Abstract
Abnormal brain activity may reflect compensation when observed in patients who perform normally on tests requiring functions usually observed as impaired. Operational criteria defining compensation have been described and aid in distinguishing compensatory from chance events. Here, we tested whether previously published functional magnetic resonance imaging data acquired in 15 recovering alcoholics and 15 controls at rest and while performing a spatial working memory task would fulfill criteria defining functional compensation. Multivariate analysis tested how well abnormal activation in the affected group predicted normal performance, despite low or no activation in brain regions invoked by controls to accomplish the same task. By identifying networks that uniquely and positively correlated with good performance, we provide evidence for compensatory recruitment of cerebellar-based functional networks by alcoholics. Whereas controls recruited prefrontal-cerebellar regions VI/Crus I known to subserve working memory, alcoholics recruited 2 other parallel frontocerebellar loops: dorsolateral prefrontal cortex (DLPFC)-cerebellar VIII system during rest and DLPFC-cerebellar VI system while task engaged. Greater synchronous activity between cerebellar lobule VIII and DLPFC at rest and greater activation within cerebellar lobule VI and DLPFC during task predicted better working memory performance. Thus, higher intrinsic cerebellar activity in alcoholics was an adequate condition for triggering task-relevant activity in the frontal cortex required for normal working memory performance.
Keywords: alcoholism, BOLD, compensation, executive, frontocerebellar circuits, salience
Introduction
Neuropsychiatric disorders are marked by cognitive dysfunctioning of selective processes characterizing each disorder. Occasionally spontaneously and sometimes with training, these impairments can be minimized or overcome (for review, see Schlaug et al. 2011). Functional magnetic resonance imaging (fMRI) studies have documented instances of these improvements in normal aging (Voss et al. 2010), traumatic brain injury (Voytek et al. 2010), anxiety disorder (Goldin and Gross 2010), dyslexia (Hoeft et al. 2011), and chronic alcoholism (Pfefferbaum et al. 2001). Typically, fMRI identifies differences between groups in brain activation patterns, measured as local activation differences between experimental and control tasks in the blood oxygen level–dependent (BOLD) response. Before examination of a specific cognitive process with functional imaging, however, the cognitive impairment is identified behaviorally. Then tasks assessing the impaired cognitive process are devised for examination with fMRI but under conditions in which the affected group performs at levels of a healthy comparison group. The goal of the functional study is to detect potential differences in activation patterns assumed to enable normal performance in the affected group. Difference in activations is interpreted as compensation and the new constellation of activated sites as the mechanisms of compensation (e.g., Marinkovic et al. 2009). Whether such differences can be considered “compensatory” remains controversial.
We pursued evidence for the operation of compensatory mechanisms by subjecting previously reported fMRI data to new analyses to examine whether alternative processes were invoked and whether they met criteria to deem them compensatory (cf., Davis et al. 2008). One criterion requires that abnormal brain activity, that is, activity not seen in an unaffected comparison group, be associated with normal level performance. A second criterion requires that abnormal increases of activity in the supposed compensatory region occur with lower activity in processing by the damaged regions (cf., Davis et al. 2008). Thus, we tested the hypothesis that, if compensatory, the “abnormal” brain activities would support good cognitive test performance. Our analysis focused on alcoholics whom we had previously shown to draw on different frontocerebellar circuits from controls to rescue processes otherwise impaired because of their reliance on circuitry with alcoholism-related damage.
The harmful effects of alcoholism are especially prominent in circuits involving the prefrontal cortex (Harper et al. 2003) and one frontocerebellar loop in particular. Frontocerebellar circuitry has multiple parallel loops, as revealed structurally by viral tracing studies (Kelly and Strick 2003) and functionally with in vivo imaging (Habas et al. 2009; Krienen and Buckner 2009). The structural loops involve multisynaptic connections between Crus II and area 46, which support cognitive functions, including motor sequencing in spatial working memory and connections between cerebellar lobules IV-VI-VIII and primary motor cortex, which support motor functions, including motor tracking (Kelly and Strick 2003). Functional systems have been identified with task-activated fMRI (Krienen and Buckner 2009) and resting state connectivity analysis (Habas et al. 2009) and are characterized by synchronous BOLD activity between selective cerebellar regions and cortical sites. Cerebellar cortical functional connectivity revealed with task-activated synchrony is consistent with the cognitive and motor loops identified in the primate structural tracing study, linking Crus II and prefrontal cortex as a cognitive loop and lobules IV-VI-VIIIB and primary motor cortex as a motor loop (Krienen and Buckner 2009). Although the structural and functional circuits are largely closed, there is potential for structural (Kelly and Strick 2003) and functional (Habas et al. 2009) interchange between loops. Because compensatory mechanisms are thought to rely on shift in function from one to another brain circuit, the circuits involved in the shift usually have a pre-existing functional relation (Matthews et al. 2004) and structural proximity (Dosenbach et al. 2008; Schlaug et al. 2011). Thus, we hypothesized that functional compensatory mechanisms recruited by alcoholics could involve other, possibly undamaged, frontocerebellar loops that are parallel to the prefrontal loop structurally damaged in alcoholics and that underlie spatial working memory in controls.
