Abstract
Several approaches exist for quantitative assessment of human immunodeficiency virus (HIV) associated distal sensory polyneuropathy (DSP). While useful, each has some limitations. This study evaluated non-invasive, in vivo reflectance confocal microscopy (RCM) of Meissner Corpuscles (MCs) as a measure of HIV-DSP. Forty-eight adults (29 HIV-infected, 19 controls) underwent RCM of MC density (MCs/mm2) at the arch, fingertip and thenar eminence (TE), ankle skin biopsy to measure epidermal nerve fiber density (ENFD), electrophysiologic studies, and tactile, vibration and thermal threshold testing. HIV+ subjects were clinically categorized as having DSP signs or no signs. MC densities were lower in HIV+ subjects with DSP signs than in controls (arch, p = 0.0003; fingertip, p < 0.0001; TE, p = 0.0002). Tactile thresholds in the TE and foot were worse in HIV-DSP than in controls, but in this mild DSP cohort, sural amplitudes, ENFD and vibration and thermal thresholds didn't differ significantly from controls. Fingertip MC densities and tactile thresholds at the foot were also lower in HIV+ subjects without DSP signs than in controls. Other sensory measures were not significantly different in HIV+ subjects without DSP signs than in controls. MC density correlated inversely with tactile thresholds at each imaging location. The results suggest that RCM of MC density complements existing sensory DSP measures and discriminates mild HIV-DSP from controls at a stage when sural amplitudes do not. Further studies are required to determine whether RCM of MC density can establish quantitative changes in DSP, in response to treatment or disease progression.
Keywords: In-vivo confocal reflectance microscopy, Meissner’s corpuscle, peripheral neuropathy, skin biopsy, outcome measure
INTRODUCTION
Sensory nerve terminals are involved early or preferentially in many forms of peripheral neuropathy, yet non-invasive microscopic approaches for their in vivo imaging in humans have been lacking. In vivo reflectance confocal microscopy (RCM) is a painless, non-invasive technique that is increasingly being used for the imaging of human skin lesions, including cancer (Rajadhyaksha et al, 1999, Selkin B et al, 2001). The approach involves application of a low level laser to illuminate a point within the skin. Due to naturally occurring variations in the refractive index in the skin, a portion of the light is reflected off the tissue and through a pinhole aperture onto a detector. RCM does not require the use of contrast agents or fluorophores and has a lateral resolution of 0.5– 1um (Rajadhyaksha et al, 1999). We recently described the feasibility of RCM of Meissner corpuscles (MCs), the main touch-pressure sensation receptors in glabrous skin (hands, feet, digits) (Bolton et al. 1966; Dyck et al. 1966; Herrmann et al. 2007; Almodovar et al. 2011). In order to explore whether MC imaging might yield a novel measure of distal sensory neuropathy, we selected as a model HIV-associated distal sensory polyneuropathy (DSP), the most common neurological complication of HIV infection (Herrmann et al. 2004; Schifitto et al. 2002; So et al. 1988; Tyor et al. 1995; Cornblath et al. 1988; Tagliati et al. 1999; Shy et al. 2003; Holland et al. 1997). Several methods (nerve conduction studies (NCS), quantitative sensory testing (QST), and skin biopsy from hairy skin for assessment of epidermal nerve fiber density (ENFD)) have been developed for monitoring of the small and large-fiber sensory components of DSP (Holland et al. 1997; Polydefkis et al. 2002; Zhou et al. 2007; Herrmann et al. 1999; Cherry et al. 2005). While useful, each has limitations with respect to detection and objective monitoring of DSP (Shy et al. 2003; Polydefkis et al. 2002; Zhou et al. 2007; Herrmann et al. 1999; Cherry et al. 2005). Non-invasive approaches toward early detection and monitoring of HIV-DSP are needed to complement these measures and help establish quantitative changes in DSP, below the clinical threshold of detection, in response to treatment or disease progression.
The aims of the present study were to: 1. compare MC density (MC/mm2) by in vivo RCM at the hand and foot between HIV-infected subjects with clinically-defined DSP and healthy control subjects; 2. compare MC density by in vivo RCM at the medial sole and hand (digit 5 and the thenar eminence) between HIV-infected subjects without clinical DSP and healthy control subjects; and 3. determine relationships among in vivo RCM of MC density at the hand and foot and standard peripheral sensory measures.
PATIENTS AND METHODS
Forty eight subjects (29 with HIV infection and 19 healthy controls) between the age of 18 and 65 years were recruited following written informed consent under an RSRB approved, NINDS supported protocol. HIV seropositivity was based on self-report and confirmed by ELISA and Western blot or viral load.
The inclusion/exclusion criteria for the HIV group were: age 18–65 years; no history of alcohol abuse; no B12 deficiency, hypothyroidism or diabetes mellitus, based on screening serum B12 levels, thyroid function studies and fasting blood glucose or hemoglobin A1C; no active opportunistic CNS infection or other chronic neurologic disorders; no familial neuropathy or other systemic disease or neurotoxin exposure (other than antiretroviral therapy) known to predispose to peripheral neuropathy; no signs or symptoms of a compression mononeuropathy; and no signs or symptoms of a myelopathy.
The inclusion/exclusion criteria for normal control subjects were: age 18–65 years; no history of diabetes mellitus, HIV infection or other conditions known to predispose to neuropathy; no clinical signs or symptoms of polyneuropathy or compression mononeuropathy; no history of neurotoxin exposure or alcohol abuse; no foot deformities suggestive of a hereditary neuropathy or history of a familial polyneuropathy; no abnormality of fasting glucose, renal, liver or thyroid function, serum vitamin B12, HIV seropositivity, serum protein electrophoresis or immunofixation. MC density, touch-pressure and vibration thresholds at the hand for the control group have been described in part (Almodovar et al. 2011).
