Abstract
Head movement imposes the additional burdens on the visual system of maintaining visual acuity and determining the origin of retinal image motion (i.e., self-motion vs. object-motion). Although maintaining visual acuity during self-motion is effected by minimizing retinal slip via the brainstem vestibular-ocular reflex, higher order visuovestibular mechanisms also contribute. Disambiguating self-motion versus object-motion also invokes higher order mechanisms, and a cortical visuovestibular reciprocal antagonism is propounded. Hence, one prediction is of a vestibular modulation of visual cortical excitability and indirect measures have variously suggested none, focal or global effects of activation or suppression in human visual cortex. Using transcranial magnetic stimulation-induced phosphenes to probe cortical excitability, we observed decreased V5/MT excitability versus increased early visual cortex (EVC) excitability, during vestibular activation. In order to exclude nonspecific effects (e.g., arousal) on cortical excitability, response specificity was assessed using information theory, specifically response entropy. Vestibular activation significantly modulated phosphene response entropy for V5/MT but not EVC, implying a specific vestibular effect on V5/MT responses. This is the first demonstration that vestibular activation modulates human visual cortex excitability. Furthermore, using information theory, not previously used in phosphene response analysis, we could distinguish between a specific vestibular modulation of V5/MT excitability from a nonspecific effect at EVC.
Keywords: human V5/MT, response entropy, TMS, vestibular
Introduction
Much of what we know of the cerebral cortical function underlying human visual perception has been obtained during the head static condition. Ecologically however, the visual system is more accustomed to functioning during head motion, and here a visuovestibular interaction is required to optimize visual acuity and to resolve self-motion versus object-motion ambiguity. The problem for the visual system is exemplified by the situation of climbing a tree with the aim of picking ripe fruit. Successful fruit picking requires adequate visualization of the fruit despite motion of oneself or the fruit. The task would not be considered a success if we fell out of the tree, and to avoid this, it is imperative that we distinguish between our own motion (passive or active) from that of the swaying branches (from which we might infer, sometimes erroneously, our own movement). The vestibular system contributes to the visual processes used in locating the fruit and avoiding falling by 1) stabilizing gaze and 2) helping to resolve self-motion from background motion (of the branches). Conventionally, visuovestibular interaction is thought of primarily as a brainstem process; first during self-motion, the vestibular-ocular reflex (VOR) improves visual acuity by stabilizing gaze upon the fovea, and second, visual inputs synapse upon primary vestibular neurons (Waespe and Henn 1977) to enable the detection of low frequency or constant velocity self-motion since the vestibular system is only sensitive to head accelerations.
Despite the eloquence of brainstem visuovestibular interaction, sensory signals may conspire to defeat a correct brain decision regarding self- versus object-motion and thus engender false sensations of self-motion (called “vection”); for example, we may erroneously perceive ourselves to be swaying when faced with the swaying of branches when climbing a tree. One solution for resolving the self- versus object-motion problem is to invoke a reciprocal antagonism between visual and vestibular signals of motion. Evidence for a visuovestibular reciprocal inhibition is derived from psychophysical experiments assessing perception of self- or object-motion during concurrent visuovestibular stimulation (Probst et al. 1985, 1986) and at cerebral cortical level by reduced visual cortex responses during vestibular stimulation in functional imaging (Wenzel et al. 1996; Bense et al. 2001; Bottini et al. 2001; Stephan et al. 2005; Dieterich and Brandt 2008) and visual evoked potentials (Probst and Wist 1990). Despite conflicting evidence on visual cortical activation (some imaging and neurophysiological studies showing no effect of vestibular stimulation; Iida et al. 1997; Lobel et al. 1998; Suzuki et al. 2001; Engelhardt et al. 2007), an inhibition of visual motion cortex function could be functionally beneficial during excessive vestibular stimulation, by attenuating disorientating visual/self-motion percepts (Seemungal et al. 2011). Conversely, suppressing early visual cortex (EVC), which includes V1, could have wider, potentially inimical effects on visual processing since EVC is the primary entry route for visual signals to the cerebral cortex. Additionally EVC may be sine qua non for the conscious awareness of all visual percepts, including motion (Pascual-Leone and Walsh 2001; Silvanto et al. 2005). Indeed there is evidence for a higher order contribution to the maintenance of visual acuity above that explained by VOR gaze stabilization, during vestibular activation (Guedry and Ambler 1973; Guedry 1974; Tong et al. 2006). Thus a simple visual cortical inhibition could compromise visual discrimination during self-motion. To resolve the apparently competing brain excitation–suppression requirements between visual discrimination and motion processing during vestibular activation, one solution for the brain would be a selective down-modulation of visual motion areas, specifically V5/MT (Zeki 1974; Born and Bradley 2005), during vestibular activation. Current data showing a vestibular-mediated suppression of visual cortex are disparate however, suggesting variously a global suppression of visual cortex including V1 (Wenzel et al. 1996; Bottini et al. 2001) or focal suppression at V5/MT (Bense et al. 2001) or even V5/MT activation (Fasold et al. 2002).
Critically direct measures of human visual cortical excitability during vestibular activation have not been previously reported. Here, we describe the use of single pulse transcranial magnetic stimulation (TMS) to probe visual cortical excitability during vestibular activation (see Materials and Methods and Fig. 1). Change in cortical excitability in response to a sensory input is not, however, sufficient to establish specific functional relevance between stimulus and response, for example, cat primary visual cortex neuronal excitability was equally potentiated by vestibular and nociceptive inputs implying a nonspecific effect (Gorgiladze and Smirnov 1967). To further interrogate the functional relevance of excitability changes accompanying vestibular activation, we assessed changes in 1) phosphene percepts—specifically phosphene size and intensity and 2) response entropy derived from an information theoretic analysis (see also Supplementary Materials). We first assessed TMS responses in area V5/MT since this region is critically important in visual motion processing (Zeki 1974; Born and Bradley 2005). We then assessed EVC responses during vestibular activation since, if vestibular signals modulate V5/MT excitability, one question is whether such modulation occurs via V1-dependent or V1-independent pathways. Certainly, modulation of human EVC excitability by magnetic or electrical stimulation (Pascual-Leone and Walsh 2001; Antal et al. 2003) induces parallel changes in V5 excitability. Recent reports (Schmid et al. 2010), however, have confirmed the existence of direct subcortical inputs to V5/MT bypassing V1, thus excitability changes in V5/MT divergent from those in EVC could allude to the pathways by which vestibular signals could modulate visual cortex.
