Abstract
How exteroceptive attention (EA) alters neural representations of the external world is well characterized, yet little is known about how interoceptive attention (IA) alters neural representations of the body's internal state. We contrasted visual EA against IA toward respiration. Visual EA modulated striate and extrastriate cortices and a lateral frontoparietal “executive” network. By contrast, respiratory IA modulated a posterior insula region sensitive to respiratory frequency, consistent with primary interoceptive cortex, and a posterior limbic and medial parietal network, including the hippocampus, precuneus, and midcingulate cortex. Further distinguishing between EA and IA networks, attention-dependent connectivity analyses revealed that EA enhanced visual cortex connectivity with the inferior parietal lobule and pulvinar of the thalamus, while IA enhanced insula connectivity with the posterior ventromedial thalamus, a relay of the laminar I spinothalamocortical pathway supporting interoceptive afference. Despite strong connectivity between the posterior and the anterior insula, anatomical parcellation of the insula revealed a gradient of IA to EA recruitment along its posterior–anterior axis. These results suggest that distinct networks may support EA and IA. Furthermore, the anterior insula is not an area of pure body awareness but may link representations of the outside world with the body's internal state—a potential basis for emotional experience.
Keywords: attention, exteroception, fMRI, insula, interoception
Introduction
Attention allows for the selection and maintenance of behaviorally relevant signals, while suppressing contextually irrelevant information. In each of vision (Brefczynski and DeYoe 1999), hearing (Hall et al. 2000), touch (Bauer et al. 2006), taste (Veldhuizen et al. 2007), and olfaction (Zelano et al. 2005), attending toward a particular sensory stimulus selectively enhances domain-specific cortical representations, suggesting that attentional modulation of sensory representation is a fundamental principle of the human nervous system. Attention may be exogenous, cued reflexively by a flashing light or sudden pain, or endogenous, intentionally directed to savor an aroma or search for misplaced keys. Both exogenous and endogenous attention powerfully affect the contents of awareness (Berger et al. 2005; Chong and Blake 2006), and it is generally believed that both forms of attention recruit a common dorsal frontoparietal neural network (Rosen et al. 1999; Peelen et al. 2004), a single neural network for the modulation of sensory awareness. In contrast to this rich history of studying the exteroceptive senses, much less is known about the role of attention in interoception, the faculty of sensing the body's internal state, proposed by William James (1890) to be the basis for the experience of emotions. It is unknown whether the systems for directing IA are shared with the exteroceptive senses or even whether attention directed toward interoceptive sensation can modulate primary interoceptive cortex.
While the exteroceptive senses have well-defined sensory cortical regions, it was once argued that interoceptive afferents were too diffuse to support a primary sensory interoceptive region affording interoceptive awareness (Cannon 1927). This argument runs contrary to common experience, in which interoception affords awareness of specific visceral states of the body, including sensations such as breathing, nausea, blushing, swallowing, and satiety (James 1884; Cameron 2002). Like the exteroceptive senses, IA may be cued exogenously, as when one's stomach begins to ache following a spicy meal, or endogenously, as when one focuses on the feelings of one's breathing or heartbeat. Supporting the theory that an interoceptive network provides awareness of the body's internal state, a lamina I spinothalamocortical pathway was recently proposed to carry sympathetic afferents that signal the physiological condition of all tissues of the body (Craig 2002). By way of the brainstem parabrachial nucleus and posterior part of the ventromedial thalamic nuclei, these inputs project to the posterior granular and middysgranular regions of the insular cortex, serving as primary interoceptive cortex (Flynn et al. 1999). While primary interoceptive signals travel to mid/posterior granular and dysgranular insular representational fields (Hua et al. 2005; Frot et al. 2007), interoceptive awareness is thought to depend upon further propagation of interoceptive signals to the anterior agranular insula, which has been argued to reflect the integration of afferent physiological signals with higher order contextual information (Damasio et al. 2000; Craig 2002, 2009; Critchley 2005; Mutschler et al. 2009). For example, in an elegant examination of interoceptive monitoring, participants decided if their heartbeats matched the frequency of auditory tones during functional magnetic resonance imaging. Successful heartbeat matching was associated with activity in the right dysgranular anterior insula and anterior cingulate cortex (ACC) (Critchley et al. 2004), consistent with attentional modulation of later viscerosensory and visceromotor cortical representations but not earlier, primary interoceptive cortex.
However, despite evidence for its role in interoceptive awareness (Critchley et al. 2004; Pollatos et al. 2007), anterior insula activity is also commonly associated with exteroceptive tasks requiring cognitive control (Duncan and Owen 2000; Derfuss et al. 2005), such as the suppression of irrelevant (Aron et al. 2004) or maintenance of relevant (Bunge et al. 2001) information. Anterior insula activity during interoception may therefore reflect endogenous attention demands, manipulating interoception at the level of conscious awareness. Like the anterior insula, the ACC is linked to both interoceptive (Medford and Critchley 2010) and exteroceptive awareness (Posner and Petersen 1990). Together these regions may form a rapid feedback loop between sensory integration in the insula and behavioral responses in the cingulate cortex (Allman et al. 2005), regardless of whether this sensory information is interoceptive or exteroceptive in origin. Another potential complication is that anterior insula activity may simply reflect feelings of uncertainty during interoception (Singer et al. 2009), since most visceromotor activity, such as the heartbeat, is poorly detected (Khalsa et al. 2008).
To control for these confounding explanations in characterizing the neural basis of IA, the present study considered a more powerful generator of viscerosensory signals: respiration. Respiration is unique among interoceptive sources as it is generally amenable to direct voluntary control and conscious inspection. We investigated whether interoception is represented in distinct neural regions from exteroception. Using the mechanical respiratory signal to confirm the posterior insula's role as primary viscerosensory cortex, we examined whether attention to respiration-related sensations modulated signal strength and connectivity in primary interoceptive cortex, relative to when it is unattended during visual attention. We further examined whether the anterior insula was activated during IA, when contrasted with visual attention suppression and maintenance tasks that share the cognitive demands of interoception. This approach afforded a comparison of the networks supporting interoception with a well-characterized network for exteroception.
Materials and Methods
The present study contrasts IA to the internal sensations of respiration against exteroceptive attention (EA) to visually presented words. EA was operationalized through a combination of 2 tasks that involved processing of external stimuli selected to control for different potential executive function demands related to IA: 1) 1-back maintenance: maintaining a stimulus representation online in working memory and 2) cognitive suppression: suppressing the processing of internal responses to external stimulus representations. Maintenance is necessary for observing changes in stimulation from one breath to the next, while suppression is necessary for inhibiting competing sources of external stimulation to focus attention on interoceptive signals. Together, these 2 EA tasks may allow for better discrimination of interoception-related neural activity by controlling for the major attentional demands required for sustained IA.
Procedures
Participants
Twenty-seven participants (20 women; mean age 45 ± 12.63) were recruited from a community sample through an information session on stress reduction at St. Joseph's Hospital in Toronto. All participants were right-handed, healthy volunteers, who provided informed consent according to the Canadian Tri-council Policy Statement on Ethical Conduct for Research Involving Humans, and the procedures were approved by the Research Ethics Boards of the University of Toronto, the Centre for Addiction and Mental Health, and Sunnybrook and Women's College Health Sciences Centre.