Subjects recruited to the HIV cohort were classified as having either (1) HIV infection with signs of DSP or (2) HIV infection with no DSP signs, whether they were symptomatic or not. Signs of DSP were defined by presence of one or more of the following clinical criteria: (1) bilaterally absent ankle reflexes, (2) reduced vibration sense at the toes, (3) abnormal proprioception at the toes or (4) a distal stocking or stocking and glove reduction in pinprick perception. Symptoms of DSP included length-dependent complaints of pain, burning, dysesthesia or paresthesia.
In vivo RCM of MC Density
In vivo RCM of MCs (VivaScope 1500, Lucid. Inc., Rochester, NY) was performed over the volar surface of left hand digit 5, the left thenar eminence and arch of the sole in all subjects. A single trained microscopist performed MC imaging at each site within a 6×6 mm area from the basal layer of the epidermis through the depth of the dermal papillae as previously described (Herrmann et al. 2007; Almodovar et al. 2011). A 6×6 mm mosaic image of individual adjacent 500×500 um imaging fields was acquired within in a horizontal plane near the basal layer of the epidermis, so that the most superficial MCs were visible. Following the initial imaging sequence, seven mosaic images were captured at successive 20um depths within this 6×6 mm region, so that the volume of tissue was sampled through the depth of the dermal papillae. Systematic random sampling was used to count MCs in a blinded manner, within an 18 mm2 area within each mosaic image. Mosaic images at successive depths were compared to avoid double counting of MCs. MC density was expressed as MCs/mm (Dyck et al. 1966; Herrmann et al. 2007; Almodovar et al. 2011).
Quantitative Sensory Testing
Quantitative Sensory Testing (QST) of vibration, cooling and heat-pain (HP) thresholds was performed using the Case IVB system (WR Medical Electronics Company, Rochester, MN) at the great toe/foot and digit V/hand. Cooling (CDT) and vibration detection thresholds (VDT) were assessed using the 4-2-1 algorithm and results expressed as a normal deviate (ND) based on age, height and gender matched normative data (Dyck et al. 1993A, 1993B). Heat-pain thresholds were assessed using the non-repeating ascending with null stimuli (NRA-NS) algorithm. Both a threshold (HP 0.5) and an intermediate response of pain from graded heating pulses (HP 5.0) were assessed using a visual analog scale and results expressed in JNDs (just noticeable differences) and converted to ND based on age, height and gender matched normative data (Dyck et al. 1993B).
Touch pressure sensory thresholds were assessed at each RCM imaging site using a series of 9 monofilaments of different bending forces. A 4-2-1 testing algorithm was applied including variable null stimuli. The touch pressure threshold was defined as that stimulus that was correctly identified at least 50% of the time (Almodovar et al. 2011).
Electrophysiologic Studies
Each subject underwent left sural nerve, medial plantar nerve and ulnar nerve (digit V) sensory testing using standard antidromic surface recording and stimulation techniques for the sural and ulnar sensory nerves, and orthodromic methods for the medial plantar nerve. Limb temperature was maintained above 32° Celsius during electrophysiologic testing.
Skin Biopsy (Epidermal Nerve Fiber Density)
All subjects underwent a 3mm distal leg skin biopsy, 10cm proximal to the left lateral malleolus. Skin biopsies were processed and immunostained with polyclonal antibodies to the panaxonal marker, protein gene product 9.5, according to previously published methods (Herrmann et al. 1999, 2004). A blinded observer assessed ENFD for each biopsy from four 50um sections according to established methodology.
Statistical Methods
Pair-wise group comparisons of demographic variables among healthy controls and HIV infected subjects with (HIV-DSP) and without DSP signs were performed using either Wilcoxon rank sum tests or Fisher’s exact tests, as appropriate. Analysis of covariance based on ranks was used in the primary analyses to compare MC density between HIV DSP subjects and healthy control subjects at each of the three imaging sites (Conover and Iman 1982). The models for digit V adjusted for age and hand area; those for thenar eminence adjusted for age; and those for medial sole adjusted for age and height. A Bonferroni-adjusted significance level of 1.7% (two-tailed) was used for each imaging site. Secondary analyses involved similar comparisons between HIV+ subjects without DSP signs and healthy control subjects. Conventional sensory measures, including monofilament thresholds, QST thresholds for vibration, cooling, and heat-pain, distal leg ENFD and sural, medial plantar and ulnar sensory NCS amplitudes, were compared among the groups (HIV-DSP and HIV without DSP signs versus control subjects) using analysis of covariance adjusting for age and gender.
Relationships between in vivo RCM of MC density and conventional sensory measures were examined at each imaging site in the overall study cohort using Spearman’s rank correlation coefficients. A significance level of 1% was used for analyses involving correlation coefficients.