Accordingly, we reanalyzed data from a sample of chronic alcoholics who performed at control levels on a spatial working memory task (Chanraud et al. 2011). Our initial analysis revealed task-related functional activity differences between alcoholics and controls when achieving similar accuracy and reaction time (RT) performance levels (Chanraud et al. 2011). The between-group contrasts of BOLD activity revealed the same pattern of brain activation differences regardless of experimental condition: the alcoholics exhibited greater activation than controls, most profoundly in bilateral insular and right middle frontal cortices, whereas the controls exhibited greater activation than alcoholics in left cerebellar regions Crus I and lobule VI, left parietal and cingulate cortices, and right postcentral and lingual gyri. Our initial analysis identified concurrent activity in local brain regions, which is an initial step in establishing compensatory mechanisms. To expand our understanding of the multiple components of cognitive processes employed in complex tasks requires knowledge about activity within and between networks enabling communication among brain loci. Therefore, in complement to a description of localized activation, the current analysis employed effective functional connectivity analysis, which permits exploration of normal and abnormal causal interactions between neuronal sites (Friston et al. 1997) in response to a task.
In addition to task activation of brain regions effectively enhancing performance, functional networks are active while the brain is at rest, that is, when the brain is not involved in externally guided activity (Greicius et al. 2003; Fox and Raichle 2007; Krienen and Buckner 2009; Van Dijk et al. 2010). The brain’s functional intrinsic architecture may serve as an index of resources that predict the success of compensatory efforts (Etkin et al. 2009). Thus, we expanded our analysis by questioning whether diagnostic differences in task-related activation patterns and performance levels can be predicted by differences in the pattern or degree of synchronous activity at rest.
Efficiency of intrinsic functional networks and its relations with performance on tasks was evaluated with graph analysis (e.g., Salvador et al. 2005; Hua et al. 2008; Wang et al. 2009), which measures network connectivity locally (efficient information processing) and globally (efficient communication across the network and integration of information). Our previous analysis indicated that, at rest, cerebellar region VIII efficiency predicted alcoholics’ performance on the spatial working memory task (Chanraud et al. 2011). We posited that the intrinsic function of this region would be participating in compensatory mechanisms recruited for the task. Accordingly, we tested whether alcoholics took advantage of an intact functional frontocerebellar loop to overcome impairment associated with a parallel frontocerebellar loop and whether this activity met criteria to be considered compensatory.
Materials and Methods
Participants
Study participants, described previously (Chanraud et al. 2011), were 15 men with alcohol dependence recruited from community treatment centers, outpatient clinics, hospitals, and by word of mouth, and 15 healthy control men, recruited through flyers, announcements, and word of mouth. All participants were right-handed, as determined with a quantitative test (Crovitz and Zener 1962). Alcoholics (mean ± standard deviation [SD] age = 40.1 ± 10.9 years) and controls (mean ± SD age = 47.7 years) did not differ significantly in age (t28 = 1.775, P = 0.083). Although alcoholics had fewer years of education (13.3 ± 1.6 years) than controls (14.9 ± 2.4 years) (t28 = 2.09, P = 0.046), both groups reached on average an education beyond high school and did not differ significantly in estimated verbal intelligence quotient (IQ) (alcoholic IQ = 108.9 ± 8.104; control IQ = 111.1 ± 9.6) (t28 = 0.651, P = 0.521) (Nelson 1982).
The psychiatric history of each participant was reviewed to exclude those with a previous history of any major psychiatric disorder, head trauma, neurological disorders, and drug or alcohol use disorders for healthy participants. Diagnosis was made according to DSM-IV-TR criteria. All participants were interviewed by a clinical research psychologist or research nurse using the Structured Clinical Interview for the DSM-IV (First et al. 1998), and diagnosis was determined by consensus of at least 2 calibrated interviewers. Approximate lifetime alcohol consumption was quantified using a modification (Pfefferbaum et al. 1988) of a semistructured time-line interview (Skinner 1982; Skinner and Sheu 1982). Drinks of each type of alcoholic beverage (wine, beer, and spirits) were standardized to units containing approximately 13.6 g of alcohol and summed over the lifetime. As expected, alcoholics reported significantly higher lifetime alcohol consumption (1078.2 ± 1161.0 kg) than controls (28.1 ± 35.9 kg) (t28 = 3.252, P = 0.003). Alcoholics had been abstinent from alcohol for at least 30 days, except for one participant, who had been abstinent for 5 days but exhibited no withdrawal signs.
All participants provided written informed consent to participate in studies assessing the impact of alcohol on brain structure and function and received a modest stipend for study participation.