Some participants had recently completed a course in Mindfulness-Based Stress Reduction at the time of scanning, which involved training in the monitoring of interoceptive signals. For this reason, course completion was modeled as a bivariate covariate in all analyses presented herein, partitioning out training-related variance from the experimental task contrasts. Subanalyses of training-naive participants are available in the Supplementary Material, providing a highly similar pattern of results to those discussed below.
Training Procedure
Participants were trained on the distinction between the 3 experimental tasks, breath monitoring (“Breathe”), working-memory maintenance (“Maintain”), and cognitive suppression (“Suppress”) prior to functional magnetic resonance imaging (fMRI) data acquisition. For the Breathe task, participants were instructed to focus on a fixation cross presented in the center of their visual field and to attend to the sensory aspects of their breath, that is, in the nose, throat, chest, and diaphragm, without intentionally altering their respiratory rhythm. For the Suppress task, participants were instructed to read sequentially presented words, but to inhibit any sort of subsequent cognitive or emotional response, to “keep their minds blank” while attending to the visually presented stimulus. For the Maintain task, participants were asked to make a key press whenever the same word was repeated in a sequence of visually presented word stimuli (a “1-back” task).
Experimental Task
The block design experiment was composed of alternating blocks of interoceptive (Breathe) and exteroceptive (Maintain or Suppress) attention tasks. Each condition was 36 s in block duration and was immediately preceded by a 10 s instruction screen consisting of a visual cue picture above the task name. One run in the scanner consisted of 2 repetitions of each condition, and each participant completed 2 runs of randomly ordered condition blocks.
In the EA tasks, a single word appeared on the screen for 4 s in duration, followed by a 2 s interstimulus interval during which a blank screen was presented, a 6 s total stimulus duration which approximates the average respiration rate in humans (Sherwood 2006), matching the durations of word and breath stimuli. In the Maintain task, one randomly positioned word in each block was repeated.
Verbal Stimuli
To approximate the self-focus demands of the breath monitoring task, personality-trait adjectives were chosen as word stimuli for the EA tasks (Farb et al. 2007). Eight sets of word lists were constructed from a well-established list of personality-trait words (Anderson 1968), which lend themselves to deep levels of processing (e.g., Craik and Lockhart 1972). Word lists were randomly assigned to each condition.
Imaging Setup
Imaging data were collected with a Signa 3-T MRI system (CV/i hardware, LX8.3 software; General Electric Medical Systems, Waukesha, WI) with a standard quadrate birdcage head coil. Stimulus presentation was controlled by the Presentation software package (version 9.81; Neurobehavioral Systems, Inc., Albany, CA). Stimuli were presented on a rear-mounted projection screen, set at a (native) 1024 × 768 resolution.
Structural Imaging
For each participant, a 3D magnetization-prepared rapid acquisition gradient echo pulse sequence was used to obtain a high-resolution T1-weighted structural volume. The imaging parameters were as follows: repetition time (TR) = 2000 ms; echo time (TE) = 2.63 ms; matrix = 256 × 192; field of view (FOV) = 256 × 256; slice thickness = 1.3 mm thick; 192 oblique axial slices; total acquisition time = 6.5 min.
Functional Imaging
fMRI was conducted using T2*-weighted single-shot spiral in–out k-space trajectories optimized for sensitivity to the blood oxygenation level–dependent (BOLD) effect (TE/TR/flip angle = 30 ms/2000 ms/70°, 20 cm FOV, 5 mm slice thickness, 64 by 64 matrix, 26 slices in oblique axial orientation), improving the capability to acquire fMRI signals in regions of high magnetic susceptibility (Glover and Law 2001). The first 10 images in each run were discarded to remove scanner equilibration effects.
Data Analysis
Interoceptive Sensitivity Pilot Study
Pilot analyses were conducted to ensure high interoceptive sensitivity to the respiratory signal. Seven pilot participants performed a breath monitoring task in which they were asked to monitor their respiration and to respond with a button press on every eighth breath. Participants performed this monitoring task in 3 min blocks, providing a more rigorous test of sustained attention to respiration than would be required in the main study breath monitoring blocks. Participants completed 4 monitoring blocks, for a combined 12 min of breath monitoring per participant. Breath monitoring accuracy was measured relative to the objective signal obtained from a respiration belt during this task. Monitoring error was computed as the deviation from 8 breaths at each button press, summed across all blocks. Monitoring accuracy was averaged across all participants to provide a behavioral index of interoceptive sensitivity.
Respiration Analysis
During IA, participants were directed to attend to the internal sensations associated with breathing. However, attention toward respiration may result in respiratory slowing (e.g., Jevning et al. 1992), potentially affecting the BOLD response (Birn et al. 2006). To control for confounding effects of respiratory change between IA and EA conditions, respiration was measured using a respiration belt. Respiratory signal was sampled at 40 Hz and analyzed for each participant in Matlab (R2009b). Respiratory signal was linearly detrended, and a low-pass Butterworth filter was applied to remove respiratory frequency greater than 0.5 Hz (patterns of breathing faster than a breath every 2 s), based on the absence of high power peaks above this frequency across participants. Respiratory peaks and troughs were estimated using a counting method, which was corroborated with visual inspection of peak and trough placement, and whose primary frequency estimates correlated highly with a fast Fourier transform of the data (r > 0.8 for all participants).
At the first level of analysis (within participant), respiratory phase (the cycle of inspiration and expiration) was modeled as a nuisance regressor, as it is linked to motion-related noise in fMRI data. Respiratory phase was modeled by coding TRs containing peaks as “1,” troughs as “−1,” and TRs containing either both peaks and troughs or neither as “0.”
At the second level of analysis (group level), we modeled respiratory rate and efficiency (respiratory volume per time; RVT) for each task block. To assess respiratory rate, the mean time between respiration signal troughs was calculated for each TR using a 5 TR sliding window centered on the TR in question. To assess RVT, respiratory signal change from peak to the average of the 2 surrounding troughs was computed and divided by the time between the 2 troughs (Porges and Byrne 1992). Respiratory rate and RVT were both included in all group-level models as covariates, controlling for average respiration in each of the Breathe, Suppress, and Maintain conditions in the factorial model.
Preprocessing
Functional activation was determined from the BOLD signal using Statistical Parametric Mapping (SPM5, University College London, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm5). Following reconstruction (SPM5 DICOM import utility), the time series functional data were spatially coregistered and realigned to correct for motion within and between functional scans (translational motion parameters were less than 1.5 mm for all participants) and coregistered with their T1-weighted structural image. The T1 image was bias corrected and segmented using template (International Consortium for Brain Mapping) tissue probability maps for gray–white matter and cerebrospinal fluid. Warping parameters were obtained from the tissue segmentation procedure and subsequently applied to the time series data (resampling to 3 mm3 voxels). Images were warped to normalize the data into a common stereotactic reference space (Montreal Neurological Institute) and then spatially smoothed to a 6 mm3 full-width at half-maximum Gaussian kernel. Finally, to control for changes in the global BOLD response across the time series, we applied a global mean detrending procedure (Macey et al. 2004).
