Abstract
Objective
Previous studies have demonstrated the ability of non-lethal doses of radiation to alter the phenotype of tumor cells to facilitate immune mediated killing. This pilot study evaluated the tolerability of a vector-based vaccine targeting CEA in combination with radiation therapy in patients with gastrointestinal malignancies metastatic to the liver.
Methods
Patients enrolled had progressive CEA+ tumors with metastatic liver lesions. Patients received a median of 3 previous chemotherapy regimens, with a median of 2 months since their last chemotherapy regimen. Only 58% had metastatic disease limited to the liver. Vaccination commenced day 1 with biweekly boosters and split course radiation (total 32 Gy) starting on day 21. Blood was collected at baseline and day 91 for immunologic analysis.
Results/conclusion
Twelve patients were enrolled in this trial. There were no grade ≥ 3 toxicities or grade ≥ 2 hepatic toxicities. Median time on-study was 3 months, with longest time on treatment being 5 months (n = 2). Immunologic analysis was limited to 2 patients; neither showed an increase above baseline in CEA-specific T cells post-therapy. CEA/TRICOM vaccination in combination with low-dose radiation therapy is safe. However, there is limited evidence of activity in this advanced patient population.
Keywords: CEA/TRICOM, fowlpox, immunotherapy, poxviral vector, therapeutic vaccine, vaccinia
1. Introduction
Carcinoembryonic antigen (CEA) is a 180-kDa immunoglobulin-like molecule that is expressed on the cell surface and primarily functions in cellular adhesion [1]. CEA is also commonly overexpressed on adenocarcinomas arising from the breast, cervix, lung, and gastrointestinal tract [2, 3]. Increased expression of CEA may increase cellular adhesion, which has been implicated in the proliferation and metastasis of malignant cells. In colon cancer cell lines, the increased adhesion from increased CEA expression distorts normal cellular architecture, decreasing cellular differentiation and inhibition of cellular proliferation [4]. The increase in cellular adhesion may also allow for cells that have broken off from the main tumor to establish metastatic sites of disease [5].
Increased expression of CEA in malignant cells makes it an attractive focus for targeted therapies, including immunotherapy. Therapeutic cancer vaccine strategies can enhance immune recognition of CEA as part of immune cell-mediated destruction of tumor cells. Several vaccine strategies have been developed targeting CEA including DNA electroporation, peptide, adenovirus, and Saccharomyces cerevisiae (yeast)-based approaches [6–9]. An additional vaccine platform used in this study is CEA/TRICOM, which employs recombinant poxvirus vectors [10]. Poxviruses are a safe and effective delivery vehicle for vaccines. The large poxviral genome allows for the incorporation of multiple transgenes into the vector, including the transgenes for CEA and 3 costimulatory molecules (B7.1, ICAM-1 and LFA-3, designated TRICOM) that enhance T-cell activation [11–14]. When injected subcutaneously (s.c.), the immunogenic viruses attract immune cells to the injection site [15, 16]. The viruses can infect a wide variety of cells, including dendritic cells. The tumor-associated antigen (TAA) transgene is expressed, processed and presented to T-cells via the MHC molecule. The T-cell costimulatory molecule transgenes lead to upregulation of these proteins on the cell surface and enhanced T-cell activation to the TAA [11]. Since poxviruses do not enter the cell nucleus or integrate into human DNA, there is no risk of induced genetic alterations in the host [11]. The existence of multiple species of poxviruses is a further advantage of this approach. The vaccine schema consists of a vaccinia-based vaccine as an initial priming dose, and subsequent boosting with fowlpox results in enhanced immune activation [17, 18].
Previous clinical trials utilizing a poxviral vaccine targeting CEA in this manner have demonstrated minimal toxicity and enhanced immune response [18–20]. An initial phase I trial with a recombinant poxviral-based vaccine targeting CEA demonstrated that 7 of 9 evaluable patients had enhanced CEA-specific T-cell responses with no significant attributable toxicity. This study also suggested that vaccinia priming followed by avipox boosters led to enhanced clinical benefit relative to avipox followed by vaccinia [18]. A subsequent trial was conducted using vaccinia priming CEA/TRICOM and fowlpox booster CEA/TRICOM. Of the 58 patients enrolled, 40 had stable disease for 4 months and 14 had stable disease beyond 6 months. CEA stabilization or decline was seen in 11 patients, and the majority of evaluable patients had enhanced CEA-specific T-cell responses [19].
