Abstract
Anxious emotion can manifest on brief (threat response) and/or persistent (chronic apprehension and arousal) timescales, and prior work has suggested that these signals are supported by separable neural circuitries. This fMRI study utilized a mixed block-event–related emotional provocation paradigm in 55 healthy participants to simultaneously measure brief and persistent anxious emotional responses, testing the specificity of, and interactions between, these potentially distinct systems. Results indicated that components of emotional processing networks were uniquely sensitive to transient and sustained anxious emotion. Whereas the amygdala and midbrain showed only transient responses, the ventral basal forebrain and anterior insula showed sustained activity during extended emotional contexts that tracked positively with task-evoked anxiety. States of lesser anxiety were associated with greater sustained activity in the ventromedial prefrontal cortex. Furthermore, ventromedial prefrontal recruitment was lower in individuals with higher scores on intolerance of uncertainty measures, and this hyporecruitment predicted greater transient amygdala responding to potential threat cues. This work demonstrates how brain circuitries interact across temporal scales to support brief and persistent anxious emotion and suggests potentially divergent mechanisms of dysregulation in clinical syndromes marked by brief versus persistent symptoms of anxiety.
Keywords: amygdala, BNST, emotion, fMRI, insula, intolerance of uncertainty, unpredictability
Introduction
Theoretical accounts have motivated a division between manifestations of anxious emotion based on the source and timescale of affective experience (Barlow 1988). On one hand, the classically characterized “fear” response entails a transient alarm reaction to environmental inputs that predict or embody threat (LeDoux 1998). By contrast, “anxiety” describes a persistent and diffuse mood state marked by defensive preparedness, sustained arousal, and vigilance (Barlow 2000; Lang et al. 2000; Davis et al. 2010). Prior work has asserted that fear-like and anxiety-like emotions are subserved by distinct but interactive neural circuitries (Davis et al. 2010; Alvarez et al. 2011). Human imaging research has broadly supported this distinction. Whereas fear-like paradigms have focused on the amygdala's role in processing salient environmental cues (LaBar et al. 1998; Phelps et al. 2001; Etkin et al. 2004), lengthy anxiety-like contexts tend to engage prefrontal regions and regions of the basal forebrain neighboring the amygdala (Chua et al. 1999; Simpson et al. 2001; Hasler et al. 2007; Somerville et al. 2010).
Though these findings are generally consistent with a division of the neural representation of fear and anxiety, a number of outstanding issues remain. Studies to date targeting anxious emotion tend to assay fear-like or anxiety-like emotion separately, precluding assessment of the specificity of, or interactions between, these neural circuitries. Functional imaging designs such as the mixed block-event–related design and analysis scheme enable transient neural responses to be decomposed from neural responses that are sustained persistently across lengthy contexts (Visscher et al. 2003). Using this design, the present study targeted both event-related neural responses engaged by brief emotional cues as well as neural responses that persist throughout contexts in which participants are made to feel anxious. This enables analysis of the specificity of systems that are sensitive to brief emotional triggers, maintain greater engagement during anxious states, and maintain greater activity during states of lesser anxiety.
The driving hypothesis, based on animal and human work, was that the representation of brief emotional responses would be supported by an amygdala–hypothalamic–periaqueductal gray network biologically suited to modulate rapid “fight-or-flight” behavior via descending glutamatergic projections (McNaughton and Corr 2004; Kober et al. 2008). Conversely, persistent neural signals observed throughout anxiogenic contexts would manifest in sustained signaling in the insular cortex and ventral basal forebrain (VBF) (including the bed nucleus of the stria terminalis [BNST]), key modulatory structures for stress and arousal maintenance (Davis 1988; Paulus and Stein 2006; Somerville et al. 2010). In addition, the present work assesses biased sensitivity of these systems in individuals with high trait-anxious characteristics.
On a behavioral level, brief and persistent emotions are not experienced in isolation. Extended states of heightened anxiety potentiate sensitivity to emotion cues (Seligman 1968; Grillon et al. 2004), especially in individuals with high trait anxiety (Fox et al. 2001; Robinson et al. 2011). However, the neural mechanisms by which persistent affective states influence brief emotional responding remain largely unaddressed. This experiment assesses interactions between brief and persistent affective responding, evaluating how sustained engagement of “anxiety” systems influence transient “fear” responses. Understanding the specificity, interactions and individual differences–based biases amongst these circuitries holds potential to specify novel contributors to the pathophysiology of psychiatric illnesses characterized by isolated fear (e.g., specific phobias) versus anxiety (e.g., generalized anxiety disorder [GAD]; Davis and Whalen 2001; Craske et al. 2009).
Materials and Methods
Participants
Sixty-one healthy adult participants completed this experiment. Participants were right handed, reported no abnormal neurological history, and were native speakers of English. Participants were verified to be absent of clinically diagnosable levels of current anxiety disorders and current or past mood disorders using the Structured Clinical Interview for Diagnostic and Statistical Manual-IV Axis I Disorders (First et al. 1995), and no participant was using psychotropic medications. The potential for covarying mood effects was minimized by excluding any participant scoring >10 on the Beck Depression Inventory (Beck et al. 1961). One participant's response data during functional magnetic resonance imaging (fMRI) scanning was lost due to technical error, leaving a final sample of n = 60 for behavioral analyses (n = 36 females, mean age = 19 years, standard deviation [SD] = 1.2 years). fMRI data from 6 participants were excluded for movement of >2 mm and/or signal artifacts, leaving a final sample of n = 55 for imaging analyses (n = 32 females, mean age = 19 years, SD = 1.2 years). All participants provided informed consent for their participation in accordance with the Committee for Protection of Human Subjects at Dartmouth College.
Individual Differences in Anxiety
Participants completed several scales assessing a range of anxiety symptoms, including the Spielberger State-Trait Anxiety Inventory (STAI)(Spielberger et al. 1988), Behavioral Inhibition Scale (BIS)/Behavioral Activation Scale (Carver and White 1994), NEO Personality Inventory Neuroticism and Extraversion subscales (Costa and McCrae 1991), Intolerance of Uncertainty Scale (IUS)(Buhr and Dugas 2002), Penn State Worry Questionnaire (PSWQ) (Meyer et al. 1990), and Anxiety Sensitivity Index (ASI) (Peterson and Reiss 1987). Individual difference analyses focused on the total scores on the IUS. IU, defined as, “a tendency of an individual to consider it unacceptable that a negative event may occur, however small the probability of its occurrence (Buhr and Dugas 2002, p. 932).” This construct holds particular relevance to tonic or long-lasting anxiety, a primary target of this study. The mean (M = 57.5) and SD (15.3) of IU scores in the present sample are consistent with published normative data (Buhr and Dugas 2002) and represents a wide range of scores (sample: 29–97; possible scores: 27–135). IU also demonstrated substantial overlapping variance with other individual difference measures targeting different facets of trait anxiety (correlations with: Trait STAI r60 = 0.32, P = 0.01; BIS r60 = 0.57, P < 0.001; NEO neuroticism r60 = 0.44, P < 0.001; PSWQ r60 = 0.45, P < 0.001; ASI r60 = 0.26, P < 0.05).