Working Memory Task Conditions during Image Acquisition
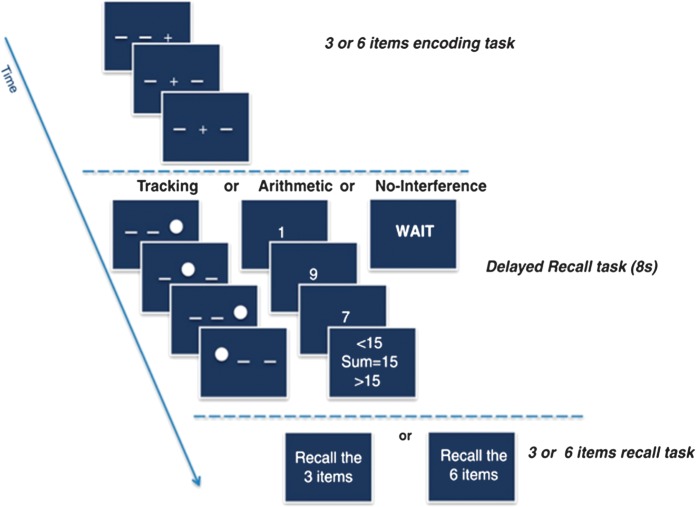
The spatial working memory task had 2 memoranda load conditions: 3 and 6 items. Subjects memorized spatial memoranda and recalled spatial sequences after a retention interval. Stimuli consisted of crosses presented in the right, center, or left part of the screen. The retention interval was either without interference (control) or with interference from a secondary task, which was either cognitive interference with an arithmetic task or motor interference with a tracking task (Fig. 1). Before going to the scanner, all the participants practiced the working memory task until they understood how to perform the task. Stimuli were presented via E-Prime software (Psychology Software Tools Inc., Pittsburgh, PA). For their responses, subjects used a keypad connected to the laptop running E-prime (http://www.pstnet.com). Recorded within-scanner behavioral measures included RT and accuracy (see Chanraud et al. 2011 for further details).
Figure 1.
Dual-tasks paradigm: Subjects watched and memorized a sequence of spatial positions; and after an 8-s retention interval during which they had to perform either a tracking or an arithmetic task or just wait, subjects tapped out the sequence of spatial positions on a keypad.
Data Acquisition
The scanning was conducted on a GE (General Electric Medical Systems, Signa, Waukesha, WI) 3T whole body MRI scanner equipped with an 8-channel head coil. A T2-weighted fast spin-echo anatomical scan (axial acquisition; time echo [TE] = 17 ms; time repetition [TR] = 5000 ms; field of view [FOV] = 24 cm; 256 × 192 matrix; NEX = 1.0; slice thickness = 5 mm; 36 slices) was acquired together with the fMRI scans. A field map was generated from a gradient recalled echo (GRE) sequence pair (TE = 3/5 ms, TR = 460 ms, slice thickness = 2.5 mm, 62 slices). Whole-brain fMRI data were acquired with a gradient echo planar pulse sequence (axial, mode = 2D, scan timing: TE = 30 ms, TR = 2200 ms, flip angle = 90°, matrix = 64 × 64, slice thickness = 5 mm, 36 slices).
For the “spatial working memory task,” 4 blocks of 3 min and 10 s each, synchronized with the beginning of fMRI volume acquisitions were acquired. Four sessions of 3096 functional images were acquired for each subject with the following pseudorandomized conditions (5 dummy scans + 10 pseudorandomised trials): 3 retention conditions × 2 loads = 6 trials + 2 interference trials without memoranda + 2 blocks of rest.
The “rest condition” fMRI session followed the spatial working memory task fMRI session. During the rest scan, subjects were instructed to lie still with eyes closed, be relaxed, and remain awake. This session lasted 4 min and 57 s (135 TR, 4860 images).
Image Preprocessing
Spatial preprocessing and statistical analysis of functional images were performed using SPM8 (Wellcome Department of Cognitive Neurology). Motion artifacts were assessed in individual subjects by visual inspection of realignment parameter estimations to confirm that the maximum head motion in each axis was less than 2 mm, without any abrupt head motion. Functional images were realigned for motion corrections and unwarped (correction for fields distortions) using the gradient echo field maps (constructed from the complex difference image between 2 echoes [3 and 5 ms] of the GREs series). Unwarped functional images were registered to structural images for each subject. The anatomical volume was then segmented into gray matter, white matter, and cerebrospinal fluid. The gray matter image was used for determining the parameters of normalization onto the standard Montreal Neurological Institute (MNI) gray matter template. The spatial parameters were then applied to the realigned and unwarped functional volumes that were finally resampled to voxels of 3 × 3 × 3 mm and smoothed with an 8 mm full-width at half-maximum kernel.
Statistical Analyses
Localized Functional Activation: fMRI Contrast Analysis with the Task
First, contrast images for each of the 3 memory task conditions (No-interference, Arithmetic interference, and Tracking interference) versus rest were computed for each load in every subject (for details, see Chanraud et al. 2011). The results of these contrasts were submitted to a factorial analysis with the group (controls vs. alcoholics), the interference (no vs. arithmetic vs. tracking), and the memory load (3 vs. 6 items) entered as main factors. A complementary between-subgroup comparison was conducted for alcoholics with good performance in contrast to poor performance in order to test for abnormal brain activations to fulfill criteria of association with performance. The alcoholic group was divided into good and poor performers using their performance median, which corresponded to an accuracy score of 50% of correct responses.