Additional strategies can augment immune recognition of CEA-expressing tumor cells. Low, non-cytolytic doses of radiation are sufficient to up-regulate major histocompatibility complex (MHC) determinants, costimulatory/adhesion molecules, and Fas ligand on tumor cells [21, 22]. These phenotypic alterations enhance T-cell recognition and eradication of CEA-expressing tumor cells in vitro [23]. Preclinical models have demonstrated that when vaccine and low-dose radiation are combined, they more effectively decrease tumor growth than either modality alone [24]. A previous clinical trial utilizing a poxviral-based vaccine targeting prostate-specific antigen (PSA) demonstrated that poxviral-based vaccine in combination with radiation generated an antigen-specific immune response [25, 26].
The role of radiation therapy in the treatment of liver metastasis has evolved significantly. Initially, its use was limited by the technical challenge of delivering tumoricidal doses of radiation therapy without causing radiation-induced liver disease. Advances in our understanding of liver tolerance and treatment delivery and planning, including stereotactic body radiation therapy and respiratory gating, have made it possible to deliver tumoricidal doses to liver metastases with encouraging control rates [27, 28]. However, while eligibility criteria for stereotactic radiation therapy trials vary, they often limit enrollment to patients with lower numbers and smaller sizes of metastatic lesions to allow appropriate sparing of normal liver from high doses of radiation. Using relatively low-dose radiation therapy for the immune-enhancing effects described above, and not primarily for its tumoricidal effects, could potentially skirt the problem of normal liver tissue tolerance.
Despite the increasing use of screening colonoscopies, almost 50,000 deaths were attributed to colon and rectal cancer in 2008 [29]. Although the treatment of metastatic disease has advanced significantly in recent years, additional strategies are required to reduce mortality from advanced colorectal cancer. Patients with late-stage colon cancer and other CEA-expressing tumors may develop multiple metastatic lesions in the liver that cannot be surgically removed. Given the potential synergy between radiation and immunotherapy, we initiated a pilot study at the National Cancer Institute (NCI) to investigate the tolerability of combined vaccine and low-dose radiation to liver metastases in patients with CEA-expressing tumors.
2. Patients and methods
2.1 Eligibility
All patients enrolled had CEA+ tumors with radiographically detectable metastatic lesions in the liver. CEA expression was determined by immunohistochemical staining (≥ 20% of cells), or elevated serum CEA of > 5 ng/mL at any point during the course of disease. Patients were required to have a > 6-month life expectancy, ECOG performance status of 0 to 1, previous treatment with at least one chemotherapy regimen for metastatic disease, and normal hepatic, renal, and hematologic functions. Exclusion criteria included a history of autoimmune disease, HIV or hepatitis B/C infection, any disease requiring systemic steroid therapy, allergy to eggs, and known brain metastasis. Patients who had prior whole liver radiation or radiation to > 50% of total body nodal groups were also excluded. All patients reviewed and signed an informed consent approved by the Institutional Review Board of the NCI.
2.2 Study design and treatment
The primary objective of this pilot single-cohort study was to evaluate the clinical safety of the combination of CEA/TRICOM vaccine and radiation in patients with CEA+ solid tumors metastatic to the liver. Secondary endpoints included evaluation of immunologic response and phenotypic changes in the tumor after radiation, compared to a baseline sample. Patients received treatment at the NCI’s Warren G. Magnuson Clinical Center in Bethesda, Maryland.
Patients were monitored monthly by physical examination and laboratory tests of serum chemistries, hematologic parameters, serum CEA, and serum vascular endothelial growth factor (VEGF). Computed tomography (CT) scans of the chest/abdomen/pelvis to assess the size and extent of tumors were performed at baseline and 3 months, and approximately every 2 months thereafter.
2.3 Vaccine
CEA/TRICOM vaccine consists of a recombinant vaccinia (rV)-CEA/TRICOM primary vaccination and recombinant fowlpox (rF)-CEA/TRICOM boosts. The vaccinia is derived from the New York City Board of Health vaccine and is replication-competent while the fowlpox is replication-defective in mammalians. The vaccine contains the CEA transgene, which also contains an agonist epitope to enhance immunogenicity [30], and transgenes for 3 human T-cell costimulatory molecules (B7-1, ICAM-1, and LFA-3). Vaccines were supplied by the Cancer Therapy Evaluation Program of the NCI.