Stimuli
Negative and neutral images were primarily drawn from the International Affective Picture System (Lang et al. 1998). Negative images included pictures of overt threats (attacking animals and weapons), disasters (bombings and plane crashes), graphic depictions of sick or injured individuals, and other arousing negative imagery, whereas neutral images depicted innocuous social and nonsocial scenes. As in prior work (Dolcos et al. 2004, 2005), we sought to equate negative and neutral pictures on certain higher-order features that may engage the circuitry of interest (i.e., presence of humans). To attain a sufficient sample set of neutral images depicting people, 50 supplementary neutral images featuring people were compiled by experimenters and shared from prior work (Yamasaki et al. 2002), which were normed for valence and arousal by a separate group of n = 45 participants and added to the pool of images (data and images available on request). Two sets of 30 negative pictures with comparable valence and arousal, and 2 sets of 30 neutral pictures with comparable valence and arousal were selected as stimuli for the 4 experimental conditions. In addition, all 4 sets were matched for relevant aspects of scene content (proportion of images depicting people with visible faces, peoples' bodies without visible faces, proportion of pictures taking place indoors and outdoors [P 's> 0.3]), see Supplementary Table 1.
Task Structure
A mixed block-event–related design and optimized analysis specifications enabled independent detection of transient and sustained blood oxygen level–dependent (BOLD) responses within a single experiment and modeling analysis (Visscher et al. 2003; Dosenbach et al. 2006). This design incorporates both brief and persistent experimental manipulations to evoke transient and sustained neural responses, respectively. Comparison of brief responses to negative versus neutral images isolated transient emotional responses (Hariri et al. 2003; Britton et al. 2006; MacNamara and Hajcak 2009), serving as generators of cued affect that experimentally model the time course of fear-like emotion (Fig. 1B, top panel for schematic version).
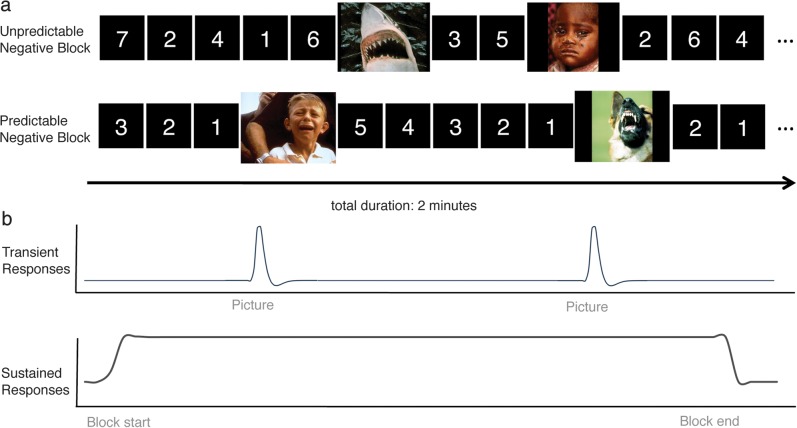
Figure 1.
Experimental design and analysis schematic. (A) Within a task block, negative or neutral pictures are presented (3 s duration) embedded within variable-duration predictable or unpredictable timings. (B) Schematic images depicting canonical transient event responses (top) that are estimated separately from sustained responses that remain persistently active throughout the blocks (bottom). This figure is for illustration purposes only and is not intended to accurately represent the actual experimental parameters.
Design provisions additionally permitted the independent detection of sustained neural signals that remained persistently active throughout task blocks (see Fig. 1B, bottom panel for schematic version). Historically, the mixed design approach has targeted neurocognitive representations of “task sets”—long-duration upregulation of localized BOLD signals that maintain a cognitive state superordinate to moment-to-moment stimulus features (Logan and Gordon 2001; Dosenbach et al. 2006). The present study targeted cognitive sets related to the persistent subjective experience of anxiety by isolating components of the BOLD response that were engaged and remained active throughout task blocks with varying levels of task-evoked anxiety.
To evoke differential levels of anxiety across blocks, 2 affective manipulations were crossed, each of which has been validated to evoke heightened anxiety. One manipulation was presenting either negative or neutral images in a given task block, based on evidence that blocked exposure to negative images induces state anxiety and psychophysiological responses that persist superordinate to image presentations (Bradley et al. 1996; Smith et al. 2005). Thus, we predicted heightened anxiety during lengthy blocks containing only negative image presentations relative to blocks in which participants knew they would view only neutral pictures.
The second manipulation involved modulating temporal properties of picture presentations, presenting the images within either predictable “countdowns” or with unpredictable random timings (Fig. 1A). This design feature was chosen based on prior work demonstrating the inherent anxiogenic properties of temporal uncertainty (Ladouceur et al. 2000; Carleton et al. 2007; Herry et al. 2007) and its contribution to the pathophysiology of anxiety disorders (Dugas et al. 1998; Ladouceur et al. 2000). We predicted that persistent task-evoked anxiety would be induced by temporally unpredictable picture presentations, particularly when the pictures are negatively valenced. According to participant reports, the 2 × 2 crossing of valence and unpredictability manipulations effectively induced significant differences in task-evoked anxiety across blocks (see Results and Fig. 2).
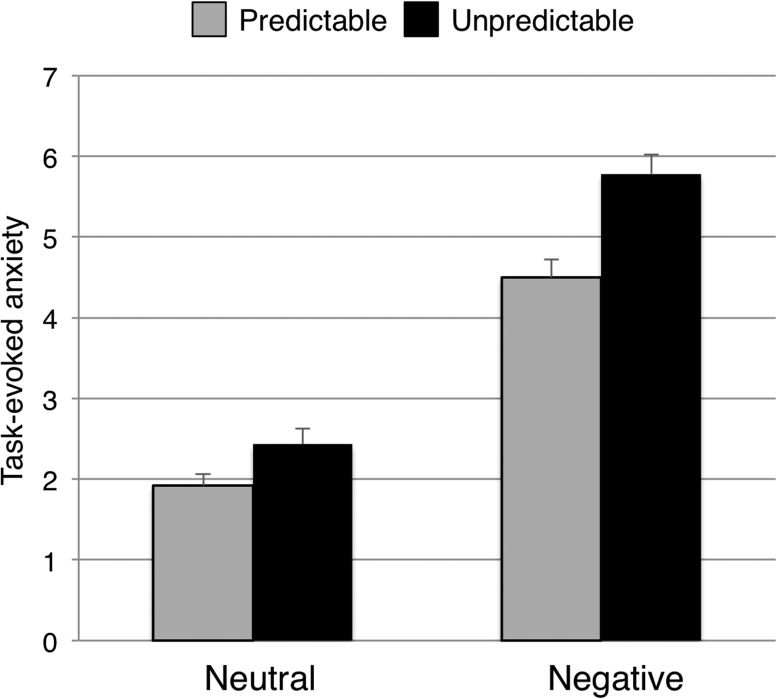
Figure 2.
Task-evoked anxiety. Blocks containing only negative pictures evoked greater anxiety than blocks containing neutral pictures, and blocks with unpredictable timings evoked greater anxiety than blocks containing predictable timings. The effect of unpredictability was exaggerated when presented with negative pictures. Error bars denote standard error of the mean.