Analyses of fMRI data were thresholded at PFDR < 0.05 for the whole brain volume with a minimum cluster extent of 10 contiguous voxels.
Activations were located anatomically using MRIcro software (http://www.mricro.com) for the cerebrum. For the cerebellum, peaks of activation were referenced with a supplemental manual delineation of probabilistic atlas of human cerebellar nuclei (Diedrichsen et al. 2009) implemented with MRIcro software and also with the cerebellar atlas of Schmahmann et al. (1999) after MNI coordinates were converted into Talairach coordinates (http://www.bioimagesuite.org/Mni2Tal/index.html).
Effective Connectivity: Psychophysiological Interaction during the Task
Psychophysiological interaction (PPI) analysis measures differences in regression slopes between activities in 2 brain regions under different behavioral contexts. This measure detects responses in specific brain regions in terms of the interaction between inputs from another brain region time series and its variation with changes in psychological context (Friston et al. 1997). PPI analysis has been described as a measure of effective connectivity, that is, the direct influence of one region on another while engaged in a specific activity (experimental condition). PPI does not measure intrinsic functional connectivity unrelated to task demands. Rather, PPI analysis can provide information about the direction of the connectivity during task engagement.
Based on results revealed in fMRI contrast analyses (see Results section), we extracted the deconvolved time course of activity in the right middle frontal cortex (a 10-mm radius sphere centered on the peak activity) found to be more activated by alcoholics than controls to test for evidence of compensatory activity. PPI analyses were conducted for each alcoholic on BOLD data acquired during the most challenging condition, arithmetic interference, and 6 items load because it yielded the largest between-group differences in brain activation (see Results section). Then, associated connectivities were entered into a one-sample group analysis (thresholded at P < 0.001 and a cluster size of >10 voxels).
Intrinsic Connectivity: Rest Analysis
We used resting-state functional connectivity analysis for each group separately to explore the intrinsic connectivity between the right middle frontal cortex with all other brain regions. The connectivity analysis on preprocessed data was conducted with the “conn” toolbox, implemented in SPM (Benjamin et al. 2010), following the steps described previously (Chanraud et al. 2011). Correlational analyses conducted between the BOLD signal from the selected seed (right middle frontal cortex) to every other brain voxel provided seed-to-voxel connectivity estimations during the rest session.
Correlations
Correlational analyses were conducted between localized functional activation levels, functional connectivity, and performance. Spearman rank order correlations (PASWStatistics 18.0) were used to test relations between level of change in functional activation within brain regions of interest, Fisher-transformed Z-value measures of functional connectivity between brain regions of interest and performance.
Results
Localized Functional Activations
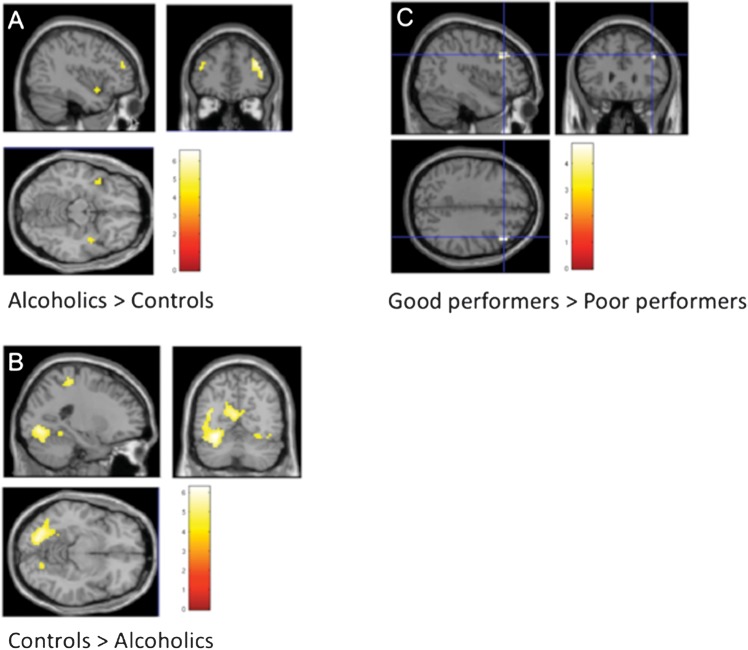
The between-group contrasts (alcoholics > controls and controls > alcoholics) and the within-group contrast (good performers > poor performers in the alcoholic group) are presented as t-test maps (P < 0.05 false discovery rate [FDR] corrected, k > 10). Results of the between-group contrast as presented in Table 1 are taken from Chanraud et al. (2011) (Fig. 2).
Table 1.