First-Level Statistical Models
Following preprocessing, individual participant time series were submitted to a first-level general linear statistical model (Friston et al. 1995). Using the SPM5 design specification, task-specific boxcar stimulus functions were convolved with the canonical hemodynamic response function, separately modeling the onsets of the Breathe, Suppress, and Maintain tasks. Each participant's model included within-session global scaling and the AR1 method of estimating temporal autocorrelation as well as the corrections for respiratory phase.
Second-Level Statistical Models
Experimental contrasts and controls.
To examine differential effects of attentional focus, positive effect t contrasts for each experimental condition (Breathe, Suppress, and Maintain) were examined at the second level using a repeated-measures analysis of variance (ANOVA). Within this model, the IA Breathe condition was doubly weighted against the EA Suppress and Maintain conditions to form the Attention contrast (IA − EA). To clarify the contribution of the IA and EA conditions, we additionally modeled a task-free baseline condition, obtained by averaging together the 10-s cue periods that preceded each task block. Additional analyses of the experimental task profiles relative to baseline and of the separate EA tasks relative to IA are available in the Supplementary Material.
Group-level t contrasts for Attention effects used a voxel height threshold of PFDR < 0.01 (T ≥ 3.46) and voxel extent of K > 10 as base thresholds. Exploratory analyses, that is, examination of respiration rate–related activity and psychophysiological interactions, used a reduced height threshold of Punc < 0.005 (T ≥ 2.68), but an increased voxel extent threshold of K > 30, equivalent to a familywise error rate of PFWE < 0.05 in a Monte Carlo simulation (AlphaSim, http://afni.nih.gov/afni/docpdf/AlphaSim.pdf).
Meditation course completion, mean respiratory rate, and RVT in each of the 3 attention tasks were all included as nuisance covariates in the second-level ANOVA models. We examined the neural activity associated with the respiration covariates to model the neural correlates of mechanical respiration.
Primary representation cortical regions of interest.
The MRIcron software package (http://www.sph.sc.edu/comd/rorden/mricron/) was used to generate a priori gray matter masks for visual and interoceptive cortices to probe recruitment of these regions by the IA and EA conditions. To characterize the visual regions, a conjunction image was made between the visual Brodmann atlas regions 17, 18, and 19. To characterize the interoceptive regions of interest (ROIs), a conjunction image was made between right and left Automated Anatomical Labeling atlas insula regions, a digital anatomical atlas delineating macroscopic brain structures (Tzourio-Mazoyer et al. 2002). EA and IA conditions were examined in their hypothesized ROIs, inclusively masking for the visual region in the EA > IA comparison and inclusively masking for the insula region for the IA > EA comparison. Mean percent signal changes for IA, EA, and the rest condition were extracted from the primary clusters of activity in each of these regions using the MarsBar toolbox for SPM (http://marsbar.sourceforge.net). Parallel supplementary analyses of the ACC, a region related to both IA and EA (Posner and Petersen 1990; Medford and Critchley 2010), are available in the Supplementary Material.
Insular cortex subregional ROIs.
Eight gray matter ROIs were selected according to the anatomical divisions of the insular gyri as defined on a high-resolution T1-weighted template anatomical volume, based on well-characterized cytoarchitectonic divisions (Mesulam and Mufson 1982; Chikama et al. 1997). Insular gyri ranged from the anterior accessory gyrus, through the short and long gyri of the middle insula, and into the short and long gyri of the posterior insula (Craig 2009). To account for dysgranular and agranular cellular layer divisions of the anterior and middle insular gyri (Chikama et al. 1997), the accessory and short gyri were further partitioned into dorsal and ventral subregions. Insular ROIs were hand drawn on each anatomical slice using MRIcron, selecting only gray matter aspects of the gyri and were exclusively masked to ensure that no overlapping voxels were selected (Fig. 1). All ROIs covered a functional imaging volume of at least 27 voxels comparable to 3-mm spherical ROIs but mapped along the cortical surface.
Figure 1.
ROI locations along the insula cortex. ROIs were drawn to fit each visible gyrus of the template brain, yielding 8 anatomically defined regions. These regions conform to the anatomical divisions outlined in Craig (2009) and illustrated in Mesulam and Mufson (1982) and Chikama et al. (1997), representing the posterior, anterior, and accessory gyri and further subdividing these regions into dorsal and ventral subregions to capture the 3 major divisions in insular cellular layers, the granular, dysgranular, and agranular regions.
In the anatomical ROI analyses, mean time courses were extracted from each ROI using the MarsBar toolbox. For each participant, mean percent signal change was extracted separately for the IA, EA, and rest conditions. Difference scores between attention conditions (IA − EA) were calculated within subject for each region to represent the attentional tuning of that region. This attentional tuning score for each ROI was compared across the dorsal and ventral posterior–anterior axis of the insula as an a priori analysis of interest. Attentional tuning scores were divided into a 5-seed dorsal zone and a 3-seed ventral zone for trend analysis. The dorsal zone ROIs were subjected to a 2 (left vs. right hemisphere) × 5 (seed location) repeated-measures ANOVA, while the ventral zone ROIs were subjected to a 2 (left vs. right hemisphere) × 3 (seed location) repeated-measures ANOVA.
Whole-brain analysis.
The IA versus EA contrast was replicated without anatomical masks to more widely examine larger scale IA and EA networks. Additionally, to determine the functional connectivity of the visual and interoceptive ROIs, 5-mm spherical seed regions were selected using the peak IA > EA voxel found in the interoceptive region and the peak EA > IA voxel found in the visual region. Time series activity was extracted from each of these volumes using the first eigenvariates of each volume's beta weights; each seed's data were used as a regressor to predict activation in the visual and interoceptive ROIs. The IA and EA connectivity maps were compared using a one-way ANOVA in SPM, allowing for identification of regions significantly more correlated with one seed than the other.
Psychophysiological interaction analyses (PPIs) were also performed to examine the effects of attention on functional connectivity (Friston et al. 1997; Gitelman et al. 2003). In a PPI analysis, seed voxel signal, experimental condition, and the interaction between them are entered as simultaneous regressors for brain activity in each subject; the interaction term is defined as the product of the condition and seed signal regressors. PPI analysis better estimates the relationship between seed regions than functional connectivity alone, as it identifies task-related changes in connectivity while controlling for the main effects of connectivity and condition. In PPI analyses for both the visual and the interoceptive ROIs, IA was coded as the positive variable in the IA versus EA condition regressor.
Results
Respiration Behavioral Pilot
We employed a respiratory belt as an objective measure of respiratory frequency in the main study but did not require that participants count their breaths or respond with a button press to avoid altering the neural expression of pure IA. During debriefing following the scanning session, no participant reported difficulty in being able to sense their respiration during the breath monitoring task. In a separate pilot study of respiratory monitoring ability, participants demonstrated near perfect accuracy in monitoring their respiration over an extended period of time (average of 97%, standard deviation = 0.03%).
