Abstract
Both cognitive and affective processes require mental resources. However, it remains unclear whether these 2 processes work in parallel or in an integrated fashion. In this functional magnetic resonance imaging study, we investigated their interaction using an empathy-for-pain paradigm, with simultaneous manipulation of cognitive demand of the tasks and emotional valence of the stimuli. Eighteen healthy adult participants viewed photographs showing other people's hands and feet in painful or nonpainful situations while performing tasks of low (body part judgment) and high (laterality judgment) cognitive demand. Behavioral data showed increased reaction times and error rates for painful compared with nonpainful stimuli under laterality judgment relative to body part judgment, indicating an interaction between cognitive demand and stimulus valence. Imaging analyses showed activity in bilateral anterior insula (AI) and primary somatosensory cortex (SI), but not posterior insula, for main effects of cognitive demand and stimulus valence. Importantly, cognitive demand and stimulus valence showed a significant interaction in AI, SI, and regions of the frontoparietal network. These results suggest that cognitive and emotional processes at least partially share common brain networks and that AI might serve as a key node in a brain network subserving cognition–emotion integration.
Keywords: cognition, emotion, empathy, fMRI, insula
Introduction
The nature of the relationship between cognition and emotion has puzzled philosophers (Descartes 1649) and scientists (Gray et al. 2002; Blair et al. 2007; Duncan and Barrett 2007; Pessoa 2008) for centuries. A widely held view “functional specialization” assumes that mental faculties are instantiated by distinct brain areas. An example of this approach is the identification of certain brain regions as primarily “cognitive,” like the dorsolateral prefrontal cortex (dlPFC) (Goldman-Rakic 1996) and others as primarily “emotional,” like the amygdala (LeDoux 2000). Grounded in such cognition–emotion duality, much research has focused on either the modulatory effect of cognition on the “emotional brain” or the influence of emotional valence on the “cognitive brain” (Ochsner and Gross 2005; Pessoa 2009). However, functional localization is likely an oversimplification that should be augmented with the principle of “functional integration” (Friston 2002), which bears greater evolutionary value, especially in the case of higher level cognitive and emotional processes. Virtually, every complex behavior has both cognitive and emotional components (James 1884; Dolan 2002; Pessoa and Adolphs 2010). Some have suggested that the distinction between cognition and emotion is phenomenological (i.e., reflecting subjective experience) rather than ontological (i.e., reflecting nature or “reality”) (Duncan and Barrett 2007), in part, based on evidence of conjoint disruption of these processes in mental disorders (Amaral et al. 2008).
Based on functional integration, cognitive and emotional processes should share neural pathways (Gray et al. 2002; Pessoa 2008). Thus, the 2 processes will interfere with each other when executed simultaneously due to competition among operations for common anatomical resources of domain general pathways (Posner and Petersen 1990; Desimone and Duncan 1995; Fan et al. 2003; Buhle and Wager 2010). For instance, it has been suggested that pain and task processing engage overlapping executive resources (Buhle and Wager 2010). Given the complex nature of both processes, the brain circuitry underlying such integrated computation must be able to conjoin both types of information and, thus, to serve as an interface—and possibly also as a bottleneck (Pashler 1994)—to perception. The anterior insula (AI) and the anterior cingulate cortex (ACC) have been considered as potential candidates for such integrative processing (Craig 2009; Shackman et al. 2011) given their neuroanatomical features and connectivity (Vogt et al. 1992; Butti and Hof 2010) and their functional roles in many psychological processes (Bush et al. 2000; Craig 2009; Shackman et al. 2011). The AI, in particular, has been shown to participate in polymodal sensory integration (Critchley et al. 2004), subjective awareness (Craig 2009), pain (Lamm et al. 2011), and empathy and social emotions (Lamm and Singer 2010). Accordingly, examination of these structures holds considerable potential to inform questions about integrative processing of cognition and emotion.
In this functional magnetic resonance imaging (fMRI) study, we investigated the neural mechanisms underlying cognition–emotion integration with simultaneous manipulation of cognitive demand of the tasks (low demand: body part judgment; high demand: laterality judgment) and emotional valence of the stimuli (pain, no pain). The current study is different from our previous study (Gu et al. 2010) in that the present paradigm represents 2 tasks of different levels of cognitive demand, instead of 2 tasks of the same level of cognitive demand (as in our previous study). The purpose of the current design is to see how emotional processing interacts with cognitive processing by manipulating both emotional valence of the stimuli and cognitive demand of the tasks. High cognitive demand has been shown to activate AI and ACC (Fan et al. 2008; Nelson et al. 2010), both structures of primary interest to our hypotheses. Additionally, we used empathetic pain as the emotional stimulus because unpleasant emotional feelings are an indispensable part of pain (Price 2000), and empathetic pain has been shown to consistently activate both AI and ACC (Kurth et al. 2010; Lamm et al. 2011). Furthermore, empathetic pain seemingly has a cognitive component in addition to a more prominent affective component, making it an excellent candidate to probe cognition–emotion interactions (Lamm et al. 2011). Thus, assuming common involvement of AI and/or ACC in processing cognition and emotion, there should be behavioral and neural interaction effects between cognitive demand and empathetic pain. If cognition and emotion were processed in parallel, we would observe activation of different brain regions for cognitive demand and empathetic pain or activation of the same brain regions for both but no interaction effect. Alternatively, if the 2 operations were implemented via partially shared pathways, interaction would be observed in some “interface” regions. We favored the latter hypothesis and predicted that the AI and its related networks would serve as a site for cognition–emotion integration.
