Abstract
The Drosophila testis harbors two types of stem cells: germ line stem cells (GSCs) and cyst stem cells (CySCs). Both stem cell types share a physical niche called the hub, located at the apical tip of the testis. The niche produces the JAK/STAT ligand Unpaired (Upd) and BMPs to maintain CySCs and GSCs, respectively. However, GSCs also require BMPs produced by CySCs, and as such CySCs are part of the niche for GSCs. Here we describe a role for another secreted ligand, Hedgehog (Hh), produced by niche cells, in the self-renewal of CySCs. Hh signaling cell-autonomously regulates CySC number and maintenance. The Hh and JAK/STAT pathways act independently and non-redundantly in CySC self-renewal. Finally, Hh signaling does not contribute to the niche function of CySCs, as Hh-sustained CySCs are unable to maintain GSCs in the absence of Stat92E. Therefore, the extended niche function of CySCs is solely attributable to JAK/STAT pathway function.
Keywords: Hh, Smo, Upd, JAK/STAT, Cyst stem cell (CySC), Germline stem cell (GSC), Hub, Niche, Testis, Self-renewal
INTRODUCTION
Drosophila has proven a useful model system in the study of stem cell biology, particularly in identifying the importance of the physical niche in maintaining stem cells and some of the signaling pathways involved. The germ line niche is well studied and has helped inform our understanding of niche-stem cell interactions (Losick et al., 2011; Spradling et al., 2011). The ovarian niche harbors two or three germ line stem cells (GSCs) and produces bone morphogenetic proteins (BMPs), which are essential for their maintenance (Xie and Spradling, 1998; Chen and McKearin, 2003). Proper development of oocytes requires two additional populations of somatic cells: the escort cells, which are dependent on JAK/STAT signaling for normal function (Decotto and Spradling, 2005; Morris and Spradling, 2011); and the follicle cells, which envelop the developing cysts. Follicle cells are produced from stem cells that require Hedgehog (Hh) signaling for self-renewal and proliferation (Zhang and Kalderon, 2000; Zhang and Kalderon, 2001).
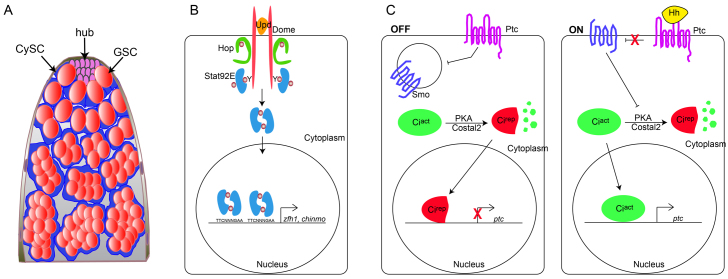
The male germ line niche shares some features with the ovary, but it possesses a different architecture and different signals have been implicated in the self-renewal of resident stem cells (Losick et al., 2011). The niche, called the hub, supports two stem cell populations that are easily identifiable by their position relative to the hub and by molecular markers. GSCs give rise to sperm, whereas the somatic cyst stem cells (CySCs) divide to give rise to postmitotic cyst cells that envelop and support the development of germ cell cysts (Fig. 1A) (de Cuevas and Matunis, 2011).
Fig. 1.
The JAK/STAT and Hh pathways. (A) The Drosophila testis. See text for details. (B) JAK/STAT signaling in Drosophila. Binding of the secreted ligand Unpaired (Upd) to its receptor Domeless (Dome) causes phosphorylation and activation of the Janus kinase (JAK) Hopscotch (Hop). Hop in turns phosphorylates the transcription factor Stat92E, which dimerizes and enters the nucleus to promote the transcription of target genes, including zfh1 and chinmo. (C) The Hh pathway in the absence (‘OFF’) or presence (‘ON’) of ligand. When Hh is not present, Patched (Ptc) inhibits Smoothened (Smo), rendering it inactive. In the absence of Smo activity, the transcription factor Cubitus interruptus (Ci) is cleaved from a full-length transcriptional activator form (Ciact) to a repressor (Cirep) by a complex that includes the Protein kinase A (PKA) enzyme and scaffolding proteins such as Costal2. Hh ligand (‘ON’) prevents Ptc activity, allowing Smo to inhibit Ci cleavage, meaning that Ciact enters the nucleus to activate transcription of target genes, such as ptc. GSC, germ line stem cell; CySC, cyst stem cell; Y, tyrosine residues.