Respiration Analyses
Mechanical Respiration
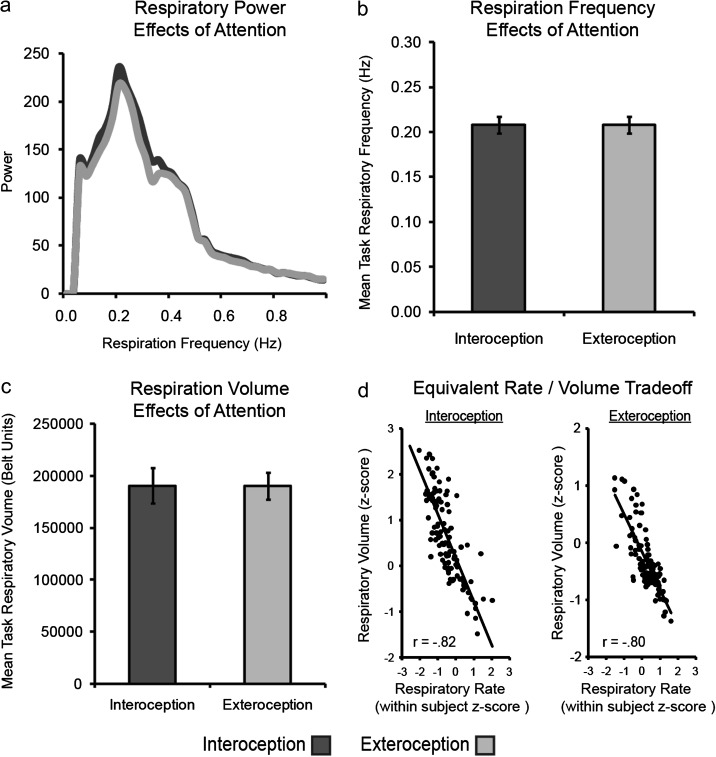
Overall, participants respired at a frequency of 0.21 Hz, or 12.5 breaths per minute, similar to the population average observed respiration rate (Sherwood 2006). Participant respiratory frequency distributions were nearly identical between attention conditions (Fig. 2a). To formally test for any differences, mean respiratory signals were analyzed as a function of attention condition, with 1) respiration rate, 2) volume, and 3) RVT, each analyzed separately in repeated-measures ANOVAs. No effects of attention were found for respiratory rate or volume (Fig. 2b,c). Respiratory efficiency (RVT), which is linked to gray matter activity independently of respiratory motion (Birn et al. 2006), was also equivalent across attention conditions.
Figure 2.
Respiration signal analyses. (a) Average power and frequency of respiration as a function of attention condition, as derived from a fast Fourier transform of the respiration data across all participants. (b) Mean respiratory frequency (Hz) as a function of attention condition, as derived from a breath counting algorithm. No differentiation between attention conditions was found for respiratory rate. (c) Mean respiratory volume (arbitrary respiration belt units) as a function of attention condition, as derived from a breath counting algorithm. No differentiation between attention conditions for respiratory volume was observed. (d) Rate/volume tradeoffs for respiration as a function of attention condition. The slopes for the 2 conditions did not significantly differ. Error bars represent standard errors. Interoception plots are in dark gray and exteroception plots in light gray.
To confirm that there were no differences in depth of respiration driving BOLD signal differences, the relationship between rate and volume was examined in a correlation analysis, using rate and volume estimates from each participant task block (Fig. 2d). Respiratory rate was strongly negatively correlated with respiratory tidal volume (r214 = −0.85, P < 0.001), such that faster breaths were shallower; however, this rate/volume relationship was equivalent between interoceptive and exteroceptive tasks (rinteroception = −0.82, rexteroception = −0.80, Fisher's z106,106 = 0.36, not significant [n.s.]). The equivalent respiratory pattern between attention conditions suggests that any BOLD differences observed between IA and EA are more likely to reflect changes due to attention than mechanical differences in respiration.
Respiration-Related Neural Activity
We first attempted to localize putative primary interoceptive cortex using an objective and observable viscerosomatic signal. To do so, we examined activation associated with changes in respiratory rate between task blocks. The area most reliably associated with respiratory rate during IA was found in the right long gyrus of the posterior insula (t1,50 = 4.0, P < 0.001 at peak x = 39; y = −21; z = 21, k = 221), such that faster respiration was linked to greater activation (Fig. 3a). Post hoc analysis of this correlation as a function of attention condition revealed that breathing rate and neural activity was more correlated during IA when breathing was attended (rinsula25 = 0.63, P < 0.05) rather than during EA when breathing was unattended (rinsula52 = 0.23, n.s.; rdiff25,52 = 0.40, P < 0.05). The area most reliably associated with respiratory rate during EA was the right somatosensory cortex (t1,50 = 3.9, P < 0.001 at peak x = 45; y = −30; z = 54, k = 180) (Fig. 3b). Respiration was reliably associated with somatosensory activity during EA (rsomatosensory52 = 0.59, P < 0.001) but not during IA (rinsula25 = 0.38, n.s.), although the IA and EA correlations did not significantly differ from each other (rdiff25,52 = 0.35, n.s.). Analysis of respiratory correlates within the anatomical insula ROIs confirmed that the peak IA-associated region was part of the dorsal posterior long gyrus of the right insula. Several other regions were correlated with respiratory rate, including the posterior cingulate, which was negatively correlated with respiration in both attention conditions (Supplementary Table 1).
Figure 3.
Between-subject correlates of respiratory signal for IA and EA. Neural correlates of respiratory rate were modeled at the group level. (a) The most robust positive correlate of respiration during IA was found in the right posterior insula, demonstrating enhanced coupling during IA relative to EA. (b) The most robust positive correlate of respiration during EA was found in the right somatosensory cortex, demonstrating strong coupling during EA and moderate coupling during IA.
IA versus EA: Cortical ROI Analysis
To investigate the effects of attention on primary cortical recruitment, we compared signal change between the IA and the EA conditions within the interoceptive insular cortex ROI (all subregions of bilateral insula) and the visual cortex ROI (Brodmann areas [BAs] 17, 18, and 19). Comparison of attention conditions within these clusters of activation suggested a strong dissociation between IA and EA in their primary representation regions. Within the interoceptive cortex, IA was associated with greater bilateral posterior and middle insula activity (granular zone, P < 1 × 10−9, t1,35 = 6.8 at peak x = 33; y = −21; z = 21, k = 129), whereas within the visual cortex, EA was associated with greater recruitment of bilateral ventral occipital regions (BAs 17–19, t1,35 = 5.6, P < 1 × 10−8, at peak x = −42; y = −66; z = −21, k = 153) (Fig. 4).
Figure 4.

Effects of attention on primary representation cortices for vision and interoception. IA recruitment (red) within the insula ROI (red border) and EA recruitment (blue) within the visual cortex ROI (blue border), as defined by the contrast between IA and EA. Peak regions in each ROI (labeled circles) were used as seeds regions to explore whole-brain functional connectivity. The breathe (red), maintain (green), suppress (blue), and baseline period (gray) % signal change plots are displayed in a bar graph for the insula and visual ROIs. Error bars represent standard errors.