Materials and Methods
Participants
Eighteen healthy adults (9 women; 22–34 years old, mean age of 25.2 years) participated in the study. All participants were right-handed, had normal color vision, and reported no previous or current psychiatric or neurological conditions. Participants were informed of the study requirements and provided written consent prior to participation. The study was approved by the Institutional Review Board of Mount Sinai School of Medicine (MSSM).
Stimuli and Procedure
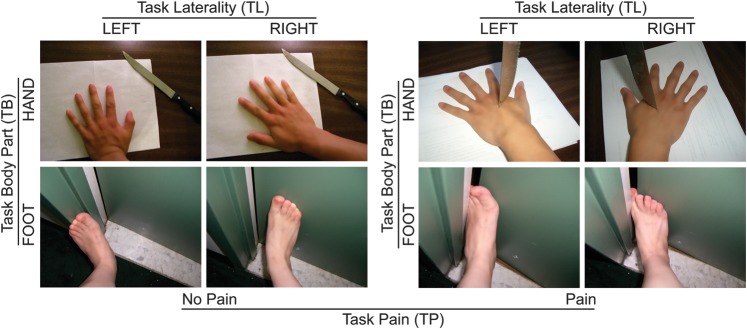
Experimental stimuli included 144 digital color photographs showing another person's left or right hand or foot in painful or nonpainful situations, a subset of stimuli used in our previous study (Gu et al. 2010). There were 18 photographs in each of the 8 permutations of 3 variables with 2 conditions each (pain: pain, no pain; laterality: left, right; and body part: hand, foot), creating 8 categories: painful-left-hand, painful-left-foot, painful-right-hand, painful-right-foot, nonpainful-left-hand, nonpainful-left-foot, nonpainful-right-hand, and nonpainful-right-foot (Fig. 1). The painful and nonpainful photographs were identical in physical properties (i.e., context, brightness, contrast). Painful stimuli were rated as significantly more painful than the nonpainful stimuli by an independent group of subjects (Gu et al. 2010).
Figure 1.
Sample stimuli of the experimental stimuli set of 144 digital color photographs. There were 18 photographs in each of the 8 permutations of 3 variables with 2 conditions each (pain: pain, no pain; laterality: left, right; and body part: hand, foot). Participants were asked to choose between “hand” and “foot” for body part judgment (TB), “left” and “right” for laterality judgment (TL), and “no pain” and “pain” for pain judgment (TP).
There were 2 different tasks: a body part identification (i.e., hand, foot) task (task body part, TB) and a laterality (i.e., left, right) identification task (task laterality, TL). The tasks relied on physically identical stimuli, differed only in the task instructions. During TB, participants were instructed to judge whether the body part shown in the photograph was a hand or a foot. During TL, participants were instructed to judge the laterality (i.e., left or right) of the hand/foot. We chose the laterality judgment task (instead of an emotionally laden task) to avoid explicit emotional judgment and to ensure that the contrast between TL and TB reflected a difference in cognitive demand. TL requires more cognitive resources than TB as confirmed by behavioral data (longer reaction time (RT) and lower accuracy; Treisman 1969; Posner 1980; see Results for details). This yielded a 2 × 2 factorial design with 2 levels of cognitive demand (TB and TL) and 2 levels of stimulus valence (no pain and pain). Additionally, a pain judgment task (task pain, TP) was used as a localizer for regions of interest (ROIs) related to cognitive demand (contrast between TP and fixation baseline) and emotional valence (contrast between TP-pain vs. TP-no pain) involved in empathetic pain processing. Subjects made explicit judgments about whether the person in the photograph was suffering from pain or not. The rationale for using TP as a localizer was that 1) TP has been shown to effectively activate both insula and ACC (Gu et al. 2010), 2) TP involves both the cognitive evaluation of pain (task-dependent response) and the emotional responses elicited by the stimuli (stimulus-dependent response), and 3) subjects' responses to TP do not depend on their responses to TB or TL, which measure the implicit processing of empathetic pain (orthogonality between TP and TB/TL).