Results of the factorial analysis (Group by condition by load effect) at *P < 0.05 FDR corrected)
| Brain regions with FDR significant activations† | Peak F and T values | MNI coordinates | |||
| x | y | z | |||
| Analysis of variance | Right middle frontal cortex | 42.69 | 48 | 47 | 7 |
| Left cerebellum CrusI/region VI | 39.8 | −27 | −70 | −17 | |
| Left parietal cortex | 39.41 | −42 | −25 | −43 | |
| Left middle cingulate cortex | 38.68 | −6 | −40 | 37 | |
| Left middle frontal cortex | 25.64 | −33 | 50 | 25 | |
| Right postcentral gyrus | 23.09 | 45 | −19 | 46 | |
| Right lingual gyrus | 21.54 | 18 | −70 | −11 | |
Figure 2.
Left: Brain regional activation in A alcoholics relative to controls at the top and B controls relative to alcoholics at the bottom, while performing on the task. (C) Brain regional activation in good alcoholics performers (8 subjects) relative to poor alcoholics performers (7 subjects). Cutoff for performance estimated with accuracy = 50% of good responses; P < 0.05 FDR corrected and k > 10 voxels.
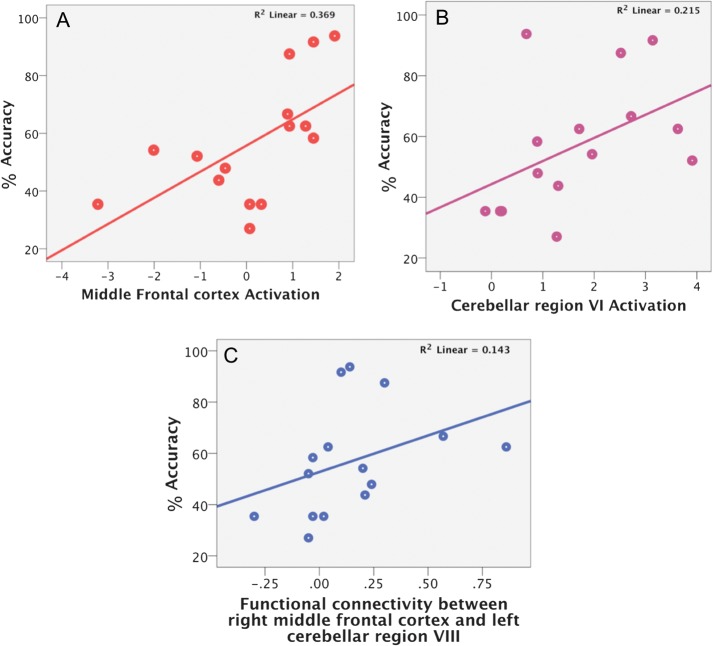
The comparison at P < 0.05 FDR corrected between good and poor performers in the group of alcoholics revealed stronger activation, specifically in the middle frontal cortex (MRICro coordinates x = 45, y = 29, z = 34; or dorsolateral prefrontal cortex [DLPFC] in Brodmann terminology), in the good performers relative to the group of poor performers (Fig. 2C). Consistent with this result, higher activation in the DLPFC positively correlated with greater accuracy (r = 0.706; P = 0.003; Fig. 3A) but not processing speed (r = −0.317; P = 0.25) in alcoholics. Also in alcoholics, whole brain correlation analyses revealed that the level of activation of the cerebellar region VI correlated significantly with greater accuracy (r = 0.463; P = 0.041; Fig. 3B) but not RT. No other brain region presented a significant relation between level of activation and performance. In controls, better performance correlated with higher activation levels in the bilateral cerebellar regions Crus I/VI, left inferior parietal cortex, and right postcentral gyrus. There was no significant relation between level of activation in any brain region and RT in controls.
Figure 3.
Correlation in the group of alcoholics between accuracy and A middle frontal cortex activation while engaged in the working memory task; (B) cerebellar region VI activation while engaged in the working memory task; (C) strength of intrinsic functional connectivity between middle frontal cortex and cerebellar region VIII at rest.
Effective Functional Connectivity of the DLPFC: Task-Related Connectivity
To test whether the DLPFC contributed to a compensatory functional brain network, we focused the PPI analysis in the alcoholic group on the most challenging condition, that is, arithmetic interference and 6-item load (Table 2) (Chanraud et al. 2010). Results revealed significant positive connectivity of the right middle frontal cortex with the calcarine gyrus, precuneus, right caudate, and left cerebellar region VI (Fig. 3).
Table 2.