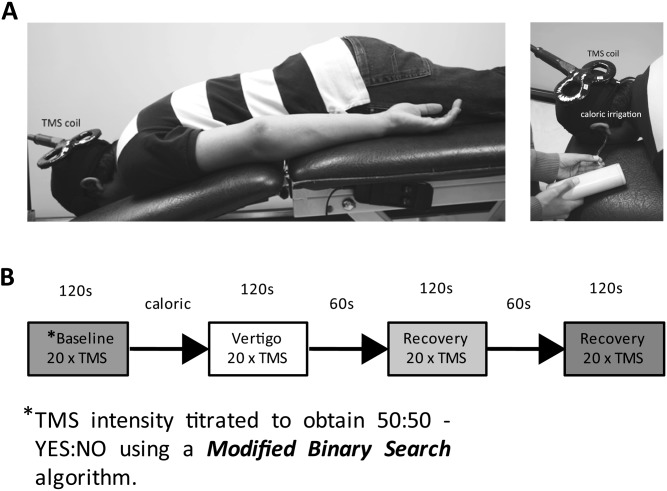
Figure 1.
Apparatus and experimental protocol. (A) Apparatus. Subjects lay prone to facilitate TMS access to the occiput. The subjects placed their face in an aperture that allowed free breathing and comfortable placement of a blindfold. This configuration also meant that the subject's head was stabilized within the aperture and thus restricted any head movement as a consequence of the elicited vestibular-colic response. The figure also shows the water irrigation tube inserted into the external auditory meatus. The water outflow was captured by a semirigid channel that sat below the pinna. The water-capture channel and coil holder are not shown for clarity. (B) Protocol. A single experimental RUN, consisting of 4 phases, is shown schematically. Each volunteer was subject to 4 RUNS. The entire experiment was conducted in low ambient light and with the subjects' eyes blindfolded. Phosphene threshold in terms of percentage of maximal TMS stimulator output was obtained prior to each “run” using a modified binary staircase algorithm (Mobs—Tyrell and Owens 1988). The average TMS threshold intensity obtained from 2 Mobs trials was then used for obtaining baseline responses (i.e., no vestibular activation) during which 20 TMS pulses were applied at threshold level at 6 s intervals. “Subjects' report of phosphene presence”—subjects were instructed to answer “yes” or “no” according to whether they perceived a phosphene immediately after each TMS pulse. The Mobs thresholding was repeated if the number of “yes” responses did not fall between the range 8–12 (inclusive) for 20 TMS pulses during the baseline. The TMS intensity was then kept constant for a given run. On average, each run took 20 min (including Mobs thresholding), with each run followed by a 10 min break. All subjects had both hemispheres and both ears irrigated (4 runs). The order of the ear/hemisphere sequence was balanced across 12 subjects. “Subjects' phosphene SIZE and INTENSITY reporting”—after every successful baseline sequence (of 20 TMS pulses), subjects were told to apply a subjective average rating of 3/5 for phosphene size and intensity elicited during the baseline. Immediately following each of the postbaseline phases, each with 20 TMS pulses (i.e., vestibular activation and 2 recovery phases), subjects were asked to rate phosphene size and intensity out of 5 as compared with the baseline rating of 3/5. This rating was updated for each new baseline for each of the 4 “runs.” Consistent with the literature, V1 phosphenes tended to be small and point like lying near or across the midline. In contrast, V5/MT phosphenes were usually large, for example, pizza wedges, and peripheral.
Materials and Methods
Subjects
All 20 subjects gave informed consent prior to participating in the study, which had been approved by the local National Health Service Research Ethics Committee (there were 12 subjects per experiment with 4 subjects participating in both experiments). All subjects had no prior medical history (including ear problems or vertigo) nor were they on regular medication. Safety precautions including relevant subject exclusion criteria (e.g. excluding subjects with a personal or family history of epilepsy) utilized published recommendations (Rossi et al. 2009).
Following consent but prior to inclusion, subjects were tested to ascertain whether they could reliably perceive phosphenes in both hemispheres (see below for the technique used). Next, subjects were required to tolerate caloric irrigation with no ill effects (e.g., nausea). Six subjects in Experiment 1 and 5 subjects in Experiment 2 had already experienced caloric irrigation with documented normal nystagmic and vertigo responses. Caloric-naive subjects were given 2 cold water irrigations on a day separate to the actual experiment to ensure both normal vestibular functioning and tolerability for the subject.
Using TMS to Probe Visual Cortical Excitability
Electrical field stimulation of the visual cortex, including that by TMS, produces illusory percepts of light called “phosphenes” (Brindley and Lewin 1968). The probability of evoking a phosphene is related to the instantaneous visual cortical excitability (Aurora and Welch 1998; Boroojerdi et al. 2000; Rauschecker et al. 2004; Romei et al. 2010). Thus the TMS intensity required to elicit a phosphene can be used to probe visual cortical excitability at the location of the applied magnetic pulse. Our experimental strategy consisted first of determining an individual's 50% phosphene threshold (i.e., the TMS intensity which evoked a phosphene 50% of the time) at a given cortical location using a modified binary search algorithm–“MOBs” (Tyrell and Owens 1988). We chose a 50% phosphene detection rate since 1) many previous papers have used a 50% phosphene detection rate as a measure of visual cortical excitability (e.g., Aurora and Welch 1998; Boroojerdi et al. 2000), so our use of a similar measure allows an easier comparison with previous data; 2) a 50% detection rate allowed us to observe equally, increases or decreases on rate of phosphene detection with our intervention (i.e., vertigo).