Functional Imaging
During fMRI scanning, participants viewed negative and neutral images embedded within contexts of predictable or unpredictable timings (Fig. 1A). Half of blocks contained only negative pictures; the remaining half contained only neutral pictures. A total of 15 pictures were presented per block. The valence manipulation was crossed with a predictability manipulation. Half of the blocks contained predictable timings, consisting of a 1–8 s “countdown” in which numbers were consecutively presented, accurately representing the number of seconds remaining until picture onset (Fig. 1A, bottom panel). The other half of blocks contained unpredictable timings consisting of random numbers that provided no predictive information with regard to picture onset (Fig. 1A, top panel). For all blocks, number presentations varied pseudorandomly with 1–8 s between pictures. Each picture was presented once, and the assignment of pictures to unpredictable and predictable blocks was pseudorandomized across participants.
Scan runs consisted of two 118 s blocks interleaved with 60-s resting fixation periods. Blocks began with a 3-s start cue alerting participants of the forthcoming block type to isolate activity associated with transitions into a task set (Konishi et al. 2001), to ensure sustained activity estimates were constrained to maintenance functions, and to mitigate the need for the participant to gradually decipher the block type. Following the on cue, stimulus presentation continuously alternated between number (1 s per number; jittered 1–8 numbers) and picture presentations (3 s).
Participants were instructed to passively view the number stimuli, being mindful of the information provided by the numbers. For each picture presentation, participants were instructed to press one of two buttons indicating whether the picture took place indoors or outdoors (Lane et al. 1997; Ochsner et al. 2004), a low-level task chosen to interfere minimally with affective responding (Lieberman et al. 2007) while providing a behavioral metric for assessing event-related response latency as a function of valence and predictability.
Each block ended with a 3-s stop cue to isolate transient task set release–related activity (Konishi et al. 2001). Two such blocks were presented per run, with block order pseudorandomized within and across participants resulting in 8 blocks presented across the experiment (2 repetitions of each type). Stimuli were presented using Cedrus Superlab 4.0.2 (San Pedro, CA), interfaced with the Lumina Response box, which recorded participant key presses and fMRI triggers. During scanning, visual stimuli were displayed onto a back projection screen at the head end of the scanner bore with an Epson (model ELP-7000) LCD projector.
Task-Evoked Anxiety
Immediately after the task, participants remained in the scanner and provided verbal answers to posttest questions assessing the subjective levels of anxiety evoked by different task blocks. Specifically, participants were asked to rate on a 1–9 Likert scale how nervous they felt throughout each of the 4 block types (negative pictures predictable timings; negative pictures unpredictable timings; neutral pictures predictable timings; and neutral pictures unpredictable timings). Rating effects were evaluated with a 2 × 2 mixed analysis of variance with repeated effects of predictability (unpredictable and predictable) and valence of pictures within the block (negative and neutral), with individual differences in IU input as a mean-centered covariate. Reaction times were z-scored and submitted to the analogous group analysis.
fMRI Acquisition
Imaging was performed on a Philips Intera Achieva 3.0 Tesla scanner with a SENSE head coil (Philips Medical Systems, Bothwell, Washington). Four T2* weighted scans sensitive to the BOLD response (repetition time = 2000 ms, echo time = 35 ms, flip angle = 90°, 3 × 3 in-plane resolution, SENSE factor = 2) were used to acquire 832 whole-brain volumes (36 slices, 3.5-mm slice thickness, 0.5 mm gap, anterior commissure–posterior commissure plane). A T1-weighted high-resolution image of the brain was acquired with a magnetization-prepared rapid gradient echo sequence (160 sagittal slices, echo time = 4.6 ms, repetition time = 9.9 ms, flip angle = 8°, voxel size = 1 × 1 × 1 mm).
fMRI Analysis
Processing of fMRI data took place in SPM2 (Wellcome Department of Cognitive Neurology, London, UK; Friston et al. 1995). Preprocessing steps included slice-time correction, motion correction, correction of movement-by-susceptibility interactions with unwarping routines, and spatial normalization. Normalized functional data were spatially smoothed (6 mm full-width at half-maximum Gaussian kernel).
Transient and sustained components of the BOLD signal were detected using first-level general linear modeling at the individual participant level, optimized to independently and simultaneously identify brief and persistent neural signals. The present design permitted transient responses to be disentangled from sustained responses due to specific design components including sufficiently variable jitter between transient stimuli, sufficient time spent in a sustained state and not experiencing a transient event (>60% total block time), and modeling of transients using a finite impulse response (FIR) basis function rather than a canonical response to ensure that sustained condition estimates are truly maintained and not aliased by high-frequency components of the signal. These design features enabled simultaneous modeling of transient and sustained signals for the present purposes of identifying common and distinct networks subserving emotional responses that manifest over fear-like and anxiety-like timescales.
For each participant, a general linear model incorporated regressors for the 4 transient event conditions, the 4 sustained block conditions, start cues, stop cues, and regressors of non-interest (session mean, linear trend, run regressor, and 6 movement parameters) to compute parameter estimates (β) and contrast maps (weighted parameter estimates). Trials with incorrect indoor/outdoor judgments or no response were not modeled separately due to their infrequent occurrence (∼6% of all trials). Transient conditions (neutral pictures within predictable blocks, negative pictures within predictable blocks, neutral pictures within unpredictable blocks, neutral pictures within unpredictable blocks, on cues, and off cues) were modeled with a FIR function over 10 TRs (20 s). (Though the FIR model does not assume a response shape, inspection of time courses from regions of interest [ROIs] validated that the reported transient responses conformed to standard hemodynamic properties.) Four sustained regressors served as predictors of signal changes that were recruited and remained persistently engaged throughout the blocks, independent of transient picture presentations. Sustained regressors (blocks of predictable timings with neutral pictures, predictable timings with negative pictures, unpredictable timings with neutral pictures, and unpredictable timings with negative pictures) consisted of a boxcar function beginning after the conclusion of the start cue and lasting until the onset of the stop cue.
Group fMRI Statistical Analyses
Voxelwise random effects group analyses were used to test targeted hypotheses regarding transient and sustained responses as a function of valence, predictability, and IU. All voxelwise analyses were thresholded at P < 0.05 whole-brain corrected, using a P value and cluster size threshold combination stipulated by Monte Carlo simulations to maintain a whole-brain α = 0.05. Regions showing transient responses as a function of valence were identified with a truncated area under the curve (AUC) analysis of FIR parameter estimates (as in Wig et al. 2009) focused on the expected hemodynamic peak 4–11 s following picture onset given a 3-s event duration. Truncated AUC estimates for negative relative to neutral images were statistically compared with a voxelwise paired t-test to identify brain regions transiently sensitive to emotional picture content.
Two complementary group statistical analyses were used to identify regions showing differential sustained emotional modulation. First, participant ratings were used to identify areas of the brain showing sustained activity titrating positively with heightened task-evoked anxiety. Parameter estimates (β) for each of the 4 sustained conditions were input to a linear contrast per participant that identified the extent to which sustained neural activity tracked faithfully with the differential self-reported anxiety experienced across conditions (i.e., Fig. 2). Z-scored, demeaned values of self-reported anxiety ratings were generated and used as contrast weightings for each of the 4 sustained conditions (the sustained predictable neutral β weighted −0.98, the sustained unpredictable neutral β weighted −0.54, the sustained predictable negative β weighted +0.38, and the sustained unpredictable negative β weighted +1.14). The resulting value per participant indicates to what extent sustained neural responses titrated faithfully with self-reported anxiety across the 4 conditions, with positive values representing increasing sustained activity with greater anxiety and negative values representing greater sustained activity proportional to states of lesser anxiety. A group statistical map was subsequently generated using a voxelwise group one-sample t-test, inputting the contrast estimates described above for each participant. Regions of the brain with positive t-values are candidates for the maintenance of lengthy states of heightened anxiety independent of discrete stimulus detection. Negative t-values identified regions with sustained responses that were strongest for experimental blocks during which lower level of anxiety was experienced.