RESULTS
Clinical and demographic features
Clinical and demographic features for the 48 participants are shown in Table 1. Control and HIV infected subjects were similar in age. More men were recruited in the HIV-infected groups (72%) than in the control group (42%). The mean duration of known HIV infection was 11±6 years. Thirteen HIV+ patients had signs of DSP and 16 did not. Among subjects with DSP signs, 6/13 (46%) had 2 or more DSP signs, 8/13 (62%) had bilaterally absent ankle reflexes, 10/13 (77%) had abnormal vibration sensation at the toes and 5/13 (38%) had absent or reduced pin sensation in the feet. Eight of 13 subjects with DSP signs had DSP symptoms, while 7 of 16 HIV-infected individuals without DSP signs, had sensory symptoms or pain. Among HIV+ subjects, mean (± standard deviation) CD4 cell count was 425.3 ± 277.9 cells/ml. Viral load was undetectable in 16 out of 29 HIV+ subjects, was less than 10,000 copies/ml in 8/29 subjects and was greater than 10,000 copies/ml in 5/29 subjects. Seventy nine percent of HIV+ subjects were on highly active antiretroviral therapy at the time of the study, while 10% of HIV infected subjects had a known history of prior exposure to dideoxynucleoside agents (Table 1.).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Variable | Controls (n = 19) | HIV NDSP (n = 16) | HIV DSP (n = 13) |
|---|---|---|---|
| Age (years) | 43.7 (8.1) | 45.2 (10.3) (p=0.68) |
45.6 (9.8) (p=0.55) |
| Gender (number M/F) | 8/11 | 11/5 (p=0.12) |
10/3 (p=0.051) |
| Height (cm) | 168.5 (8.9) | 170.0 (9.8) (p=0.55) |
173.3 (6.4) (p=0.071) |
| Weight (kg) | 77.1 (16.4) | 79.4 (16.5) (p=0.67) |
76.5 (22.4) (p=0.58) |
| CD4+ cell count (cells/ml) | 369.4 (227.4) | 494.1 (326.0) | |
| Viral load (%) Undetectable < 10,000 copies/ml ≥ 10,000 copies/ml |
54% 31% 15% |
56% 25% 19% |
|
| HAART (% currently taking) | 81% | 75% | |
| Known Dideoxynucleoside Drug exposure (%) |
19% | 0% |
Values are mean (standard deviation) unless otherwise indicated
P-values indicate comparisons between each of the HIV groups and the control group
HIV NDSP = HIV without signs of DSP; HIV DSP = HIV with signs of DSP; HAART highly active antiretroviral therapy
Comparisons of MC densities in HIV infected subjects with and without DSP signs versus those in controls
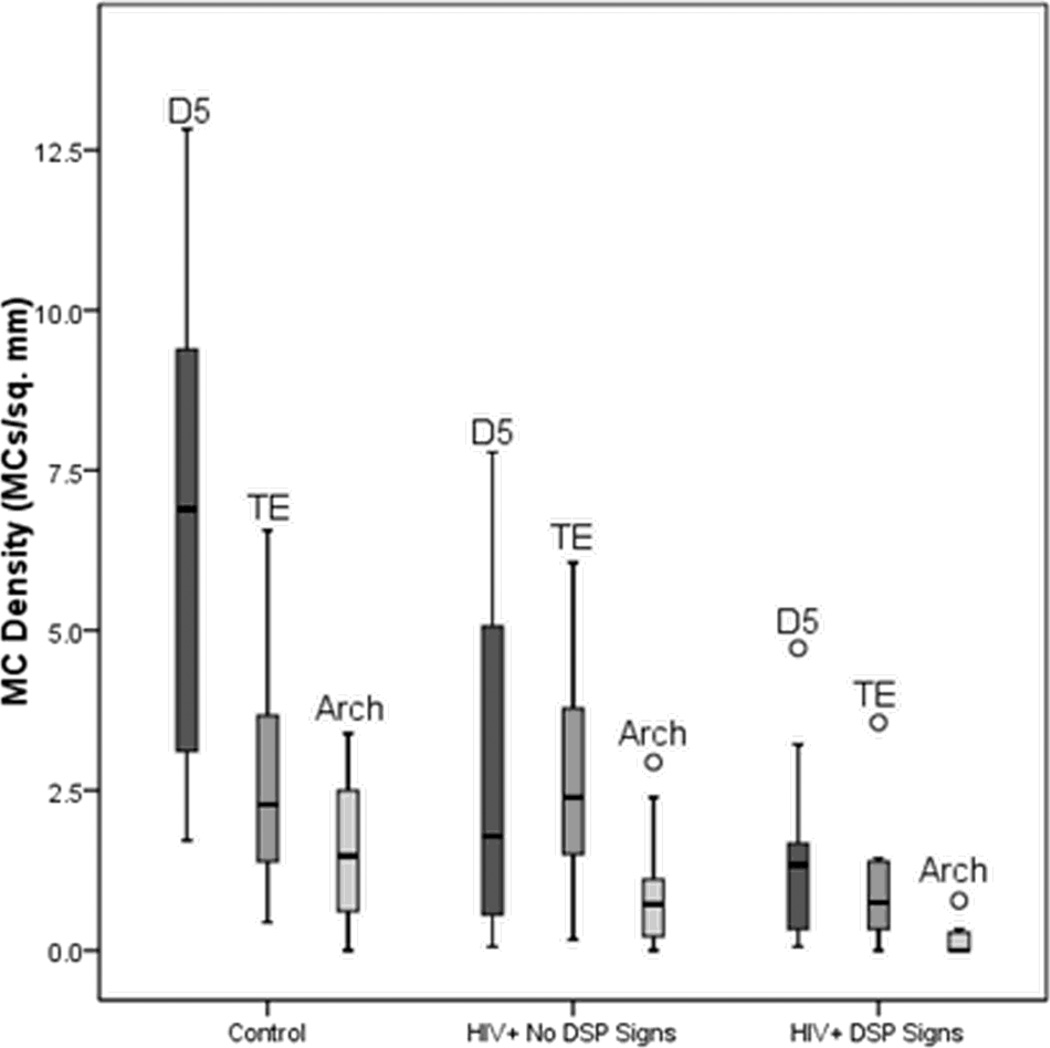
MC densities were lower in HIV+ subjects with DSP signs than in controls at all 3 imaging sites, after controlling for relevant covariates (foot: p = 0.0003; fingertip: p < 0.0001; and thenar eminence: p = 0.0002) (Table 2, Table 3, Fig. 1, Fig. 2). Among HIV+ subjects without DSP signs, MC densities at digit V were lower than in controls (p = 0.002), but those at the foot and thenar eminence did not differ significantly from those in controls. MC densities were lower at the foot (p=0.01) and thenar eminence (p=0.01) in HIV infected subjects with DSP signs, than those without DSP signs.
Table 2.
