Abstract
Purpose
The aim of this study was to determine the effects of Tai Chi exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy.
Methods
A pretest–posttest design with a nonequivalent control group was utilized to recruit 59 diabetic patients with neuropathy from an outpatient clinic of a university hospital. A standardized Tai Chi for diabetes program was provided, which comprised 1 hour of Tai Chi per session, twice a week for 12 weeks. Outcome variables were fasting blood glucose and glycosylated hemoglobin for glucose control, the Semmes-Weinstein 10-g monofilament examination scores and total symptom scores for neuropathy, single leg stance for balance, and the Korean version of the SF-36v2 for quality of life. Thirty-nine patients completed the posttest measures after the 12-week Tai Chi intervention, giving a 34% dropout rate.
Results
The mean age of the participants was 64 years, and they had been diagnosed with type 2 diabetes for more than 12 years. The status was significantly better for the participants in the Tai Chi group (n=20) than for their control (i.e., nonintervention) counterparts (n=19) in terms of total symptom scores, glucose control, balance, and quality of life.
Conclusion
Tai Chi improved glucose control, balance, neuropathic symptoms, and some dimensions of quality of life in diabetic patients with neuropathy. Further studies with larger samples and long-term follow-up are needed to confirm the effects of Tai Chi on the management of diabetic neuropathy, which may have an impact on fall prevention in this population.
Introduction
Diabetic peripheral neuropathy (DPN) is one of the most common long-term microvascular complications of diabetes, leading to considerable patient morbidity and mortality.1 Diabetes negatively affects nerve conduction in both the central and peripheral nervous systems, leading to postural instability by changing peripheral nerve function.2 Other consequences of DPN include reduced balance, strength, and gait parameters that are likely intermediaries that put individuals with DPN at a high risk of falls3; patients with DPN exhibit increased amplitude of sway and sway area due to an inability to generate proper neuromuscular responses. The reduced balance may also result in decreased physical, emotional, and social function, ultimately negatively affecting quality of life.4,5
Exercise is considered to be one of the three major interventions for diabetes, along with medication and diet.6 The positive effects of exercise intervention on glucose control, lipid metabolism, and cardiovascular risk factors for diabetic patients are well supported in the literature.7,8 However, little is known about the effects of exercise on the alterations in peripheral sensation and balance related to neuropathy in diabetic patients.2 A prospective randomized intervention study9 followed 78 diabetes patients with no neuropathy for 4 years and found that the exercise group—who performed a prescribed and supervised brisk walking exercise four times per week—developed significantly less motor and sensory neuropathy than did their sedentary controls. The potential effects of long-term aerobic exercise training may prevent the onset or modify the natural history of DPN.9 Diabetic patients, however, tend to participate in exercise less than their nondiabetic counterparts.10,11 There is a need to provide active, yet safe exercise interventions for individuals with DPN along with motivation strategies so that they can exercise for longer periods.10
Tai Chi (TC) has been introduced to health professionals as a moderate-intensity aerobic exercise.12 Previous studies have provided support for the positive effects of a TC for diabetes (TCD) program, which is easy to follow and safe to perform for diabetic patients who have physical limitations.8 A TCD program developed by Lam13 is based on a standardized form of 21 movements from the combined Yang and Sun style of Tai Chi. This program has been shown to improve glucose control that might result in positive changes in microcirculation to the lower extremities.8 Previous study with long-term Yang style Tai Chi practitioners also reported higher cutaneous microcirculatory function than did their sedentary counterparts, revealing the possibility of improving or attenuating decline of cutaneous microcirculation in older adults or those with diabetes.14
However, one systematic review found conflicting results on the effect of TC on glucose control.15 This inconsistent finding may be explained by the lack of standardization of the TC exercise prescribed, in terms of exercise intensity, type, and duration,15 which in some cases may not have been sufficient to induce the positive changes in glucose control. Moreover, only a few studies have focused on TC exercise in diabetic patients with neuropathy. The hypothesis for a positive effect on peripheral sensory function is based on the research of Wang and his colleagues,14 who showed that elderly patients who had been practicing TC exhibited a higher volume of blood circulation in the skin as assessed via peripheral vasodilation.