To validate this approach, a second set of analyses was conducted that did not rely on self-report. Specifically, whole-brain paired t-tests were generated to isolate sustained neural responses as a function of picture valence (sustained activity in blocks containing negative vs. neutral pictures) and predictability (blocks containing unpredictable vs. predictable timings).
A final objective was to target the potential interactions between tonic and phasic systems. We predicted that individual differences in persistent engagement in contexts in which participants experienced heightened anxiety would predict brief responses to affective cues. We explored this possibility with bivariate correlation analyses across amygdala and ventromedial prefrontal cortex (vmPFC) ROI data, testing whether individual differences in the magnitude of sustained network recruitment in unpredictable contexts predicted the magnitude of transient neural responses to cued events.
Though a whole-brain statistical analysis was performed, ROI selection was constrained to loci within a priori affective circuities of interest (Kober et al. 2008; Davis et al. 2010). Regional specificity to transient versus sustained responses, interactions between regions, and modulated activity with individual differences in IU were evaluated within these ROIs during off-line analyses. Four-millimeter radius spherical ROIs about activation peaks were generated with the MarsBaR 0.41 toolbox, and signal estimates were extracted for each condition for off-line statistical analysis using SPSS Statistics 18.0 and 19.0 software. Visualization of cortical activity was aided with surface reconstructions generated by Computerized Anatomical Reconstruction and Editing Toolkit (Caret) v5.5 software (Van Essen et al. 2001). Key findings beneath the cortical surface are presented on a representative spatially normalized T1-weighted image. All reported coordinates have been converted to Talairach atlas space (Talairach and Tournoux 1988).
Results
Accuracy and Reaction Times
Participants performed with 93.9% mean accuracy on indoor/outdoor judgments. Accuracy was worse for negative than neutral pictures (F1,58 = 4.10, P = 0.047), were not affected by predictability of timings (P > 0.8), and did not interact with individual differences in IU (P 's > 0.1; main effect of IU P > 0.9). Reaction times were significantly slowed by negative picture valence (F1,58 = 162.5, P < 0.001; zneg − zneu = 0.41) and by unpredictable contexts (F1,58 = 13.50, P < 0.001; zunp − zpre = 0.16). In addition, there was a significant 3-way interaction between valence, predictability, and IU (F1,58 = 4.35, P = 0.041). Post hoc comparisons revealed that individuals with above-median IU showed a particular slowing to negative pictures regardless of predictability (negative picture reaction time (RT) for above-median IU versus below median IU: t58 = 2.02, P = 0.048; zabove − zbelow = 0.09), and trend toward a greater influence of unpredictability on neutral picture RTs (unpredictable vs. predictable neutral picture RT: t58 = 1.6, P = 0.1; zabove − zbelow = 0.18).
Task-Evoked Anxiety
Blocks containing negative pictures elicited higher task-evoked anxiety than blocks containing neutral pictures (main effect of valence: F1,58 = 204.72, P < 0.001; Fig. 2). Blocks containing unpredictable timings also evoked heightened anxiety (main effect of predictability: F1,58 = 61.79, P < 0.001) and individuals with greater IU trended toward endorsing task-evoked anxiety (main effect of IU: F1,58 = 2.72, P = 0.1). There was also a significant valence by predictability interaction (F1,58 = 19.63, P < 0.001), driven by a stronger effect of unpredictability on task-evoked anxiety for negative than neutral image blocks ([unpredictable negative − unpredictable neutral] versus [predictable negative − predictable neutral] t59 = 4.46, P < 0.001). Finally, we observed a valence by IU interaction (F1,58 = 6.33, P = 0.015), with greater IU predicting higher nervousness ratings for negative image blocks (P's < 0.04) but not neutral blocks (P's > 0.6).
Transient Responses to Pictures
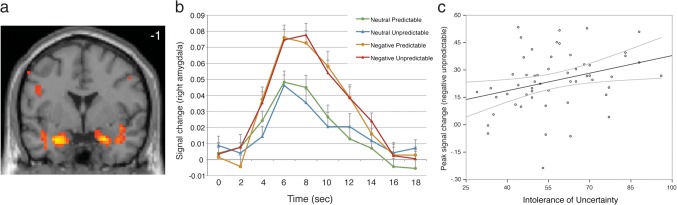
Brain regions transiently active to negative pictures (relative to neutral pictures) are listed in Table 1. Active regions included the left (x = −21, y = −7, z = −17) and right amygdala (x = 24, y = −1, z = −20; Fig. 3A–B) and an area of the midbrain consistent with the periaqueductal gray (midbrain/PAG; x = 6, y = −30, z = −13; see Supplementary Fig. 1). Time course data (derived from FIR signal estimates) was extracted from 4 mm ROIs centered on activation peaks and evaluated off-line for independent effects of predictability and anxiety. Effects of predictability on the right amygdala response were not significant (main effect, valence by predictability interaction P's > 0.3) but were qualified by a significant valence by predictability by IU interaction (F1,53 = 4.02, P = 0.05). Specifically, peak transient responding to negative pictures embedded in unpredictable timings was selectively exaggerated with greater IU (r54 = 0.31, P = 0.019, R2 = 0.1; Fig. 3C), whereas IU did not predict amygdala responses to the other conditions (P's > 0.5). Left-amygdala activity was not modulated by predictability (P's > 0.2), though yielded a trend toward overall larger responses with higher IU (F1,53 = 3.57, P = 0.06). Midbrain/PAG activity did not differ by predictability or individual differences in IU (P's > 0.3).
Table 1.
Transient neural responses modulated by emotion
| Label | BA | x | y | z | t |
| Negative > Neutral pictures | |||||
| Visual cortex | 18 | −12 | −96 | −2 | 7.50 |
| Amygdala | −21 | −7 | −17 | 6.39 | |
| Amygdala | 24 | −1 | −20 | 6.23 | |
| Extended amygdala/insula* | −42 | −1 | −15 | 3.42 | |
| Extended amygdala/insula* | 36 | 2 | −20 | 5.57 | |
| Inferior frontal gyrus | 47 | −45 | 32 | 7 | 5.64 |
| Midbrain | 6 | −29 | −6 | 5.41 | |
| Midbrain/PAG* | 6 | −30 | −13 | 4.21 | |
| Inferior frontal gyrus | 9 | 50 | 13 | 24 | 5.05 |
| Inferior frontal gyrus | 46/10 | 53 | 38 | −1 | 4.80 |
| Middle frontal gyrus | 6 | −48 | 4 | 25 | 4.58 |
| Neutral > Negative Pictures | |||||
| Inferior parietal lobule | 40 | 56 | −53 | 39 | 6.36 |
| Medial prefrontal cortex | 6 | −6 | 15 | 60 | 6.32 |
| Medial prefrontal cortex | 10/11 | 18 | 43 | −12 | 6.03 |
| Superior frontal gyrus | 9 | −15 | 45 | 20 | 4.48 |
| Superior frontal gyrus | 9 | 36 | 37 | 29 | 4.14 |
| Superior parietal lobule | 7 | 12 | −62 | 47 | 3.96 |
| Medial prefrontal cortex | 8 | 3 | 36 | 29 | 3.88 |
| Middle temporal gyrus | 21 | −57 | −14 | 3 | 3.57 |
Note: Threshold P < 0.05, whole-brain corrected. Areas with * denote subclusters encompassed within larger functional activation clusters. BA = Brodmann area.