Neuropathy Outcomes (Upper Limb)
| Variable | Controls (n = 19) | HIV NDSP (n = 16) | HIV DSP (n = 13) |
|---|---|---|---|
| MC density, digit V (MCs/mm2); median (IQR) |
6.89 (3.11, 9.39) |
1.64 (0.31, 4.73) (p = 0.002) |
1.34 (0.33, 1.67) (p < 0.0001) |
| MC density, thenar eminence (MCs/mm2); median (IQR) |
2.00 (1.17, 3.67) | 2.25 (1.34, 3.59) (p = 0.90) |
0.67 (0.33, 1.39) (p = 0.0002) |
| Ulnar SNAP amplitude (uV) | 35.1 (11.3) | 32.8 (17.7) (p = 0.66) |
30.7 (14.3) (p = 0.28) |
| Monofilament threshold, digit V; median (IQR) |
0.07 (0.02, 0.07) | 0.07 (0.07, 0.16) (p = 0.05) |
0.07 (0.07, 0.16) (p = 0.25) |
| Monofilament threshold, thenar eminence; median (IQR) |
0.07 (0.07, 0.16) | 0.12 (0.07, 0.16) (p = 0.09) |
0.16 (0.12, 0.28) (p = 0.01) |
| QST vibration, finger (ND) | 0.62 (0.82) | 0.54 (0.92) (p = 0.66) |
0.84 (1.53) (p = 0.77) |
| QST cooling, hand (ND) | 1.03 (1.32) | 1.86 (1.24) (p = 0.18) |
1.94 (1.56) (p = 0.17) |
| QST HP 0.5, hand (ND) | −0.91 (1.77) | −1.02 (1.79) (p = 0.79) |
−0.71 (1.53) (p = 0.95) |
| QST HP 5.0, hand (ND) | −1.38 (1.87) | −1.15 (2.11) (p = 0.89) |
−1.01 (1.81) (p = 0.98) |
| QST HP 0.5–5.0, hand (ND) | −0.20 (0.60) | 0.06 (1.41) (p = 0.71) |
0.41 (0.91) (p = 0.10) |
Values are mean (standard deviation) unless otherwise indicated
Monofilament thresholds are measured as log of bending force of the monofilament in tenths of a mg
P-values indicate comparisons between each of the HIV groups and the control group
HIV NDSP = HIV without signs of DSP; HIV DSP = HIV with signs of DSP; IQR = interquartile range (25th and 75th percentiles); MC = Meissner corpuscle; SNAP = sensory nerve action potential; QST = quantitative sensory testing; ND = normal deviate; HP = heat pain
Table 3.
Neuropathy Outcomes (Lower Limb)
| Variable | Controls (n = 19) | HIV NDSP (n = 16) | HIV DSP (n = 13) |
|---|---|---|---|
| MC density, foot (MCs/mm2); median (IQR) |
1.39 (0.61, 2.50) | 0.83 (0.22, 1.67) (p = 0.32) |
0.09 (0.00, 0.33) (p = 0.0003) |
| ENF density, ankle (fibers/mm) |
14.3 (4.9) | 10.1 (3.4) (p = 0.027) |
10.3 (3.8) (p = 0.08) |
| Sural SNAP amplitude (uV) | 24.1 (9.9) | 19.2 (8.7) (p = 0.37) |
14.3 (7.9) (p = 0.06) |
| Plantar SNAP amplitude (uV) | 10.6 (4.0) | 8.8 (5.2) (p = 0.65) |
6.0 (2.4) (p = 0.008) |
| Monofilament threshold, foot; median (IQR) |
0.16 (0.07, 0.16) |
0.40 (0.16, 1.00) (p = 0.006) |
0.70 (0.28, 1.00) (p = 0.0009) |
| QST vibration, toe (ND) | 0.66 (1.05) | 0.57 (1.33) (p = 0.79) |
1.82 (1.47) (p = 0.03) |
| QST cooling, foot (ND) | 0.64 (1.67) | 1.17 (1.35) (p = 0.67) |
2.03 (1.52) (p = 0.11) |
| QST HP 0.5, foot (ND) | −0.62 (1.21) | −0.75 (2.14) (p = 0.76) |
−0.02 (1.85) (p = 0.56) |
| QST HP 5.0, foot (ND) | −0.42 (1.79) | −0.35 (2.06) (p = 0.83) |
−0.42 (2.00) (p = 0.62) |
| QST HP 0.5–5.0, foot (ND) | 0.43 (1.21) | 0.29 (1.45) (p = 0.25) |
−0.30 (0.90) (p = 0.05) |
Values are mean (standard deviation) unless otherwise indicated
Monofilament thresholds are measured as log of bending force of the monofilament in tenths of a mg
P-values indicate comparisons between each of the HIV groups and the control group
HIV NDSP = HIV without signs of DSP; HIV DSP = HIV with signs of DSP; IQR = interquartile range (25th and 75th percentiles); MC = Meissner corpuscle; ENF = epidermal nerve fiber; SNAP = sensory nerve action potential; QST = quantitative sensory testing; ND = normal deviate; HP = heat pain
Fig. 1.
Meissner corpuscle (MC) densities for control subjects and HIV infected subjects with and without DSP signs. For each group, boxplots (median, quartiles and extreme values (denoted by open circles)) of MC densities at the fingertip (D5), thenar eminence (TE) and arch are shown from left to right.
Fig. 2.