Subjects’ performance in terms of accuracy and RT (from Chanraud et al. (2011) Cerebral Cortex 2011)
| Retention interval task | Delayed condition | Arithmetic condition | Tracking condition | |||
| Memory load | 3 items | 6 items | 3 items | 6 items | 3 items | 6 items |
| Accuracy (%) | ||||||
| Alcoholics | ||||||
| Mean | 81.1 | 68.3 | 61.1 | 53.0 | 63.1 | 51.9 |
| SD | 22.15 | 16.65 | 27.6 | 25.18 | 30.219 | 19.27 |
| Controls | ||||||
| Mean | 95.0 | 71.7 | 71.5 | 51.1 | 76.2 | 56.8 |
| SD | 9.85 | 24.15 | 27.6 | 15.7 | 19.46 | 19.2 |
| Reaction time (ms) | ||||||
| Alcoholics | ||||||
| Mean | 2205.5 | 5362.3 | 2905.8 | 5998.2 | 2705.5 | 5695.7 |
| SD | 650.13 | 1084.74 | 749.4 | 1539.95 | 637.28 | 1384.77 |
| Controls | ||||||
| Mean | 2938.3 | 4970.8 | 2692.6 | 5741.7 | 2626.0 | 5633.4 |
| SD | 1945.54 | 1418.61 | 858.27 | 1316.17 | 546.72 | 1337.62 |
| P value | P = 0.177 | P = 0.403 | P = 0.474 | P = 0.628 | P = 0.717 | P = 0.9 |
Intrinsic Functional Connectivity of the DLPFC: Rest-Related Connectivity
During rest, the alcoholics and controls showed a similar pattern of intrinsic connectivity using the right DLPFC as a seed, which was positively connected with frontal and parietal cortices, insula, and cerebellar region VIII (P < 0.05 FDR corrected). In alcoholics but not controls, significant synchronizations were observed between time-series of spontaneous fluctuations of the right DLPFC with left premotor cortex, middle cingulate cortex, and precuneus (significant at P < 0.05 FDR). In alcoholics, correlations between strength of intrinsic connectivity and performance revealed that greater synchronous activity between the DLPFC and cerebellar region VIII (x = −24, y = −56, z = −45) was related to greater accuracy in alcoholics (r = 0.564; P = 0.028; Fig. 3C). Thus, rest-related connectivity in the alcoholics was predictive of accuracy in the task-activated conditions. No other regional correlations were significant, and none occurred with RT in alcoholics. Rest-related connectivity correlations were not significant in the controls. As a follow-up exploratory analysis of synchronous regions meeting the FDR-corrected criterion of P < 0.5, a significant interaction was revealed between group performance and connectivity at rest involving synchrony between the right dorsolateral prefrontal and supramarginal cortices. This interaction indicated that better performance was correlated with greater regional synchrony in the alcoholics, whereas the controls showed the opposite pattern of performance and synchrony.
Discussion
An advantage of neuroimaging data is their permanence and, therefore, availability for reanalysis with new developments. Since the initial publication of the data used herein, novel approaches for connectivity analyses became available and permitted reassessment of these data to address whether initially observed findings met criteria for compensation and whether consideration of the dynamic interchange of rest and task could reveal information about diagnostic differences in functional connectivity, efficiency in connectivity, and prediction of cognitive performance. Here, we identified, in recovering alcoholics, brain abnormalities that may be involved in compensatory mechanisms. These mechanisms include the participation of cerebellar region VIII in compensatory processes even during rest. We posited a model proposing that the cerebellar region VIII launches the involvement of the middle frontal cortex for performing the task; then, once recruited the middle frontal cortex would launch the activation of the cerebellar region VI, normally engaged for performing on this task. Therefore, we identified a set of positive outcomes in the performance of alcoholics that meet criteria for inferring the operation of compensatory mechanisms enabling normal performance on a spatial working memory task, impairment on which is a common feature of alcoholism.
Are Observed Abnormal Brain Processes Involved in Compensation?
Our results fulfill the 2 criteria presented necessary for considering brain activations as compensatory (cf., Davis et al. 2008). First, we identified a relationship between a brain region that was less activated in alcoholics than controls (cerebellar region VI/Crus I) and a brain region that was more activated in alcoholics than controls (DLPFC). This relation satisfies the criterion requiring that abnormal increases of activity in the supposed compensatory region also be related to abnormally low activation in other brain regions normally associated with the task. Indeed, the effective connectivity analysis revealed that the 2 regions were related when engaged in the spatial working memory task. Specifically, greater activation in the DLPFC of alcoholics while performing the task was correlated with greater activation in cerebellar region VI. This relation provided a basis to speculate that activity in the DLPFC could launch activity in cerebellar region VI when required to boost performance. Secondly, even though activation within cerebellar region VI was related to accuracy in controls, this region was not activated at control level in alcoholics. However, the same significant relation between level of activation in cerebellar region VI and accuracy was revealed in the alcoholics. Positive correlations with accuracy in the 2 groups support involvement of this region as essential for performing the task. The abnormal activation pattern associated with normal behavioral performance in the alcoholic group fulfills the second criterion for a brain mechanism to be considered as compensatory.
Together, these results provide evidence for functional reorganization of frontocerebellar circuitry in alcoholics who performed at high levels. A biological interpretation is that the recruitment of an unaffected loop parallel to the affected one leads to rapid changes in connectivity, which depends on already present but “silent” connections rather than new ones. This assumption is supported because in controls functional connectivity between the DLPFC and the cerebellar lobule VIII was present in addition to functional connectivity between DLPFC and cerebellar lobule VI. Furthermore, results showing a relation between synchronization of the cerebellar region VIII with the DLPFC at rest and accuracy suggest that bottom-up resources recruited via cerebello-cortical connections are likely to compensate for top-down cortico-cerebellar deficits, thereby enabling accurate task performance.