Figure 3.
Mean transient responses to negative versus neutral pictures. (A) The left and right amygdala responded more strongly to negative than neutral pictures. Image threshold P < 0.05, whole-brain corrected. (B) Timecourse of right amygdala (x = 24, y = −1, z = −20) response to pictures as a function of valence and predictability. Timecourse values were derived from FIR parameter estimates; error bars denote standard error of the mean. (C) Greater IU predicts exaggerated right amygdala response to negative pictures when embedded within unpredictable timings. Gray curves denote 95% confidence interval.
To test for temporal specificity, the left and right amygdala and midbrain/PAG ROI values were evaluated for possible sustained responses—signals that remained continuously engaged throughout task blocks. Sustained beta estimates from the left and right amygdala showed no evidence of persistent responding relative to resting baseline (mean parameter estimate for sustained activity, left: −0.039; right: −0.178) or modulated sustained activity as a function of valence or predictability (P's > 0.5). The midbrain/PAG did not yield evidence of greater sustained responding relative to baseline (P > 0.7), though it did show a trend toward greater sustained responding to unpredictable relative to predictable states (F1,53 = 3.31, P = 0.07). This effect was substantially weaker than other sustained effects and would not survive multiple comparisons correction, and thus was not considered further.
Anxiogenic State Maintenance
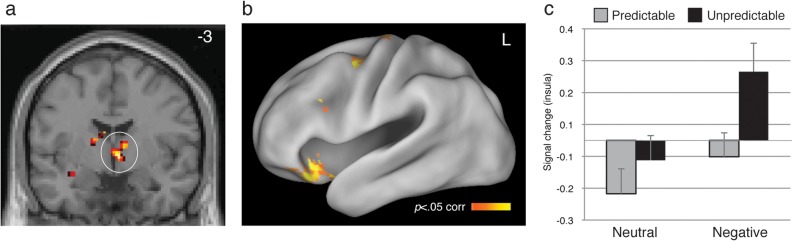
A whole-brain analysis incorporated subject reports to identify regions of the brain that remained continuously active throughout task blocks proportionally to task-evoked anxiety, which was heightened via negative valence and temporal unpredictability manipulations (e.g., Fig. 2). Positive neural predictors of sustained anxiety included the left and right inferior frontal gyrus (BA47m in the Price 2007 nomenclature and Area 12 in the primate; left x = −36, y = 20, z = −14; right x = 33, y = 29, z = −12; Fig. 4B) that extended to the insular cortex (x = −30, y = 2, z = −10), as well as the right VBF/BNST (x = 6, y = −3, z = −2 [the VBF/BNST and midbrain/PAG reported earlier are anatomically small structures. These activations achieved the cluster size/P value combination required to preserve P < 0.05 corrected thresholding in part due to spatial contiguity with a more posterior cluster outside of the VBF/BNST-proper, and in the midbrain/PAG, with a more dorsal cluster within the midbrain. For completeness, we note that the VBF/BNST cluster, alone, consists of 108 mm3 at P < 0.001, uncorrected, and the midbrain/PAG region consists of 702 mm3 at P < 0.0005, uncorrected]; Fig. 4A; see Table 2 for full list of activations). In addition, the right BA47m/insula cluster showed an interaction between responsivity to unpredictable states and IU, with greater IU associated with higher sustained activity in unpredictable blocks (F1,53 = 5.36, P = 0.024; see Supplementary Fig. 2). Aside from a trend for which the left BA47m showed marginally greater responding for negative blocks in high IU individuals (P = 0.08), individual differences in IU did not further modulate sustained activity in these regions (P 's > 0.18).
Figure 4.
Sustained responses that increase as a function of greater task-evoked anxiety. (A) The right VBF/BNST, left (B) and right insula increased in sustained activity with greater task-evoked anxiety. Image threshold P < 0.05, whole-brain corrected. (C) Mean signal estimates in right IFG/insula (x = 33, y = 29, z = −12) plotted for the 4 sustained conditions. Error bars denote standard error of the mean.
Table 2.
Sustained emotional responses modulated by task-evoked anxiety and replication with direct contrasts
| Label | BA | x | y | z | t |
| Increasing responses with increasing task-evoked anxiety | |||||
| Anterior insula/inferior frontal gyrus | 47m | −36 | 20 | −14 | 4.38 |
| Anterior medial insula* | −30 | 2 | −10 | 4.37 | |
| Ventral basal forebrain/BNST* | 6 | −3 | −2 | 3.62 | |
| Inferior frontal gyrus | 47m | 33 | 29 | −12 | 4.00 |
| Visual association cortex | 37 | 27 | −55 | 3 | 4.33 |
| Visual cortex | 18 | −15 | −67 | 9 | 3.89 |
| Superior occipital gyrus | 19 | −45 | −60 | 14 | 4.73 |
| Superior frontal gyrus | 6 | −9 | −11 | 61 | 3.89 |
| Superior temporal gyrus | 20 | 42 | −27 | −9 | 3.85 |
| Increasing responses with decreasing task-evoked anxiety | |||||
| vACC/vmPFC | 32 | −6 | 37 | −17 | 3.51 |
| vACC/vmPFC* | 32 | 3 | 29 | −12 | 3.38 |
| Replication: whole-brain contrasts | |||||
| Sustained negative versus sustained neutral | |||||
| Anterior insula | 11 | −30 | 11 | −3 | 4.09 |
| Inferior frontal gyrus | 11 | 48 | 17 | −16 | 3.83 |
| Ventral basal forebrain/BNST | 6 | −3 | −2 | 4.05 | |
| Sustained unpredictable versus sustained predictable | |||||
| Anterior insula/inferior frontal gyrus | 47 | 30 | 26 | −13 | 4.33 |
| Ventral basal forebrain/BNST | 12 | 0 | 3 | 3.66 | |
Note: Threshold P < 0.05, whole-brain corrected. Areas with * denote subclusters encompassed within larger functional activation clusters. BA = Brodmann Area.
We evaluated temporal specificity by evaluating whether these regions also showed transient responses to picture events by testing transient beta estimates within these ROIs. Notably, all regions also showed some degree of transient activation relative to resting fixation (P's < 0.02). However, unlike the transient responses reported earlier, responses in these regions showed no main effects of predictability or valence and no modulation of activity by IU (P's > 0.1).