A mixed block/event-related design was used. There were a total of 6 runs, and in each run, there were 6 blocks (2 TB blocks, 2 TL blocks, and 2 TP localizer blocks). The order of blocks was counterbalanced using a Latin square for each participant. Each block included 8 trials and each trial corresponded to 1 of the 8 categories, presented in a random order. Each photograph was displayed for 2500 ms. Interstimulus intervals were randomized and ranged from 750 to 2250 ms, with an average of 1500 ms. Participants were instructed to respond as quickly and accurately as possible.
fMRI Data Acquisition and Analysis
All magnetic resonance imaging (MRI) data were obtained using a 3-T Siemens Allegra MRI system at MSSM. Foam padding was used to minimize subject head movements. All images were acquired along axial planes parallel to the anterior commissure–posterior commissure line. A high-resolution T2-weighted anatomical volume of the whole brain was acquired with a turbo spin–echo pulse sequence. The fMRI imaging was performed using a gradient-echo echo-planar imaging (GE-EPI) sequence using the following protocol: 40 axial slices, 4-mm thick, and skip = 0 mm, time repetition = 2500 ms, time echo = 27 ms, flip angle = 82°, field of view = 240 mm, and matrix size = 64 × 64. Slices were obtained corresponding to the T2-weighted anatomical images. Six series of EPIs corresponding to the 6 runs were acquired. Each series started with 2 dummy volumes before the onset of the task to allow for equilibration of T1 saturation effects, followed by 146 image volumes for each run.
Event-related analyses of the fMRI data from the tasks were conducted using the statistical parametric mapping package (SPM5; Wellcome Department of Imaging Neuroscience, London, UK). The functional scans were adjusted for slice timing, realigned to the first volume, coregistered to the T2 image, normalized to a standard template (Montreal Neurological Institute), and spatially smoothed with an 8 × 8 × 8 mm full-width at half-maximum Gaussian kernel. General linear modeling (Friston et al. 1995) was then conducted for the functional scans from each participant by modeling the observed event-related blood oxygenation level–dependent (BOLD) signals and regressors to identify the relationship between the task events and the BOLD signal. Regressors were created by convolving a train of delta functions representing the sequence of individual events with the default SPM basis function, which consists of a synthetic hemodynamic response function composed of 2 gamma functions (Friston et al. 1998). There were seven regressors: 3 task regressors (TB: TB-pain plus TB-no pain; TL: TL-pain plus TL-no pain; and TP: TP-pain plus TP-no pain), 3 parametric regressors (TB-pain minus TB-no pain, TL-pain minus TL-no pain, and TP-pain minus TP-no pain), and 1 regressor for the first trial of each task block to model out the task-switch effect, which also elicits ACC and AI activation (Sridharan et al. 2008). Six parameters generated during motion correction were entered as covariates. Linear contrasts of the parameter estimates were made to identify the main effects of cognitive demand and empathetic pain and the interaction effect between cognitive demand and empathetic pain, resulting in images of contrast estimate for these effects for each participant. These images from all participants were entered into a second-level group analysis conducted with a random effects statistical model. Significant activations of interest were identified with voxelwise P < 0.005 in conjunction with clusterwise P < 0.05 to control for regional effects and to represent topographical inferences. This height and extent threshold combination is similar to the suggested threshold of P < 0.005 and 10 voxels (Lieberman and Cunningham 2009), reaching a desirable balance between Type I and Type II errors. These whole-brain analyses were exploratory, and our hypothesis testing was primarily built on ROI analysis (Poldrack 2007).
We selected ROIs based on previous research on empathetic pain (Lamm et al. 2011) and cognitive demand (Fan et al. 2008; Nelson et al. 2010). The coordinates of ROI centers were taken from peaks during the localizer task (cognitive demand: TP > baseline contrast for bilateral AI and ACC and emotional valence: TP-pain > TP-no pain contrast for bilateral posterior insula (PI) and somatosensory cortex (SI); for a complete listing of activated regions in these contrasts, see Supplementary Table 1). We created spherical ROIs of 6 mm radius using the MarsBaR toolbox (http://marsbar.sourceforge.net). Cognitive demand–related ROIs included bilateral AI ([−30, 20, 6] and [30, 26, 4]) and ACC ([6, 14, 46]); empathic pain–related ROIs included bilateral PI ([−40, 4, −4] and [42, −6, −8]) and bilateral SI ([−54, −28, 34] and [50, −28, 38]). These analyses were based on a priori expectations (Gu and Han 2007a; Gu et al. 2010; Lamm et al. 2011) and, therefore, were not subjected to correction for multiple comparisons (Rothman 1990). Statistical significance for the ROI analyses was set at uncorrected P < 0.05. Parameter estimates were extracted from each ROI for each of the 4 experimental conditions from each subject and then entered into a two-way repeated measures analysis of variance model for each ROI. Planned comparisons were carried out between TB-no pain and TB-pain and TL-no pain and TL-pain for each ROI.
