Abstract
Objective
One aim of this study was to investigate the effects of acupuncture on cerebral function of patients with acute cerebral infarction. Another goal was to evaluate the relationship between acupuncture treatment and motor recovery patients with stroke and to provide a foundation for using acupuncture therapy for such patients.
Design
Twenty (20) patients with recent cerebral infarction were divided randomly to an acupuncture group and a control group. The infarction area in each patient was in the basal ganglia or included the basal ganglia with an area size of > 1 cm2. Serial diffusion tensor imaging (DTI), fluid-attenuated inversion recovery (FLAIR), and T2-weighted imaging (T2WI) scans were performed on all patients and the results were evaluated using the National Institute of Health Stroke Scale and the Barthel Index each week. DTI images were postprocessed and analyzed. Apparent diffusion coefficient (ADC) and fractional anisotropy (FA) values of abnormal signals on DTI in the infarction areas and cerebral peduncles were calculated for both groups and compared with one another.
Results
(1) The ADC value of infarction lesions decreased at stroke onset; then, a significant elevation was observed after the acute stage, and a significant reduction in FA values was observed from stroke onset to the chronic stage. (2) The ADC of the bilateral cerebral peduncle was reduced on the infarction side. (3) There was a significant difference in ADC and FA values between the acupuncture and control groups. The FA value was higher in the acupuncture group than the control group.
Conclusions
ADC and FA values might correlate to patient recovery and reveal the progress of secondary degeneration. Acupuncture treatment is effective for protecting neurons and facilitating recovery.
Introduction
Acupuncture is an ancient therapeutic modality originating in ancient China; this modality has been used extensively in Oriental medicine. However, acupuncture was not widely introduced as an alterative medicine in the West. In recent years, acupuncture has gained increasing popularity in modern health care and has gotten increasing support among scientific investigators.1,2 Although the exact mechanism of acupuncture is still unknown and in need of further investigation, various animal-based data and clinical observations suggest that acupuncture modulates activities in the central nervous system (CNS), and influences designated treatment areas.3–5 In humans with acute stroke, abnormalities in water diffusion visualized with magnetic resonance imaging (MRI) may be reversible if blood flow is restored. Longitudinal alterations in poststroke brain tissue may be ideally assessed with serial in vivo MRI scans. Diffusion tensor imaging (DTI), a noninvasive MRI technique, measures the random motion of water molecules and provides information about cellular integrity and pathology.6 The current study used DTI to investigate the effect of acupuncture on the CNS by measuring changes in apparent diffusion coefficient (ADC) and fractional anisotropy (FA) values.
Materials and Methods
Patients
Twenty (20) right-handed patients (16 males, 4 females; ages 40–78, with a mean age of 55), with a recent cerebral infarction were randomly divided to either an acupuncture group or a control group. Both groups received the same Western therapies, including thrombolysis, antiplatelet aggregation, and microcirculation-improving therapies. The acupuncture group received acupuncture treatment in addition to the Western therapies, whereas the control group did not receive acupuncture treatment. There was no significant difference in age distribution between the two groups (Table 1). All patients had first-onset stroke and manifested motor deficits, with an infarction area > 1 cm2. The infarction areas were all in the basal ganglia, and completely or partially covered the internal capsules. Patients were usually admitted within 3 days after showing symptoms. Onset for the acupuncture group was from 0.5 hours to 36 hours, with an average of 10.70±11.13 hours. Onset for the control group was from 1 hour to 28 hours, with an average of 10.05±8.93 hours. The difference between the two groups was not statistically significant (p=0.91). Patients underwent MRI scans and were clinically assessed each week for 8 weeks. All subjects were informed about all aspects of the study and provided approval in the form of a written consent before the MRI examinations.
Table 1.
Age Distributions of the Groups
| Group | Age (years) | Mean age | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acupuncture | 52 | 59 | 58 | 65 | 78 | 69 | 40 | 48 | 40 | 41 | 55.00±13.14 | 0.898 |
| Control | 56 | 72 | 57 | 61 | 79 | 65 | 35 | 42 | 53 | 38 | 55.80±14.38 | |
Therapy
The acupuncture group received Xingnao Kaiqiao acupuncture treatment from the same acupuncturist for three continuous sessions. Hwato brand acupuncture needles were used (Acupuncture Suzhou Ltd., Jiangsu, China). Each patient was given acupuncture treatments for 2 weeks. The patients received 30 minutes of acupuncture daily, at 3 pm, at the following points: Shangxin (Du 23); Baihui (Du 20); Yintang (EX-HN3); Neiguan (PC 6); and Sanyingjiao (Sp 6). The needle was rotated manually clockwise at a rate of ∼ 180 times per minute.