Thus, using the same TMS intensity as that used to obtain a baseline 50% phosphene detection rate, we recorded the probability of inducing phosphenes during vestibular activation via caloric irrigation and twice more during the postcaloric period. This sequence was carried out 4 times in a subject, that is, twice for each ear and each hemisphere (Fig. 1). We assessed the change in cortical excitability with vestibular activation in 12 healthy volunteers (average age 28 years, 6 males) in area V5/MT (Experiment 1) and then in EVC (Experiment 2), in 12 healthy subjects (average age 28 years, 9 males), 4 of whom participated in Experiment 1. We also assessed the effect of perceived self-motion engendered by a non-vestibular stimulus (viz. auditory vection), on V5/MT phosphene reports in a control experiment (see Supplementary Materials).
TMS Stimulation Parameters
TMS was delivered to the cranial region of interest using a Magstim 200 stimulator (Magstim Co., UK) via a 70 mm figure-of-eight–shaped coil held in place by a dedicated coil holder. V5/MT stimulation sites were located using a functional method typically used in studies investigating phosphene perception (Aurora and Welch 1998; Guzman-Lopez et al. 2011; Schwarzkopf et al. 2011; and for a detailed discussion, see Walsh and Pascual-Leone 2003). A point was marked on the cranium, 3 cm dorsal and 5 cm lateral to the inion. Stimulation (at 80% of stimulator output) commenced at this point, and the coil was then moved around this spot (up to a maximum of 0.5 cm from the marked point), until a location from which a consistently vivid phosphene was induced. Eleven subjects saw clear and consistently moving phosphenes (one subject reported some variability in seeing the phosphenes move) in both hemispheres at this location, either at phosphene threshold or at a higher TMS intensity. For V5/MT, average phosphene thresholds, expressed as a percentage of maximal stimulator output, were as follows: all thresholds—63.1%; right and left hemispheres—60.6% and 65.5%, respectively; ipsilateral and contralateral conditions—63.3% and 62.8%.
EVC was determined by measuring 2 cm dorsal and 1 cm lateral to the inion. The coil was moved around an area of maximum radius 0.5 cm from the marked point until a vivid phosphene was obtained. No moving phosphenes were seen at this location. For EVC, average phosphene thresholds were as follows: all thresholds—68.6%; right and left hemispheres—68.6% and 68.5%, respectively; ipsilateral and contralateral conditions—68.1% and 69.0%.
Caloric Irrigation
Subjects underwent cold water caloric irrigation in the prone position to near 30 °C below the horizontal (Fig. 1). Following otoscopy, the external auditory meatus was irrigated with water at 30 °C at a rate of 500 mLs/min for 40 s. The onset of vertigo and slow-phase vestibular nystagmus typically reaches a maximum 20 s after the end of the irrigation and lasts up to 2 min (Hood and Korres 1979). Subjects' nystagmic responses were not measured during the phosphene experiments, but we confirmed that subjects experienced dizziness as in preexperiment caloric irrigations.
Entropy Estimation
Entropy is a measure of the uncertainty associated with a random variable. In the case of an equally weighted coin being flipped, the uncertainty associated with the outcome would be 1 bit—the maximum entropy possible for 2 outcomes. It was shown by Shannon (1948) that entropy is the only correct way to measure this uncertainty; it is therefore of interest to examine phosphene probabilities in entropy terms, as this allows us to directly interpret measured changes in phosphene probability as a reduction in the uncertainty of the phosphene report. To assess how much the uncertainty of phosphene reporting was reduced by caloric irrigation, we measured the yes/no response of subjects to each of 80 TMS pulses per experimental run. There were 4 runs per subject, thus this sequence of 80 pulses was repeated 4 times for each subject. In order to quantify the entropy over trials, we first calculated the probability of a yes response for each run (over subjects and repeats). This probability distribution (p) was used to calculate the Shannon entropy (Shannon 1948):
| (1) |
where the index i runs over K possible trial outcomes. In our case, i can be 0 (no phosphene) or 1 (phosphene observed). In practice, we estimated H using the NSB entropy estimation algorithm (Nemenman et al. 2004), a Bayesian technique using a Dirichlet prior, as described in Supplementary Materials. All entropy calculations were bootstrapped 200 times and have been presented as mean ± standard deviation over this bootstrapped distribution (i.e., standard error of the entropy) in the text.
Results
Effect of Vestibular Activation on Probability of Perceiving Phosphenes
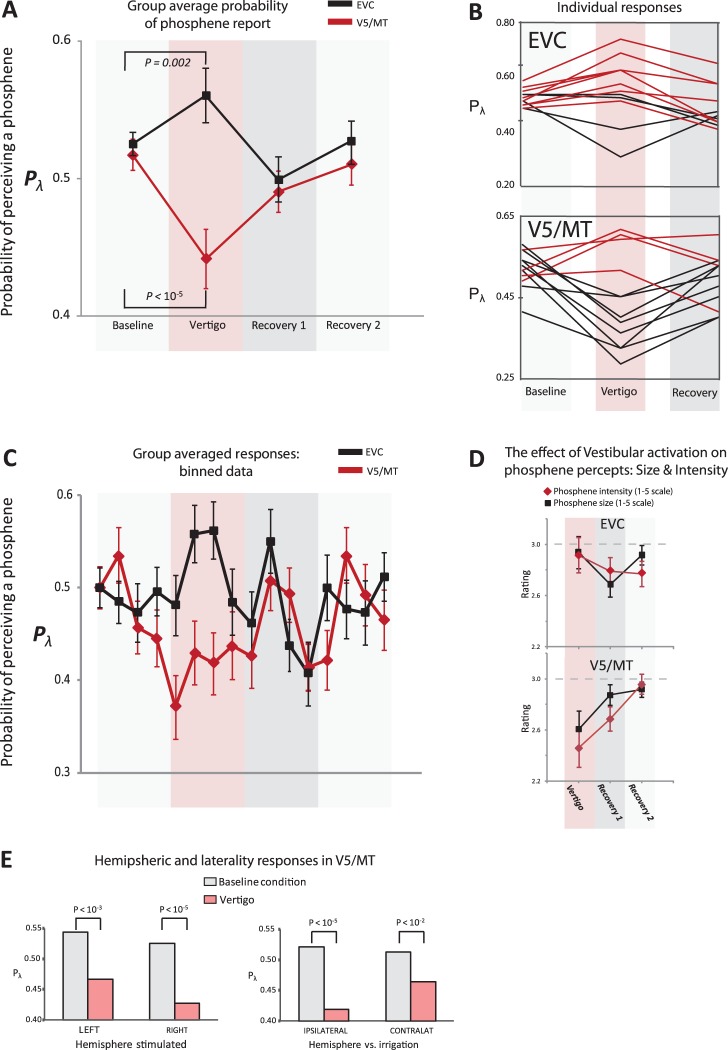
Vestibular activation significantly reduced the probability of perceiving V5/MT phosphenes as compared with baseline (P < 10−5, Binomial test; Fig. 2A). In contrast, vestibular activation “increased” the probability of perceiving V1 phosphenes, although the magnitude of the effect was less than that seen in V5/MT (P = 0.002, Binomial test; Fig. 2A). We also analyzed the phosphene responses via a repeated measures analysis of variance (ANOVA) with factors “cortex” (2 levels: V5/MT vs. EVC) and phase (4 levels: viz., “baseline,” “vertigo,” “recovery1,” and “recovery2”). Given the pattern of response shown in Figure 2A, the lack of significant (P > 0.05) main effects of cortex and “phase” were to be expected, but the significant interaction between cortex and phase (F3,9 = 4.24, P = 0.04) confirmed the dichotomous response pattern between V5/MT versus EVC. An analysis of the individual responses (Fig. 2B) showed that for V5/MT, 8 subjects perceived fewer phosphenes during vestibular activation and only 2 subjects showed a prominent increase in phosphene frequency. The pattern for EVC differed from that for V5/MT with most subjects showing increases in perceiving EVC phosphenes during vestibular activation.