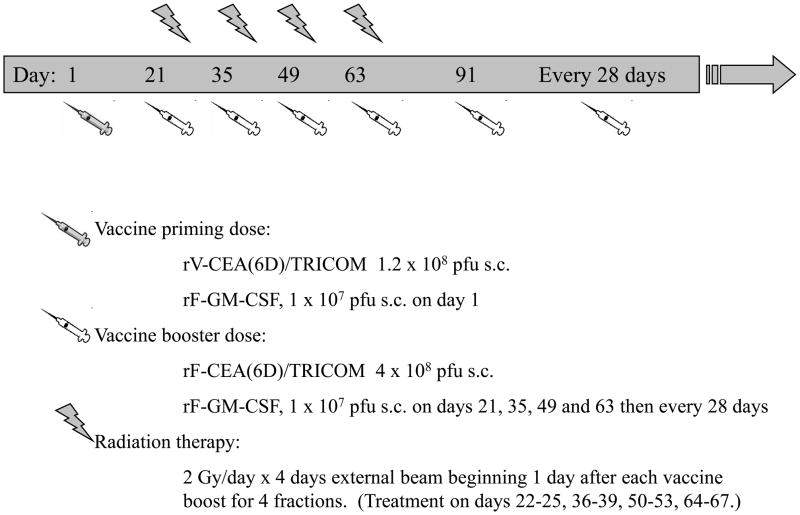
All patients were given 1.2 × 108 pfu rV-CEA/TRICOM s.c. on day 1 as the primary vaccination, followed by 4 boosts with 4 × 108 pfu rF-CEA/TRICOM s.c. on days 21, 35, 49, and 63. All vaccines were administered with 1 × 107 pfu rF-GM-CSF s.c. as an immune adjuvant. After day 63, patients with either stable disease or objective response could continue on trial and receive additional boosts at the same dosage at one-month intervals, with no further radiation therapy (Figure 1).
Figure 1.
Trial design.
2.4 Radiation therapy
CT simulation was performed for 3-dimensional conformal radiotherapy treatment planning. Contrasted abdominal CT or MRI examinations were fused for target delineation. Treatment volumes were based on liver tumor size prevaccination. A margin of 2 cm in the inferior and superior dimension and 1.5 cm in the anterior, posterior, and lateral dimensions was added to define the planning target volume. Modifications to the planning target volume were allowed to account for extension outside of the liver. Appropriate beam angles were chosen to allow coverage of the planning target volume within the 90% isodose line, while minimizing dose to normal structures and remaining liver. Where there were multiple liver metastases, up to 3 metastatic lesions were targeted if an acceptable plan resulted in < 50% of the liver volume receiving 32 Gy.
Patients received a total radiation dose of 32 Gy to sites of metastatic disease in the liver, delivered in 8-Gy courses (4 separate 2-Gy fractions) beginning one day after each vaccine boost (days 22–25, 36–39, 50–53, 64–67). Treatment was delivered with energies of 6 MV and higher. Portal films were obtained on the first day of every 8-Gy course to verify proper positioning.
2.5 Immunologic monitoring
Immunologic response was evaluated by comparing peripheral blood mononuclear cells (PBMCs) obtained via leukapheresis at baseline and around day 91. IFN-γ production was measured by ELISPOT assay as previously described [31] to determine CTL precursor frequency to CEA and MUC-1 peptide in both pre- and post-treatment PBMCs.
2.5.1 Flow cytometry analysis
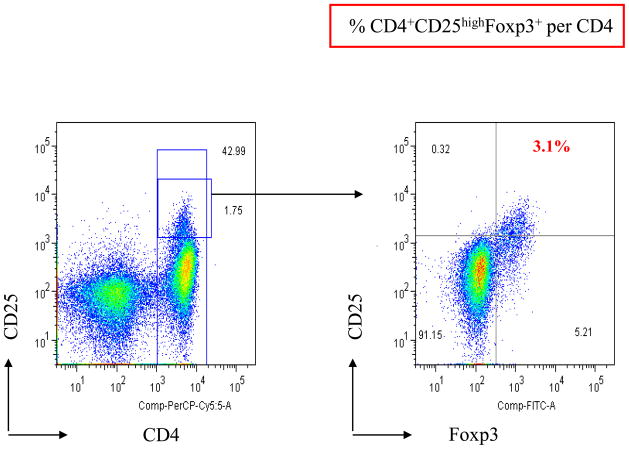
Phenotypic characterization of regulatory T-cells (Tregs) was performed by 3-color flow cytometry analysis of PBMCs, as previously described [26]. Cells were resuspended in staining buffer (PBS containing 3% fetal bovine serum) and stained for 30 min at 4°C with PerCP-Cy5.5–conjugated anti-CD4 and phycoerythrin-conjugated anti-CD25 (both from BD Biosciences; Franklin Lakes, NJ). FoxP3 intracellular staining was done on the cells stained with anti-CD4 and anti-CD25. Cells were fixed and permeabilized using a fix/perm kit (eBioscience; San Diego, CA) according to the manufacturer’s instructions and labeled with FITC-conjugated anti-FoxP3 antibody (PCH101 clone) or its isotype control antibody (eBioscience) as a negative control. Flow cytometry was done on a Becton Dickinson LSRII (BD Biosciences); 1 × 105 cells were acquired, and data were analyzed using FlowJo software (BD Biosciences). To determine the percentage of Tregs, lymphocytes were gated by plotting forward versus side scatter, followed by gating of the CD4+ population. Then the CD25high and FoxP3+ populations were gated. The CD25high population was separated from the CD25low population on the basis of the level of CD25 expression in CD4−T cells, as previously described [32] (Figure 2).