IA versus EA: Insular Cortex Subregional ROI Analysis
Each anatomically defined insula ROI was investigated for attentional tuning effects (IA − EA). For the dorsal zone, a main effect of seed location was observed (F4,104 = 25.36, P < 1 × 10−6), with IA recruitment of posterior regions giving way to EA recruitment in anterior regions (Fig. 5). A similar anterior to posterior axis was found for the 3 ventral zone ROIs (F2,52 = 21.21, P < 1 × 10−5). No effects of laterality were observed. In follow-up tests, IA resulted in significant activation relative to baseline for all insula locations except for the most anterior of the dorsal and ventral ROIs (t26 > 2.35, P < 0.05); conversely EA resulted in activation relative to baseline for all locations except for the most posterior of the dorsal and ventral ROIs (t26 > 2.7, P < 0.01).
Figure 5.

Insula attention tuning by anatomical partitions. A significant effect of insula location was found, such that interoceptive bias shifts to exteroceptive bias from posterior to anterior insula. All data points are presented relative to the baseline condition, as an average of left and right insula signal. (a) Right dorsal insula percent signal change plots for interoceptive and exteroceptive recruitment as a function of location. (b) Seed locations shown sagittally across the dorsal/ventral insula. (c) Right ventral insula attention tuning plots (left and right side averaged). Error bars represent standard errors. Asterisks represent areas of significant difference between IA and EA. AC = accessory gyrus; AS = anterior short gyrus; MS = middle short gyrus; PS = posterior short gyrus; AL = anterior long gyrus; PL = posterior long gyrus. Asterisks (*) denote significant (P < 0.01) IA (in red) versus EA (in blue) differences at a specific location.
IA versus EA: Whole-Brain Analysis
To characterize the broader attention networks implicated in IA and EA, IA and EA were compared across the whole brain (Table 1). IA was associated with bilateral activity along the posterior to midinsula as well as hippocampus, caudate, precentral, and postcentral gyrus extending into the midcingulate, precuneus, and posterior cingulate (Fig. 6a,b). EA was associated with activity in primary and secondary visual cortices, bilateral dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, inferior and superior parietal cortex, bilateral frontal opercula, anterior insula, head of the caudate, and the cerebellum (Fig. 6c,d).
Table 1.
Whole-brain differences in regional brain activity between interoception and exteroceptive attention
| Anatomic region | Cluster | ||||||
| BA | Side | Size | Peak Z | x | y | z (mm) | |
| Interoceptive attention (IA > EA) | |||||||
| Insula/cingulate/hippocampus cluster | 14 951 | ||||||
| Precuneus/posterior cingulate | 23/26/29 | L | 6.84 | −12 | −57 | 21 | |
| Posterior insula | — | R | 6.20 | 33 | −18 | 21 | |
| Anterior cingulate | 24 | R | 6.18 | 15 | 24 | 18 | |
| Precuneus/posterior cingulate | 23/26/29 | R | 6.04 | 9 | −51 | 18 | |
| Posterior insula | — | L | 5.88 | −33 | −15 | 21 | |
| Middle insula | — | R | 5.85 | 33 | −6 | 18 | |
| Parahippocampus/hippocampus | 27/37/20 | L | 5.39 | −27 | −33 | −12 | |
| Parahippocampus/hippocampus | 27/37/20 | R | 5.26 | 30 | −42 | −6 | |
| Anterior cingulate | 24 | L | 5.21 | −15 | 24 | 24 | |
| Exteroceptive attention (EA > IA) | |||||||
| Inferior parietal cortex | 40 | R | 376 | 6.21 | 33 | −60 | 54 |
| Inferior parietal Cortex | 40 | L | 180 | 5.56 | −51 | −48 | 42 |
| Fusiform | 19 | L | 113 | 5.56 | −42 | −66 | −21 |
| Dorsolateral prefrontal cortex | 44 | R | 307 | 5.42 | 51 | 12 | 36 |
| Dorsomedial prefrontal cortex | 6/32 | – | 217 | 5.38 | 3 | 15 | 57 |
| Dorsolateral prefrontal cortex | 42 | R | 269 | 5.14 | −48 | 3 | 36 |
| Caudate nucleus | — | L | 139 | 4.73 | −24 | 3 | −12 |
| Cerebellum | 19 | R | 21 | 3.34 | 39 | −63 | −21 |
| Caudate nucleus | — | R | 139 | 4.15 | 18 | 18 | −9 |
Note: Side = hemisphere (left = L, right = R), cluster size is in voxels, Z = peak Z statistic of the BOLD signal in this cluster, x, y, z refer to the 3D coordinates in Montreal Neurological Institute normalized space.
Figure 6.
Whole-brain effects of attention (interoception vs. exteroception). a and b summarize the interoceptive network regions, while c and d summarize exteroceptive network regions. (a) Posterior cingulate and precuneus. (b) Hippocampus and parahippocampus. (c) Inferior parietal lobule. (d) Dorsolateral prefrontal cortex (PFC). The breathe (red), maintain (green), suppress (blue), and baseline period (gray) % signal change plots are displayed in a bar graph for each of the peak regions displayed. Error bars represent standard errors.
IA versus EA: Connectivity
We next examined functional connectivity across the whole brain using the interoceptive and visual seed regions, across the whole time course. The posterior insula seed showed robust bilateral connectivity along the entire anterior–posterior axis of the insula cortex. Insula connectivity extended medially into the thalamus, caudally into the parahippocampus, and rostrally into the pregenual cingulate and frontal operculum (Fig. 7a). The visual ROI was positively associated with both ventral and dorsal regions of occipital cortex as well as the dorsolateral prefrontal cortex and intraparietal sulcus (Fig. 7b).
Figure 7.
Whole-brain functional connectivity. Whole-brain functional connectivity for the insula (A) and visual cortex (B) seeds. (C) Whole-brain PPI with the right insula interoceptive seed as a function of the IA/EA attention conditions. Right insula activity is more correlated with the thalamus during IA than EA. (d) Whole-brain PPI with the visual exteroceptive seed as a function of the attention conditions. The visual region is more correlated with the thalamus during EA than IA. The scatterplots are taken from a representative subject (whose PPI scores best matched the group PPI scores) and depict IA in red and EA in blue.
PPIs were then employed to examine how attention modulated whole-brain connectivity patterns with each IA and EA sensory cortical seed region (Table 2). Importantly, the PPI analysis controlled for the effects of connectivity and condition described above, modeling the effects of attention on functional connectivity. Using the right insula interoceptive seed, IA predicted increased seed connectivity with contralateral left posterior insula compared with EA (t1,35 = 4.3 at peak P = 1 × 10−4, x = −39; y = −9; z = −6, k = 69) as well as greater insula connectivity to the posterior ventromedial thalamus (t1,26 = 3.8 at peak P < 5 × 10−4, x = 6; y = −15; z = 0, k = 104) (Fig. 7c). Using the visual exteroceptive seed, EA predicted greater visual seed connectivity to the inferior parietal lobule (t1,26 = 4.6 at peak P < 5 × 10−5, x = 51; y = −45; z = 27, k = 83) and to both the ventral anterior nucleus and the pulvinar of the thalamus (t1,26 = 4.8 at peak P < 3 × 10−5, x = −6; y = 0; z = 9, k = 670) (Fig. 7d) relative to IA.