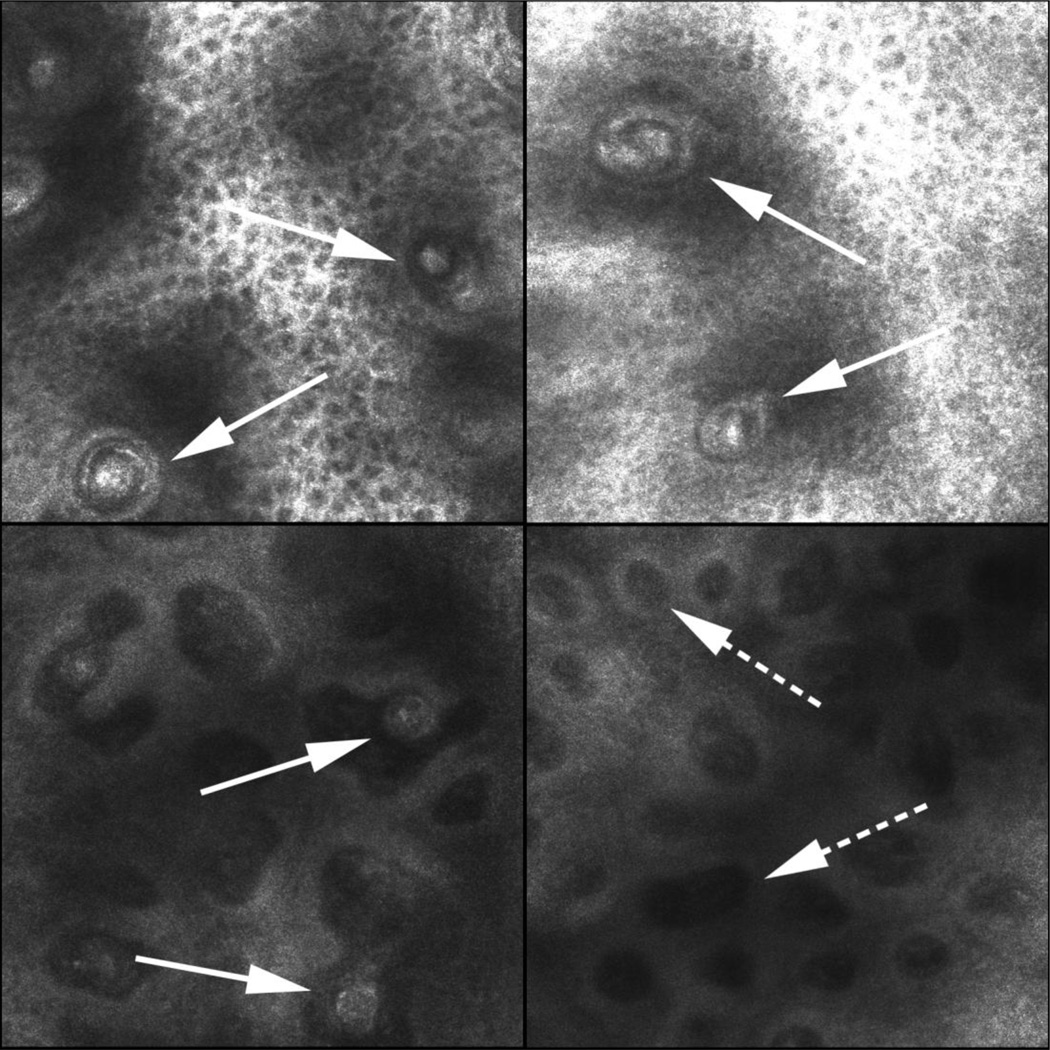
RCM of Meissner Corpuscles (MCs) in a control subject (arrows, Panel A,B – high power) and (C – lower power). Panel D shows multiple dermal papillae without MCs (broken arrow) in a subject with HIV DSP with marked depletion of MC density
Comparison of epidermal nerve fiber density among HIV infected subjects and controls
Epidermal nerve fiber density at the ankle tended to be lower in the HIV+ groups than in the control group (14.3 ± 4.9 fibers/mm), but these differences did not reach significance after controlling for age and gender. The DSP signs group had a mean ENFD of 10.3 ± 3.8 fibers/mm (p = 0.08) and those without DSP signs had a mean ENFD of 10.1± 3.4 (p= 0.027) (Table 3).
Comparison of other sensory measures among HIV infected subjects and controls
Touch-pressure thresholds at the sites of MC imaging were higher in HIV+ subjects with DSP signs than in controls at the foot (p=0.0009) and thenar eminence (p=0.01), but not at the fingertip (p=0.25), after controlling for age and gender (Table 2, Table 3). Touch-pressure thresholds in HIV+ subjects without DSP signs were higher at the foot than in controls (p= 0.006). Vibration, cooling and heat-pain thresholds did not differ significantly between the HIV-infected and control groups, although there were trends toward higher vibration (p = 0.03) and lower (hyperalgesic) HP 0.5 thresholds at the toe/foot (p=0.05) in HIV infected subjects with DSP signs compared to controls (Table 2, Table 3).
Medial plantar nerve action potential (NAP) amplitudes were lower among HIV+ subjects with DSP signs (6.0 ± 2.4 uV) than in controls (10.6 ± 4.0 uV, p = 0.008), while those in HIV+ subjects without DSP signs did not differ significantly from those in the control group. Sural SNAP amplitudes tended to be lower in HIV+ subjects with DSP signs than in controls (14.3 ± 7.9 uV vs. 24.1 ± 9.9 uV, p = 0.06). Ulnar SNAP amplitudes were similar among the three groups (Table 2).
Associations between MC densities and other sensory measures
MC density at the foot was associated with MC density at the fingertip (r = 0.47, p = 0.002) and thenar eminence (r = 0.48, p = 0.0009), and was inversely associated with touch-pressure threshold at the arch (r = −0.41, p = 0.006). There were weaker associations between MC density at the foot and sural SNAP amplitude (r = 0.32, p = 0.03) and ENFD (r = 0.34, p= 0.02). There was no significant association between MC density at the foot and medial plantar NAP amplitude, or with vibration, cooling, or HP thresholds at the foot (Table 4).
Table 4.