The present study was designed to test the effect of exercise by conducting a trial implementing 12 weeks of standardized TCD, and by using both subjective and objective instruments to detect changes in the signs and symptoms of neuropathy in diabetic patients who already had neuropathy. The purpose of this study was to determine the effects of 12 weeks of TC exercise on glucose control, neuropathy score, balance, and quality of life in diabetic patients with neuropathy that were undergoing follow-up therapy.
Methods
Study population
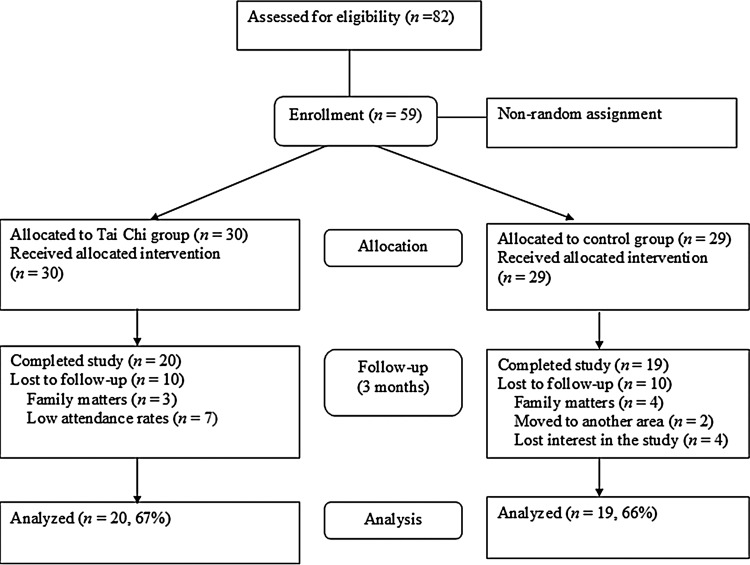
A pretest–posttest quasi-experimental design with a nonequivalent control group was used. Fifty-nine type 2 diabetic patients with neuropathy were recruited from an outpatient clinic of a university hospital in Korea with the following inclusion criteria: (1) had permission to participate in regular exercise from their primary physicians, (2) had a glycosylated hemoglobin (HbA1c) level greater than 7.0%, and (3) agreed to participate in this study (Fig. 1). A power analysis16 revealed that a sample size of 52 was required based on effect size d=0.7, α=0.05 (one-side probability), and power (1−β)=0.80. The effect size was calculated based on the study by Yeh et al.17 to examine the effect of TC exercise on changes in HbA1c with a one-group design (mean difference score 0.8, standard deviation=1.2).
FIG. 1.
Flow diagram of the study.
Intervention: the TCD program
The individuals in the TC group completed a supervised 1-hour session twice weekly for 12 weeks. The TC group performed the TCD program, consisting of 5 minutes of warm-up exercises, 5 minutes of qigong exercise, 40 minutes of TC movements, another 5 minutes of qigong exercise, and finally 5 minutes of cool-down exercise. The research team checked the participants' blood glucose levels before the exercise by a portable glucometer and prepared chairs for safety and comfort. We also carefully observed for any adverse effects during the 12-week program, but none were found.
Various motivation strategies were applied to reduce the dropout rate, such as drawing up a contract to encourage the patients to perform exercise regularly at home, providing TC shirts and TC music tapes, holding a TC contest to improve the group dynamic, and weekly follow-up calls from a research assistant to remind the participants about the home exercise and class attendance. The frequency and duration of home exercise were self-reported in the participants' exercise logs, which were assessed during every weekly session. The mean attendance rate for the study participants was 21 sessions (out of 24) during the 12-week program.
Outcome assessment
Approval for this study was obtained from an institutional review board of the university hospital. The research team recruited 59 people from a diabetes specialty outpatient clinic at a university hospital among those who had their clinician's permission to participate in a regular exercise program. The purpose, methods, and process of the research were explained to these 59 subjects, all of whom subsequently provided written consent to participate.