Figure 2.
The effect of vestibular activation on perceiving phosphenes. (A) The group effect of vestibular activation (via caloric irrigation) on EVC (black) and V5/MT (red). The probability of perceiving a phosphene (Pλ) was assessed during vestibular activation. Note that Pλ for baseline was purposefully titrated close to 0.5. The standard errors were obtained by taking the average of the probability scores for each subject for all of the phases (n = 12). Significance compared with baseline was assessed via the binomial test corrected for multiple comparisons (Bonferroni corrected significance of P < 0.016). (B) The panels show individual Pλ responses (baseline and vestibular activation) for EVC and V5 (as labeled). Here, red lines show increases and black lines decreases, in Pλ. (C) The time course of the effect of vestibular activation on Pλ (group data) for EVC (black) and V5 (red). The binary data (yes/no) was binned to produce yes probability scores (±1 standard error) for every 5 TMS pulses (viz. each score was out of 5). To aid comparison between EVC and V5 responses over time, the displayed probability for the first bin (at baseline) was normalized to a score of 0.5 for both EVC and V5. (D) Vestibular activation systematically reduced SIZE and INTENSITY of reported phosphenes at V5/MT but not EVC. (E) The left panel shows the effects of vestibular activation (pink bars) versus baseline (gray) for left and right hemispheres (labeled) irrespective of side of caloric irrigation. Although both hemispheres showed an effect, there was a more prominent inhibitory effect observed for right V5 phosphenes. A more prominent inhibitory effect was also noted for the ipsilateral condition (i.e. right hemisphere and right caloric irrigation or left hemisphere and left caloric irrigation) compared to the contralateral condition.
To illustrate the time course of the effect of vestibular activation on visual cortical excitability, the binary responses (“yes” and “no”) were converted into a “probability score” with yes (=1) and no (=0) responses summed to produce a score for every 5 stimuli. Thus the 20 responses per phase were binned into 4 epochs. Figure 2C shows the average scores obtained for each of the 4 epochs per phase across all subjects. The binned data displays the clear divergence between the scores for V5/MT (attenuation) compared with V1 (augmentation) underlying changes in cortical excitability in response to vestibular activation.
When the effect of vestibular activation from Experiment 1 (V5/MT phosphenes) was divided into ipsilateral (irrigation on same side as TMS stimulation) and contralateral, the effect of vestibular activation on attenuating phosphene perception was still significant (Fig. 2D; Bonferroni corrected significance level P = 0.01), although the size of the effect was more prominent in the ipsilateral condition. Similarly, we found a significant effect of vestibular activation on attenuating the probability of perceiving right and left hemisphere elicited V5/MT phosphenes (Fig. 2D) with a more marked effect in the right hemisphere. The same analysis for EVC found no significant difference in the size of the effect between hemispheres.
Effect of Vestibular Activation on Phosphene Percept
Subjects were told to give a rating to the phosphenes obtained during baseline of 3/5 for SIZE and INTENSITY and then to give subsequent phosphene ratings relative to baseline (and of 5) at the end of each phase for each run. The averaged data for SIZE and INTENSITY responses are shown in Fig. 2E demonstrating that vestibular activation modulated V5/MT but not V1 phosphene characteristics. We analyzed phosphene SIZE and INTENSITY ratings in response to vestibular activation for both EVC and V5/MT, in 2 ways:
First, we assumed that subjects maintained an accurate memory of the baseline rating (i.e., 3/5). We thus performed a 1-sample t-test between rating scores obtained for the “vestibular activation” condition as compared with a hypothetical mean of 3 (of 5). This showed significant effects for V5/MT for SIZE (t = 2.73, df = 47, P < 0.01) and INTENSITY (t = 3.64, df = 47, P < 0.001) but not for EVC (P > 0.05) for either rating (SIZE or INTENSITY).
The second method assumed that the recovery back toward baseline from the vestibular activation condition more accurately described subjects' internal rating of phosphene SIZE and INTENSITY. Here, we performed separate repeated measures ANOVA for INTENSITY and SIZE estimates with factors “laterality” (ipsilateral versus contralateral) and “phase” (vestibular activation, Recovery 1, Recovery 2). For V5/MT, phosphene INTENSITY showed a significant main effect of laterality (F1,23 = 9.57, P = 0.005) and phase (F2,22 = 3.53, P < 0.05) but with no interaction between laterality and phase. For EVC, we found no significant effect of vestibular activation on perceived phosphene SIZE or INTENSITY. In summary, the data show that vestibular activation attenuates V5/MT phosphene INTENSITY; the evidence for an effect on phosphene SIZE at V5/MT was however less compelling. In contrast, all the analyses showed no effect of vestibular activation on EVC phosphene characteristics.