Correlations between MC Densities and Conventional Sensory Measures
| A. MC Density, Foot | ||||||||
|---|---|---|---|---|---|---|---|---|
| ENF density, ankle |
Sural SNAP amplitude |
Plantar SNAP amplitude |
MF threshold, foot |
QST vibration, toe |
QST cooling, foot |
QST HP 0.5, foot |
QST HP 5.0, foot |
QST HP 0.5–5.0, foot |
| r = 0.34 p = 0.02 |
r = 0.32 p = 0.03 |
r = 0.26 p = 0.10 |
r = −0.41 p = 0.006 |
r = −0.003 p = 0.99 |
r = −0.05 p = 0.76 |
r = −0.11 p = 0.54 |
r = 0.03 p = 0.89 |
r = 0.15 p = 0.39 |
| B. MC Density, Digit V | ||||||
|---|---|---|---|---|---|---|
| Ulnar SNAP amplitude |
MF threshold, digit V |
QST vibration, finger |
QST cooling, hand |
QST HP 0.5, hand |
QST HP 5.0, hand |
QST HP 0.5–5.0, hand |
|
r = 0.44 p = 0.005 |
r = −0.38 p = 0.01 |
r = −0.19 p = 0.28 |
r = −0.41 p = 0.02 |
r = −0.11 p = 0.55 |
r = −0.08 p = 0.65 |
r = −0.21 p = 0.25 |
| C. MC Density, Thenar Eminence | ||||||
|---|---|---|---|---|---|---|
| Ulnar SNAP amplitude |
MF threshold, thenar eminence |
QST vibration, finger |
QST cooling, hand |
QST HP 0.5, hand |
QST HP 5.0, hand |
QST HP 0.5–5.0, hand |
| r = 0.29 p = 0.06 |
r = −0.46 p = 0.001 |
r = −0.24 p = 0.14 |
r = −0.36 p = 0.02 |
r = −0.02 p = 0.89 |
r = −0.09 p = 0.61 |
r = −0.41 p = 0.01 |
MC = Meissner corpuscle; ENF = epidermal nerve fiber; SNAP = sensory nerve action potential; MF = monofilament; QST = quantitative sensory testing; HP = heat pain
MC density at the fingertip was associated with MC density at the thenar eminence (r = 0.42, p = 0.004), the ulnar SNAP amplitude (r = 0.44, p = 0.005), and was inversely associated with touch-pressure threshold at the fingertip (r = −0.38, p = 0.01). There was a weaker inverse relationship between MC density at the fingertip and cooling threshold at the hand (r = −0.41, p= 0.02). There were no significant associations between MC density at the fingertip and vibration or HP thresholds at the hand (Table 4).
MC density at the thenar eminence was inversely associated with touch-pressure threshold at the site of imaging (r = −0.46, p = 0.001). There were weaker associations between MCD density at the thenar eminence and cooling threshold at the finger (r = −0.36, p = 0.02) and HP 5.0-0.5 at the hand (r = − 0.41, p = 0.01).
Among HIV+ subjects, MC densities at the fingertip, thenar eminence and foot were not associated with CD4+ lymphocyte count.
DISCUSSION
We undertook a prospective study of in-vivo imaging of MCs in HIV neuropathy. This study enrolled groups of HIV-infected subjects with or without signs of DSP as well as a prospective control group. HIV+ subjects with DSP signs had, as a group, very mild DSP as reflected by conventional measures of sensory neuropathy, including the sural SNAP amplitude and ankle ENFD.
In the present study, MC densities by in-vivo RCM were reduced at the hand and foot in HIV+ subjects with DSP signs compared to controls at a time when sural and ulnar SNAP amplitudes didn’t differ significantly from those in control subjects and medial plantar NAP amplitudes were only mildly reduced relative to controls. These data indicate that mechanoreceptor imaging in the skin can provide evidence of peripheral sensory pathology in HIV infection when conventional electrophysiologic assessments of large myelinated sensory nerve fiber function show minimal changes.
There has been uncertainty regarding the relative involvement of small diameter unmyelinated sensory fibers versus large myelinated sensory fibers in HIV neuropathy. Both populations of sensory fibers are known to be involved during the course of HIV neuropathy, but some studies have suggested that small-fiber involvement may predominate, given that sural SNAPs are not uncommonly normal in HIV-DSP (Zhou et al. 2007). Contrary to the concept of HIV-DSP as a predominantly small-fiber neuropathy, however, is the clinical observation that ankle reflexes and vibratory sensation at the toes (mediated via myelinated fiber pathways) are commonly abnormal in individuals diagnosed with HIV-DSP, whether or not sensory electrophysiologic abnormalities are present (Cherry et al. 2006). The lack of an easily applied, localizing assessment of myelinated sensory nerve terminals has hitherto limited study of patterns of distal sensory fiber involvement in HIV-DSP. This study suggests that the nerve terminals of large-diameter sensory fibers are affected in HIV infection. HIV neuropathy in its mildest clinical form appears to be a small and large-fiber sensory neuropathy, with preferential involvement of nerve terminals, rather than a pure small-fiber neuropathy.