Assignment to either the Tai Chi program (TC) or the control group was not random. First, 30 subjects were assigned to the TC group and participated in the 12-week TCD program. The remaining 29 subjects were assigned to the control group, who received their usual care and were given the opportunity to participate in the TCD program in 3 months. Both the TC group and the control group were provided routine education on diabetes management including diet, exercise, foot care, and medication twice during the study period.
Outcome measures were conducted at baseline (before the intervention) and after the 12-week TCD program. The same research team performed the pretest and posttest measurements for both groups.
Glucose control
Glucose control was evaluated by measuring fasting blood sugar (FBS: enzymatic assay), which reflects current glucose control, and HbA1c (turbidimetric immunoassay), which reflects average glucose level during the past 3 months.
Peripheral sensory function
Peripheral sensory function (i.e., a measure of peripheral neuropathy) was evaluated using the Semmes-Weinstein monofilament examination (SWME) and total neuropathy symptom scores. A nurse educator specializing in diabetic patients conducted the monofilament pre- and posttests and was blind to the group assignment of the participants.
The SWME was conducted at 10 noncallused sites on each foot (the big toe; the plantar aspect of the first, third, and fifth metatarsal heads; and six sites on the bottom of plantar aspect) using a 5.07/10-g monofilament, based on the practical guidelines on the management and prevention of the diabetic foot.18 The following 3-point scale was used: “normal” (score of 2), when an individual feels pressure on seven or more sites; “reduced” (score of 1), for those feeling pressure on six or fewer sites; and “absent” (score of 0), for those who feel no pressure at any of the sites.
The total symptom score (TSS) for peripheral neuropathy was used to evaluate the frequency (absent, rarely, frequent, and always) and severity (mild, moderate, and severe) of DPN symptoms such as pain, burning, paresthesia, and numbness. The possible range of TSSs was 0–14.64.
Balance
Balance was assessed by timing each individual standing on one leg with their eyes closed (in seconds). The duration of the one-leg stance was measured by the same research assistant at the pre- and posttests with a stopwatch (Casio, Tokyo, Japan).
Quality of life
The participants' perceived quality of life was measured using the Korean version of the SF-36v2 (36-Item Short Form Health Survey version 2), which assesses eight dimensions of quality of life along with physical and mental component summary scores. Each dimension was scored with weighted items out of 100 points, with higher scores representing a higher perceived quality of life. The psychometrics of the original version of the SF-36 was reported to have a validity and reliability coefficient of 0.84–0.95.19 For the present study, Cronbach's alpha values for the subscale ranges were 0.75–0.85.
Statistical analysis
Data were analyzed using SPSSWIN V.17 software (SPSS, Chicago, IL). Descriptive statistics were used for demographics and outcome variables. Independent t-tests were used to assess significant group differences in baseline measures. We calculated difference scores between post- and pretest values for each group, and independent t-tests were conducted to compare group differences in different scores. A significance level (alpha) was set as 0.05.
Results
Demographic characteristics of the subjects
The mean age of the subjects was 65 years, and their duration of diabetes mellitus was 12–13 years. Most of the participants were married (84%), not currently employed (85%), and reported their socioeconomic status as moderate (76%). Most of them (62%) perceived their health as being similar to others in their age group. Some subjects followed a controlled diet (40%), participated in regular exercise (36.8%–50%), and engaged in foot care (20%–30%). No significant group differences were found in demographic and disease-related characteristics (Table 1).
Table 1.