FIGURE 2.
Representative dot plot of the expression of FoxP3 in CD4+ CD25high Tregs. PBMCs from a patient were analyzed by flow cytometry after cell surface staining with PerCP Cy5.5-conjugated anti-CD4, phycoerythrin-conjugated anti-CD25, and intracellular staining with FITC-conjugated anti-FoxP3. CD4+CD25+ T cells were gated as CD25high and CD25low relative to the level of CD25 expression in CD4−T cells. Expression of Foxp3 in CD4+ CD25high Tregs was analyzed.
2.5.2 Measurement of CD4 antigen-specific responses
The CD4 assay used in this study was previously described by Kobayashi et al. [33]. CD4+ T cells (2 × 105/well) were mixed with irradiated antigen-presenting cells (APCs) in the presence of various concentrations of CEA protein (AspenBio Pharma; Castle Rock, CO) in 48-well culture plates. Flu protein and myoglobulin (Sigma-Aldrich; St. Louis, MO) were used as controls. Autologous dendritic cells were used as APCs (2 × 104/well). Culture supernatants were collected after 48 h for measurement of IFN-γ with ELISA kits.
3. Results
Of the 12 patients enrolled on this trial (Table 1), 11 had colon cancer and one had rectal cancer. The 6 men and 6 women had a median age of 58 years (range: 27 to 69). The patients were a median of 2 months removed from their last chemotherapy regimen (range: 1 to 8 months) and had a median of 3 previous chemotherapeutic regimens.
Table 1.
Overview of patients enrolled on-study.
| Patient # | Gender | Age | ECOG PS | Primary tumor | Time since last chemo (mos) | No. of prior chemo regimens |
|---|---|---|---|---|---|---|
| 1 | male | 53 | 1 | colon | Unknown | 3 |
| 2 | male | 58 | 0 | colon | 2 | 2 |
| 3 | female | 58 | 1 | colon | 4 | 3 |
| 4 | female | 59 | 1 | colon | 3 | 2 |
| 5 | male | 63 | 0 | colon | 1 | 3 |
| 6 | female | 27 | 1 | colon | 2 | 3 |
| 7 | male | 54 | 1 | colon | 2 | 4 |
| 8 | female | 52 | 1 | colon | 2 | 3 |
| 9 | male | 68 | 1 | colon | 1 | 3 |
| 10 | male | 59 | 0 | colon | 2 | 3 |
| 11 | female | 55 | 1 | rectal | 4 | 2 |
| 12 | female | 69 | 0 | colon | 8 | 3 |
ECOG PS = Eastern Cooperative Oncology Group performance status.
The 12 patients enrolled also had a significant amount of tumor burden as demonstrated by the target lesions followed for standard Response Evaluation Criteria in Solid Tumors (RECIST) assessment (Table 2). These lesions do not represent the entirety of the tumor volume and most patients had additional lesions that were not followed as target lesions, per RECIST guidelines [34]. Most of the target lesions were in the liver; however, 5 patients had radiographic evidence of metastatic lesions outside of the liver. Eleven of the 12 patients had at least one target lesion of 3.0 cm or greater at baseline. Four patients had baseline target lesions of ≥ 4.5 cm, including 2 patients who had hepatic lesions of ≥ 8.0 cm.
Table 2.
