cell destruction. We measured soluble FasL in lpr LN cell recipients (considered less effective than membrane bound FasL in target cell toxicity), but found no elevated levels to explain the reduced effectiveness of lpr LN cells. Although DN T cells from lpr mice have been reported to have immunosuppressive effects21, we demonstrated the contrary in our studies in vitro, in which DN T cells acted as immune effectors and caused apoptosis of C.B10 BM cells. A more plausible mechanism is the reduction of key molecules in the apoptotic pathway of target cells induced by the presence of lpr LN cells. Results from our gene expression analysis showed significant down-regulation of many genes important in Fas/FasL apoptosis and toll-like receptor pathways, which are typically over-expressed in BM failure syndromes21,29,50. These results provide novel evidence indicating that Fas-deficient effector T cells are also defective in causing cell death, possibly secondary to down regulation of pro-apoptotic genes in target cells.
Given these findings, our model may provide a useful tool for interrogating the degrees of disease severity in AA, especially since differences between mild and severe manifestations are not limited to a quantitative deficit of stem cells10. Historically, graft failure of syngeneic transplants in patients without pre-conditioning5 as well as the non-correlation of blood counts with colony-forming cells51 have suggested that the pathophysiology of AA is in actuality more complex than stem cell absence alone. In addition, the Fas/FasL pathway has been implicated in other hematological disorders including Fanconi anemia52, myelodysplastic syndrome53, and chronic myelogenous leukemia54. Our data support observations in patients with AA and other BM failure syndromes by showing that lymphocytes with abnormal Fas or FasL expression are incapable of inducing BM failure. The ready availability of genetically modified mice with well characterized functional defects like that of lpr and gld mice should further allow a full description of the molecular pathways involved in immune-mediated marrow failure. While our study focused on effectors, the lpr model could also be used to evaluate Fas-aberrant targets. Additionally, this study supports that there are alternative pathways that warrant further testing, possibly through the use of double or triple transgenic mice barring lethality.
Acknowledgments
The authors are grateful to Keyvan Keyvanfar for his expertise with the sorting experiment and Susan Wong for her knowledge and assistance with the array experiments.
Footnotes
SO designed the study, performed the experiments and wrote the paper, MJD and FME performed the experiments, MAE performed pathological examinations, JC and NSY designed the study and wrote the paper
References
- 1.Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology. 2006:72–77. doi: 10.1182/asheducation-2006.1.72. [DOI] [PubMed] [Google Scholar]
- 2.Maciejewski JP, Hibbs JR, Anderson S, Katevas P, Young NS. Bone marrow and peripheral blood lymphocyte phenotype in patients with bone marrow failure. Experimental Hematology. 1994;22:1102–1110. [PubMed] [Google Scholar]
- 3.Nistico A, Young NS. gamma-Interferon gene expression in the bone marrow of patients with aplastic anemia. Annals of internal medicine. 1994;120:463–469. doi: 10.7326/0003-4819-120-6-199403150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bacigalupo A. Bone marrow failure syndromes. American Society of Hematology; 2007. pp. 23–28. [Google Scholar]
- 5.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108(8):2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selleri C, Maciejewski JP, Sato T, Young NS. Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996;87:4149–4157. [PubMed] [Google Scholar]