The control group did not receive any acupuncture treatment. There was no difference between other medical treatments given between the 2 groups.
MRI protocol and data acquisition
For data acquisition, a 1.5T whole-body MRI system (Philips Intera Achieva, The Netherlands) equipped with an 8-element receive head coil array was used. All patients underwent weekly brain MRI scans including serial DTI, fluid-attenuated inversion recovery (FLAIR), and T2-weighted imaging (T2WI) scans for 8 weeks, and therapeutic effect was evaluated with National Institute of Health Stroke Scale (NIHSS) and the Barthel Index (BI).
First, multiecho, multislice T2WI MRI (repetition time [TR]/echo time [TE]=3000 ms/100 ms; acquisition matrix=128×128; voxel resolution=0.25×0.25×1.0 mm3) and FLAIR (TR/ TE=3000 ms/120 ms; acquisition matrix=128×128; voxel resolution=0.25×0.25×1.0 mm3) were performed to measure each edematous ischemic lesion. Then, for DTI, an echo-planar imaging (EPI) sequence were used, with a matrix size of 256×256, field of view 224×224 mm, slice thickness 2 mm without interslice gap, and voxel size 3×3×3 mm. Diffusion sensitizing gradients (b=800 s/mm2 were applied along six directions, and one image without diffusion weighting (b=0 s/mm2) was obtained. DTI images were obtained at an acquisition time of 5 minutes and 45 seconds. The characteristic changes of ADC and FA values in the area of infarction and the cerebral peduncle were analyzed and compared.
Data analysis
Imaging data were transferred to the Philips Medical Systems Extended MR Workspace (Version 7.1.5.1) and processed by the application software (Version 2.6.3.2) provided by the manufacturer. The Philips diffusion affine registration tool was used to remove shear and eddy current distortion and head motion prior to calculating FA maps with the Philips fiber-tracking software. Regions of interest (ROIs) were drawn manually, using the protocols described below, then the automated Philips 3-dimensional fiber-tracking tool used to determine fiber tracks passing through ROIs. ROIs for each area were drawn on the axial slices in which the respective structures appeared clearly and were most delineated. Philips software generated an automatic mean FA and ADC for each of the ROIs as described by Levin et al.7 Maps at the center of each lesion were used to determine ROIs to measure the ADC value (30 mm2<area<40 mm2). Elliptical ROIs of the same size were placed on infarction areas and cerebral peduncles. FA was expressed as the fraction of orientational coherence in an intravoxel level (ranging from 0 to 1.0), and mean diffusion was expressed as a ratio of area to time.
Subjective parameters were evaluated by self-assessment on a three-point scale; patients were routinely asked if paresis or hypesthesia were better, equal, or aggravated at each weekly scan for 8 weeks. Data were expressed as mean±standard deviation.
An independent samples t-test was used to assess the significance of the difference between the two groups. A p-value < 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS 13.0 statistical software (SPSS Inc., Chicago, IL).