Target lesions for patients enrolled demonstrate large tumor burden.
| Patient # | Lesion number (location) | Baseline size by RECIST (cm) |
|---|---|---|
| 1 | 1 (liver) | 3.8 |
| 2 (liver) | 3.1 | |
| 2 | 1 (liver) | 3.7 |
| 2 (liver) | 1.6 | |
| 3 (liver) | 1.6 | |
| 3 | 1 (liver) | 1.5 |
| 2 (liver) | 1.0 | |
| 4 | 1 (liver) | 8.0 |
| 2 (liver) | 2.7 | |
| 5 | 1 (liver) | 8.5 |
| 2 (liver) | 2.2 | |
| 6 | 1 (liver) | 2.2 |
| 2 (lung) | 6.2 | |
| 7 | 1 (liver) | 3.5 |
| 2 (liver) | 2.2 | |
| 8 | 1 (liver) | 2.5 |
| 2 (liver) | 2.3 | |
| 3 (lymph node) | 3.3 | |
| 9* | 1 (rectum) | 4.5 |
| 2 (adrenal gland) | 2.2 | |
| 10 | 1 (liver) | 3.3 |
| 2 (liver) | 2.5 | |
| 3 (liver) | 2.0 | |
| 4 (lymph node) | 1.7 | |
| 11 | 1 (liver) | 3.6 |
| 2 (liver) | 2.3 | |
| 3 (liver) | 2.2 | |
| 4 (lung) | 1.4 | |
| 12 | 1 (liver) | 3.0 |
| 2 (liver) | 1.6 |
Patient had metastatic liver lesions not followed as target lesions because they were not > 1 cm.
The treatment was well tolerated, with the most common toxicity being a transient dermatologic reaction at the vaccine injection site (≤ grade 2) seen in 6 of the 12 patients enrolled. One patient had a self-limiting grade 2 constitutional/flu-like reaction attributed to the vaccine. Another patient had grade 2 leukopenia possibly related to the radiation component of the therapy. There were no ≥ grade 3 toxicities attributable to the treatment and no ≥ grade 2 hepatic toxicities.
The median time on-study was 3 months, with 7 patients staying on trial for ≥ 3 months. Two patients (#1 and #12) had stable disease for 5 months, which was the greatest duration of treatment on trial. Neither of these patients had extrahepatic sites of disease. Only one patient had a decline in serum CEA, but this did not correlate with response. Given the small sample size, it is unclear if the presence of lesions beyond the radiation field affected responses or outcomes.
PBMCs could only be collected for analysis from 4 patients after treatment; therefore, the planned immunologic analysis could not be adequately carried out in this study. Similarly, planned optional biopsies to evaluate for local immune response in the tumor microenvironment and phenotypic changes in tumor post-radiation (pre-treatment vs. post-treatment) were only obtained from 3 patients pre-treatment and only from one of those post-treatment. This post-study biopsy had insufficient tumor cells for analysis. The 2 patients that were HLA-A2 positive from whom pre- and post-treatment PBMCs were collected for evaluation by ELISPOT showed no increase in CEA-specific T cells post-vaccination. Of the 4 patients evaluated for changes in the ratio of effector T cells:Tregs, 2 had decreases in the ratio and 2 had increases (Table 3). One of the patients (#1) who remained on-study the longest (5 months) had a decline in Tregs at 3 months. Patient #1 also had a nearly 50% reduction in serum VEGF levels, which may have prognostic value for some patients with colon cancer[35, 36]. Tregs in the second patient on-study for 5 months (#12) were not evaluated because peripheral blood was not able to be collected after treatment.
Table 3.
Changes in Tregs after treatment with a combination of radiotherapy and vaccine.
| Patient # | Day of sample | Tregs as % of circulating CD4+ T cells | Effector T cell:Treg ratio |
|---|---|---|---|
| 1 | 0 | 3.1 | 20.1:1 |
| 96 | 2.4 | 24.3:1 | |
| 2 | 0 | 5.9 | 6.2:1 |
| 93 | 2.8 | 14.4:1 | |
| 5 | 0 | 2.47 | 18.8:1 |
| 48 | 2.75 | 15.1:1 | |
| 8 | 0 | 2.17 | 27.1:1 |
| 48 | 3.0 | 18.6:1 |
4. Discussion
A previous phase I clinical trial using CEA/TRICOM showed promising results in patients with CEA+ tumors, the majority (60%) of which were colon cancer. In fact, 40% of the 58 patients treated had stable disease at 4 months, and 14 patients had stable disease for > 6 months. In addition, 11 of 16 evaluable patients had a ≥ 3-fold increase in CEA-specific T cells after treatment with 4 doses of vaccine [19]. More recently, a trial was conducted in patients with colon cancer who had metastectomy and were thus without detectable disease. Patients were given a poxviral vaccine that targeted MUC-1 in addition to CEA (with TRICOM). The vaccine was administered s.c. or by ex vivo priming of a patient’s own dendritic cells. Regardless of the in vivo or ex vivo approach, enhanced antigen-specific T-cell responses were seen and the patients had enhanced survival compared with historical controls [37, 38].