Results
Behavioral Results
Mean RTs and error rates of 4 experimental conditions (TB-no pain, TB-pain, TL-no pain, and TL-pain) are shown in Table 1 and Figure 2. For RT (Fig. 2A), there was a significant main effect of stimulus (F1,17 = 9.21, P < 0.01), a significant main effect of task (F1,17 = 52.85, P < 0.001), and a significant interaction (F1,17 = 19.87, P < 0.001). Simple comparisons showed that for TB, participants were significantly faster judging body part for painful than nonpainful photographs (TB-no pain vs. TB-pain; F1,17 = 5.33, P < 0.05), whereas for TL, participants were slower judging body laterality for painful than nonpainful stimuli (TL-no pain vs. TL-pain; F1,17 = 27.78, P < 0.001). For error rate (Fig. 2B), there was also a significant main effect of stimulus (F1,17 = 6.38, P < 0.05), a significant main effect of task (F1,17 = 14.68, P = 0.001), and a significant interaction (F1,17 = 4.67, P < 0.05). Simple comparisons showed that there was no significant difference in error rates of judging body part of nonpainful photographs versus painful photographs (TB-no pain vs. TB-pain; F < 1), whereas judging laterality of painful stimuli was associated with significantly more errors than judging nonpainful stimuli (TL-no pain vs. TL-pain; F1,17 = 5.81, P < 0.05). These results suggest that the concurrent processing of high cognitive demand and empathetic pain interfered with each other.
Table 1.
Behavioral performance (mean ± SD)
| TB | TL | TP | ||||
| No pain | Pain | No pain | Pain | No pain | Pain | |
| RT (ms) | 884 ± 143 | 858 ± 145 | 969 ± 163 | 1032 ± 174 | 1092 ± 154 | 1050 ± 151 |
| Error (%) | 0.6 ± 1.1 | 0.7 ± 1.2 | 1.2 ± 2.2 | 3.1 ± 3.1 | 5.4 ± 6.1 | 4.8 ± 4.9 |
Figure 2.
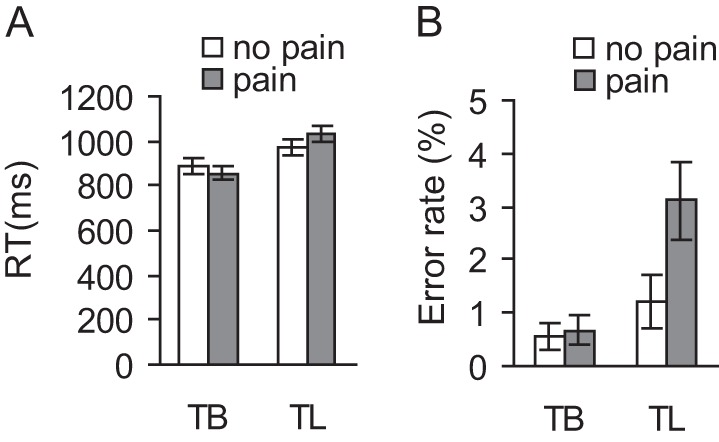
Behavioral results. (A) RTs for 4 experimental conditions. There was a significant main effect of stimulus valence, a significant main effect of cognitive demand, and a significant interaction. (B) Error rate for 4 experimental conditions. There was a significant main effect of stimulus valence, a significant main effect of cognitive demand, and a significant interaction. Error bars represent standard error of the mean.
The RTs and error rates for the 2 localizer conditions (TP-no pain and TP-pain) are also listed in Table 1. There was no significant difference between nonpainful and painful stimuli under TP in terms of RTs (t1,17 = 1.70, P > 0.05) or error rates (t1,17 = 0.33, P > 0.05).
fMRI: ROI Analysis
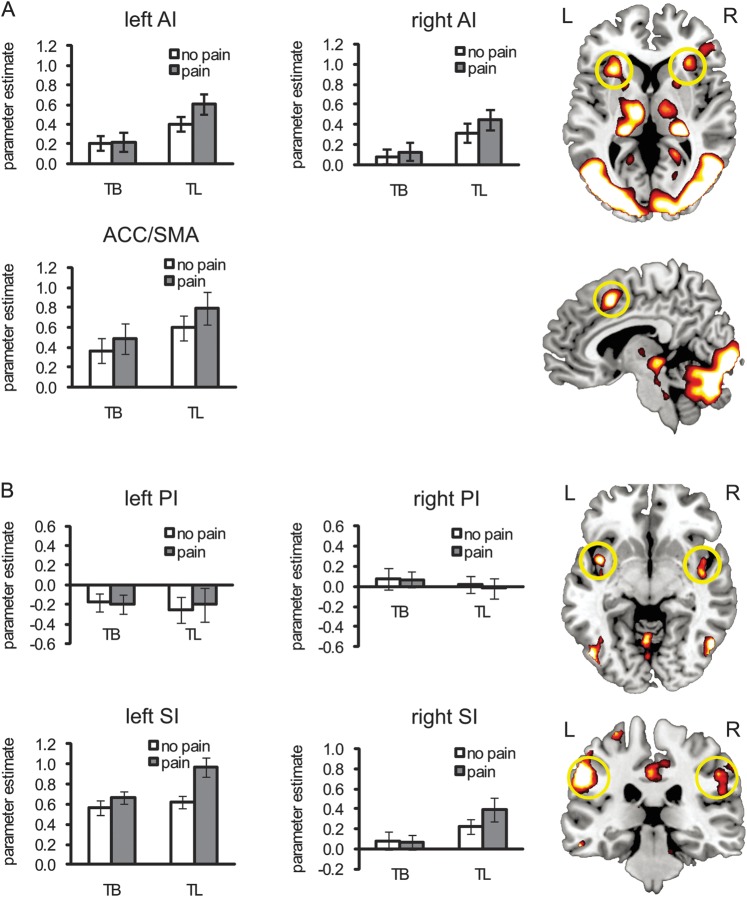
As predicted, we observed a significant interaction between cognition and emotion in left AI (F1,17 = 5.32, P < 0.05) but not in right AI or ACC (Fs > 5.5, Ps > 0.05; Fig. 3A). For left AI, there was also a significant main effect of cognitive demand (TL vs. TB, F1,17 = 19.57, P < 0.001) and stimulus valence (pain vs. no-pain, F1,17 = 4.33, P = 0.05). Planned comparisons indicated that left AI activation did not differ significantly between nonpainful and painful situations under