Results
Objective outcome
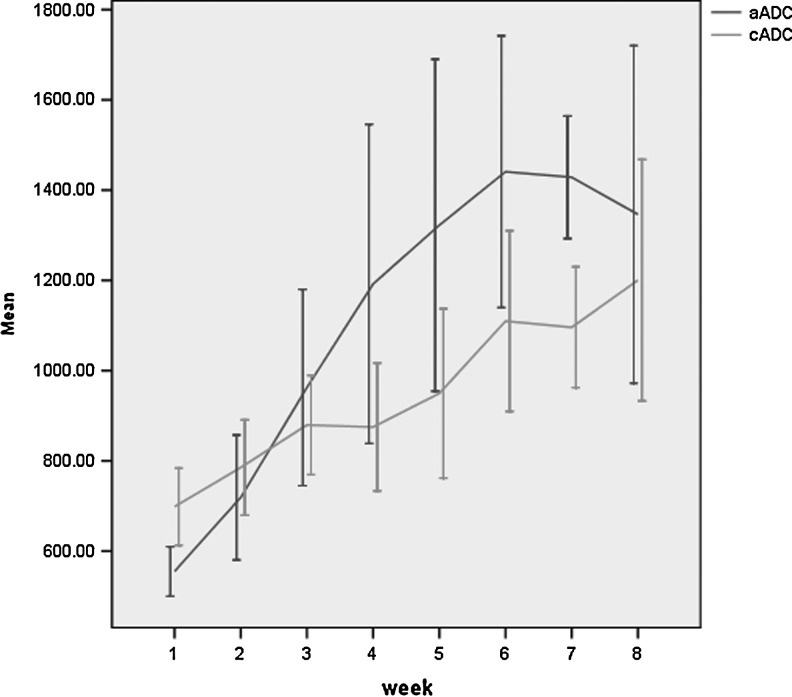
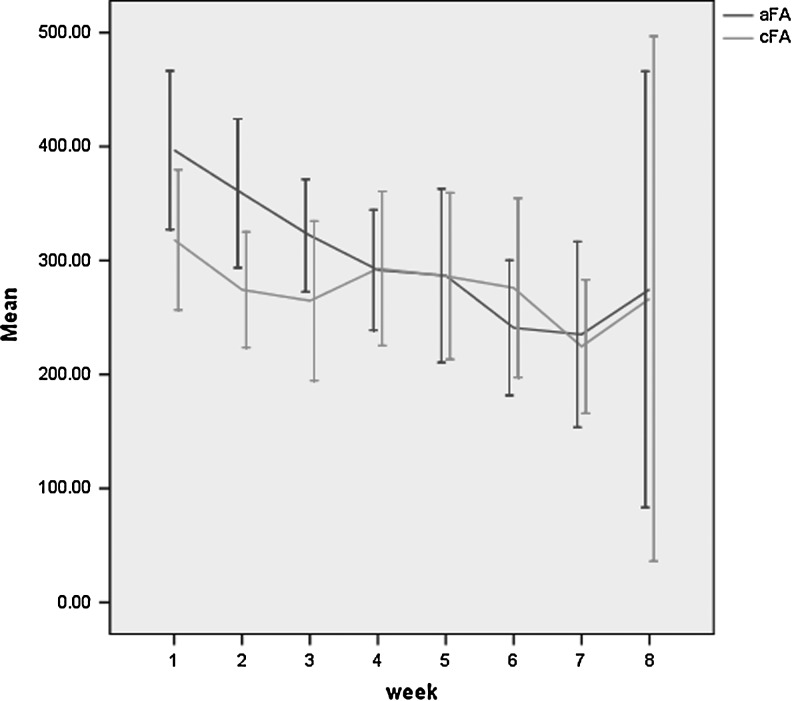
All 20 patients were included in the final analysis. ADC and FA maps presented a gradient enlargement of the ischemic region. ADC and FA values changed according to ischemia time (Figs. 1 and 2).
FIG. 1.
Change of apparent diffusion coefficient (ADC) values for acupuncture group and control groups (unit: 10–3mm2/s). The dark line represents the acupuncture group and the light-colored line represents the control group (p=0.001). aADC, acupuncture group ADC; cADC, control group ADC; CI, confidence interval.
FIG. 2.
Change of fractional anisotropy (FA) values for the acupuncture group (0.308±0.012) and the control group (0.278±0.011). The dark line represents the acupuncture group and the light-colored line represents the control group (p=0.049). aFA, acupuncture group FA; cFA, control group FA; CI, confidence interval.