Homogeneity Tests for General Characteristics at the Baseline (N=39)
| Tai Chi (n=20) Mean (SD) | Control (n=19) Mean (SD) | t (p) | ||
|---|---|---|---|---|
| Age (yr) | 66.05 (6.42) | 62.73 (7.53) | 1.80 (0.081) | |
| Duration of diabetes mellitus (yr) | 12.30 (8.81) | 13.00 (10.03) | 0.23 (0.812) | |
| Frequency (%) | Frequency (%) | χ2 (p) | ||
|---|---|---|---|---|
| Sex | Male | 12 (60.0) | 8 (40.0) | 1.24 (0.261) |
| Female | 8 (42.1) | 11 (57.9) | ||
| Marital status | Married | 18 (90.0) | 16 (84.2) | 0.29 (0.664)a |
| Other | 2 (10.0) | 3 (15.8) | ||
| Employment status | Employed | 4 (20.0) | 6 (31.6) | 0.68 (0.482)a |
| Not employed | 16 (80.0) | 13 (68.4) | ||
| Level of education | Middle school | 13 (65.0) | 11 (57.9) | 0.20 (0.653) |
| High school | 7 (35.0) | 8 (42.1) | ||
| Monthly incomeb | 1999 K | 17 (85.0) | 14 (73.7) | 0.76 (0.451)a |
| 2000 K | 3 (15.0) | 5 (26.3) | ||
| Present health status | Poor to very poor | 3 (15.0) | 1 (5.2) | 1.06 (0.791)a |
| Average | 12 (60.0) | 12 (63.2) | ||
| Good to very good | 5 (25.0) | 6 (31.6) | ||
| Hypertension | No | 10 (76.9) | 9 (76.0) | 0.20 (0.644) |
| Yes | 3 (23.1) | 3 (25.0) | ||
| Cardiac disease | No | 16 (80.0) | 17 (89.5) | 0.67 (0.412) |
| Yes | 4 (20.0) | 2 (10.5) | ||
| Insulin injection | No | 16 (80.0) | 15 (78.9) | 0.11 (1.00) |
| Yes | 4 (20.0) | 4 (21.1) | ||
| Hypoglycemic drugs | No | 4 (20.0) | 4 (21.1) | 0.10 (1.00) |
| Yes | 16 (80.0) | 15 (78.9) | ||
| Diet control | No | 10 (53.8) | 11 (57.9) | 0.24 (0.624) |
| Yes | 10 (46.2) | 8 (42.4) | ||
| Regular exercise | No | 10 (50.0) | 12 (63.2) | 0.68 (0.403) |
| Yes | 10 (50.0) | 7 (36.8) | ||
| Foot care | No | 14 (70.0) | 15 (78.9) | 0.41 (0.712)a |
| Yes | 6 (30.0) | 4 (21.1) |
Fisher's exact test.
K, Korean won (1000 KW=US$1).
Group comparisons for baseline measures
Group comparisons at the pretest revealed no significant differences in the outcome variables between the groups (Table 2).
Table 2.
Homogeneity Test of Study Variables at the Baseline (N=39)
| Tai Chi (n=20) Mean (SD) | Control (n=19) Mean (SD) | t (p) | ||
|---|---|---|---|---|
| Neuropathy scores | Monofilament test scores | 1.90 (0.31) | 1.63 (0.59)) | 1.75 (0.094) |
| Total symptom scores | 1.13 (1.95) | 1.19 (1.98) | −0.09 (0.925) | |
| Balance | Single leg stance | 22.37 (23.72) | 15.71 (18.99) | 0.96 (0.345) |
| Glucose control | Fasting blood glucose | 137.85 (45.19) | 143.47 (47.45) | −0.37 (0.701) |
| HbA1c | 7.63 (1.38) | 8.02 (1.65) | −0.79 (0.435) | |
| Quality of life | Physical functioning | 67.00 (16.88) | 61.05 (21.57) | 0.96 (0.343) |
| Bodily pain | 67.50 (28.50) | 71.71 (19.91) | −0.53 (0.595) | |
| Role limitation, physical | 59.37 (27.46) | 61.51 (32.89) | −0.22 (0.826) | |
| Role limitation, emotional | 57.75 (21.91) | 67.54 (35.23) | 1.03 (0.309) | |
| Social functioning | 70.00 (25.45) | 80.26 (20.11) | −1.39 (0.172) | |
| Vitality | 43.12 (15.56) | 45.06 (19.82) | −0.34 (0.735) | |
| General health | 46.00 (16.85) | 40.00 (17.11) | 1.10 (0.277) | |
| Mental health | 69.50 (17.83) | 61.31 (17.54) | 1.44 (0.157) | |
| PCSa | 44.53 (6.80) | 42.82 (7.08) | 0.76 (0.448) | |
| MCSa | 42.58 (10.40) | 43.59 (10.89) | −0.29 (0.769) |
PCS, physical component score; MCS, mental component score.