TB (TB-no pain vs. TB-pain; F < 1); however, this difference reached significance under TL (TL-no pain vs. TL-pain; F1,17 = 9.16, P < 0.01). For right AI, there was a significant main effect of cognitive demand (F1,17 = 19.82, P < 0.001) and stimulus valence (F1,17 = 4.82, P < 0.05). Planned comparisons show that right AI activation did not differ significantly between nonpainful and painful situations under TB (TB-no pain vs. TB-pain; F < 1), but this difference was significant under TL (TL-no pain vs. TL-pain; F1,17 = 4.28, P = 0.05). For ACC, there was a significant main effect of cognitive demand (F1,17 = 8.59, P < 0.01) but not of stimulus valence (F1,17 = 4.01, P > 0.05). ACC activation did not significantly differ between nonpainful and painful stimuli under either TB (F1,17 = 1.42, P > 0.05) or TL (F1,17 = 2.90, P > 0.05).
Figure 3.
fMRI ROI results. (A) ROI analysis of the parameter estimates of AI and ACC for 4 experimental conditions (TB-no pain, TB-pain, TL-no pain, and TL-pain) derived from the task localizer (TP vs. baseline). (B) ROI analysis of the parameter estimates of PI and primary somatosensory cortex (SI) for 4 experimental conditions derived from the empathy localizer (TP-pain vs. TP-no pain). TB: task body part. TL: task laterality. TP: task pain. Error bars represent standard error of the mean.
We also found a significant interaction effect in left SI (F1,17 = 5.53, P < 0.05) but not in bilateral PI or right SI (Fs > 5.5, Ps > 0.05; Fig. 3B). Additionally, for left SI, there was a significant main effect of cognitive demand (F1,17 = 11.90, P < 0.05) and stimulus valence (F1,17 = 18.19, P < 0.05). Planned comparisons showed that left SI activation did not differ significantly between nonpainful and painful situations under TB (TB-no pain vs. TB-pain; F1,17 = 1.88, P > 0.05); however, this difference reached significance under TL (TL-no pain vs. TL-pain; F1,17 = 21.07, P < 0.01). For right SI, there was a significant main effect of cognitive demand (F1,17 = 11.90, P < 0.05), although the main effect of stimulus valence was not significant (F1,17 = 3.82, P > 0.05). Planned comparisons indicated that the difference between nonpainful and painful situations was not significant under TB (TB-no pain vs. TB-pain; F < 1, P > 0.05) but was significant under TL (TL-no pain vs. TL-pain; F1,17 = 5.47, P < 0.05). For either left or right PI, there was no significant main effect of stimulus valence or cognitive demand (Fs < 1, Ps > 0.05). None of the planned comparisons showed significance (Fs < 1, Ps > 0.05).
fMRI: Exploratory Whole-Brain Analysis
Interaction between Cognitive Demand and Stimulus Valence
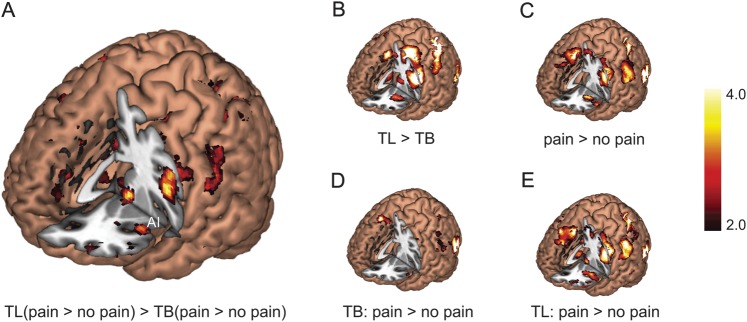
We then explored the interaction effects between task and stimulus (see Supplementary Table 2 and Fig. 4A). Task-related activation was enhanced by pain compared with no-pain in dlPFC and superior parietal lobule (SPL). We did not find deactivation related to the interaction effect under the same threshold.