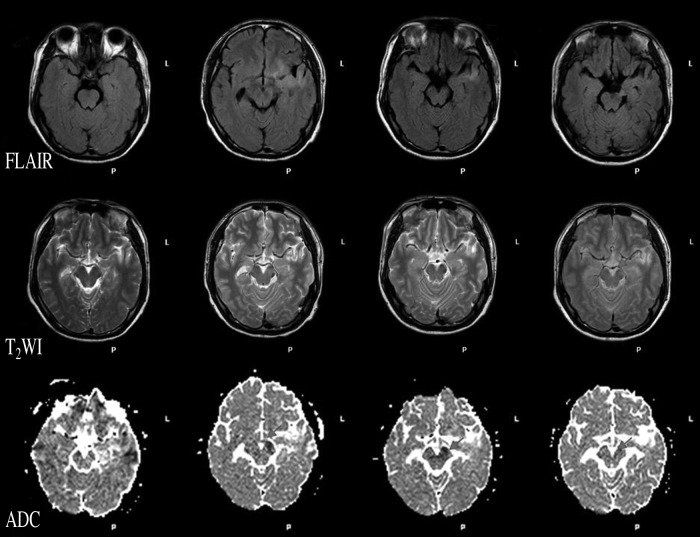
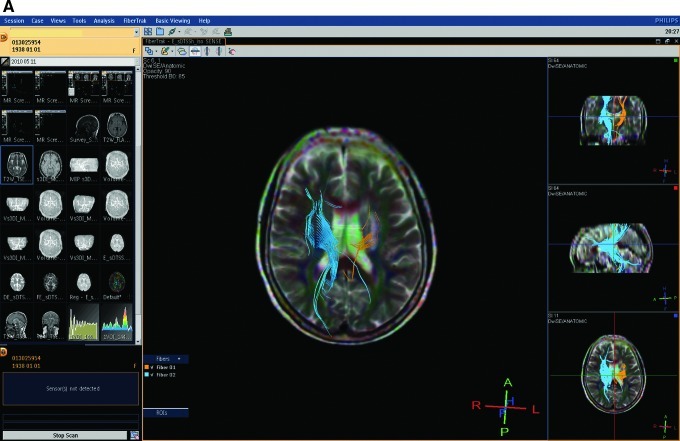
No obvious signal alterations were observed in T2WI or FLAIR in the first week. Corresponding structural changes were clearly visible on the coregistered high-resolution ADC image in the late chronic stage, during a hypointensity resulting from degeneration of descending tracts in the mediolateral cerebral peduncle was easily identifiable; at the same time, the T2WI and FLAIR images did not reveal obvious changes (Fig. 3). DTI showed obviously less fiber in the lesion side than the normal side (Fig. 4).
FIG. 3.
Signal alterations of cerebral peduncle in different serial scan images during 8 weeks. No obvious signal alterations were observed in T2-weighted imaging (T2WI) or fluid-attenuated inversion recovery (FLAIR) during the first 4 weeks. A small level of hypointensity in the cerebral peduncle was found on the ADC map in the first week, and an outstanding hypointensity was found during the second week to the eighth week.
FIG. 4.
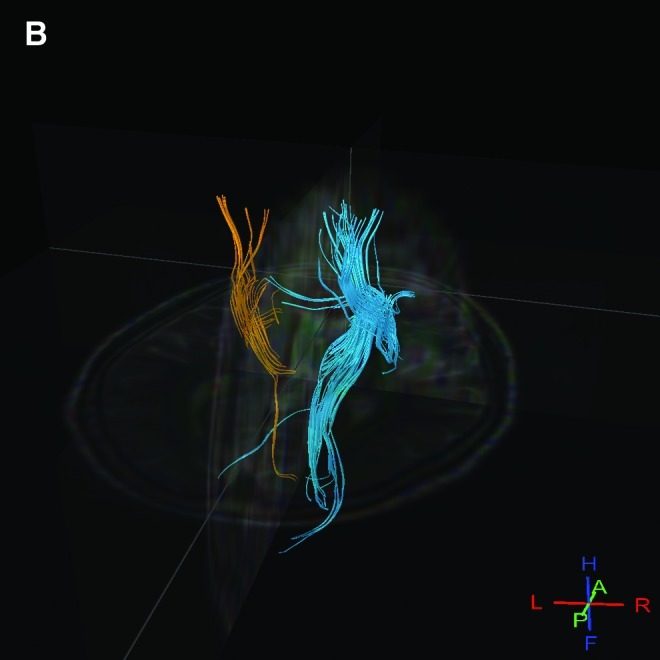
Results for a patient in the acupuncture group. (A) The number of fibers in the infarction area of a patient decreased significantly. There is less fiber in the lesion than in the normal side. Yellow represents the infarction side; blue represents the normal side. A and B images show the nerve fibers of the same patient, and, in B, the rest of brain tissue was subtracted to highlight the nerve fibers. ROIs, regions of interest.