A second approach to evaluating sustained emotional responses was to test for replication of BA47m/insula and BNST engagement using basic contrasts that did not rely on self-report. Whole-brain paired t-tests targeting differential sustained effects of valence (sustained activity in blocks containing negative vs. neutral pictures) and predictability (blocks containing unpredictable vs. predictable timings) also identified the regions described above at P < 0.05, whole-brain corrected thresholding. Specifically, greater activity was observed in area BA47m/insula and VBF/BNST to negative relative to neutral sustained states and to unpredictable relative to predictable sustained states (Table 2).
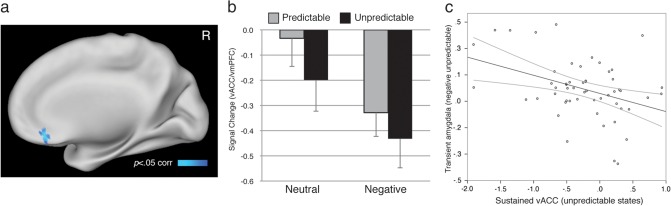
Sustained Responses to States of Lesser Anxiety
The current design permitted identification of regions showing maximal sustained activity in contexts of low anxiety. To do so, we queried for greater sustained activity with lesser task-evoked anxiety by using subject ratings of task-evoked anxiety as inverse contrast weightings in a group whole-brain analysis (see Materials and Methods). Results yielded a single region located in the ventral anterior cingulate cortex bordering on ventromedial prefrontal cortex (vACC/vmPFC; x = 3, y = 29, z = −12; BA25/32; Table 2, Fig. 5A). Signal estimates derived from ROI analyses of the vACC/vmPFC (4 mm sphere surrounding peak activation) are plotted in Figure 5B for descriptive purposes. Testing ROI parameter estimates yielded a significant predictability by IU interaction (F1,52 = 11.28, P = 0.0014) (one participant with questionable signal coverage was excluded from all vmPFC analyses), such that greater IU predicted sustained vACC/vmPFC hyporecruitment during unpredictable contexts (r53 = −0.30, P = 0.03, R2 = 0.087; see Supplementary Fig. 3), an effect not observed for predictable contexts (P > 0.4).
Figure 5.
Greater responding in less anxious states in the right ventral anterior cingulate/vmPFC (A). Image threshold P < 0.05, whole-brain corrected. (B) Signal estimates in (x = 3, y = 29, z = −12) plotted for the 4 sustained conditions. Error bars denote standard error of the mean. (C) Greater recruitment of vACC/vmPFC to unpredictable states predicts lesser transient amygdala response to unpredictable negative events. Gray curves denote 95% confidence interval.
Sustained-Transient Interactions in Emotion Response and Regulation
Prior research assaying limbic/prefrontal circuitry (Milad and Quirk 2002; Phelps et al. 2004; Johnstone et al. 2007; Soliman et al. 2010) suggests an inverse relationship between the vACC/vmPFC and the amygdala. The design of the current study permitted testing for inverse interactions between sustained signals and the magnitude of stimulus-wedded transient responses. Bivariate correlation analyses of parameter estimates from previously defined amygdala and vmPFC ROIs (see above) evaluated whether the degree of sustained vACC/vmPFC engagement predicted the magnitude of transient amygdala response to negative events. Because the construct of IU isolates uncertainty-related anxiety, we focus analyses on interactions between these regions in the unpredictable phases of the experiment. Results indicated that sustained under recruitment of the vACC/vmPFC during unpredictable states predicted the degree of exaggeration of transient amygdala responses to negative pictures in unpredictable contexts (r53 = −0.39, P = 0.004; R2 = 0.15; Fig. 5C). This relationship was specific to transient negative picture responses, as the inverse correlation was not evident when considering neutral pictures (P > 0.1).
Discussion
Anxious emotion can be experienced along brief and persistent timescales. Using an fMRI paradigm sensitive to both transient “fear”-like and sustained “anxiety”-like neural responses, we demonstrate that these components of anxious emotion are spatially and temporally distinct at the neurobiological level, and organized in a manner consistent with regional downstream modulatory effects on behavior. Rapid glutamatergic output centers including the amygdala and midbrain/PAG were uniquely sensitive to transient emotional provocation. Conversely, brain regions critical to modulating sustained arousal maintenance such as the BNST and insula remained continuously engaged throughout lengthy emotional contexts, showing greater activity during states of heightened anxiety. Furthermore, downregulation of transient amygdala responses was predicted by heightened sustained activity in ventromedial prefrontal regions, suggesting regulation of momentary emotional responses may be accomplished via sustained prefrontal engagement. These results provide evidence supporting the specificity of “fear” and “anxiety” systems and offer a mechanism by which persistently anxious states influence brief emotional sensitivity.
Behavioral Findings
During fMRI scanning, participants viewed negative and neutral images, embedded within predictable and unpredictable timings. Negative (vs. neutral) image presentations constituted a cued threat manipulation, which selectively slowed reaction times in individuals with high IU, the anxiety measure we focused on given the relationship of this metric to unpredictable anticipatory states (Buhr and Dugas 2002; see Materials and Methods). This response profile is consistent with assertions that trait anxiety, which correlates with IU (Buhr and Dugas 2002, 2006), enhances attention toward potential threats (Bar-Haim et al. 2007; Bishop 2009) perhaps due to compromised capacity to engage in attentional control during threat processing (MacLeod and Mathews 1988; Bishop 2007).
Sustained emotional modulation was achieved by blocking the valence of images such that a given task block contained only negative or neutral pictures, and by embedding the images within contexts varying in temporal unpredictability. Task-evoked anxiety was greater during blocks containing negative pictures relative to neutral pictures and in contexts involving temporal unpredictability. Though the present findings rely on participant report, they are consistent with studies reporting that temporal uncertainty elicits startle responses (Grillon et al. 2004) and physiological upregulation (Grupe and Nitschke 2011) in humans, as well as anxiety-like behavior in rodents (Herry et al. 2007). Furthermore, blocks in which negative pictures were presented within unpredictable timings elicited an exaggerated rise in anxiety relative to either condition alone. Thus, the effects of unpredictability are especially evident when uncertainty relates to negative outcomes (Whalen 1998; Grillon and Baas 2003).
Transient Emotional Responses
As in prior work (Hariri et al. 2003; Britton et al. 2006; Urry et al. 2006), we observed transient amygdala responding to negative relative to neutral images. Furthermore, the peak height of this response was positively predicted by greater IU. This is consistent with other findings documenting the influence of healthy variation in anxiety on amygdala activity (Etkin et al. 2004; Somerville et al. 2004; Bishop et al. 2007; Stein et al. 2007). Here, amygdala response upregulation with IU was constrained to unpredictable temporal contexts. Other work has shown heightened amygdala response to emotional cues when the contingency schedule between a cue and aversive stimulus is made uncertain by being partially reinforced or ambiguous (Belova et al. 2007; Sarinopoulos et al. 2010). The specificity of IU effects to temporally unpredictable contexts also suggests that event-related fMRI designs with variable-duration jittering, due to the less predictable temporal presentation, maybe incidentally more suited to evoke biased amygdala recruitment with greater trait anxiety.