Figure 4.
fMRI whole-brain analysis. (A) Interaction between cognitive demand and stimulus valence. (B) Main effect of cognitive demand. (C) Main effect of stimulus valence. (D) Pairwise comparison between TB-pain versus TB-no pain. (E) Pairwise comparison between TL-pain versus TL-no pain. TB: task body part. TL: task laterality.
Main Effect of Cognitive Demand
TL elicited greater activation than TB (see Supplementary Table 3 and Fig. 4B) in bilateral AI, supplementary motor area (SMA, extending into dorsal ACC), areas near/along the intraparietal sulcus, temporooccipital visual areas, frontal eye field (FEF)/precentral gyrus, dlPFC, thalamus, cerebellum, and midbrain.
Main Effect of Stimulus Valence
Consistent with previous findings, painful stimuli activated bilateral occipitotemporal visual areas, AI and frontal operculum, SI, FEF/precentral gyrus, SMA, dlPFC, and cerebellum (see Supplementary Table 4 and Fig. 4C).
Simple Comparisons of Stimulus Valence under Each Task Condition
As shown in Supplementary Table 5 and Figure 4D,E, judging body parts of painful stimuli (TB-pain vs. TB-no pain) elicited significant activation in bilateral inferior occipital gyrus, inferior and middle temporal gyri, as well as right dlPFC. On the other hand, judging laterality of painful stimuli (TL-pain vs. TL-no pain) activated left AI/frontal operculum, right SI, bilateral SMA and FEF/precentral gyrus, dlPFC, bilateral occipital and temporal gyri, and bilateral cerebellum.
Discussion
We observed a significant behavioral interaction between cognitive demand and emotional valence, associated with cortical activation in AI and SI, together with attentional control regions (dlPFC, FEF, and SPL). We also identified a functional segregation of subregions within the insular cortex in that AI was involved in the interaction, whereas activation of PI was only associated with explicit pain judgment. These findings suggest functional integration between cognition and emotion in AI.
Anterior Insula as an Interface for Cognition–Emotion Integration
Our primary finding is that cognitive demand and stimulus valence are integrated in a neural network where AI is a key node. We observed behavioral interaction between cognitive demand and stimulus valence: Judging laterality of painful stimuli was significantly more difficult and time consuming than judging laterality of nonpainful stimuli, whereas judging body part of painful stimuli did not elicit greater error rates or slower RTs than judging body part of nonpainful stimuli. These results suggest that stimulus valence interfered with information processing under high cognitive demand and facilitated information processing under low cognitive demand (Gu et al. 2010).
To show that any brain region is involved in integrating multiple mental operations, one needs to provide evidence of 1) corepresentation of each of the operations in that brain region and 2) a positive interaction between operations in the same region (Gray et al. 2002). A similar logic has been adopted in the study of multisensory integration (Calvert 2001). In the context of cognition–emotion integration, empirical evidence is rare (Pessoa 2008). In the current study, we showed that together with SI, dlPFC, FEF, and SPL, AI was super-additively activated by cognitive demand and stimulus valence, suggesting that the concurrent processing of cognitive demand and emotional valence resulted in competition for neuroanatomical resources. These findings confirmed the role of AI in integrating different streams of information from goal-directed cognitive demand and stimulus-driven emotional valence. Compared with other brain structures that showed an interaction effect (e.g., SPL and IFG), the AI has been shown to participate in a wide range of mental computations involving the rerepresentation of interoception including temperature (Craig et al. 2000) and pain perception (Treede et al. 1999), self recognition (Devue et al. 2007), risk and uncertainty (Seymour et al. 2004), error detection (Klein et al. 2007), attention (Fan, Byrne, et al. 2007), emotional awareness (Jabbi et al. 2007), time perception (Livesey et al. 2007), music comprehension (Platel et al. 1997), and decision making (Thielscher and Pessoa 2007). As such, the AI has been suggested to play a central role in subjective awareness (Craig 2009).
It should be noted that the effect size of cognition–emotion interaction in the AI was modest in our ROI analysis and did not survive the chosen threshold in the exploratory whole-brain analysis. This may, in part, be due to modest sample size, though it could also be an inflated estimate of the effect. Future studies should employ larger samples and more powerful paradigms in attempt to replicate the interaction effect and to provide a more accurate estimate of the effect size.