The ADC value and FA value comparison between the two groups at different time points are shown in Table 2. At the eighth week, the ADC value was higher in the acupuncture group than in the control group (p=0.001), and the FA value was higher in the acupuncture group than in the control group (p=0.049).
Table 2.
Comparison of ADC and FA Values Between Control Group and Acupuncture Group
| |
ADC |
FA |
||||
|---|---|---|---|---|---|---|
| Week | Acupuncture | Control | p-Value | Acupuncture | Control | p-Value |
| 1 | 0.555±0.076 | 0.698±0.119 | 0.021 | 0.397±0.097 | 0.318±0.086 | 0.045 |
| 1–2 | 0.637±0.166 | 0.742±0.138 | 0.045 | 0.378±0.094 | 0.296±0.080 | 0.003 |
| 1–3 | 0.745±0.267 | 0.788±0.156 | 0.383 | 0.359±0.089 | 0.286±0.086 | 0.001 |
| 1–4 | 0.857±0.384 | 0.810±0.169 | 0.419 | 0.342±0.090 | 0.287±0.087 | 0.003 |
| 1–5 | 0.950±0.448 | 0.838±0.196 | 0.056 | 0.331±0.095 | 0.287±0.089 | 0.011 |
| 1–6 | 1.018±0.467 | 0.875±0.221 | 0.010 | 0.319±0.097 | 0.286±0.089 | 0.037 |
| 1–7 | 1.062±0.461 | 0.899±0.224 | 0.001 | 0.310±0.099 | 0.279±0.088 | 0.038 |
| 1–8 | 1.079±0.455 | 0.916±0.232 | 0.001 | 0.308±0.099 | 0.278±0.091 | 0.049 |
ADC, apparent diffusion coefficient; FA, fractional anisotropy.
Subjective outcome
Eighty percent (80%) of the acupuncture-treated patients (n=8) reported an improvement after 3 courses of treatments. Twenty percent (20%) reported no change (n=2). In the control group, 30% of the patients (n=3) reported a reduction of their symptoms, and 70% reported no change (n=7; p<0.05). The 2 groups of patients did not show significant pretreatment differences in neurologic function as assessed by NIHSS and BI (p>0.05). After treatment, the BI difference between the two groups was statistically significant (p<0.05; Table 3), whereas the NIHSS difference was not statistically significant.
Table 3.
Outcome Assessed by Physician
| |
Before treatment (mean) |
After treatment (mean) |
||
|---|---|---|---|---|
| Group & p-value | NIHSS | BI | NIHSS | BI |
| Acupuncture | 7.20±3.97 | 47.50±18.89 | 0.80±1.40 | 95.50±11.17 |
| Control | 7.80±3.23 | 47.50±18.14 | 2.40±2.01 | 72.50±13.79 |
| p | 0.7 | 1.0 | 0.054 | 0.001 |
NIHSS, National Institute of Health Stroke Scale; BI, Barthel Index.
Discussion
Instead of using objective measurements, most, if not all, previous studies relied on subjective scales to assess the symptomatic state of patients to evaluate the effects of acupuncture in stroke. Lee et al. used single-photon emission computed tomography (SPECT) and T2WI to investigate the cerebrovascular response to acupuncture.8 However, SPECT is an invasive method. Functional magnetic resonance imaging (fMRI) has also been used to evaluate the effects of acupuncture on stroke.9–11 However, this method is affected by patients' thinking or motion, and the image data analysis is inconsistent. First, the study designs varied considerably; second, fMRI is blood oxygenation level–dependent, and blood flow can be increased by the task as well as by thinking or moving. The current study showed that acupuncture treatment of stroke led to a significant improvement of FA, compared with a control group condition of no acupuncture.