- 7.Barnes DWH, Mole RH. Aplastic anaemia in sublethally irradiated mice given allogeneic lymph node cells. Br J Haematol. 1967;13:482–491. doi: 10.1111/j.1365-2141.1967.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Lipovsky K, Ellison F, Calado RT, Young NS. Bystander destruction of hematopoietic progenitor and stem cells in a mouse model of infusion-induced bone marrow failure. Blood. 2004;104(6):1671–1678. doi: 10.1182/blood-2004-03-1115. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Ellison FM, Eckhaus MA, Smith AL, Keyvanfar K, Calado RT, Young NS. Minor antigen H60-mediated aplastic anemia is ameliorated by immunosuppression and the infusion of regulatory T cells. The Journal of Immunology. 2007;178:4159–4168. doi: 10.4049/jimmunol.178.7.4159. [DOI] [PubMed] [Google Scholar]
- 10.Knopse WH, Husseini SG, Chiu KM, Fried W. Immunologically mediated aplastic anemia in mice: evidence of hematopoietic stromal injury and injury to hematopoietic stem cells. Experimental Hematology. 1994;22:573–581. [PubMed] [Google Scholar]
- 11.Bloom ML, Wolk AG, Simon-Stoos KL, Bard JS, Chen J, Young NS. A mouse model of lymphocyte infusion-induced bone marrow failure. Experimental Hematology. 2004;22:1163–1172. doi: 10.1016/j.exphem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Callera F, Garcia AB, Falcão RP. Fas-mediated apoptosis with normal expression of bcl-2 and p53 in lymphocytes from aplastic anaemia. Br J Haematol. 1998;100:698–703. doi: 10.1046/j.1365-2141.1998.00625.x. [DOI] [PubMed] [Google Scholar]
- 13.Ismail M, Gibson FM, Gordon-Smith EC, Rutherford TR. Bcl-2 and Bcl-x expression in the CD34+ cells of aplastic anaemia patients: relationship with increased apoptosis and upregulation of Fas antigen. Br J Haematol. 2001;113:706–712. doi: 10.1046/j.1365-2141.2001.02810.x. [DOI] [PubMed] [Google Scholar]
- 14.Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br J Haematol. 1995;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 15.Nagata S, Goldstein P. The Fas death factor. Science. 1995;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 16.Davidson WF, Dumont FJ, Hendrick GB, Fowlkes BJ, Morse HC., III Phenotypic, functional and molecular comparisons of the abnormal lymphoid cells of C3H-lpr/lpr and C3H-gld/gld mice. The Journal of Immunology. 1986;136(11):4075–4084. [PubMed] [Google Scholar]
- 17.French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. The Journal of Cell Biology. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nose M, Nishihara M, Kamogawa J, Terada M, Nakatsuru S. Genetic basis of autoimmune disease in MRL/lpr mice: dissection of the complex pathological manifestations and their susceptibility loci. Rev in Immunogenetics. 2000;2:154–164. [PubMed] [Google Scholar]
- 19.Craft J, Peng S, Fuji T, Okada M, Fatenejad S. Autoreactive T cells in murine lupus. Immunologic Research. 1999;19(2–3):245–257. doi: 10.1007/BF02786492. [DOI] [PubMed] [Google Scholar]
- 20.Theofilopoulos AN. The basis of autoimmunity: Part II Genetic predisposition. Immunology Today. 1995;16(3):150–159. doi: 10.1016/0167-5699(95)80133-2. [DOI] [PubMed] [Google Scholar]
- 21.Hamad AR, Mohamood AS, Trujillo CJ, Huang CT, Yuan E, Schneck JP. B220+ Double-Negative T Cells Suppress Polyclonal T Cell Activation by a Fas-Independent Mechanism That Involves Inhibition of IL-2 Production. The Journal of Immunology. 2003;171:2421–2426. doi: 10.4049/jimmunol.171.5.2421. [DOI] [PubMed] [Google Scholar]
- 22.Davidson WF, Calkins C, Hugins A, Giese T, Holmes KL. Cytokine secretion by C3H-lpr and -gld T cells: Hypersecretion of IFN-gamma and tumor necrosis factor-alpha by stimulated CD4+ T cells. The Journal of Immunology. 1991;146:4138–4148. [PubMed] [Google Scholar]