Information Theoretic Analysis of Effect of Vestibular Activation on Visual Cortical Excitability
We found that vestibular activation affected the excitability of both EVC and V5/MT, but this does not necessarily prove that vestibular signals have a specific functional interaction with visual cortex. To further interrogate the specificity of the vestibular effect on phosphene reports, we hypothesized that a functionally relevant visuovestibular interaction would be indicated by a significant reduction in the uncertainty associated with phosphene reports as assessed by an entropy analysis.
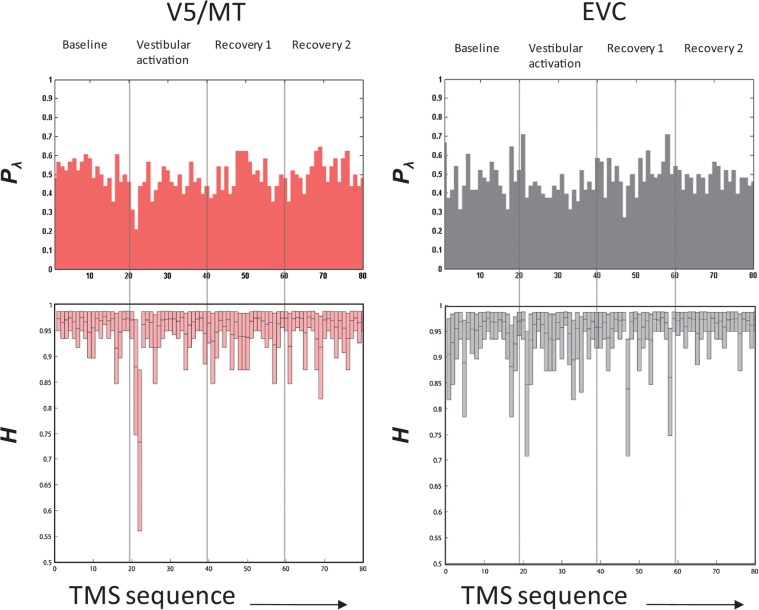
We titrated the baseline TMS intensity to yield a phosphene response rate of close to 50%, that is, the probability of perceiving a phosphene was 0.5, which, for a binary choice situation, is the state of maximum uncertainty (or maximum “Entropy” = 1 bit). We obtained the response entropy (pooled across subjects and repeats) throughout the 80 TMS stimulus sequence (Fig. 3) for baseline (stimuli 1–20), vestibular activation (stimuli 22–40), and recovery phases (stimuli 41–60 and 61–80). As the simple “plugin” estimate of entropy is biased by limited sampling (Miller 1955; Panzeri and Treves 1996), we used the NSB entropy estimator (Nemenman et al. 2004) for our calculations. Using surrogate data, we were able to demonstrate that the expected bias using this estimator with our sample set size (80 trials) is limited to a few percent (see Materials and Methods and Supplementary Materials). The key observable effect was a significant reduction in entropy for V5/MT stimulation responses during the 21st and 22nd trials (from 0.96 ± 0.03 bits at baseline to 0.73 ± 0.10 bits in the 22nd trial, significant at p ≈ 10−140 with two-sample Kolmogorov-Smirnov test), corresponding to the onset of vestibular activation.
Figure 3.
Response entropy. The 4 panels (V5/MT on the left and EVC on the right) relate to response data pooled from the subjects for each TMS stimulus in a run and ordered in the sequence of presentation; that is, each run consisted of 4 phases and 20 stimuli/phase so there were 80 TMS pulses per run (x-axis of panels = 1–80). The top panels show the pooled Pλ (probability of seeing a phosphene), and the bottom panels show information entropy (H). For V5/MT, the baseline entropy is stable but a clear decline in H occurs with vestibular activation. In contrast, for EVC, the moderate decline in H with vestibular activation is comparable to the entropy spikes that seem to occur spontaneously throughout the record.
Discussion
Our main finding was that vestibular activation resulted in opposing effects on phosphene reports at area V5/MT versus EVC; specifically, a reduction at V5/MT versus an increase in EVC phosphene reports were observed during vestibular activation.
Specific and Nonspecific Effects of Vestibular Activation on Visual Cortex
Our results additionally suggest a specific vestibular effect on visual cortex area V5/MT. First, that EVC and V5/MT results were divergent excludes a generalized effect of vestibular activation (e.g. arousal) on visual cortical excitability. Second, using a similar protocol, we have recently shown that visual motion adaptation increases V5/MT excitability (Guzman-Lopez et al. 2011). Third, V5/MT phosphene intensity and size were attenuated by vestibular modulation. Note that the task required subjects to both detect phosphenes and assess their characteristics (i.e., phosphene size and intensity) and thus divided their attention. This is a potential reason for attenuating phosphene percepts, but this was the case for all phases, including baseline (where subjects were also required to focus on phosphene presence and characteristics in applying a 3/5 size and intensity score) and was the same for both V5/MT and EVC phosphenes where we obtained opposing responses. Fourth, that we found a larger vestibular effect on cortical excitability in the right hemisphere is consistent with reports of a right hemisphere bias for vestibular cortical processing (Dieterich et al. 2003; Seemungal et al. 2008) and provides further evidence against a nonspecific effect for our results. Last, we found that V5/MT response entropy (Fig. 3) changed markedly during the initial period of vestibular activation (where vestibular activation is maximal), in stark contrast to highly stable response entropy in the pre- and postvestibular epochs.
Theoretically, caloric-evoked eye movements could contribute to the observed vestibular effects on V5/MT excitability (i.e., suppressive), since vestibular effects were more prominent when the fast phase was directed toward the stimulated (with TMS) hemisphere. Such an eye movement–related effect could not however account for “all” of our findings since we also found significant inhibitory effects when the evoked nystagmus was directed away from the stimulated hemisphere. Additionally, 2 previous studies (Thilo et al. 2004; Boulay and Paus 2005) failed to find an eye-movement modulation of visual cortical excitability (measured by TMS-induced phosphenes).