Abnormal Brain Processes Observed in Alcoholics: Recruitment of an Emergent Functional Loop
Group comparisons of functional activation measured with the BOLD response revealed differences in nodes of 2 known frontocerebellar loops. Cerebellar and frontal nodes of these 2 loops have been recently described using functional connectivity analysis (Habas et al. 2009). The executive loop mainly involves cerebellar regions Crus I and II and dorsolateral–dorsomedial prefrontal cortex; the salience loop involves cerebellar lobule VI, the DLPFC, and frontoinsular cortices in the frontal lobe. We observed lower activation of cerebellar regions VI/Crus I in alcoholics than controls, denoting functional difference within the executive network of alcoholics. Cerebellar lobule VI and Crus I, which together form the superior/lateral cerebellum, are both activated during working memory tasks in healthy subjects (Chen and Desmond 2005; Kirschen et al. 2010), for review see Marvel and Desmond (2010). Furthermore, the activity in these regions can be modified with variation in working memory demands (Desmond et al. 1997; Kirschen et al. 2005). Mental rotation and spatial transformation tasks also activate these posterior cerebellar regions (Bonda et al. 1995; Parsons et al. 1995; Vingerhoets et al. 2002; Zacks et al. 2002). Taken together, our results comport with the concept of a favorable functional alteration and recruitment of the extended executive loops in high-performing chronic alcoholics (Chanraud et al. 2010; Tessner and Hill 2010).
When engaged in tasks of attention, response selection, and working memory, nodes of the executive network (cerebellar regions Crus I and Crus II and prefrontal and posterior parietal cortices) can be coactivated with nodes of salience network (Curtis and D'Esposito 2003; Menon and Uddin 2010). The salience network, as identified with functional activation analysis, includes cerebellar lobule VI (Habas et al. 2009), anterior cingulate cortex, and orbital frontoinsular cortices (Seeley et al. 2007). As integrated activation sites, the executive and salience networks constitute a “task activation ensemble” (Seeley et al. 2007). This integration is consistent with the separate and complementary purposes of these networks: the executive loop is invoked to carry out a specific process or task, whereas the salience network is activated to maintain a goal-directed behavior (Dosenbach et al. 2006), typically in response to sensory salience (Seeley et al. 2007). Thus, the salience network plays a role in recruiting relevant brain regions for processing task-relevant sensory information (White et al. 2010). The insula, in particular, has been associated with “bottom–up” detection of salient events and mediating information flow across brain networks involved in attention and cognition (Menon and Uddin 2010). The finding herein of bilateral coactivation of insula and DLPFC in alcoholics adds support to the insula as involved in the transient detection of salient stimuli and in the initiation of attentional control signals, which are then taken over by other regions specialized in the activity at hand (Menon and Uddin 2010). The nonspecificity of this network’s function makes it a potential candidate for compensatory roles because it is well positioned to participate in cognitive control processes (Johnston et al. 2007)
To coordinate these functional alterations, we present a model (Fig. 4) that describes the 2 known anatomical and functional loops of frontocerebellar circuitry (depicted in blue and red). We illustrate (in green) the recruitment by alcoholics of a hypothesized compensatory third loop. The functioning of this hypothetical loop is supported by the involvement of the cerebellar lobule VIII even during rest (involvement being therefore not task-specific) enabling, as necessary, the launching of the activation of the middle frontal cortex, which in turn triggers the activation of the cerebellar lobule VI. This possibility comports with our earlier observation that the efficiency of the cerebellar region VIII connectivity at rest was predictive of alcoholics’ performance (Chanraud et al. 2011). Thus, we speculate that this region is available at rest for triggering compensatory processes when needed to enhance performance.
Figure 4.
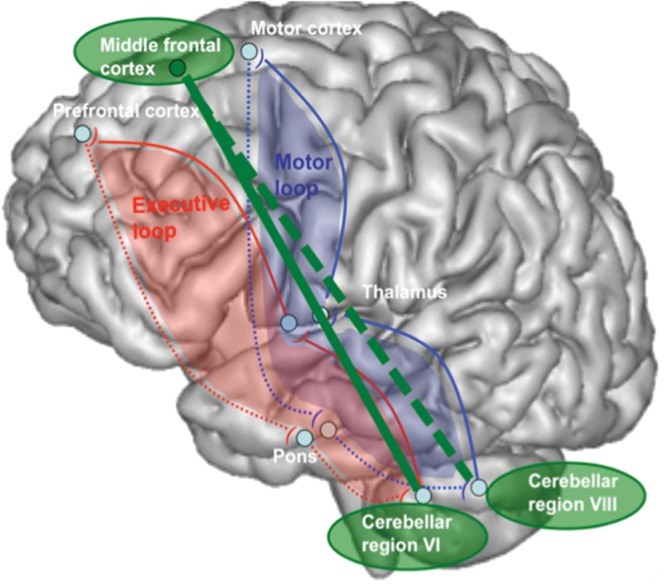
Hypothetical functional model explaining normal performance in alcoholics on a spatial working memory task. The red loop describes the anatomical and functional executive loop and the blue loop describes the anatomical and functional motor loop. The green loop describes the hypothetical functional loop underlying compensation in alcoholism.