There was a significant difference in ADC and FA values between the acupuncture group and the control group after 8 weeks. According to the modern understanding of acupuncture mechanisms, acupuncture may elicit vegetative reflexes, thereby changing the flow of blood and enhancing functional properties of connective tissue and organs.12,13 DTI has been used to study the white matter architecture and integrity of normal and diseased brains (multiple sclerosis, stroke, aging, dementia, schizophrenia, etc).6 The FA index is the most widely used parameter of DTI for representing the motional anisotropy of water molecules, being sensitive to the presence and integrity of white-matter fibers. DTI describes the degree of FA of the tissue, which is influenced by a number of factors, including the degree of myelination, density, diameter, distribution, and orientational coherence of the axons, as well as the diffusion barriers presented by the glia.6,14 Therefore, FA provides an indirect marker of the microstructural properties of the white matter. ADC values change dynamically following brain injury. The current study's results showed that ADC values initially decrease and then begin to increase within days, return transiently to normal (pseudonormalization), and ultimately remain higher than normal in the injured area. Inside the permanent chronic lesion T2 and ADC increased significantly, indicating loss of tissue structures. A larger area of T2 prolongation was evident subacutely after stroke, most likely resulting from extensive vasogeinc edema formation. The current study showed that acupuncture treatment of stroke results in a significant improvement of FA, compared with no acupuncture.
Wallerian degeneration (WD)14–18 of descending fiber tracts after ischemic stroke is a well-known phenomenon reflecting severe fiber-tract damage. WD represents a uniform answer to injury within the central and peripheral nervous systems, and disintegration of axonal structures within the first days after injury is followed by infiltration of macrophages and degradation of myelin after several weeks, and finally followed by fibrosis and atrophy of the affected fiber tracts.17,18 After ischemic stroke, it usually takes 2–4 weeks before WD can be detected by conventional MRI, for which the main pathologic finding is a hyperintensity on T2WI along the affected tracts in the chronic stage, weeks to months after stroke. DTI was used to assess Wallerian degeneration of the pyramidal tract within 8 weeks after ischemic stroke, and correlated the extent of Wallerian degeneration with the motor deficit. The current study showed that diffusion imaging may detect Wallerian degeneration earlier than T2WI.
In its early stage, WD is characterized histopathologically by axonal swelling without myelin loss.15 Disintegration of axonal structures and myelin, as occurs in WD, results in loss of anisotropy, which can be detected by DTI. The current study longitudinally assessed the course of WD in all patients by DTI and found a pattern of progressive structural degeneration, which corresponds well to findings from experimental studies of WD.
The findings on imaging reflect the different stages of WD that are well known from experimental and histologic studies.19 Thus, DTI offers a way to detect and monitor the time course of severe degeneration of the pyramidal tract and may be a helpful tool for forecasting and monitoring recovery in patients with ischemic stroke.
Replacement of the intact anisotropic microstructure by disorganized glial proliferation may also underlie the marked reduction in FA. In a longstanding cerebral infarct, it is thought that cell lysis and loss of normal tissue architecture expand the extracellular space allowing water molecules to diffuse more freely.20 These changes would account for the increased diffusivity and reduced anisotropy that were demonstrated in regions of infarction. The difference in diffusion properties between the primary lesion and the degenerated tract may allow DTI to distinguish between the primary lesion and associated WD. In an experimental model of cerebral ischemia in rats, degeneration of descending axons was detected in the brainstem in histologic stains as early as 2–7 days after the injury.21
Conclusions
This study demonstrated the same results as reported by Yu et al.20 that longitudinal multiparametric MRI can provide unique in vivo information on structural-tissue changes related to damage and recovery after stroke. The dynamic evolution of WD was observed in vivo, using DTI; This may promote understanding of the effects of acupuncture treatment in stroke. The current study results provide significant evidence demonstrating the efficacy of acupuncture treatment for structural reorganization in and beyond ischemic lesions, which may contribute to functional recovery after stroke. The results might contribute to the identification of optimal strategies for stroke treatment and rehabilitation at an early poststroke stage.
Limitations
There were limitations to this study. Only 6 orientations were used to estimate the diffusion tensor, more orientations will be used in a future study. Because of the small sample size, the power of statistical analysis was restricted. These findings must be interpreted carefully and require detailed histologic validation in future studies. Furthermore, a selected group of patients were examined. Signs of early WD were found in all of these patients, and this may be explained by the inclusion criteria: only patients with a marked motor deficit on admission to the hospital and with a lesion visible on MRI that affected the pyramidal tract were included.
Acknowledgments
This work was supported by the grants from the Department of Science and Technology of Shenzhen City, China (No: 200902163).
Disclosure Statement
No competing financial interests exist.