We did not observe a significant cognitive demand by stimulus valence interaction in ACC, although there was a significant main effect of cognitive demand. Both AI and ACC have dense projections to frontal and temporal cortex as well as thalamus, amygdala, and subcortical autonomic regions (Vogt et al. 1992; Flynn et al. 1999; Butti and Hof 2010). Interestingly, AI and ACC are the only regions in the human brain that contain a morphologically highly specialized neuronal subtype, the von Economo neurons (Nimchinsky et al. 1995, 1999; Allman et al. 2010), which are thought to be involved in the fast and intuitive processing of information (Allman et al. 2005). However, AI has been suggested to serve a more domain-specific role in empathetic pain than ACC (Gu et al. 2010). We hypothesize that AI might initially identify and integrate externally salient information with changes in interoceptive processes; ACC can then transform this evaluative signal into voluntary control over behaviors (Gu et al. 2010). Indeed, it has been shown that AI has a strong causal influence on ACC and serves as the causal outflow interface at the junction of the central executive network and default mode network (Sridharan et al. 2008). It is noteworthy that functional integration of cognitive and emotional processing may also exist in the ACC (Kalisch et al. 2010; Shackman et al. 2011). We speculate that the absence of an interaction effect in dorsal ACC is likely due to the insufficient negative affect elicited by seen-pain compared with felt-pain as used in most studies (Shackman et al. 2011). Future investigations are needed to explore cognition–emotion interactions in other subdivisions of ACC (i.e., subgenual and pregenual ACC) (Fan et al. 2011), especially using paradigms focusing on types of cognitive–emotional processing other than empathy.
We also observed activation in dlPFC, FEF, and SPL associated with cognition–emotion interaction (see Results and Supplementary Table 4). These regions have been implicated in the dorsal attention stream (Shulman et al. 2009), which exerts top-down control and conflict processing (Fan, Kolster, et al. 2007). A previous study suggested that dlPFC is part of a network that integrates cognition and emotion (Gray et al. 2002). Our finding extended the previous proposal by incorporating AI in the brain circuitry subserving such an interface function. The difference in functional roles of AI and control regions such as dlPFC could be that AI represents interoceptive changes of unique relevance to subjective experience, whereas dlPFC, FEF, and SPL maintain online representations of cognitive demand and stimulus features as well as goal-directed implementation (Dosenbach et al. 2008), all of which are operations required in cognition–emotion integration.
Involvement of the Somatosensory Cortex in Emotion and Cognition
Similar to AI, SI was also associated with the interaction between cognitive demand and stimulus valence. Although not predicted, this finding supplements the well-established role of SI as a neural substrate for somatic sensation (Khalsa et al. 2009). Unlike previous experiments, however, the present study cannot inform the possibility of a direct somatic route of SI activation (Khalsa et al. 2009); interoceptive changes in the present findings result indirectly from subjective interpretation of visual information rather than tactile feedback. SI, as suggested by the current findings, also participates in high-level cognitive and emotional functions. The notion that SI encodes somatic information and is highly modulated by cognitive factors is not new (Hyvarinen et al. 1980; Sterr et al. 2007). It has been reported that BOLD signal changes in SI were greater for attended painful stimuli than unattended stimuli (Sterr et al. 2007). Evidence from animal research suggests that neuronal activity in monkey SI can be enhanced by attention to tactile stimuli (Hyvarinen et al. 1980).
Beyond its roles in basic sensory processing, the somatosensory cortex has also been shown to participate in the recognition (Adolphs et al. 2000), self-generation (Damasio et al. 2000), and evaluation (Gu and Han 2007b) of emotions as well as social cognition such as empathy (Keysers et al. 2004; Avenanti et al. 2005). In a previous study where SI was found to participate in emotion recognition (Adolphs et al. 2000), it has been proposed that subjects need to retrieve information from a past scenario that bears similar emotional valence in order to make successful judgments about a current emotional event. Such information includes the past somatosensory experience of the observer (somatic record). It is also possible that subjects generate online somatic representation when viewing emotional pictures (Gallese and Goldman 1998). Our current finding, consistent with a previous study (Gu et al. 2010), suggests that SI activation most likely represents contributions to a “somatic marker” (Damasio 1996) and that such “somatic record” or online somatic presentation can only be achieved when visual stimuli exceed a certain depth of processing. We speculate that laterality judgment might elicit a stronger somatic representation in the subject.
Functional Distinction between Anterior and Posterior Insula
We also identified that the activation of PI was only associated with explicit pain judgment, whereas activation of AI was related to the interaction between cognitive demand and stimulus valence. Anatomically, the posterior granular portion of the insula receives stronger input from several sensory regions including the thalamus and parietal, occipital, and temporal association cortices, whereas the anterior agranular portion has stronger connection with prefrontal regions including ACC and orbitofrontal cortex as well as the amygdala and the ventral striatum (Flynn et al. 1999). These features serve as the anatomical basis for the functional distinction between AI and PI. Resting state data have also suggested differential functional connectivity of AI and PI, with AI being connected to PFC and ACC, while PI is connected with the sensorimotor cortex (Cauda et al. 2011). A recent meta-analysis of functional neuroimaging studies on the insula revealed 4 functionally distinct regions: anterior-dorsal portion—cognitive domain, anterior-ventral portion—social–emotional processes, middle portion—olfactory–gustatory function, and middle-posterior portion—sensorimotor information (Kurth et al. 2010). Lesion studies reported that patients with damage to the PI, but not to AI, showed diminished sensitivity to somatosensory pain (Veldhuijzen et al. 2010). In conjunction with these findings, our study provides further evidence for a proposed model of awareness where a posterior–anterior processing gradient exists in the insular cortex to sequentially integrate interoceptive, homeostatic, environmental, hedonic, motivational, and cognitive conditions (Craig 2009).