This study incorporated a range of QST assessments. Vibration and thermal thresholds has been employed in longitudinal studies and therapeutic trials in HIV-DSP (Berger et al. 1993; Herrmann et al. 2006; McArthur et al. 2000). In the current sample, while vibration and thermal thresholds showed normal deviates that were in the direction of worse sensory function in HIV infection with DSP signs, significant differences from controls were not observed although the small sample sizes may have contributed to this. We also included assessment of touch-pressure thresholds. Touch-pressure thresholds have not been systematically studied in HIV neuropathy. Among all QST measures, touch-pressure thresholds best distinguished HIV+ subjects with DSP signs from controls. Monofilament thresholds were significantly elevated at the foot and thenar eminence in HIV+ subjects with DSP signs. Touch-pressure is the main sensory modality transduced by MCs (Baba et al. 2001). Touch-pressure thresholds provide information on the functional integrity of MCs and associated peripheral and central sensory pathways. Touch-pressure thresholds can be inexpensively assessed and as such may prove a useful adjunct in the clinical and research monitoring of HIV-DSP, particularly in resource constrained settings. However touch-pressure thresholds are not localizing to either the peripheral or central nervous system and are a psychophysical modality that can be impacted by cognitive impairment in HIV infection. In vivo RCM, by contrast, provides non-invasive, quantitative, localizing and more objective information regarding the sensory peripheral nervous system (PNS). For clinical and research questions where rigorous, quantitative assessment of different components of the sensory PNS is desirable, addition of MC imaging has advantages over assessment of touch-pressure thresholds alone.
HIV infected subjects without DSP signs were less distinct from control subjects with respect to their peripheral sensory structure and function than were subjects with DSP signs. In vivo RCM of MC density among the HIV+ group without DSP signs was reduced at the fingertip as compared to controls, but not at the sole or palm. Medial plantar and sural SNAP amplitudes in HIV subjects without DSP signs did not differ significantly from those in controls. Touch-pressure sensation thresholds were worse at the foot in HIV+ subjects without DSP signs relative to controls. The occurrence of sub-clinical abnormalities of peripheral sensory structure and function in HIV+ subjects without DSP signs is consistent with previous investigations (Herrmann et al. 2004; McCarthy et al. 1995). In vivo RCM of MC density extends observations with regard to the spectrum of sub-clinical peripheral sensory involvement in HIV infection.
This study incorporated extensive evaluations of large and small fiber sensory structure and function so as to develop a detailed understanding of relationships between in vivo RCM of MC density and standard sensory neuropathy measures. The most consistent structure-function correlations were observed between MC densities and touch-pressure thresholds. Specifically, MC densities were inversely associated with touch-pressure thresholds at the site of imaging at all 3 locations. As MCs primarily subserve touch pressure sensation, these inverse associations make biological and clinical sense and support the functional meaningfulness of RCM of MC density (Baba et al. 2001). By contrast, weaker or insignificant associations were seen between MC densities and other measures of sensory perception including vibration and thermal thresholds as well as ENFD. This indicates that MC density assessments are complementary in the evaluation of the sensory PNS, providing quantitative information that is distinct from other sensory measures.
In this study, a clinical, rather than a neurophysiologic or pathologic approach was used to categorize HIV+ subjects. This was done in order to analyze relationships between RCM of MC density and standard neuropathy measures in a cohort of HIV-infected individuals as they present clinically.
Investigations of neuropathy measures in HIV infection have variously categorized subjects according to whether or not they had “at least one clinical sign of DSP” in some studies versus “presence of 2 or more DSP signs” in other studies (Simpson et al 2006; Ellis et al, 2010). A threshold of “one clinical sign” can result in over-diagnosis of DSP, while the criterion of 2 or more signs under-diagnoses DSP (Simpson et al 2006; Ellis et al, 2010). In our study the presence of “one or more DSP signs” was used to categorize subjects. This could potentially have resulted in misclassification of some individuals with regard to DSP. The impact however on the findings of this study was likely small, as approximately half of subjects in the DSP signs group had 2 or more signs, and the overall inference from this study, that MC density might serve as a potential marker of early peripheral sensory involvement in HIV infection (whether clinical or subclinical) would not have been influenced by the method of categorization of subjects.
A further potential limitation of this study is that the HIV+ cohort did not contain subjects with advanced DSP. In part this was by design, in order to evaluate whether MC assessments could serve as a marker of early peripheral sensory involvement in HIV infection. The paucity of subjects with advanced DSP also reflects the changing spectrum of disease seen in our medical center in the era of HAART. Future studies will need to assess changes in MC structure and function across the full severity spectrum of HIV-DSP.
In summary, structural assessment of myelinated sensory terminals with in vivo RCM of MC density coupled with functional assessment of MCs (touch-pressure thresholds) appear to identify sensory PNS involvement in HIV infection at a time when changes in sensory NCS and conventional QST are sparse. RCM of MC density thus provides a structural marker of early sensory neuropathy in HIV infection and, in combination with standard measures of neuropathy, a more complete profile of the sensory involvement in HIV infection.
Additional longitudinal studies are required and to determine whether MC density assessment coupled with touch-pressure thresholds can be used reliably to follow progression or improvement of HIV neuropathy through the course of disease and therapy.