Table 2.
Psychophysiological interaction summary
| Anatomic region | Cluster | ||||||
| BA | Side | Size | Peak Z | x | y | z (mm) | |
| Insula seed PPI: IA > EA | |||||||
| Posterior insula | — | L | 352 | 3.88 | −36 | −33 | 12 |
| Thalamus (ventromedial) | — | R | 104 | 3.36 | 6 | −15 | 0 |
| Parahippocampus | 37 | R | 55 | 3.36 | 39 | −36 | −6 |
| Middle temporal | 21 | L | 41 | 3.35 | −51 | −36 | −9 |
| Cerebellum (vermis 3) | — | — | 36 | 3.31 | 0 | −42 | −12 |
| Thalamus (pulvinar) | — | R | 49 | 2.91 | 18 | −30 | 15 |
| Insula seed PPI: EA > IA | |||||||
| Precuneus | 7 | — | 74 | 3.60 | 0 | −69 | 45 |
| Insula seed PPI: IA > EA within insula ROI | |||||||
| Posterior insula | — | L | 69 | 3.72 | −39 | −9 | −6 |
| Visual seed PPI: IA > EA | |||||||
| Middle occipital/calcarine | 17/18 | L | 621 | 4.31 | −27 | −81 | −15 |
| Middle occipital | 19 | L | 161 | 3.98 | −24 | −84 | 18 |
| Hippocampus | 20 | R | 35 | 3.67 | 36 | −3 | −27 |
| Cerebellum | 30 | R | 92 | 3.61 | 12 | −45 | −6 |
| Visual seed PPI: EA > IA | |||||||
| Thalamus (lateral) | — | L | 670 | 4.02 | −6 | 0 | 9 |
| Temporal/parietal junction | 42 | R | 83 | 3.92 | 51 | −45 | 27 |
| Occipitotemporal cortex | 37 | L | 48 | 3.60 | −42 | −39 | −9 |
| Posterior cingulate | 23 | R | 39 | 3.25 | 15 | −36 | 33 |
| Supplementary motor area | 6 | L | 60 | 3.24 | −15 | −6 | 45 |
| Visual seed PPI: IA > EA within visual ROI | |||||||
| Middle occipital/calcarine | 17/18 | L | 540 | 4.32 | −27 | −81 | −15 |
| Middle occipital | 19 | L | 137 | 4.00 | −24 | −84 | 18 |
Note: Side = hemisphere (left = L, right = R), cluster size is in voxels, Z = peak Z statistic of the BOLD signal in this cluster, x, y, z refer to the 3D coordinates in Montreal Neurological Institute normalized space.
Discussion
The present study offers 4 major findings. First, changes in respiratory rate directly modulated signal in the right posterior granular insula, evidencing its function as primary interoceptive cortex. Second, while EA modulated primary and secondary visual cortical regions, IA modulated primary and secondary interoceptive regions in the posterior granular and middle dysgranular insular cortices, supporting the hypothesis that attention serves to increase the “gain” of viscerosensory receptive fields for bodily sensation. Third, exteroception and interoception appear to rely on dissociable attention networks: EA was associated with a well-characterized lateral frontoparietal network (e.g., Corbetta and Shulman 2002) and increased attention-dependent connectivity between the visual cortex and both the inferior parietal cortex and the visual thalamus. By contrast, IA was characterized by a posterior limbic, insula, and medial parietal network and increased attention-dependent connectivity with the homologous posterior insular cortical fields and putative interoceptive thalamus. Finally, this study demonstrated novel dissociable recruitment of the anterior and posterior insula in a single paradigm: there was a graded attentional tuning of insular response, with interoceptive responses in the posterior insula shifting toward exteroceptive responses in more anterior gyri. While posterior insula activation was predicted by attention to the breath, consistent with a role in supporting interoceptive sensory awareness (Critchley et al. 2004), and moment to moment self-awareness (Farb et al. 2007), anterior insula activation was better predicted by attention to external visual stimuli, consistent with its role in the attribution of stimulus salience (Seeley et al. 2007) or attributing motivational significance to pain (e.g., Price 2000; Lamm et al. 2011), requiring an awareness of environmental context beyond the body. This internal/external distinction suggests that the middle, dysgranular portions of the insula might serve as a region of multisensory integration between interoceptive and exteroceptive inputs, affording an integrated representation of present moment experience (Craig 2009).
Right Insula Representation and Respiratory Signal
Consistent with its anatomical innervations by afferent viscerosensory pathways, posterior insula activity correlated directly with respiratory rate. However, this coupling was dependent upon attentional framing: task-averaged respiratory rate predicted right posterior insula activity when respiration was attended during IA, whereas it instead predicted somatosensory activity during EA. IA therefore appears to alter representation of respiratory stimulation by affording a deeper visceral representation of respiratory signal than the surface-based representations promoted in the absence of such attention. Indeed, while IA appears to increase the gain of interoceptive signal through increased magnitude of posterior insula activation, it also appears to increase signal conduction: connectivity analyses of the right insula demonstrated greater connectivity with the posterior ventromedial thalamus, potentially reflecting increased relay of the lamina I spinothalamocortical pathway hypothesized to support interoceptive afference (Craig 2002). A similar pattern of enhanced coupling was observed between the visual thalamus and visual cortices during EA, suggesting that privileged connectivity between attended primary representation cortices and thalamic relays may be a common property of the attentional system.
In addition, we found that insula sensitivity to respiratory frequency changes was enhanced during IA to respiration. It would thus appear that IA serves 2 functions: first, to increase the gain of neural response in the attended interoceptive sensory field, enhancing viscerosomatic signal amplitude and second, to increase the selectivity of interoceptive neurons, coupling their sensitivity to changes in the attended dimension, that is, respiratory frequency, to better meet the task demand of attention to respiratory signal throughout the entire respiratory cycle. Altered signal gain, combined with altered selectivity of neuronal reactivity, has already been demonstrated in the case of exteroceptive visual attention (Murray and Wojciulik 2004) and appears to be a property that extends to IA as well.
The Exteroceptive Anterior Insula?
Despite strong IA activation in the posterior insula, and robust attention-independent connectivity between posterior and anterior insula regions, IA was still a weaker predictor of anterior insula activity than EA. Resting-state connectivity analyses of the insula suggest that the posterior and anterior insula may be functionally distinct, with only anterior regions implicated in exteroceptive focal attention (Nelson et al. 2010). Within the context of exteroceptive paradigms, the anterior insula has been argued to play a critical role in attention switching (Menon and Uddin 2010), particularly, in switching between executive control of tasks similar to the maintenance task employed in this study and the “default mode network” associated with task-free brain activity (Sridharan et al. 2008). Recent applications of graph theory have distinguished multiple subsystems for the “top down” control of behavior, distinguishing between the maintenance of control in the medial prefrontal cortex and the integration of feedback and adjustment of behavior in a lateral frontoparietal network (Dosenbach et al. 2008). While anterior insula activity consistent with switching between task blocks was apparent during EA, this effect was independent of IA, which activated a distinct network that included the posterior insula. Such results reinforce the idea that anterior insula activation may require the integration of sensory information within an external task context rather than interoceptive sensory information in particular.