Conclusions
The current study provides the first empirical evidence to show that AI is a key node in a neural network that serves as the anatomical basis for cognition–emotion integration. We speculate that functional integration of cognition and emotion is adaptively advantageous and parsimonious. Such integration does not exclude the distinction between cognition and emotion, but rather, suggests that the difference might be phenomenological instead of ontological. With further exploration of the integrative dynamic nature of cognition and emotion, we may acquire a better understanding of neurobehavioral function and as a result of its deficits in psychiatric illnesses.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institute of Health (NIH) (R21 MH083164 to J.F.); National Center for Research Resources (NCRR) (UL1RR029887), NARSAD Young Investigator Award (X.L.); James S. McDonnell Foundation (22002078 to P.R.H.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCRR.
Supplementary Material
Acknowledgments
Conflict of Interest : None declared.
References
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci. 2005;8:955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010;149:19–26. doi: 10.1016/j.pain.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Butti C, Hof PR. The insular cortex: a comparative perspective. Brain Struct Funct. 2010;214:477–493. doi: 10.1007/s00429-010-0264-y. [DOI] [PubMed] [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Descartes R. Les passions de l'âme (Passions of the soul) Amsterdam: Louys Elzevier; 1649. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, Bredart S. Here I am: the cortical correlates of visual self-recognition. Brain Res. 2007;1143:169–182. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. Affect is a form of cognition: a neurobiological analysis. Cogn Emot. 2007;21:1184–1211. doi: 10.1080/02699930701437931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI. The relation of brain oscillations to attentional networks. J Neurosci. 2007;27:6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fan J, Gu X, Liu X, Guise KG, Park Y, Martin L, de Marchena A, Tang CY, Minzenberg MJ, Hof PR. Involvement of the anterior cingulate and frontoinsular cortices in rapid processing of salient facial emotional information. Neuroimage. 2011;54:2539–2546. doi: 10.1016/j.neuroimage.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Hof PR, Guise KG, Fossella JA, Posner MI. The functional integration of the anterior cingulate cortex during conflict processing. Cereb Cortex. 2008;18:796–805. doi: 10.1093/cercor/bhm125. [DOI] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD. Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. J Neurosci. 2007;27:2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn FG, Benson DF, Ardila A. Anatomy of the insula—functional and clinical correlates. Aphasiology. 1999;13:55–78. [Google Scholar]
- Friston K. Beyond phrenology: what can neuroimaging tell us about distributed circuitry? Annu Rev Neurosci. 2002;25:221–250. doi: 10.1146/annurev.neuro.25.112701.142846. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RSJ. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci U S A. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007a;36:256–267. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Gu X, Han S. Neural substrates underlying evaluation of pain in actions depicted in words. Behav Brain Res. 2007b;181:218–223. doi: 10.1016/j.bbr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J Neurosci. 2010;30:3739–3744. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen J, Poranen A, Jokinen Y. Influence of attentive behavior on neuronal responses to vibration in primary somatosensory cortex of the monkey. J Neurophysiol. 1980;43:870–882. doi: 10.1152/jn.1980.43.4.870. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;os-IX:188–205. [Google Scholar]
- Kalisch R, Mechias ML, Etkin A. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009;12:1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey AC, Wall MB, Smith AT. Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45:321–331. doi: 10.1016/j.neuropsychologia.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci U S A. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Vogt BA, Morrison JH, Hof PR. Spindle neurons of the human anterior cingulate cortex. J Comp Neurol. 1995;355:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel H, Price C, Baron JC, Wise R, Lambert J, Frackowiak RS, Lechevalier B, Eustache F. The structural components of music perception. A functional anatomical study. Brain. 1997;120(Pt 2):229–243. doi: 10.1093/brain/120.2.229. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Dayan P, Koltzenburg M, Jones AK, Dolan RJ, Friston KJ, Frackowiak RS. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, Corbetta M. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Shen S, Zaman A, Roberts N, Szameitat A. Activation of SI is modulated by attention: a random effects fMRI study using mechanical stimuli. Neuroreport. 2007;18:607–611. doi: 10.1097/WNR.0b013e3280b07c34. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Pessoa L. Neural correlates of perceptual choice and decision making during fear-disgust discrimination. J Neurosci. 2007;27:2908–2917. doi: 10.1523/JNEUROSCI.3024-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Treisman AM. Strategies and models of selective attention. Psychol Rev. 1969;76:282–299. doi: 10.1037/h0027242. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen DS, Greenspan JD, Kim JH, Lenz FA. Altered pain and thermal sensation in subjects with isolated parietal and insular cortical lesions. Eur J Pain. 2010;14:535.e1–535.e11. doi: 10.1016/j.ejpain.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.