There are several possible explanations for why similar results were not seen in this study. Although no radiation was administered in the previous phase I trial, preclinical data indicate that it is unlikely the radiation used in the present trial prevented clinical response. Differences in patient population may be a more likely explanation. Patients in the trial reported here were heavily pretreated. Most had received ≥ 3 prior chemotherapy regimens and most had completed their last regimen within 2 months of enrollment. The number and timing of previous therapies may have affected patients’ ability to mount an immune response to vaccine. An analysis of patients treated in a previous trial with a similar poxviral-CEA vaccine demonstrated a negative association between the number of previous chemotherapy regimens and the magnitude of CEA-specific T-cell response (p = 0.032), as well as a positive association between the magnitude of T-cell response and time since last chemotherapy administration (p = 0.005) [39].
The tumor burden of patients enrolled in this study may also have reduced the likelihood of response to vaccine therapy. Studies in both animal and human models have demonstrated that Treg numbers increase in proportion to the volume of disease [40, 41]. Tregs can limit the effect of cancer vaccines by decreasing T-cell activation and expansion [42, 43]. In addition, bulky tumors have been shown to produce TGF-β, IL-10, and indoleamine-2,3-dioxygenase, which can also inhibit T-cell activation [44, 45]. The association between greater clinical benefit and smaller disease volume has been seen in 2 poxviral vaccine studies targeting PSA in prostate cancer, where patients with smaller disease volume appeared to have an overall survival advantage after treatment with vaccine [46, 47]. A report of clinical outcomes following therapeutic vaccines and relation to tumor volume has recently been reviewed [48]. Therefore, the requirement that eligible patients have unresectable hepatic lesions large enough to radiate may have decreased these patients’ likelihood of responding to vaccine treatment. It is possible that this approach of vaccine with radiation therapy could still be successful in adjuvant settings, where patients have minimal previous chemotherapy and where tumor burden would also likely be minimal or undetectable.
A planned endpoint of this trial was to compare pre- and post-treatment biopsies of liver lesions to determine if radiation therapy induced phenotypic changes in tumor, such as increased expression of tumor-associated antigens or MHC, as seen in preclinical models. It was anticipated that these samples would yield valuable immunologic data on T-cell trafficking to the tumor after vaccine, as well as changes in and effects of Tregs in the tumor microenvironment. However, procurement of post-treatment tissue samples became problematic, as many patients elected not to undergo the voluntary procedure, and others were too ill at progression to tolerate it. In the end, investigators felt it was unethical to mandate post-treatment liver biopsy, even though such tissue procurement may have proven vital to understanding immune response to vaccines. Improved strategies are needed to explore this important endpoint.
An evaluation of radiographic imaging after treatment was also met with limitations. In patients who received the complete course of radiation, radiographic images at one month post-treatment typically showed a hypodensity in the irradiated liver tissue. Most tumors showed an enlargement at one month post-treatment, with only one patient exhibiting increased enhancement. Although it is likely that these results are consistent with progression, it is difficult to determine if the enlargement signified progression, localized edema, or cystic degeneration, given the small number of patients available for analysis. Newly developed response criteria have been proposed for trials involving immunotherapy and are being implemented in newer clinical investigations. These criteria take into account delayed responses and minimal initial progression, both of which are characteristic of previous successful immunotherapy trials [49]. Such criteria may be of great value in future trials involving immunotherapy and radiation.
The design and outcome of this study were not optimal for assessing the ability of radiation to enhance the clinical benefit of vaccine treatment strategies. As mentioned previously, patients with lower tumor volume and earlier-stage disease may be better candidates for this type of study. In a recent study, a cohort of newly diagnosed prostate cancer patients were all treated with standard definitive radiation therapy, while some also received concurrent poxviral-based vaccine targeting PSA. Of 19 evaluable patients, 17 had increases in PSA-specific T cells, while patients treated with radiation alone showed no increase in PSA-specific T cells [25]. Other methods of delivering radiation are also being investigated. Samarium-153 is a radioactive agent that binds to areas of osteoblastic metastatic activity in the bone, providing palliation and ultimately delivering low levels of radiation to metastatic sites of disease [50, 51]. A trial at the NCI is currently investigating the clinical benefits of combining a second generation pox-viral vaccine, PSA-TRICOM, with Samarium-153 to determine if this combination may also enhance immune response and clinical benefit [52].