Despite not activating for a purely interoceptive task, the anterior insula may still be important for representing a broader environmental context in which to situate interoceptive sensation. Together, posterior and anterior insula regions may integrate interoceptive and exteroceptive information to constitute the individual's sense of self in the present moment (e.g., Farb et al. 2007; Craig 2009). Such a suggestion finds support in neuroanatomy: function across insular gyri is likely heterogeneous, commensurate with variations in connectivity along the insular posterior–anterior axis. Posterior regions of the insula receive inputs from somatosensory association cortices (Mesulam and Mufson 1982; Friedman et al. 1986) and have been linked to multiple forms of interoceptive representation, including cutaneous stimulation (Schneider et al. 1993), pain and temperature sensation (Casey et al. 1994), and sense of agency in limb movement (Karnath et al. 2005). Unlike surface bodily sensation that is somatotopically represented in somatosensory cortices, interoceptive representation such as pain is first somatotopically organized in dorsal posterior insula regions (e.g., Brooks et al. 2005), implicating the posterior insula as a primary viscerosomatic cortex. Connectivity, however, shifts from somatosensory cortices in the posterior insula to the striatum in the middle insula (Menon and Levitin 2005) and finally to the ACC and orbitofrontal cortices in anterior insula nuclei (Craig 2003). The anterior and middle insula may therefore integrate viscerosomatic signals into a motivational space that no longer directly represents bodily sensation (Critchley 2005). For example, while the physical temperature of thermal stimulation predicts posterior and middle insula activity, subjective ratings of thermal intensity are associated with the anterior insula and orbitofrontal cortex (Craig et al. 2000). Thus, while we find that attention to interoceptive inputs modulates activity in the caudal aspects of the insula, rostral insular regions may bind visceral signals with exteroceptive representations, linking external stimuli with their resultant effects on the internal state of the body.
The anterior insula's unique neurophysiology may help to explain its importance for the integration of disparate sensations into a broader motivational context. Together with the ACC, the anterior insula is host to a specialized subset of cortical neurons known Von Economo neurons, whose function may be the rapid integration of sensory feedback information in the insula to inform behavioral responses in the ACC (Allman et al. 2005; Craig 2009). Since IA in the present study task was endogenously cued and did not require an external response, the recruitment of this motivational integration network may not have been necessary. In supplementary analyses available online, we observed that while rostral regions of the ACC did show preferential recruitment during IA relative to EA, only EA modulated ACC connectivity: during EA, the ACC demonstrated increased task-related connectivity with the visual ROI, whereas no modulation of ACC connectivity was observed during IA with the insula. Thus, while both the ACC and the anterior insula may be recruited by IA, a need for integrating sensation into a broader motivational context may be necessary to fully engage this network of Von Economo neurons.
The present research supports the idea of the insula acting as a bridge between IA and EA networks. Strong IA tuning was observed in the posterior insula, while the anterior insula was primarily tuned toward EA rather than acting as a marker of visceral and somatic awareness (e.g., Damasio et al. 2000). Nevertheless, attention-independent connectivity was observed between posterior insula IA regions and anterior insula EA regions. Furthermore, prior research indicates that while endogenous IA appears to recruit posterior insula regions classically associated with involuntary visceral sensation (e.g., Aziz et al. 2000; Dupont et al. 2003), IA may also promote recruitment of anterior insula regions when interoceptive information must be integrated in a broader exteroceptive context (e.g., Singer et al. 2009). Further research examining the effects of integrating interoceptive signal into external task demands would directly test this integration hypothesis.
Exteroceptive versus Interoceptive Networks
The present study suggests that the frontoparietal “executive” attention network (Seeley et al. 2007) may be specialized toward tasks requiring exteroceptive monitoring. This well-characterized frontoparietal network was recruited during EA, regardless of whether exteroception was defined as attention toward external visual stimuli (maintenance) or away from internal signals (suppression). Maintenance and suppression recruited common neural networks that include regions, such as the lateral prefrontal and posterior parietal cortices (Bunge et al. 2001; Wendelken et al. 2008), and connectivity analyses demonstrated that EA increased connectivity between visual regions and the inferior parietal cortex. In addition, the ability to regulate processing of exteroceptive signals has been associated with the frontal operculum (for review, see Aron et al. 2004), whereas working-memory maintenance tasks commonly recruit the dorsolateral prefrontal cortex and superior parietal cortices (Wendelken et al. 2008) as well as caudate (McNab and Klingberg 2007) and cerebellum (Desmond et al. 1997). All of these regions were more active during EA than IA, supporting their exteroception-specific role. Moreover, this EA network has been linked to nonvisual exteroceptive sensory systems, such as hearing (Johnson and Zatorre 2005).
By contrast, IA recruited a distinct limbic and paralimbic network. IA-related activity extended beyond primary interoceptive and somatosensory regions to include the anatomically connected posterior cingulate and hippocampus (Eckert et al. 2009), which may be critical for the representation of broader contextual information (Vogt et al. 2006), as well as the paracentral cortex, which is associated with sensorimotor engagement (Jantzen et al. 2007). Together, these contextual and viscerosomatic regions may constitute a basis for our physical sense of embodiment in the world, a hypothesis consistent with the high association between anasognosia and posterior insula lesions (Karnath et al. 2005). Furthermore, IA promoted greater insula connectivity with thalamic relays of the viscerosomatic spinothalmaic tract, suggesting that the deployment of the IA network directly impacts sensory afference to the neocortex.
Several critiques of this interpretation of the data might be offered. One might argue that the observed effects are indicators of mental effort rather than attentional focus, that a lack of prefrontal recruitment during IA could denote a lack of effort rather than a different type of attention. Although we do not have a measure of relative cognitive effort in the IA and EA tasks, prior work suggests that focusing on interoceptive states actually requires greater cognitive effort than exteroception, as exteroceptive signals often mask competing interoceptive cues (Ludwick-Rosenthal and Neufeld 1985; Moltner and Holzl 2002). Greater frontoparietal activation for EA is therefore less likely a reflection of the amount of resources needed but rather the kind, supporting a distinct network from IA. A second objection may arise about the specificity of this IA network activation: even if IA can be associated with a distinct neural network, perhaps IA networks arise from attending to internal sensation broadly, including thoughts and memories, rather than interoceptive activity specifically; however, the present findings differentiate IA networks from those supporting internally generated and stimulus-independent thought, which recruit medial and dorsolateral prefrontal cortical circuits rather than the interoceptive network demonstrated here (e.g., Christoff et al. 2003; Gilbert et al. 2005), making it unlikely that mind wandering is driving the observed task differences. Finally, one might question the plausibility of the proposed dissociation: why would the neural networks supporting EA differ from those supporting IA? We conjecture that exteroceptive sensory systems contain a critical allocentric spatial component to locate the distal stimulus in the external environment relative to the organism. The exception to this is taste, whose primary sensory cortical fields reside in the insula as well (Faurion et al. 1999); indeed, taste may be considered a bridge between exteroception and interoception. An IA system for mapping internal body sensations radically differs from EA lateral frontal and posterior parietal spatial representations, in that, it relates sensation within the internal context of the self rather than spatially relating external objects to a separable self and deriving their significance. In sum, the dissociation between IA and EA suggests a common attentional modulation of primary representation but originating from distinct attentional modulatory sources. Interoception may arise from anatomically distinct, evolutionarily older limbic regions that provide a present-centered context for sensations originating from the inside of the body (Farb et al. 2007; Craig 2009).