Group comparisons of outcomes
At the completion of the 12-week TCD program, FBS was significantly better controlled in the TC group than in the controls (mean value changed from 137 to 125 mg/dL versus 143 to 155 mg/dL for the controls; t=2.23, p=0.036). HbA1c was also significantly better controlled in the TC group (mean value changed from 7.63% to 7.20% versus 8.02% to 8.32% for the controls, t=3.11, p=0.004). The balance of the diabetic patients in the TC group also improved compared to the control group (Table 3).
Table 3.
Group Comparison on Neuropathy Scores, Balance, Glucose Control, and Quality of Life at the Posttest (N=39)
| |
Tai Chi (n=20) |
Control (n=19) |
|
|
||
|---|---|---|---|---|---|---|
| Post test mean (SD) | Pre–post difference mean (SD) | Post test mean (SD) | Pre–post difference mean (SD) | ta | p | |
| Glucose control | ||||||
| Fasting blood glucose | 125.50 (45.57) | 12.85 (40.66) | 155.31 (44.88) | −11.84 (26.36) | 2.23 | 0.036 |
| HbA1c | 7.20 (1.32) | 0.43 (.57) | 8.32 (1.76) | −0.30 (0.87) | 3.11 | 0.004 |
| Balance | ||||||
| Single leg stance | 30.02 (28.08) | −7.65 (16.78) | 14.27 (16.31) | 1.44 (9.97) | −2.04 | 0.044 |
| Neuropathy scores | ||||||
| 10-g monofilament test | 1.95 (0.22) | −0.05 (0.22) | 1.73 (0.56) | −0.10 (0.31) | 0.63 | 0.535 |
| Total symptom scores | 0.91 (1.87) | 0.21 (1.44) | 2.83 (3.29) | −1.64 (3.61) | 2.09 | 0.042 |
| Quality of life | ||||||
| Physical functioning | 71.75 (19.95) | 4.75 (16.58) | 55.26 (19.75) | −5.78 (11.69) | 2.28 | 0.028 |
| Bodily pain | 79.37 (19.98) | 11.87 (26.74) | 60.36 (24.49) | −11.34 (26.17) | 2.73 | 0.009 |
| Role limitation, physical | 76.62 (20.72) | 17.25 (28.64) | 57.48 (27.80) | −4.02 (14.20) | 2.96 | 0.006 |
| Role limitation, emotional | 75.25 (22.84) | 17.50 (24.78) | 60.17 (32.13) | −7.36 (20.73) | 3.38 | 0.002 |
| Social functioning | 80.89 (18.11) | 10.89 (30.29) | 61.97 (28.89) | −18.28 (18.85) | 3.58 | 0.001 |
| Vitality | 49.00 (15.12) | 5.87 (12.74) | 42.63 (18.29) | −2.43 (18.39) | 1.64 | 0.107 |
| General health | 48.65 (13.79) | 2.65 (17.46) | 38.84 (13.50) | −1.15 (12.83) | 0.77 | 0.446 |
| Mental health | 68.35 (21.72) | −1.15 (28.49) | 53.42 (19.58) | −7.89 (18.05) | 0.87 | 0.387 |
| PCS | 45.36 (8.04) | .83 (7.31) | 43.57 (7.00) | .74 (6.08) | 0.04 | 0.967 |
| MCS | 44.52 (9.19) | 1.93 (13.50) | 41.38 (12.90) | −2.20 (10.77) | 1.05 | 0.298 |
t values were calculated from the difference scores (posttest – pretest scores).
The effect of the TCD program on peripheral neuropathy scores was only partially supported. The TSS was significantly higher in the TC group (mean change from 0.86 to 0.91, versus 1.19 to 2.83 for the controls, t=2.09, p=0.042), but the SWME scores did not differ significantly between the groups.