Responses in the amygdala and midbrain/PAG were constrained to transient “fear”-like responses, as signal change maintained across lengthy blocks did not differ from rest, and largely were not modulated by valence, predictability, or individual differences in anxiety. Anatomically, the PAG is a key convergence point for rapid amygdala and hypothalamic signaling of environmental salience, serving to orchestrate defense responses via downstream reflex modulation (Fanselow 1991; Brandão et al. 1999). Recent human neuroimaging work documents a role for the human PAG in mediating physiological responses during social anxiety (Wager et al. 2009) and representing imminent danger relative to less threatening phases of exposure to a simulated predator (Mobbs et al. 2007; Mobbs et al. 2009). The response specificity to brief threat events in the present study constrains the role for the human PAG to transient signaling.
Sustained Anxious Responses
This study applied design and analysis parameters from validated studies of task-set maintenance to target circuitry demonstrating persistent neural activity during anxious states. The inferior frontal gyrus (BA47m) and anterior insula, subpeaks of a larger activation increasing in activity with more anxiogenic states, are heavily interconnected with one another as well as with a number of structures in the temporal and orbital cortex (Saleem et al. 2008). The insula is physiologically suited to maintain sustained changes in arousal given its role in supporting the continuous updating of a subjective sense of “feeling” (Critchley et al. 2002; Craig 2003) and has been implicated in prior studies measuring brain activity during anxiogenic contexts (Chua et al. 1999; Hasler et al. 2007; Alvarez et al. 2011; Carlson et al. 2011). The present study demonstrates temporal persistence of insular signals during contexts involving heightened anxiety, with additionally exaggerated activity observed in high IU individuals during contexts of temporal unpredictability (see also Simmons et al. 2008). A role for the insula in maintaining states of anxious arousal fits well with its proposed role as an integrator of body state information, which is thought to support physiological upregulation associated with anxiety (Paulus and Stein 2006). Evidence that antianxiety medications such as benzodiazepines reduce insular activity in a dose-dependent fashion (Paulus et al. 2005) and that symptom reduction in GAD correlates with lesser insula sensitivity (Hoehn-Saric et al. 2004) supports this conceptualization. Interestingly, the inferior frontal gyrus has been implicated in cognitive task-set maintenance in nonemotional contexts (e.g., Dosenbach et al. 2006). Further research is needed to determine the exact nature of these adjacent regions' relative contributions to emotional and nonemotional task sets, and to further probe whether the cognitive operations subserved by this region are anxiety-specific or domain-general processes drawn on during anxious states.
The present study identified the BNST as maintaining persistent states of heightened anxiety. The BNST has been widely implicated in sustained anxiety using animal models (Davis et al. 1997; Hammack et al. 2004), though has remained elusive in human imaging results until recently. Human (Straube et al. 2007; Somerville et al. 2010; Alvarez et al. 2011) and non-human primate (Fox et al. 2008; Oler et al. 2009) studies have begun to target this structure, finding increased activity during sustained anticipatory states. Our prior work has demonstrated that VBF/BNST and insula activity tracks sustained threat monitoring using an event-free paradigm in which threat level slowly and continuously fluctuated (Somerville et al. 2010). It is notable that VBF/BNST and insula recruitment in a prior study (Somerville et al. 2010) was exaggerated in individuals with high trait anxiety, whereas the present study observed IU modulation in the insula, but not the VBF/BNST. It is possible that the experimental context contributed to these differential effects. Whereas the present study utilized subtle valence and predictability manipulations, Somerville et al. (2010) reported findings from a shock-threat paradigm, likely a more powerful and salient manipulation of sustained anxiety. Thus, while both studies report engagement of insula and VBF/BNST circuitry, potential physical threat evoked further VBF/BNST upregulation in more anxious individuals. Taken together, these findings constitute accumulating evidence that the inferior frontal gyrus (47m), insula, and VBF/BNST support the maintenance of anxious emotion via tonic signaling.
Sustained-Transient Interactions as a Function of Trait Anxiety
In contrast to circuitry showing greater task-evoked anxiety, we observed greater activity with lesser anxiety along the cortical midline spanning the vACC and vmPFC. The vmPFC has been implicated in the extinction of conditioned fear (Milad and Quirk 2002; Phelps et al. 2004) and in predicting positive interpretations of ambiguous information (Kim et al. 2003). The present findings suggest that the vmPFC may also accomplish regulation of brief affective responses via greater sustained signaling throughout contexts in which environmental cues instruct an individual that nothing negative will happen (e.g., neutral blocks) and that nothing unexpected will happen (e.g., predictable blocks). Indeed, predictive knowledge relating to current and future environmental events is thought to buffer individuals from experiencing state anxiety by blunting cognitions associated with intolerance of uncertainty, a key generator of anxious arousal (Ladouceur et al. 2000).
Accordingly, individuals with higher IU showed reduced engagement of the vACC/vmPFC during unpredictable contexts. This reduction may influence or reflect anxious individuals' inherent sensitivity to ambiguity (Eysenck 1992; Ladouceur et al. 2000), such that signals of “safety” are weakened in contexts in which environmental inputs are temporally ambiguous. This idea converges with work demonstrating differential engagement of this circuitry in clinically anxious adolescents while processing uncertainty (Krain et al. 2008). Furthermore, Nitschke et al. (2009) reported that treatment response efficacy in individuals with clinically significant anxiety is positively predicted by vACC responsivity. The present results suggest that blunted sustained vACC/vmPFC activity may be one mechanism by which individual differences in IU can influence tonic anxiety.
Several studies have documented an inverse relationship between the vACC/vmPFC and amygdala, with greater vACC/vmPFC involvement predicting a reduction of amygdala response (Kim et al. 2003; Urry et al. 2006; Johnstone et al. 2007) and comprising a functional network supported by direct reciprocal anatomical projections (Amaral 1986; Ghashghaei and Barbas 2002). In the present study, lesser sustained vACC/vmPFC recruitment predicted the degree of exaggeration of amygdala response to negative pictures with greater IU, which converges with other recent findings (Indovina et al. 2011). It is notable that in the current study, the level of “sustained” vACC/vmPFC response negatively predicted “transient” amygdala sensitivity. This suggests that tonic signals of safety may orchestrate moment-to-moment emotional sensitivity, analogous to tonic–phasic interactions thought to coordinate cognitive operations such as sustained attention (Posner and Petersen 1990) and cognitive control (Carter et al. 1998; Botvinick et al. 1999; Dosenbach et al. 2008). Though outside of the core “default mode” network demonstrating spontaneous and correlated activity at rest (Fox and Raichle 2007), the locus of vACC/vmPFC activity has recently been identified as a subsystem of the default mode network with strong intrinsic connectivity with regions of the medial temporal lobe (Andrews-Hanna et al. 2010). This network is thought to support self-oriented cognitions and the constructive representation of future events, though its precise role in emotional or anxiogenic contexts remains unexplored. In the present study, persistent vACC/vmPFC activity is suspended in contexts in which participants are made to feel anxious. Future research incorporating affective tasks and resting state connectivity data may further specify affective contributions to “default-mode” activity.