Another issue is whether vertigo, which itself creates illusions of self-motion, could be more distracting to the perception of moving V5 phosphenes than to static phosphenes and thus explain the discrepancy in response between the V5 and EVC. An additional control experiment (see Supplementary Materials) where we assessed V5/MT phosphene reports during illusory self-motion engendered by a rotating sound source (called “auditory vection”) failed to show any suppressive effect on phosphenes (there was a trend for increased phosphene reports during vection). These data thus suggest that it is vestibular activation and not a sensation of self-motion per se that results in an attenuation of V5/MT phosphene reports.
Our data suggest that vestibular activation augments EVC excitability in a nonspecific manner. We found no effect of vestibular activation on reported phosphene size and intensity, and no laterality effect (right versus left hemisphere) on reported phosphene probability, implying a more general effect for EVC. EVC response entropy did dip slightly with vestibular activation but this appeared unremarkable in the context of repeated entropy “spikes” (Fig. 3) perhaps alluding to the multimodal sensory inputs to EVC. For example, vestibular, pain, and auditory stimulation all enhance V1 neuronal excitability (Gorgiladze and Smirnov 1967; Romei et al. 2007).
Single cell data in primates (Chowdhury et al. 2009) demonstrate a modulation of V5/MT neuronal activity by rotations in the light but with marked attenuation during dark rotations with a light point fixation. These data suggest an optic flow modulation rather than a direct vestibular drive to the sampled V5/MT neurons but do not however exclude the possibility that V5/MT neurons could show a vestibular modulation only during concurrent visual motion stimulation. Such a modulation was found in the lateral geniculate nucleus (LGN) (Papanioannou 1973), where the caloric effects on LGN light-evoked neuronal responses were independent of caloric effects upon spontaneous activity.
Although area V1 is the major input to V5/MT, hence explaining the finding that changes in human V1 excitability (e.g., by magnetic or electrical stimulation) generally match changes in V5/MT excitability (Antal et al. 2003; Silvanto et al. 2005), our data showing a dichotomous effect on EVC versus V5/MT excitability imply a vestibular modulation of V5/MT activity via direct subcortical pathways to V5. Indeed, there is recent evidence supporting direct LGN and collicular inputs to V5/MT (Sincich et al. 2004; Nassi and Callaway 2006; Berman and Wurtz 2008; Lanyon et al. 2009; Lyon et al. 2010; Schmid et al. 2010). This data allied with evidence for vestibular reactivity in both subcortical loci (Papanioannou 1973; Bisti et al. 1974; Magnin et al. 1974), provides a potential anatomical explanation for our findings.
A Role for Vestibular Modulation of Visual Signals?
One role for a vestibular modulation of visual motion signals at cortical level would be optimizing visual form perception. The magnocellular system conveying visual motion information to V5/MT via a low latency pathway (Bullier 2001; Born and Bradley 2005) could be suited to a vestibular modulation, since vestibular signals possess low latencies themselves. Beckers and Zecki (1995) found evidence of a fast direct non-V1-dependent pathway relaying visual motion signals to V5/MT in humans. Furthermore, recurrent (and rapid) V5/MT-V1 pathways modulate the earliest components of striate neuronal responses that affect motion processing, including direction and motion selectivity responses (Hupé et al. 1998; Galuske et al. 2002). Such recurrent pathways could also benefit visual form processing if head motion predictive signals were utilized to optimize vision during head turns when minor degrees of retinal image slip and thus defoveation could occur. Supporting this notion is the observation in humans that visual discrimination is enhanced during vestibular activation (Guedry and Ambler 1973; Guedry 1974; Tong et al. 2006) and in animals that inactivating V5/MT impaired both visual motion and global visual form responses in V1 (Hupé et al. 1998). It is tempting to speculate that vestibular signals, with latencies in the order of 5 ms, could specifically modulate those fast V1-independent pathways which could be employed in a motion predictive mechanism to enable a rapid retuning of visual perception to improve visual form perception. Indeed Vannier-Mercier and Magnin (1982) considered such a role for vestibular signals following their demonstration of a vestibular modulation of V1 neuronal activity in cats.
A role for vestibular signals in optimizing visual form perception does not obviate a vestibular role in resolving the self- versus object-motion conflict. Indeed, neuronal centre-surround inhibition, a prominent feature of V5/MT neurons, is one suggested neuronal mechanism (Huang et al. 2008) mediating motion perception discrimination (for review, also see Born and Bradley 2005). The classical neuronal receptive fields, where increased neuronal firing rate occurs in response to visual targets within a specific retinotopic location, are attenuated by centre-surround inhibition mechanisms (prominent in V5/MT), which are engaged by large visual targets. Thus small high-contrast stimuli would engender overall neuronal activation and large visual targets overall neuronal inhibition. Centre-surround inhibition may play a role in visual-vestibular phenomenon, such as vection, whereby large field visual stimuli (e.g., moving clouds) elicit sensations of self-motion. Vection carries the risk of an erroneous interpretation of self-motion. Centre-surround mechanisms, by modulating the integration or segmentation of a moving visual scene, may help resolve this object/self-motion ambiguity (Huang et al. 2008). Hence, the centre-surround mechanism could mediate the reported visual-vestibular antagonism suggested by human imaging studies (for review, see Dieterich and Brandt 2008).
Bimodal Sensory Interaction, State Dependent Excitability, and Information Content
Recent neurophysiological data demonstrate that bimodal stimulation engenders cortical neuronal responses with more information content than with unimodal stimulation (Kayser et al. 2010). Additionally, when the result of such bimodal combination is a reduction in neuronal activity, the information content is further increased. Indeed, we have recently quantified the state-dependency of TMS-induced V5/MT responses (Guzman-Lopez et al. 2011) showing that adaptation to a random dot visual motion stimulus induces a facilitation of TMS responses. Conversely, we suggest that vestibular activation might reduce the level of neuronal excitability in V5/MT by suppressing visually evoked activity that is incompatible with the vestibular cue, resulting in an overall more sparse V5/MT activity. In contrast, a nonspecific vestibular effect on area EVC would be equivalent to adding noise thus making EVC more likely to be excited by TMS.