Quality of life scores in five out of eight domains were significantly higher in the TC group than in the control group. The domains of physical functioning (t=2.28, p=0.028), bodily pain (t=2.73, p=0.009), physical role limitation (t=2.96, p=0.006), emotional role limitation (t=3.38, p=0.002), and social functioning (t=3.58, p=0.001) were improved in the TC group compared to the controls (Table 3).
Discussion
We examined the effect of a 12-week TCD program on neuropathy scores, balance, glucose control (FBS and HbA1c), and quality of life in type 2 diabetes mellitus patients with neuropathy. DPN is frequently associated with pain, infection, and sensory loss in affected patients, leading to a variety of complications including falls.20 The present study found significant improvements in the TSS for neuropathy and balance at the completion of the 12-week TCD program. In previous studies, diabetic patients who practiced TC exercise for more than 12 weeks reported higher cutaneous vascular conductance14 as well as better peripheral nerve conduction velocities of the bilateral median and tibial nerves.20 These findings suggest that sensory function and neuropathy-related symptoms can be improved by TC exercise. The previous study also showed plantar sensation improvement after 24 weeks of Tai Chi exercise among older adults with peripheral neuropathy.21 Along with the improvements in plantar sensory perception, TC training can help subjects to gain better control over their posture, leading to better balance.2 The effects of TC exercise on balance in older adults or those with diabetes are also well supported in the literature.22
However, we were unable to find any significant differences in SWME scores after the 12-week TCD program. Although the SWME is a useful measure for identifying diabetic neuropathy and is easily applicable in clinical practice, the limited range of scores from 0 to 2 probably makes it difficult to realize group differences due to the small variance.
The pathogenesis of DPN is multifactorial, and prolonged hyperglycemia is known to be the primary factor.20 We provided the 12-week TCD program along with diabetic counseling and education to diabetic patients at the outpatient clinic, and their FBS and HbA1c decreased by 12.85 mg/dL and 0.43%, respectively. The differences shown in the TC group differed significantly from those of their control. The low-to-moderate intensity of TC exercise is expected to improve glucose metabolism, resulting in lower levels of HbA1c and insulin resistance.17
Previous studies looking at the effects of TC exercise on glucose control in diabetic patients have produced conflicting results. The findings of several studies support the beneficial effects of TC exercise on glucose control,20,23 but a randomized study with the TCD program found no significant improvement on either glucose homeostasis or insulin sensitivity.22 Tsang et al.22 found a small and nonsignificant effect size (−0.07±0.4%) on HbA1c levels after a 16-week TCD program. This finding may be explained either by a lack of statistical power due to the small sample, or perhaps because the intensity of the TCD program was not sufficient to induce significant changes to HbA1c.22
The quality of life of the diabetic patients who participated in the 12-week TCD program was significantly improved, especially in the dimensions of physical functioning, bodily pain, physical and emotional role limitation, and social functioning. The severity of DPN symptoms was found to be predictive of poor health-related quality of life.1 There has been consistent evidence that supports the finding that TC exercise has a positive effect on quality of life in individuals with various health conditions.23,24
However, several limitations should be considered when interpreting these data. First, the potential confounding variables were not controlled by nonrandom assignment of the groups. We conducted homogeneity tests on demographic characteristics and baseline study variables to confirm that the distributions were similar in the groups (Appendix 1). We also used the differences between posttest and pretest scores for group comparisons to control for the differences at the pretest. Second, relatively high dropout rates (33%–34% in both the TC and control groups) may have contributed to changes in the demographic characteristics of the groups. We conducted secondary analysis to compare demographic characteristics and study variables between the participants and those who dropped out. No significant group differences were found in neuropathy scores, blood glucose levels, and balance. However, the physical functioning and bodily pain scores of quality of life were significantly higher among the dropouts than among the participants, showing that those with better quality of life in some dimensions dropped out from the study. Furthermore, younger subjects with jobs who perceived their health to be better than others and who were taking oral medication tended to drop out. Further studies with random assignment are needed to control for these potential confounding variables.