The current findings may hold particular relevance to the neural substrates of mood and anxiety disorders. Whereas the symptoms of some anxiety disorders are thought to be modulated by exaggerated sensitivity of the threat-detection system (Barlow 1988), the symptomatology of other anxiety disorders (such as GAD) are marked by chronic and higher-order apprehension of future threats that are not necessarily triggered by an external threat cue (Ohman 1993; Lang et al. 2000). Though the present study only assesses variation within the healthy range of trait anxiety, these data may inform predictions regarding the specificity of transient and sustained affective networks on “fear”-based and “anxiety”-based features of mood and anxiety dysregulation. For example, a meta-analysis (Etkin and Wager 2007) reported exaggerated amygdala responses in anxiety disorders with core “fear”-like features (e.g., specific phobias and social anxiety disorder). However, such a clear pattern of hyperresponse is less evident in other anxiety disorders such as GAD. Rather, the literature reports mixed results, with some showing exaggerated amygdala responses in GAD participants to threat cues (McClure et al. 2007; Monk et al. 2008), some showing diminished responses (Blair et al. 2008), some reporting no differences (Whalen et al. 2008), and others demonstrating comparable responses to aversive cues but indiscriminate and exaggerated responses in GAD to anticipatory cues (Nitschke et al. 2009). Etkin et al. (2009) have suggested that the connections between subregions of the amygdala and areas of the frontal and parietal cortices show selective aberrant patterns of activity in GAD, implying a broad network of pathophysiology in disorders such as GAD marked by chronic and less cue-driven symptoms of anxiety. The present findings offer the suggestion that the heterogeneity of anxiety symptoms seen across disorders may predict selective dysregulation of (or interactions between) brief and persistent affect circuitries identified in the present study. More generally, this work demonstrates that evaluating affective processes across broader timescales offers new insights into how emotional brain systems can support the wide variety of emotional behaviors humans exhibit, in healthy and pathological states.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by the National Institute of Mental Health (K99MH087813 to L.H.S.), the National Science Foundation (NSF0746220 to W.M.K.; Graduate Research Fellowship to L.H.S), and the Dartmouth Brain Imaging Center.
Supplementary Material
Acknowledgments
We acknowledge B.J. Casey, Christine Colyer, Todd Heatherton, Tammy Moran, Lisa Shin, and George Wolford for assistance and helpful comments, Kevin LaBar for sharing stimulus materials, and Antonia Hamilton for sharing fMRI quality assurance tools. Conflict of Interest : None declared.
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. Amygdalohippocampal and amygdalocortical projections in the primate brain. Adv Exp Med Biol. 1986;203:3–17. doi: 10.1007/978-1-4684-7971-3_1. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakerman-Kranenburg MJ, van Izendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders. New York: Guilford Press; 1988. [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Morrison SE, Salzman CD. Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala. Neuron. 2007;55:970–984. doi: 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Picture media and emotion: effects of a sustained affective context. Psychophysiology. 1996;33:662–670. doi: 10.1111/j.1469-8986.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Brandão ML, Asnseloni VZ, Pandóssio JE, De Araújo JE, Castilho VM. Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev. 1999;23:863–875. doi: 10.1016/s0149-7634(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Buhr K, Dugas MJ. The Intolerance of Uncertainty Scale: psychometric properties of the English version. Behav Res Ther. 2002;40:931–945. doi: 10.1016/s0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- Buhr K, Dugas MJ. Investigating the construct validity of intolerance of uncertainty and its unique relationship with worry. J Anxiety Disord. 2006;20:222–236. doi: 10.1016/j.janxdis.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Sharpe D, Asmundson GJ. Anxiety sensitivity and intolerance of uncertainty: requisites of the fundamental fears? Behav Res Ther. 2007;45:2307–2316. doi: 10.1016/j.brat.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular responses predict the feeling of anxious anticipation. Soc Cogn Affect Neurosci. 2011;6:74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Neo Five-Factor Inventory (NEO-FFI) professional manual. Odessa (FL): Psychological Assessment Resources; 1991. [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE. What is an anxiety disorder? Depress Anxiety. 2009;26:1066–1085. doi: 10.1002/da.20633. [DOI] [PubMed] [Google Scholar]
- Critchley HD, McImed RN, Featherstone E, Mathias CJ, Dolan R. Volitional control of autonomic arousal: a functional magnetic resonance study. Neuroimage. 2002;16:909–919. doi: 10.1006/nimg.2002.1147. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1988;15:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Ann N Y Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas MJ, Gagnon F, Ladouceur R, Freeston MH. Generalized anxiety disorder: a preliminary test of a conceptual model. Behav Res Ther. 1998;36:215–226. doi: 10.1016/s0005-7967(97)00070-3. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck M. Anxiety: the cognitive perspective. Hillsdale (NJ): Erlbaum; 1992. [Google Scholar]
- Fanselow MS. The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: DePaulis A, Bandler R, editors. The midbrain periaqueductal gray matter. Functional, anatomical and immunohistochemical organization. New York: Plenum Press; 1991. pp. 151–173. [Google Scholar]
- First MB, Spitzer MRI, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-V (SCID) Washington (DC): American Psychiatric Association; 1995. [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J Exp Psychol Gen. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application to psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion. 2011;11:413–424. doi: 10.1037/a0022583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger D. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. J Neurosci. 2007;27:6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Luthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res. 2004;131:11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Nunez-Elizale AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69:563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Donaldson DI, Buckner RL. Transient activation during block transition. Neuroimage. 2001;13:364–374. doi: 10.1006/nimg.2000.0691. [DOI] [PubMed] [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, Milham MP. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry. 2008;63:563–568. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Ladouceur R, Gosselin P, Dugas MJ. Experimental manipulation of intolerance of uncertainty: a study of a theoretical model of worry. Behav Res Ther. 2000;38:933–941. doi: 10.1016/s0005-7967(99)00133-3. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. 1998. International Affective Picture System (IAPS): technical manual and Affective Ratings. Gainsville (FL): The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention toward threat. Q J Exp Psychol A. 1988;40:653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Hajcak G. Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia. 2009;47:2975–2980. doi: 10.1016/j.neuropsychologia.2009.06.026. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ohman A. Fear and anxiety as emotional phenomena: clinical phenomenology, evolutionary perspectives, and information-processing mechanisms. In: Lewis M, Haviland JM, editors. Handbook of emotions. New York: Guilford Press; 1993. pp. 511–536. [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Christian BT, Murali D, Oakes TR, Davidson RJ, Kalin NH. Serotonin transporter availability in the amygdala and bed nucleus of the stria terminalis predicts anxious temperament and brain glucose metabolic activity. J Neurosci. 2009;29:9961–9966. doi: 10.1523/JNEUROSCI.0795-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by Lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view on anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Reiss S. Test manual for the anxiety sensitivity index. Orland Park (IL): International Diagnostic Systems; 1987. [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, Grillon C. The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cogn Affect Behav Neurosci. 2011;11:217–227. doi: 10.3758/s13415-011-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman MEP. Chronic fear produced by unpredictable electric shock. J Comp Physiol Psychol. 1968;66:402–411. doi: 10.1037/h0026355. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Bradley MM PJ. State anxiety and affective physiology: effects of sustained exposure to affective pictures. Biol Psychol. 2005;69:247–260. doi: 10.1016/j.biopsycho.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Bed nucleus of the stria terminalis indexes tonic hypervigilance in humans. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI-manual for the State Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1988. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H-J, Milner WHR. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software system for surface-based analysis of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidsons ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, part II: prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–188. [Google Scholar]
- Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, Davidson RJ, Kalin NH. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. J Neurophysiol. 2009;101:2632–2648. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.