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Canadian Institute of Health Research (#MT81164; www.cihr-irsc.gc.ca); National Institute of Mental Health (#MH066992; www.nimh.nih.gov); Ontario Mental Health Foundation (Studentship; www.omhf.on.ca); Mind and Life Institute (Varela grant; www.mindandlife.org).
Supplementary Material
Acknowledgments
The authors thank Eve De Rosa and 2 anonymous reviewers for helpful comments on this manuscript. Conflict of Interest: None declared.
References
- Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. J Pers Soc Psychol. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. J Clin Neurophysiol. 2000;17:604–612. doi: 10.1097/00004691-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci. 2006;26:490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Henik A, Rafal R. Competition between endogenous and exogenous orienting of visual attention. J Exp Psychol Gen. 2005;134:207–221. doi: 10.1037/0096-3445.134.2.207. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlate of the ‘spotlight’ of attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Brooks JCW, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Visceral sensory neuroscience: interoception. New York: Oxford University Press.; 2002. [Google Scholar]
- Cannon WB. The James-Lange theory of emotions: a critical examination and an alternative theory. Am J Psychol. 1927;39:106–124. [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious stimuli. J Neurophysiol. 1994;71:802–807. doi: 10.1152/jn.1994.71.2.802. [DOI] [PubMed] [Google Scholar]
- Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SC, Blake R. Exogenous attention and endogenous attention influence initial dominance in binocular rivalry. Vision Res. 2006;46:1794–1803. doi: 10.1016/j.visres.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Geddes LPT, Gabrieli JDE. Evaluating self-generated information: anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117:1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craig A. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig A. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: a framework for memory research. J Verb Learn Verb Behav. 1972;11:671–684. [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Derfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JDE, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surg Radiol Anat. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faurion A, Cerf B, Van De Moortele P-F, Lobel E, Mac Leod P, Le Bihan D. Human taste cortical areas studied with functional lateralization related to handedness. Neurosci Lett. 1999;277:189–192. doi: 10.1016/s0304-3940(99)00881-2. [DOI] [PubMed] [Google Scholar]
- Flynn FG, Benson DF, Ardila A. Anatomy of the insula—functional and clinical correlates. Aphasiology. 1999;13:55–78. [Google Scholar]
- Friedman DP, Murray EA, O'Neill JB, Mishkin M. Cortical connections of the somatosensory fields on the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol. 1986;252:323–347. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Firth CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frot M, Magnin M, Mauguière F, Garcia-Larrea L. Human SII and posterior insula differently encode thermal laser stimuli. Cereb Cortex. 2007;17:610–620. doi: 10.1093/cercor/bhk007. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. Eur J Neurosci. 2005;21:1423–1431. doi: 10.1111/j.1460-9568.2005.03981.x. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Summerfield AQ, Palmer AR, Bowtell AR. Modulation and effects in auditory processing measured using fMRI. Hum Brain Mapp. 2000;10:107–119. doi: 10.1002/1097-0193(200007)10:3<107::AID-HBM20>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua LH, Strigo IA, Baxter LC, Johnson SC, Craig AD. Anteroposterior somatotopy of innocuous cooling activation focus in human dorsal posterior insular cortex. Am J Physiol Regul Integr Comp Physiol. 2005;289:R319–R325. doi: 10.1152/ajpregu.00123.2005. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- James W. Principles of psychology. Vol. 1. New York: Henry-Holt and Co; 1890. [Google Scholar]
- Jantzen KJ, Oullier O, Marshall M, Steinberg FL, Kelso JAS. A parametric fMRI investigation of context effects in sensorimotor timing and coordination. Neuropsychologia. 2007;45:673–684. doi: 10.1016/j.neuropsychologia.2006.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevning R, Wallace RK, Beidebach M. The physiology of meditation: a review. Neurosci Biobehav Rev. 1992;16:415–424. doi: 10.1016/s0149-7634(05)80210-6. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Zatorre RJ. Attention to simultaneous unrelated auditory and visual events: behavioral and neural correlates. Cereb Cortex. 2005;15:1609–1620. doi: 10.1093/cercor/bhi039. [DOI] [PubMed] [Google Scholar]
- Karnath H-O, Baier B, Nägele T. Awareness of the functioning of one's own limbs mediated by the insular cortex? J Neurosci. 2005;25:7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, Tranel D. Interoceptive awareness in experienced meditators. Psychophysiology. 2008;45:671–677. doi: 10.1111/j.1469-8986.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Ludwick-Rosenthal R, Neufeld RWJ. Heart beat interoception: a study of individual differences. Int J Psychophysiol. 1985;3:57–65. doi: 10.1016/0167-8760(85)90020-0. [DOI] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. A method for removal of global effects from fMRI time series. Neuroimage. 2004;22:360–366. doi: 10.1016/j.neuroimage.2003.12.042. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2007;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28:175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Mufson EJ. Insula of the old world monkey. I: architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Moltner A, Holzl R. Interoception, body perception and awareness. Acta Biol Hung. 2002;53:515–536. doi: 10.1556/ABiol.53.2002.4.12. [DOI] [PubMed] [Google Scholar]
- Murray SO, Wojciulik E. Attention increases neural selectivity in the human lateral occipital complex. Nat Neurosci. 2004;7:70–74. doi: 10.1038/nn1161. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human anterior insular cortex. Neuroscience. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlagger BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Heslenfeld DJ, Theeuwes J. Edogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage. 2004;22:822–830. doi: 10.1016/j.neuroimage.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Porges SW, Byrne EA. Research methods for measurement of heart rate and respiration. Biol Psychol. 1992;34:93–130. doi: 10.1016/0301-0511(92)90012-j. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffarra P, Scaglioni A, Woodley SJ, Hammeke TA, Cunningham JM, Prieto TE, Binder JR. Neural basis of endogenous and exogenous spatial orienting: a functional MRI study. J Cogn Neurosci. 1999;11:135–152. doi: 10.1162/089892999563283. [DOI] [PubMed] [Google Scholar]
- Schneider RJ, Friedman DP, Mishkin M. A modality-specific somatosensory area within the insula of the rhesus monkey. Brain Res. 1993;621:116–120. doi: 10.1016/0006-8993(93)90305-7. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood L. Fundamentals of physiology: a human perspective. Toronto (Canada): Thomson; 2006. [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Bender G, Constable RT, Small DM. Trying to detect taste in a tasteless solution: modulation of early gustatory cortex by attention to taste. Chem Senses. 2007;32(6):569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laurey S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA, Carter CS. Maintaining structured information: an investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia. 2008;46:665–678. doi: 10.1016/j.neuropsychologia.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8:114–120. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.