Conclusion
In summary, we found that vestibular activation specifically suppresses V5/MT excitability but engenders a nonspecific increase in Early Visual Cortical excitability. Such visuovestibular cortical interaction could underlie the perceptual mechanisms that attempt to resolve visual motion from self-motion percepts while simultaneously optimizing visual form perception under natural conditions of vestibular activation such as during locomotion.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
B.M.S. is supported by a Health Foundation/Academy of Medical Sciences Clinician Scientist Fellowship; S.R.S. by the Engineering and Physical Research Sciences Council (United Kingdom) and a Royal Society Industry Fellowship; V.W. by a Royal Society Wolfson Research Merit award and the Medical Research Council (United Kingdom); N.Y. by a BUPA Foundation award; J.G. by the National Council of Science and Technology of Mexico.
Supplementary Material
Acknowledgments
We thank Dr Paresh Malhotra for his comments on the manuscript. Conflict of Interest: None declared.
References
- Antal A, Kincses TZ, Nitsche MA, Paulus W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia. 2003;41:1802–1807. doi: 10.1016/s0028-3932(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Aurora SK, Welch KM. Brain excitability in migraine: evidence from transcranial magnetic stimulation studies. Curr Opin Neurol. 1998;11:205–209. doi: 10.1097/00019052-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Beckers G, Zecki S. The consequences of inactivating areas V1 and V5 on visual motion perception. Brain. 1995;118:49–60. doi: 10.1093/brain/118.1.49. [DOI] [PubMed] [Google Scholar]
- Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI) J Neurophysiol. 2001;85(2):886–899. doi: 10.1152/jn.2001.85.2.886. [DOI] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH. Exploring the pulvinar pathway to visual cortex. Prog Brain Res. 2008;171:467–473. doi: 10.1016/S0079-6123(08)00668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisti S, Maffei L, Piccolino M. Visuovestibular interactions in the cat superior colliculus. J Neurophysiol. 1974;37:146–155. doi: 10.1152/jn.1974.37.1.146. [DOI] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;18:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Prager A, Muellbacher W, Cohen LG. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology. 2000;54:1529–1531. doi: 10.1212/wnl.54.7.1529. [DOI] [PubMed] [Google Scholar]
- Bottini G, Karnath HO, Vallar G, Sterzi R, Frith CD, Frackowiak RS, Paulesu E. Cerebral representations for egocentric space: functional-anatomical evidence from caloric vestibular stimulation and neck vibration. Brain. 2001;124:1182–1196. doi: 10.1093/brain/124.6.1182. [DOI] [PubMed] [Google Scholar]
- Boulay C, Paus T. Modulation of phosphene perception during saccadic eye movements: a transcranial magnetic stimulation study of the human visual cortex. Exp Brain Res. 2005;167(2):297–300. doi: 10.1007/s00221-005-0103-1. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol. 1968;196(2):479–493. doi: 10.1113/jphysiol.1968.sp008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullier J. Integrated model of visual processing. Brain Res Brain Res Rev. 2001;36(2–3):96–107. doi: 10.1016/s0165-0173(01)00085-6. [DOI] [PubMed] [Google Scholar]
- Chowdhury SA, Takahashi K, DeAngelis GC, Angelaki DE. Does the middle temporal area carry vestibular signals related to self-motion? J Neurosci. 2009;29(38):12020–12030. doi: 10.1523/JNEUROSCI.0004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13(9):994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain. 2008;131:2538–2552. doi: 10.1093/brain/awn042. [review] [DOI] [PubMed] [Google Scholar]
- Engelhardt H, Feddersen B, Boetzel K, Noachtar S. The influence of caloric nystagmus on flash evoked transient and steady-state potentials. Clin Neurophysiol. 2007;118(10):2282–2286. doi: 10.1016/j.clinph.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17(3):1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Galuske RA, Schmidt KE, Goebel R, Lomber SG, Payne BR. The role of feedback in shaping neural representations in cat visual cortex. Proc Natl Acad Sci U S A. 2002;99(26):17083–17088. doi: 10.1073/pnas.242399199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgiladze GI, Smirnov GD. The effect of vestibular stimulation on the neuronal activity of the visual cortex of the cat brain. Zh Vyssh Nervn Deyat Im I P Pavlova. 1967;17(2):345–352. [PubMed] [Google Scholar]
- Guedry FE., Jr . Psychophysics of vestibular sensation. In: Kornhuber HH, editor. Handbook of sensory physiology, Vol. VI, Pt 2. Berlin (Germany): Springer-Verlag; 1974. pp. 3–154. [Google Scholar]
- Guedry FE, Ambler RK. Assessment of reactions to vestibular disorientation stress for purposes of aircrew selection. AGARD-CP-109. London: Technical Editing and Reproduction Ltd; 1973. [Google Scholar]
- Guzman-Lopez J, Silvanto J, Seemungal BM. Visual motion induction increases the susceptibility of area V5/MT to phosphene induction by transcranial magnetic stimulation. Clin Neurophysiol. 2011;122(10):1951–1955. doi: 10.1016/j.clinph.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Huang X, Albright TD, Stoner GR. Stimulus dependency and mechanisms of surround modulation in cortical area MT. J Neurosci. 2008;28(51):13889–13906. doi: 10.1523/JNEUROSCI.1946-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupé JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons. Nature. 1998;394(6695):784–787. doi: 10.1038/29537. [DOI] [PubMed] [Google Scholar]
- Hood JD, Korres S. Vestibular suppression in peripheral and central vestibular disorders. Brain. 1979;102(4):785–804. doi: 10.1093/brain/102.4.785. [DOI] [PubMed] [Google Scholar]
- Iida M, Sakai M, Igarashi M. Visual-vestibular interaction-an evoked potential study in normal human subjects. Tokai J Exp Clin Med. 1997;22(3):137–139. [PubMed] [Google Scholar]
- Kayser C, Logothetis NK, Panzeri S. Visual enhancement of the information representation in auditory cortex. Curr Biol. 2010;20:19–24. doi: 10.1016/j.cub.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Lanyon LJ, Giaschi D, Young SA, Fitzpatrick K, Diao L, Bjornson BH, Barton JJ. Combined functional MRI and diffusion tensor imaging analysis of visual motion pathways. J Neuroophthalmol. 2009;29(2):96–103. doi: 10.1097/WNO.0b013e3181a58ef8. [DOI] [PubMed] [Google Scholar]