Conclusion
In conclusion, the standardized program with 21 movements combined from the Yang and Sun style of TC was applied to type 2 diabetic patients with neuropathy both safely and effectively for 12 weeks. The 12-week TCD program was effective in reducing blood glucose and HbA1c levels, neuropathy TSSs, and in improving balance and some dimensions of quality of life in these patients. The moderate-intensity, 12-week (minimum) TCD program was sufficient to exert positive effects on glucose control and quality of life in individuals with DPN. Further studies with larger samples are required to confirm the effects on neuropathy index with more objective and sensitive measures.
Appendix
Appendix 1.
Comparison of Study Variables and General Characteristics at the Baseline Between the Study Participants and the Dropouts
| Variables | Participants (n=39) Mean (SD) | Dropouts (n=20) Mean (SD) | χ2/t | p |
|---|---|---|---|---|
| Age | 64.07 (7.19) | 59.25 (8.63) | 2.27 | 0.026 |
| Employment status | ||||
| Employed | 10 (25.6) | 10 (50.0) | 3.50 | 0.084 |
| Not employed | 29 (74.4) | 10 (50.0) | ||
| Present health status | ||||
| Poor to very poor | 4 (10.3) | 0 (0.0) | 7.34 | 0.015a |
| Average | 24 (61.5) | 7 (36.8) | ||
| Good to very good | 11 (28.2) | 12 (63.2) | ||
| Hypoglycemic drugs | ||||
| None | 8 (20.5) | 1 (5.0) | 2.46 | 0.148a |
| Yes | 31 (79.5) | 19 (95.0) | ||
| Neuropathy scores | ||||
| Monofilament test scores | 1.76 (0.48) | 1.90 (0.29) | −1.22 | 0.224 |
| Total symptom scores | 1.16 (1.94) | 1.43 (1.77) | −0.52 | 0.604 |
| Balance | 19.12 (21.53) | 20.99 (25.52) | −0.287 | 0.775 |
| Glucose control | ||||
| Fasting blood glucose | 140.58 (45.78) | 133.57 (51.42) | 0.526 | 0.601 |
| HbA1c | 7.82 (1.51) | 7.26 (1.12) | 1.42 | 0.159 |
| Quality of life | ||||
| Physical functioning | 64.10 (19.29) | 77.63 (18.05) | −2.558 | 0.013 |
| Bodily pain | 69.55 (24.46) | 82.73 (20.56) | −2.10 | 0.039 |
Fisher's exact test.
Acknowledgment
The study was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (Grant No. 531-2008-1-E00092).
Disclosure Statement
No competing financial interests exist for all authors.
References
- 1.Currie CJ. Poole CD. Woehl A, et al. The health-related utility and health-related quality of life of hospital-treated subjects with type 1 or type 2 diabetes with particular reference to differing severity of peripheral neuropathy. Diabetologia. 2006;49:2272–2280. doi: 10.1007/s00125-006-0380-7. [DOI] [PubMed] [Google Scholar]
- 2.Richerson S. Rosendale K. Does Tai Chi improve plantar sensory ability? A pilot study. Diabetes Technol Ther. 2007;9:276–286. doi: 10.1089/dia.2006.0033. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz AV. Vittinghoff E. Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396. doi: 10.2337/dc07-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau CY. Qureshi AK. Scott SG. Association between glycaemic control and quality of life in diabetes mellitus. J Postgrad Med. 2004;50:189–193. discussion 194. [PubMed] [Google Scholar]
- 5.Richardson JK. Factors associated with falls in older patients with diffuse polyneuropathy. J Am Geriatr Soc. 2002;50:1767–1773. doi: 10.1046/j.1532-5415.2002.50503.x. [DOI] [PubMed] [Google Scholar]
- 6.Praet SF. van Rooij ES. Wijtvliet A, et al. Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2008;51:736–746. doi: 10.1007/s00125-008-0950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong IS. Lee HJ. Kim MH. The effect of the Taeguk Gi-Gong exercise on insulin resistance and blood glucose in patients with type II diabetes mellitus. J Korean Acad Fundam Nurs. 2007;14:44–52. [Google Scholar]