5. Conclusions
Although it may be challenging to determine the appropriate clinical setting for vaccine therapy combined with low-dose radiotherapy, an emerging body of both preclinical and clinical data supports further investigation. This is illustrated by the comparison of both clinical and immunologic responses as mentioned above in patients with less advanced disease receiving the exact same vaccine [19]. Targeted radiotherapeutics and more appropriate patient selection (lower tumor burden or more indolent disease, lower number of prior chemotherapies) may lead to more successful investigations of the potentially dynamic therapeutic combination of radiation and vaccine.
Acknowledgments
This study was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland. The authors gratefully acknowledge the participation of patients and their families in this study, the assistance of the research fellows of the Medical Oncology Branch, NCI, and Bonnie L. Casey for editorial assistance in the preparation of this manuscript.
Footnotes
Declaration of interest
The authors have no conflict of interest.
Bibliography
- 1.Benchimol S, Fuks A, Jothy S, et al. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327–34. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 2.Robbins PF, Eggensperger D, Qi CF, Schlom J. Definition of the expression of the human carcinoembryonic antigen and non-specific cross-reacting antigen in human breast and lung carcinomas. Int J Cancer. 1993;53:892–7. doi: 10.1002/ijc.2910530604. [DOI] [PubMed] [Google Scholar]
- 3.Tendler A, Kaufman HL, Kadish AS. Increased carcinoembryonic antigen expression in cervical intraepithelial neoplasia grade 3 and in cervical squamous cell carcinoma. Hum Pathol. 2000;31:1357–62. [PubMed] [Google Scholar]
- 4.Ilantzis C, DeMarte L, Screaton RA, Stanners CP. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia. 2002;4:151–63. doi: 10.1038/sj.neo.7900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hostetter RB, Campbell DE, Chi KF, et al. Carcinoembryonic antigen enhances metastatic potential of human colorectal carcinoma. Arch Surg. 1990;125:300–4. doi: 10.1001/archsurg.1990.01410150022004. [DOI] [PubMed] [Google Scholar]
- 6.Mori F, Giannetti P, Peruzzi D, et al. A therapeutic cancer vaccine targeting carcinoembryonic antigen in intestinal carcinomas. Hum Gene Ther. 2009;20:125–36. doi: 10.1089/hum.2008.116. [DOI] [PubMed] [Google Scholar]
- 7.Cho HI, Kim HJ, Oh ST, Kim TG. In vitro induction of carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses. Vaccine. 2003;22:224–36. doi: 10.1016/s0264-410x(03)00569-3. [DOI] [PubMed] [Google Scholar]
- 8.Babatz J, Rollig C, Lobel B, et al. Induction of cellular immune responses against carcinoembryonic antigen in patients with metastatic tumors after vaccination with altered peptide ligand-loaded dendritic cells. Cancer Immunol Immunother. 2006;55:268–76. doi: 10.1007/s00262-005-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madan RA, Bilusic M, Hodge JW, et al. A phase I trial of a yeast-based therapeutic cancer vaccine targeting CEA [abstract]. ASCO Annual Meeting; 2011. p. 2604. [Google Scholar]
- 10.Greiner JW, Zeytin H, Anver MR, Schlom J. Vaccine-based therapy directed against carcinoembryonic antigen demonstrates antitumor activity on spontaneous intestinal tumors in the absence of autoimmunity. Cancer Res. 2002;62:6944–51. [PubMed] [Google Scholar]
- 11.Essajee S, Kaufman HL. Poxvirus vaccines for cancer and HIV therapy. Expert Opin Biol Ther. 2004;4:575–88. doi: 10.1517/14712598.4.4.575. [DOI] [PubMed] [Google Scholar]
- 12.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62:5770–7. [PubMed] [Google Scholar]
- 13.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61:4497–505. [PubMed] [Google Scholar]
- 14.Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–7. [PubMed] [Google Scholar]
- 15.Kantor J, Abrams S, Irvine K, et al. Specific immunotherapy using a recombinant vaccinia virus expressing human carcinoembryonic antigen. Ann N Y Acad Sci. 1993;690:370–3. doi: 10.1111/j.1749-6632.1993.tb44034.x. [DOI] [PubMed] [Google Scholar]
- 16.Kass E, Schlom J, Thompson J, et al. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999;59:676–83. [PubMed] [Google Scholar]
- 17.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15:759–68. doi: 10.1016/s0264-410x(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 18*.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–73. doi: 10.1200/JCO.2000.18.23.3964. Study showing clinical utility for a diversified prime-and-boost strategy (priming with vaccinia-CEA and boosting with fowlpox-CEA) [DOI] [PubMed] [Google Scholar]
- 19**.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–31. doi: 10.1200/JCO.2005.10.206. Clinical trial showing safety and preliminary evidence of activity (immunologic and clinical) of CEA-TRICOM vaccine. Included a patient with a pathologic CR. [DOI] [PubMed] [Google Scholar]