- Lobel E, Kleine JF, Bihan DL, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol. 1998;80(5):2699–2709. doi: 10.1152/jn.1998.80.5.2699. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Nassi JJ, Callaway EM. A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron. 2010;65(2):270–279. doi: 10.1016/j.neuron.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnin M, Jeannerod M, Putkonen P. Vestibular and saccadic influences on dorsal and ventral nuclei of the lateral geniculate body. Exp Brain Res. 1974;21(1):1–18. doi: 10.1007/BF00234255. [DOI] [PubMed] [Google Scholar]
- Miller GA. Problems and methods II-B. IL: Free Press; 1955. Note on the bias on information estimates Information Theory in Psychology; pp. 95–100. [Google Scholar]
- Nassi JJ, Callaway EM. Multiple circuits relaying primate parallel visual pathways to the middle temporal area. J Neurosci. 2006;26(49):12789–12798. doi: 10.1523/JNEUROSCI.4044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemenman I, Bialek W, de Ruyter van Steveninck R. Entropy and information in neuronal spike trains: progress on the sampling problem. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:056111–056116. doi: 10.1103/PhysRevE.69.056111. [DOI] [PubMed] [Google Scholar]
- Panzeri S, Treves A. Analytical estimates of limited sampling biases in different information measures. Network. 1996;7:87–107. doi: 10.1080/0954898X.1996.11978656. [DOI] [PubMed] [Google Scholar]
- Papanioannou JN. Changes in the light-evoked discharges from lateral geniculate neurones in the cat, induced by caloric labyrinthine stimulation. Exp Brain Res. 1973;17:10–17. doi: 10.1007/BF00234560. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast back projections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292(5516):510–512. [Google Scholar]
- Probst T, Brandt T, Degner D. Object-motion detection affected by concurrent self-motion perception: psychophysics of a new phenomenon. Behav Brain Res. 1986;22(1):1–11. doi: 10.1016/0166-4328(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Probst T, Straube A, Bles W. Differential effects of ambivalent visual-vestibular somatosensory stimulation on the perception of self-motion. Behav Brain Res. 1985;16(1):71–79. doi: 10.1016/0166-4328(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Probst T, Wist ER. Electrophysiological evidence for visual-vestibular interaction in man. Neurosci Lett. 1990;108(3):255–260. doi: 10.1016/0304-3940(90)90650-x. [DOI] [PubMed] [Google Scholar]
- Rauschecker AM, Bestmann S, Walsh V, Thilo KV. Phosphene threshold as a function of contrast of external visual stimuli. Exp Brain Res. 2004;157(1):124–127. doi: 10.1007/s00221-004-1910-5. [DOI] [PubMed] [Google Scholar]
- Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J Neurosci. 2010;30(25):8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Murray MM, Merabet LB, Thut G. Occipital transcranial magnetic stimulation has opposing effects on visual and auditory stimulus detection: implications for multisensory interactions. J Neurosci. 2007;27(43):11465–11472. doi: 10.1523/JNEUROSCI.2827-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M, Peters AJ, Ye FQ, Leopold DA. Blindsight depends upon the lateral geniculate nucleus. Nature. 2010;466:373–377. doi: 10.1038/nature09179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf DS, Silvanto J, Rees G. Stochastic resonance effects reveal the neural mechanisms of transcranial magnetic stimulation. J Neurosci. 2011;31(9):3143–3147. doi: 10.1523/JNEUROSCI.4863-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal BM, Masaoutis P, Green DA, Plant GT, Bronstein AM. Symptomatic recovery in Miller Fisher syndrome parallels vestibular-perceptual and not vestibular-ocular reflex function. Front Neurol. 2011;2:2. doi: 10.3389/fneur.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemungal BM, Rizzo V, Gresty MA, Rothwell JC, Bronstein AM. Posterior parietal rTMS disrupts human Path Integration during a vestibular navigation task. Neurosci Lett. 2008;437(2):88–92. doi: 10.1016/j.neulet.2008.03.067. [DOI] [PubMed] [Google Scholar]
- Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27:379–423. [Google Scholar]
- Silvanto J, Cowey A, Lavie N, Walsh V. Striate cortex (V1) activity gates awareness of motion. doi: 10.1038/nn1379. 2005. Nat Neurosci. 8(2): 143–144. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci. 2004;10:1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Stephan T, Deutschländer A, Nolte A, Schneider E, Wiesmann M, Brandt T, Dieterich M. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage. 2005;26(3):721–732. doi: 10.1016/j.neuroimage.2005.02.049. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, Shiino A, Kitajima K. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2001;12(3):441–449. doi: 10.1016/s0926-6410(01)00080-5. [DOI] [PubMed] [Google Scholar]
- Thilo KV, Santoro L, Walsh V, Blakemore C. The site of saccadic suppression. Nat Neurosci. 2004;7(1):13–14. doi: 10.1038/nn1171. [DOI] [PubMed] [Google Scholar]
- Tong J, Patel SS, Bedell HE. The attenuation of perceived motion smear during combined eye and head movements. Vision Res. 2006;46(26):4387–4397. doi: 10.1016/j.visres.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrell RA, Owens DA. A rapid technique to assess the resting states of the eyes and threshold phenomena: the Modified binary search (Mobs) Behav Res Methods Instrum Comput. 1988;20:137–141. [Google Scholar]
- Vannier-Mercier G, Magnin M. Single neuron activity related to natural vestibular stimulation in the cat's visual cortex. Exp Brain Res. 1982;45:451–455. doi: 10.1007/BF01208606. [DOI] [PubMed] [Google Scholar]
- Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res. 1977;27(5):523–538. doi: 10.1007/BF00239041. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation: a neurochronometrics of mind. Cambridge (MA): MIT Press; 2003. [Google Scholar]
- Wenzel R, Bartenstein P, Dieterich M, Danek A, Weindl A, Minoshima S, Ziegler S, Schwaiger M, Brandt T. Deactivation of human visual cortex during involuntary ocular oscillations. A PET activation study. Brain. 1996;119:101–110. doi: 10.1093/brain/119.1.101. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol. 1974;236:549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.