- 23.Bettinardi A, Brugoni D, Quiros-Roldan E, Malagoli A, La Grutta S, Correra A, Notarangelo LD. Missense mutations in the Fas gene resulting in autoimmune lymphoproliferative syndrome: A molecular and immunological analysis. Blood. 1997;89:902–909. [PubMed] [Google Scholar]
- 24.Klinman DM, Steinberg AD. Inquiry into murine and human lupus. Immunology Rev. 1995;144:157–193. doi: 10.1111/j.1600-065x.1995.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 25.Schneider E, Moreau G, Arnould A, Vasseur F, Khodabaccus N, Dy M, Ezine S. Increased fetal and extramedullary hematopoiesis in Fas-deficient C57BL/6-lpr/lpr mice. Blood. 1999;94(8):2613–2621. [PubMed] [Google Scholar]
- 26.Li W, Fu J, Wang F, Yu G, Wang Y, Zhang X. Distinct overexpression of Fas Ligand on T lymphocytes in aplastic anemia. Cellular & Molecular Immunology. 2004;1(2):142–147. [PubMed] [Google Scholar]
- 27.Luther-Wyrsch A, Nissen C, Wodnar-Filipowicz A. Intracellular Fas ligand is elevated in T lymphocytes in severe aplastic anaemia. Br J Haematol. 2001;114:884–890. doi: 10.1046/j.1365-2141.2001.03026.x. [DOI] [PubMed] [Google Scholar]
- 28.Killick S, Cox CV, Marsh J, Gordon-Smith EC, Gibson FM. Mechanisms of bone marrow progenitor cell apoptosis in aplastic anemia and the effect of anti-thymocyte globulin: examination of the role of the Fas-Fas-L interaction. Br J Haematol. 2000;111(4):1164–1169. doi: 10.1046/j.1365-2141.2000.02485.x. [DOI] [PubMed] [Google Scholar]
- 29.Maciejewski J, Selleri C, Anderson S, Young NS. Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates cytokine-mediated hematopoietic suppression in vitro. Blood. 1995;85(11):3183–3190. [PubMed] [Google Scholar]
- 30.Kakagianni T, Giannakoulas NC, Thanopoulou E, Galani A, Michalopoulou S, Kouraklis-Symeonidis A, Zoumbos NC. A probable role for trail-induced apoptosis in the pathogenesis of marrow failure. Implications from an in vitro model and from marrow of aplastic anemia patients. Leuk Res. 2006;30:713–721. doi: 10.1016/j.leukres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100(4):1185–1191. doi: 10.1182/blood-2002-01-0035. [DOI] [PubMed] [Google Scholar]
- 32.Horikawa K, Nakakuma H, Kawaguchi T, Iwamoto N, Nagakura S, Kagimoto T, Takatsuki K. Apoptosis resistance of blood cells from patients with paroxysmal nocturnal hemoglobinuria, aplastic anemia, and myelodysplastic syndrome. Blood. 1997;90:2761–2722. [PubMed] [Google Scholar]
- 33.Kim SC, Min YH, Lee S, Chung SY, Yoo NC, Lee JW, Hahn JS, Ko YW. Delayed activation-induced T lymphocytes death in aplastic anemia: related with abnormal Fas system. Korean J Intern Med. 1998;13:41–46. doi: 10.3904/kjim.1998.13.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinohara K, Takahahi T, Nawata R, Oeda E. Decreased expression of the Fas ligand on peripheral blood mononuclear cells and undetectable levels of soluble Fas ligand in the serum of patients with aplastic anemia and myelodysplastic syndrome. Am J Hematol. 1999;62:124–125. doi: 10.1002/(sici)1096-8652(199910)62:2<124::aid-ajh16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: As good as it gets? Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 36.Boyton RJ, Altmann DM. Transgenic models of autoimmune disease. Clin Exp Immunology. 2002;127:4–11. doi: 10.1046/j.1365-2249.2002.01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunninghame Graham DS, Vyse TJ. The candidate gene approach: have murine models informed the study of human SLE? Clin Exp Immunology. 2004;137:1–7. doi: 10.1111/j.1365-2249.2004.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Every AL, Kramer DR, Mannering SI, Lew AM, Harrison LC. Intranasal vaccination with proinsulin DNA induces regulatory CD4+ T cells that prevent experimental autoimmune diabetes. J Immunol. 2006;176:4608–4615. doi: 10.4049/jimmunol.176.8.4608. [DOI] [PubMed] [Google Scholar]