The results reported here should be considered in light of certain limitations. First, although commonly used, the criterion chosen for movement parameters, that is, , 2 mm, could have biased the observed results. It seems unlikely, however, that functional connectivity results were significantly affected by movement because such influence has been revealed to weaken measured connectivity in long-range networks and enhance shorter range connections, therefore in the opposite direction to the results revealed in this study. Second, the compensatory mechanisms identified here might be limited to alcoholism or to conditions that affect a common set of neural systems to those affected by alcoholism.
In summary, our results suggest that alcoholics recruited pathways parallel to those used by controls to compensate for impairment in the normally invoked executive frontocerebellar loop. We provide evidence that the pattern of brain activity and associated behavioral performance meet criteria for deeming the abnormal activation pattern as compensatory. Evaluation of differences in activation and connectivity in relation to performance has important implications for concepts of neuroplasticity and reorganization in therapeutic settings. If, for example, compensatory responses can be brought under external or internal control, our results may be useful in therapeutic efforts involving cognitive remediation following brain insult. Our model of the novel recruitment of a parallel loop employed to produce normal function highlights the potential gain of region-to-region synchronization. Further studies need to explore factors affecting cognitive reserve that could enhance or hamper brain reorganization, whether it is functional or structural in nature. The specific pattern of synchronization observed in the alcoholic men might also serve to distinguish those who can maintain abstinence from those who cannot.
Funding
National Institute of Health grants (AA010723, AA012388, AA017168, and AA017923).
Acknowledgments
Financial Disclosures: None of the authors—Sandra Chanraud, Anne-Lise Pitel, Eva M. Müller-Oehring, Adolf Pfefferbaum, and Edith V. Sullivan—has any financial conflicts of interest with the reported data or their interpretation. Conflict of Interest : None declared.
References
- Benjamin C, Lieberman DA, Chang M, Ofen N, Whitfield-Gabrieli S, Gabrieli JD, Gaab N. The influence of rest period instructions on the default mode network. Front Hum Neurosci. 2010;4:218. doi: 10.3389/fnhum.2010.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Frey S, Evans A. Neural correlates of mental transformations of the body-in-space. Proc Natl Acad Sci U S A. 1995;92:11180–11184. doi: 10.1073/pnas.92.24.11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, Pfefferbaum A, Sullivan EV. Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35:1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener KA. Group test for assessing hand and eye dominance. Am J Psychol. 1962;75:271–276. [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders (SCID) version 2.0. New York: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, Lyytinen H, Whitfield-Gabrieli S, Glover GH, Reiss AL, et al. Neural systems predicting long-term outcome in dyslexia. Proc Natl Acad Sci U S A. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua QP, Zeng XZ, Liu JY, Wang JY, Guo JY, Luo F. Dynamic changes in brain activations and functional connectivity during affectively different tactile stimuli. Cell Mol Neurobiol. 2008;28:57–70. doi: 10.1007/s10571-007-9228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Desmond JE. Modality specific cerebro-cerebellar activations in verbal working memory: an fMRI study. Behav Neurol. 2010;23:51–63. doi: 10.3233/BEN-2010-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Chen SH, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O'Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. Functional topography of the cerebellum in verbal working memory. Neuropsychol Rev. 2010;20:271–279. doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PM, Johansen-Berg H, Reddy H. Non-invasive mapping of brain functions and brain recovery: applying lessons from cognitive neuroscience to neurorehabilitation. Restor Neurol Neurosci. 2004;22:245–260. [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) 1982. test manual. Windsor (UK): NFER-Nelson. [Google Scholar]
- Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA, Lancaster JL. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–58. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Crusan K, Jernigan TL. Brain CT changes in alcoholics: the effects of age and alcohol consumption. Alcohol Clin Exp Res. 1988;12:81–87. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Wan CY. The use of non-invasive brain stimulation techniques to facilitate recovery from post-stroke aphasia. Neuropsychol Rev. 2011;21:288–301. doi: 10.1007/s11065-011-9181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA. Development and validation of a lifetime alcohol consumption assessment procedure. Toronto (Canada): Addiction Research Foundation; 1982. [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: the lifetime drinking history and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychol Rev. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets G, de Lange FP, Vandemaele P, Deblaere K, Achten E. Motor imagery in mental rotation: an fMRI study. Neuroimage. 2002;17:1623–1633. doi: 10.1006/nimg.2002.1290. [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00032. pii: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Davis M, Yago E, Barcelo F, Vogel EK, Knight RT. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron. 2010;68:401–408. doi: 10.1016/j.neuron.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q, Chen Z, Zhu C, He Y. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123:105–115. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Ollinger JM, Sheridan MA, Tversky B. A parametric study of mental spatial transformations of bodies. Neuroimage. 2002;16:857–872. doi: 10.1006/nimg.2002.1129. [DOI] [PubMed] [Google Scholar]