- 8.Song R. Lee EO. Bae SC, et al. Effects of Tai Chi self-help program on glucose control, cardiovascular risks, and quality of life in type II diabetic patients. J Musc Joint Health. 2007;14:13–25. [Google Scholar]
- 9.Balducci S. Iacobellis G. Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20:216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Kavookjian J. Elswick BM. Whetsel T. Interventions for being active among individuals with diabetes: a systematic review of the literature. Diabetes Educ. 2007;33:962–988. doi: 10.1177/0145721707308411. discussion 989–990. [DOI] [PubMed] [Google Scholar]
- 11.Sohn TS. Lee JM. Chang SA, et al. The appropriate distance and duration of walking for exercise in patients with type 2 diabetes mellitus. J Korean Diabetes Assoc. 2007;31:157–162. [Google Scholar]
- 12.Wang C. Collet JP. Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med. 2004;164:493–501. doi: 10.1001/archinte.164.5.493. [DOI] [PubMed] [Google Scholar]
- 13.Lam P Tai chi. Diabetes Self Manag. 2004;21:7–10. 12, 14. [PubMed] [Google Scholar]
- 14.Wang JS. Lan C. Wong MK. Tai Chi Chuan training to enhance microcirculatory function in healthy elderly men. Arch Phys Med Rehab. 2001;82:1176–1180. doi: 10.1053/apmr.2001.24305. [DOI] [PubMed] [Google Scholar]
- 15.Lee MS. Pittler MH. Kim MS, et al. Tai chi for type 2 diabetes: a systematic review. Diabet Med. 2008;25:240–241. doi: 10.1111/j.1464-5491.2007.02325.x. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J, editor. 2nd. Philadelphia: Lawrence Erlbaum; 1998. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 17.Yeh SH. Chuang H. Lin LW, et al. Tai chi chuan exercise decreases A1C levels along with increase of regulatory T-cells and decrease of cytotoxic T-cell population in type 2 diabetic patients. Diabetes Care. 2007;30:716–718. doi: 10.2337/dc06-1507. [DOI] [PubMed] [Google Scholar]
- 18.Apelqvist J. Bakker K. van Houtum WH, et al. Practical guidelines on the management and prevention of the diabetic foot: based upon the International Consensus on the Diabetic Foot (2007) Prepared by the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev. 2008;24(Suppl 1):S181–187. doi: 10.1002/dmrr.848. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE. SF-36 health survey update (vol 25, pg 3130, 2000) Spine. 2001;26:2062–2062. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 20.Hung JW. Liou CW. Wang PW, et al. Effect of 12-week tai chi chuan exercise on peripheral nerve modulation in patients with type 2 diabetes mellitus. J Rehabil Med. 2009;41:924–929. doi: 10.2340/16501977-0445. [DOI] [PubMed] [Google Scholar]
- 21.Li L. Manor B. Long term Tai Chi exercise improves physical performance among people with peripheral neuropathy. Am J Chin Med. 2010;38:449–459. doi: 10.1142/S0192415X1000797X. [DOI] [PubMed] [Google Scholar]
- 22.Tsang T. Orr R. Lam P, et al. Effects of Tai Chi on glucose homeostasis and insulin sensitivity in older adults with type 2 diabetes: a randomised double-blind sham-exercise-controlled trial. Age Ageing. 2008;37:64–71. doi: 10.1093/ageing/afm127. [DOI] [PubMed] [Google Scholar]
- 23.Song R. Ahn S. Roberts BL, et al. Adhering to a t'ai chi program to improve glucose control and quality of life for individuals with type 2 diabetes. J Altern Complement Med. 2009;15:627–632. doi: 10.1089/acm.2008.0330. [DOI] [PubMed] [Google Scholar]
- 24.Park IS. Song R. Oh KO, et al. Managing cardiovascular risks with Tai Chi in people with coronary artery disease. J Adv Nurs. 2010;66:282–292. doi: 10.1111/j.1365-2648.2009.05134.x. [DOI] [PubMed] [Google Scholar]