- 39.Jones RE, Bourdette D, Moes N, Vandenbark A, Zamora A, Offner H. Epitope spreading is not required for relapses in experimental autoimmune encephalomyelitis. J Immunol. 2003;170:1690–1698. doi: 10.4049/jimmunol.170.4.1690. [DOI] [PubMed] [Google Scholar]
- 40.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 41.Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25− regulatory T cells. J Immunol. 2006;176:4748–4756. doi: 10.4049/jimmunol.176.8.4748. [DOI] [PubMed] [Google Scholar]
- 42.Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, Ziegler SF, Bradley LM. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
- 43.Yu M, Johnson JJ, Tuohy VK. A predictable sequential determinant spreading cascade invariably accompanies progression of experimental autoimmune encephalomyelitis: a basis forpeptide-specific therapy after onset of clinical disease. J Exp Med. 1996;183:1777–1788. doi: 10.1084/jem.183.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salbeko-Downes KA, Russell JH, Cross AH. Role of Fas-FasL interactions in the pathogenesis and regulation of autoimmune demyelinating disease. Journal of Neuroimmunology. 1999;100:42–52. doi: 10.1016/s0165-5728(99)00191-5. [DOI] [PubMed] [Google Scholar]
- 45.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75(2):203–205. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 46.Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 47.Solomou EE, Gibellini F, Stewart B, Malide D, Berg M, Visconte V, Green S, Childs R, Chanock SJ, Young NS. Perforin gene mutations in patients with acquired aplastic anemia. Blood. 2007;109:5234–5237. doi: 10.1182/blood-2006-12-063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merger M, Viney JL, Borojevic R, Steele-Norwood D, Clark DA, Riddell R, Maric R, Podack ER, Croitoru K. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor a in T cell induced mucosal damage in the mouse intestine. Gut. 2002;51(2):155–163. doi: 10.1136/gut.51.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng W, Kajigaya S, Chen G, Risitano AM, Nunez O, Young NS. Transcript profile of CD4+ and CD8+ T cells from the bone marrow of acquired aplastic anemia patients. Experimental Hematology. 2004;32:806–814. doi: 10.1016/j.exphem.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clinical Cancer Res. 2005;13(4):1154–1160. doi: 10.1158/1078-0432.CCR-06-2108. [DOI] [PubMed] [Google Scholar]
- 51.Maciejewski J, Selleri C, Sato T, Anderson S, Young NS. A severe and consistent deficit in marrow and circulating primitive hematopoietic cells (long-term culture-initiating cells) in acquired aplastic anemia. Blood. 1996;88:1983–1991. [PubMed] [Google Scholar]
- 52.Baruque GA, Bitencourt MA, Pasquini R, Castelo-Branco MT, Llerena JC, Jr, Rumjanek VM. Apoptosis and expression of anti- and pro-apoptotic proteins in peripheral blood mononuclear cells of Fanconi anaemia patients: a study of 73 cases. Eur J Haematol. 2005;75:384–390. doi: 10.1111/j.1600-0609.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 53.Sloand EM, Kim S, Fuhrer M, Risitano AM, Nakamura R, Maciejewski JP, Barrett AJ, Young NS. Fas-mediated apoptosis is important in regulating cell replication and death in trisomy 8 hematopoietic cells but not in cells with other cytogenetic abnormalities. Blood. 2002;100:4427–4432. doi: 10.1182/blood-2002-01-0096. [DOI] [PubMed] [Google Scholar]
- 54.Selleri C, Sato T, Del Vecchio L, Luciano L, Barrett AJ, Rotoli B, Young NS, Maciejewski JP. Involvement of Fas-mediated apoptosis in the inhibitory effects of interferon-alpha in chronic myelogenous leukemia. Blood. 1997;89:957–964. [PubMed] [Google Scholar]