- 20.Tsang KY, Zaremba S, Nieroda CA, et al. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–90. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 21.Hodge JW, Guha C, Neefjes J, Gulley JL. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology (Williston Park) 2008;22:1064–70. discussion 75, 80–1, 84. [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara TA, Hodge JW, Gulley JL. Combining radiation and immunotherapy for synergistic antitumor therapy. Curr Opin Mol Ther. 2009;11:37–42. [PMC free article] [PubMed] [Google Scholar]
- 23.Garnett CT, Palena C, Chakraborty M, et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 24**.Chakraborty M, Abrams SI, Coleman CN, et al. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. Preclinical study showing that radiation therapy can make it easier for the immune system to recognize and kill tumor cells. [DOI] [PubMed] [Google Scholar]
- 25*.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. Clinical trial showing the ability to mount immune responses despite external beam radiation therapy. [DOI] [PubMed] [Google Scholar]
- 26.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14:5284–91. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–8. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 28.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279–83. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 30.Zaremba S, Barzaga E, Zhu M, et al. Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. Cancer Res. 1997;57:4570–7. [PubMed] [Google Scholar]
- 31.Gulley JL, Arlen PM, Tsang KY, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–9. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokokawa J, Cereda V, Remondo C, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi H, Omiya R, Ruiz M, et al. Identification of an antigenic epitope for helper T lymphocytes from carcinoembryonic antigen. Clin Cancer Res. 2002;8:3219–25. [PubMed] [Google Scholar]
- 34.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama Y, Sako T, Shibao K, et al. Prognostic value of plasma vascular endothelial growth factor in patients with colorectal cancer. Anticancer Res. 2002;22:2437–42. [PubMed] [Google Scholar]
- 36.Werther K, Christensen IJ, Nielsen HJ. Prognostic impact of matched preoperative plasma and serum VEGF in patients with primary colorectal carcinoma. Br J Cancer. 2002;86:417–23. doi: 10.1038/sj.bjc.6600075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morse M, Niedzwiecki D, Marshall JL, et al. Survival rates among patients vaccinated following resection of colorectal cancer metastases in a phase II randomized study compared with contemporary controls [abstract]. ASCO Annual Meeting; 2011. p. 3557. [Google Scholar]
- 38.Lyerly H, Hobeika A, Niedzwiecki D, et al. A dendritic cell-based vaccine effects on T-cell responses compared with a viral vector vaccine when administered to patients following resection of colorectal metastases in a randomized phase II study [sbstract]. ASCO Annual Meeting; 2011. p. 2533. [Google Scholar]
- 39**.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–91. Clinical trial suggesting that more recent prior chemotherapy and more cycles of prior chemotherapy may blunt an immune response to a therapeutic vaccine. [PubMed] [Google Scholar]
- 40.Fu T, Shen Y, Fujimoto S. Tumor-specific CD4(+) suppressor T-cell clone capable of inhibiting rejection of syngeneic sarcoma in A/J mice. Int J Cancer. 2000;87:680–7. [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 42.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 43.Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 44.Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res. 2007;13:6247–51. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- 45.Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug Targets. 2007;7:31–40. doi: 10.2174/156800907780006896. [DOI] [PubMed] [Google Scholar]
- 46.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–31. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–74. doi: 10.1007/s00262-009-0782-8. Clinical trial suggesting improved clinical outcomes compared with expected, with greatest improvement over predicted in patients with predicted survival (based largely on tumor volume) greater than median. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Gulley J, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol. 2011;18:e150–57. doi: 10.3747/co.v18i3.783. Review of clinical outcomes following therapeutic cancer vaccines in relation to tumor volume from available clinical studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–97. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goeckeler WF, Edwards B, Volkert WA, et al. Skeletal localization of samarium-153 chelates: potential therapeutic bone agents. J Nucl Med. 1987;28:495–504. [PubMed] [Google Scholar]
- 51.Anderson P. Samarium for osteoblastic bone metastases and osteosarcoma. Expert Opin Pharmacother. 2006;7:1475–86. doi: 10.1517/14656566.7.11.1475. [DOI] [PubMed] [Google Scholar]
- 52.153Sm-EDTMP With or Without a PSA/TRICOM Vaccine To Treat Men With Androgen-Insensitive Prostate Cancer. 2011 Jul; Available from: http://clinicaltrials.gov/ct2/show/NCT00450619?term=NCT00450619&rank=1.