ACKNOWLEDGEMENTS
Michele Gaugh for assistance with subject recruitment. Janet Sowden, BS, Ming Ji, MD, and Kai Bekar MD for technical assistance. Jennifer Rafferty, BS, for blinded quantitation of epidermal nerve fiber densities. Christi Alessi-Fox (Lucid Inc.) for technical support with in vivo confocal microscopy. Dr. Herrmann had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study supported by: This study was supported by NINDS NS058259-02A1 (D Herrmann, PI), and the Bioinformatics core of the CTSI of the University of the Rochester (NIH/NCRR 2UL1RR024160-06 (T Pearson, PI)
REFERENCES
- Almodovar J, Ferguson M, McDermott MP, Lewis RA, Shy ME, Herrmann DN. In vivo confocal microscopy of meissner corpuscles as a novel sensory measure in CMT1A. J Peripher Nerv Syst. 2011;16:169–174. doi: 10.1111/j.1529-8027.2011.00342.x. [DOI] [PubMed] [Google Scholar]
- Baba M, Simonetti S, Krarup C. Sensory potentials evoked by tactile stimulation of different indentation velocities at the finger and palm. Muscle Nerve. 2001;24:1213–1218. doi: 10.1002/mus.1134. [DOI] [PubMed] [Google Scholar]
- Berger AR, Arezzo JC, Schaumburg HH, Skowron G, Merigan T, Bozzette S, Richman D, Soo W. 2',3'-Dideoxycytidine (ddC) toxic neuropathy: A study of 52 patients. Neurology. 1993;43:358–362. doi: 10.1212/wnl.43.2.358. [DOI] [PubMed] [Google Scholar]
- Bolton CF, Winkelmann RK, Dyck PJ. A quantitative study of Meissner's corpuscles in man. Neurology. 1996;16:1–9. doi: 10.1212/wnl.16.1.1. [DOI] [PubMed] [Google Scholar]
- Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology. 2005;65:1778–1781. doi: 10.1212/01.wnl.0000187119.33075.41. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38:715–724. [PubMed] [Google Scholar]
- Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38:794–796. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, O'Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993A;43:1508–1512. doi: 10.1212/wnl.43.8.1508. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Winkelmann RK, Bolton CF. Quantitation of Meissner’s corpuscles in hereditary neurologic disorders Charcot-Marie-Tooth Disease, Roussy-Levy syndrome, Dejerine-sottas, hereditary sensory neuropathy, spinocerebellar degenerations, and hereditary spastic paraplegia. Neurology. 1966;16:10–17. doi: 10.1212/wnl.16.1.10. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Zimmerman I, Gillen DA, Johnson D, Karnes JL, L’Brien PC. Cool, warm, and heat-pain detection thresholds: testing methods and inferences about anatomic distribution of receptors. Neurology. 1993B;43:1500–1508. doi: 10.1212/wnl.43.8.1500. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I CHARTER Study Group. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann DN, Boger JN, Jansen C, Alessi-Fox C. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology. 2007;69:2121–2127. doi: 10.1212/01.wnl.0000282762.34274.94. [DOI] [PubMed] [Google Scholar]
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- Herrmann DN, McDermott MP, Henderson D, Chen L, Akowuah K, Schifitto G North East AIDS Dementia (NEAD) Consortium. Epidermal nerve fiber density, axonal swellings and QST as predictors of HIV distal sensory neuropathy. Muscle Nerve. 2004;29:420–427. doi: 10.1002/mus.10567. [DOI] [PubMed] [Google Scholar]
- Herrmann DN, McDermott MP, Sowden JE, Henderson D, Messing S, Cruttenden K, Schifitto G. Is skin biopsy a predictor of transition to symptomatic HIV neuropathy? A longitudinal study. Neurology. 2006;66:857–861. doi: 10.1212/01.wnl.0000203126.01416.77. [DOI] [PubMed] [Google Scholar]
- Holland NR, Stocks A, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Intraepidermal nerve fiber density in patients with painful sensory neuropathy. Neurology. 1997;48:708–711. doi: 10.1212/wnl.48.3.708. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, Marra C, Rubin M, Cohen BA, Tucker T, Navia BA, Schifitto G, Katzenstein D, Rask C, Zaborski L, Smith ME, Shriver S, Millar L, Clifford DB, Karalnik IJ. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. Neurology. 2000;54:1080–1088. doi: 10.1212/wnl.54.5.1080. [DOI] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Yiannoutsos CT, Cohen BA, Hollander H, Schifitto G, Clifford DB, Simpson DM, Katzenstein D, Shriver S, Hauer P, Brown A, Haidich AB, Moo L, McArthur JC. Reduced intraepidermal nerve fiber density in HIV-associated sensory neuropathy. Neurology. 2002;58:115–119. doi: 10.1212/wnl.58.1.115. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha M, Gonzalez S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- Selkin B, Rajadhyaksha M, Gonzalez S, Langley RG. In vivo confocal microscopy in dermatology. Dermatol Clin. 2001;19:369–377. doi: 10.1016/s0733-8635(05)70274-6. [DOI] [PubMed] [Google Scholar]
- Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, Epstein L, Kieburtz K Dana Consortium of the Therapy of HIV Dementia and Related Cognitive Disorders. Incidence of and risk factors for HIV-associated distal sensory polyneuropathy. Neurology. 2002;58:1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- Shy ME, Frohman EM, So YT, Arezzo JC, Cornblath DR, Giulianai MJ, Kincaid JC, Ochoa JL, Parry GJ, Weimer LH Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2003;60:898–904. doi: 10.1212/01.wnl.0000058546.16985.11. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, So Y, Marra CM, Diaz-Arrastia R, Shriver S, Millar L Clifford DB and the ACTG A5117 Study Group. HIV neuropathy natural history cohort study: Assessment measures and risk Factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- So YT, Holtzman DM, Abrams DI, Olney RK. Peripheral neuropathy associated with Acquired Immunodeficiency Syndrome. Prevalence and clinical features from a population-based survey. Arch Neurol. 1988;45:945–948. doi: 10.1001/archneur.1988.00520330023005. [DOI] [PubMed] [Google Scholar]
- Tagliati M, Grinnell J, Godbold J, Simpson DM. Peripheral nerve function in HIV infection: clinical, electrophysiologic and laboratory findings. Arch Neurol. 1999;56:84–89. doi: 10.1001/archneur.56.1.84. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Wesselingh SL, Griffin JW, McArthur JC, Griffin DE. Unifying hypothesis for the pathogenesis of HIV-associated dementia complex, vacuolar myelopathy, and sensory neuropathy. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:379–388. [PubMed] [Google Scholar]
- Zhou L, Kitch DW, Evans SR, Hauer P, Raman S, Ebenezer GJ, Gerschenson M, Marra CM, Valcour V, Diaz-Arrastia R, Goodkin K, Millar L, Shriver S, Asmuth DM, Clifford DB, Simpson DM, McArthur JC NARC and ACTG A5117 Study Group. Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology. 2007;68:2113–2119. doi: 10.1212/01.wnl.0000264888.87918.a1. [DOI] [PubMed] [Google Scholar]