References
- 1.Mutasem AH. Zi XH. Clinical comparison of acupuncture treatment on acute and subacute phase of cerebral infarction. J Clin Res. 2007;34:443–446. [Google Scholar]
- 2.Yang ZX. Shi XM. Systematic evaluation of the therapeutic effect and safety of Xingnao Kaiqiao needling method in treatment of stroke. Chinese Acupunct. 2007;27:601–607. [PubMed] [Google Scholar]
- 3.Adibhatla RM. Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 4.Sozmen EG. Kolekar A. Havton LA, et al. A white matter stroke model in the mouse: Axonal damage progenitor responses and MRI correlates. J Neurosci Methods. 2009;180:261–272. doi: 10.1016/j.jneumeth.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder S. Liepert J. Remppis A, et al. Acupuncture treatment improves nerve conduction in peripheral neuropathy. Eur J Neurol. 2007;14:276–281. doi: 10.1111/j.1468-1331.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 6.Assaf Y. Pasternak O. Diffusion tensor imaging (DTI)–based white matter mapping in brain research: A review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 7.Levin H. Wilde E. Chu Z, et al. Diffusion tensor imaging in relation to cognitive, functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 2008;23:197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JD. Chon JS. Jeong HK, et al. The cerebrovascular response to traditional acupuncture after stroke. Neuroradiology. 2003;45:780–784. doi: 10.1007/s00234-003-1080-3. [DOI] [PubMed] [Google Scholar]
- 9.He Y. Wang L. Huang L, et al. Effects of acupuncture on the cortical functional areas activated by index finger motion in the patient with ischemic stroke. Chinese Acupunct Moxibustion. 2006;26:357–361. [PubMed] [Google Scholar]
- 10.Schocket T. Schnitker R. Boroojerdi B, et al. Cortical activation by Yamamoto New Scalp Acupuncture in the treatment of patients with a stroke: A sham-controlled study using functional MRI. Acupunct Med. 2010;28:212–214. doi: 10.1136/aim.2010.002683. [DOI] [PubMed] [Google Scholar]
- 11.Li G. Jack C. Yang E. An fMRI Study of somatosensory implicated acupuncture points in stable somatosensory stroke patients. J Magn Reson Imaging. 2006;24:1018–1024. doi: 10.1002/jmri.20702. [DOI] [PubMed] [Google Scholar]
- 12.Jiangang D. Ming L. Effect of acupuncture on content of serum myelin basic protein and remyelination of ischemic focus in rats of focal cerebral ischemia. Chinese J Rehabil Med. 2007;22:118–121. [Google Scholar]
- 13.Dhong RP. Kettner N. Napadow V. Neuroimaing acupuncture effects in the human brain. J Altern Complement Med. 2007;13:603–616. doi: 10.1089/acm.2007.7040. [DOI] [PubMed] [Google Scholar]
- 14.Thomalla G. Glauche V. Weiller C, et al. Time course of Wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2005;76:266–268. doi: 10.1136/jnnp.2004.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valencia MP. Castillo M. MRI findings in posttraumatic spinal cord Wallerian degeneration. Clin Imaging. 2006;30:431–433. doi: 10.1016/j.clinimag.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Devetten G. Coutts SB. Hill MD, et al. Acute corticospinal tract Wallerian degeneration is associated with stroke outcome. Stroke. 2010;41:751–756. doi: 10.1161/STROKEAHA.109.573287. [DOI] [PubMed] [Google Scholar]
- 17.Sylaja RN. Goyal M. Warson T, et al. Wallerian-like degeneration after ischemic stroke revealed by diffusion-weighted imaging. Can J Neural Sci. 2007;34:243–244. doi: 10.1017/s0317167100006120. [DOI] [PubMed] [Google Scholar]
- 18.Gupta RK. Saksena S. Hasan KM, et al. Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: Serial diffusion tensor imaging. J Magn Reson Imaging. 2006;24:549–555. doi: 10.1002/jmri.20677. [DOI] [PubMed] [Google Scholar]
- 19.Qian J. Herrera JJ. Narayana PA. Neuronal and axonal degeneration in experimental spinal cord injury: In vivo proton magnetic resonance spectroscopy and histology. J Neurotrauma. 2010;27:599–610. doi: 10.1089/neu.2009.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu C. Zhu C. Zhang Y. Chen H. Qin W. Wang M. Li K. A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage. 2009;47:451–458. doi: 10.1016/j.neuroimage.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 21.Durukan A. Tatlisumak T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]