Abstract
The identity and proportional distribution of immunoglobulin A1 (IgA1) protease-producing streptococci in the oral and pharyngeal microflora were studied. A collection of 459 streptococcal strains, including reference strains of Streptococcus species, and fresh isolates from human dental plaque and buccal and pharyngeal mucosa were identified by biochemical means and were examined for IgA1 protease production. IgA1 protease production was demonstrated in some, but not all, strains of Streptococcus sanguis and Streptococcus mitior and in a group of strains of uncertain taxonomic affiliation. The property was not associated with particular biotypes within the two species. Strains of S. sanguis and S. mitior isolated from Macaca fascicularis also cleaved human IgA1. A significantly different proportion of streptococcal populations in different ecosystems produced IgA1 protease. The enzyme was released by 62.7% of streptococcal isolates from buccal mucosa in contrast to only 7.8% from pharyngeal mucosa. In samples from initial and mature dental plaque 38 to 40% of streptococcal isolates produced IgA1 protease. This difference was largely a result of the frequency by which IgA1 protease activity was present in S. mitior, the predominant streptococcal species in all samples. Among otherwise identical isolates of S. mitior, 67.8% from buccal mucosa in contrast to only 5.9% from pharyngeal mucosa produced IgA1 protease. The results indicate that IgA1 protease may confer an ecological advantage to streptococci colonizing surfaces exposed to a secretory IgA-mediated selection pressure.
Full text
PDF



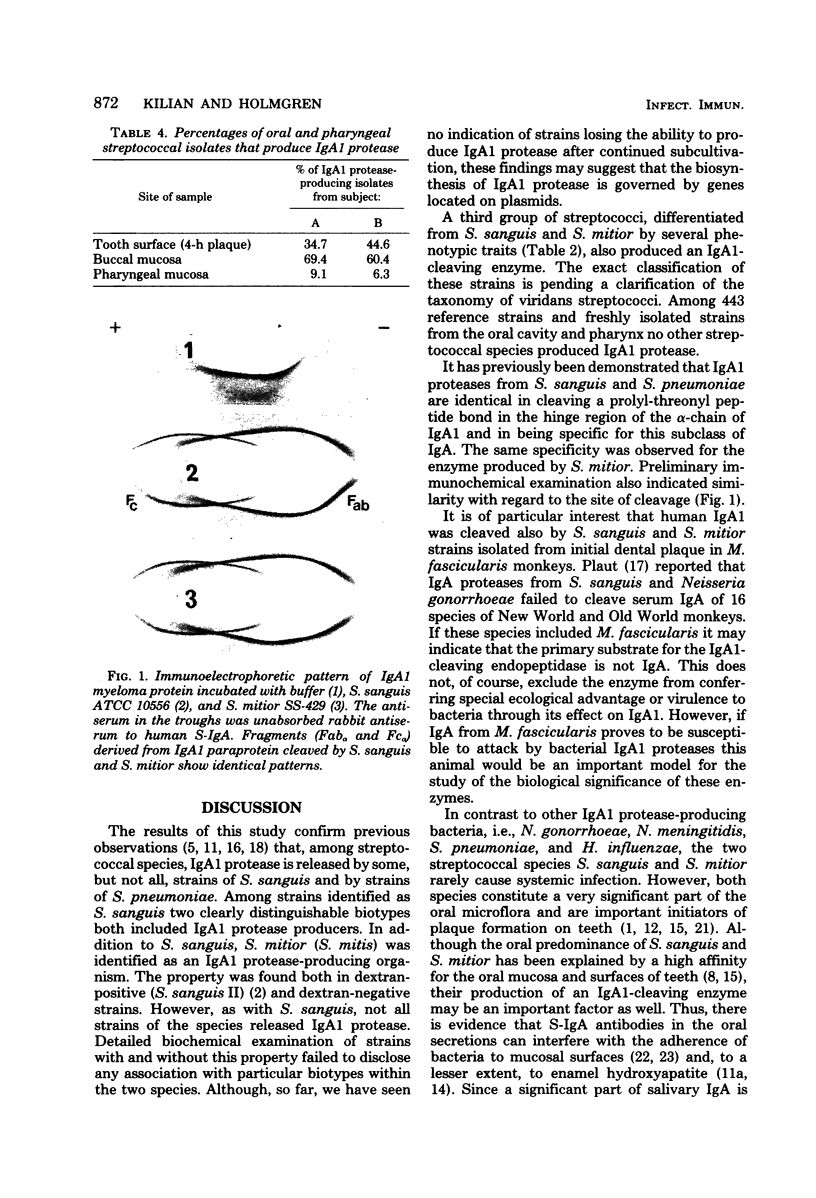

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson J. A numerical taxonomic study of human oral streptococci. Odontol Revy. 1968;19(2):137–160. [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Abel C. A., Yount W. J., Kunkel H. G. A subclass of human gamma-A-globulins (gamma-A2) which lacks the disulfied bonds linking heavy and light chains. J Exp Med. 1968 Dec 1;128(6):1223–1236. doi: 10.1084/jem.128.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J. M., Marsh P. D. Streptococci and the human oral flora. Soc Appl Bacteriol Symp Ser. 1978;7:157–206. [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Schrohenloher R. E. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979 Oct;26(1):143–149. doi: 10.1128/iai.26.1.143-149.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Roland K., Mestecky J. Interference of secretory immunoglobulin A with sorption of oral bacteria to hydroxyapatite. Infect Immun. 1981 Mar;31(3):942–951. doi: 10.1128/iai.31.3.942-951.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Rölla G. Initial colonization of teeth in monkeys as related to diet. Infect Immun. 1976 Oct;14(4):1022–1027. doi: 10.1128/iai.14.4.1022-1027.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Thylstrup A., Fejerskov O. Predominant plaque flora of Tanzanian children exposed to high and low water fluoride concentrations. Caries Res. 1979;13(6):330–343. doi: 10.1159/000260423. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Ofstehage J. C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979 Dec;26(3):1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male C. J. Immunoglobulin A1 protease production by Haemophilus influenzae and Streptococcus pneumoniae. Infect Immun. 1979 Oct;26(1):254–261. doi: 10.1128/iai.26.1.254-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. Microbial IgA proteases. N Engl J Med. 1978 Jun 29;298(26):1459–1463. doi: 10.1056/NEJM197806292982608. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Wistar R., Jr, Capra J. D. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J Clin Invest. 1974 Dec;54(6):1295–1300. doi: 10.1172/JCI107875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Svanborg-Edén C., Svennerholm A. M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978 Dec;22(3):790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]