The hub secretes the cytokine Unpaired (Upd; Upd1 or Os – FlyBase) to activate the JAK/STAT signaling pathway in both stem cell populations (Kiger et al., 2001; Tulina and Matunis, 2001). Upd activates Stat92E, which is the only Drosophila STAT transcription factor (Arbouzova and Zeidler, 2006), in both CySCs and GSCs (Fig. 1B). Clonal analyses revealed that GSCs and CySCs lacking Stat92E are not maintained in the niche and subsequently differentiate (Kiger et al., 2001; Tulina and Matunis, 2001; Leatherman and Dinardo, 2008). Furthermore, a hetero-allelic Stat92E temperature-sensitive mutant (Stat92Ets) loses both stem cell populations – GSCs because they lose adhesion to the niche within 16 hours of Stat92E inactivation, and CySCs because they fail to self-renew (Leatherman and Dinardo, 2008; Leatherman and Dinardo, 2010). Remarkably, restoration of wild-type Stat92E function only in the soma of Stat92Ets flies raised at the restrictive temperature rescues both germ line and somatic lineages (Leatherman and DiNardo, 2010). In the soma-rescued Stat92Ets flies, CySCs are found at locations next to the hub normally occupied by GSCs, presumably owing to increased Stat92E activation in the rescued CySCs. Regardless, these displaced GSCs are supported by CySCs, most likely through BMP ligands secreted by Stat92E-rescued CySCs, and give rise to mature spermatocytes.
In the CySCs, zfh1 and chinmo are targets of the JAK/STAT pathway that have important roles in self-renewal (Leatherman and Dinardo, 2008; Flaherty et al., 2010). In fact, Zfh1 is currently the best marker for the CySC state (Leatherman and Dinardo, 2008; Issigonis et al., 2009; Flaherty et al., 2010; Leatherman and Dinardo, 2010; Inaba et al., 2011). Hyperactivation of Stat92E or misexpression of either Chinmo or Zfh1 in the somatic lineage gives rise to stem cell tumors consisting of GSC-like and CySC-like cells (Leatherman and Dinardo, 2008; Flaherty et al., 2010; Leatherman and Dinardo, 2010), similar in constitution to the tumors observed when the ligand Upd is overexpressed (Kiger et al., 2001; Tulina and Matunis, 2001). The ability of CySCs to support and expand GSCs is most likely due to Stat92E-dependent production of BMPs, which act locally on GSCs to promote their self-renewal. Thus, a new model has emerged in which CySCs form part of the niche for GSCs together with the hub, and JAK/STAT signaling in CySCs is responsible for this support role (see Fig. 6L). Stat92E activation in GSCs is dispensable for self-renewal, and instead BMPs produced by the hub and by CySCs downstream of Stat92E and its targets Chinmo and Zfh1 regulate GSC self-renewal (Shivdasani and Ingham, 2003; Kawase et al., 2004; Flaherty et al., 2010; Leatherman and Dinardo, 2010). This is reminiscent of the female germ line niche, where BMP production also appears to be downstream of JAK/STAT signaling, albeit in different somatic cells (López-Onieva et al., 2008; Wang et al., 2008).
Fig. 6.
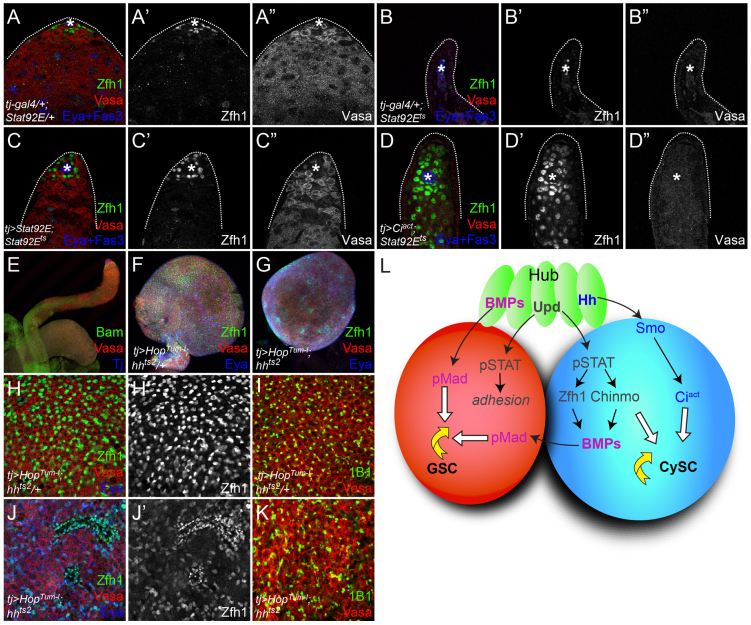
Hh does not contribute to CySC niche function. (A-D″) tj-gal4/+; Stat92E/+ control testes contain a normal complement of CySCs (determined by Zfh1 staining) and germ cells (as assessed by Vasa staining). CySCs and GSCs are lost in a Stat92E temperature-sensitive mutant (Stat92Ets) (B). Expression of Stat92E in the somatic lineage using tj-gal4 can rescue both stem cell types (C). Activation of the Hh pathway in these cells by expressing Ciact leads to rescue of Zfh1-expressing CySCs (D′), but not of GSCs (D″). Zfh1 is green (single channel in A′-D′), Vasa is red (single channel in A″-D″) and Eya and Fas3 are blue. (E-G) A wild-type testes taken at 10× magnification. HopTum-l overexpression in the somatic lineage causes tumors in both hh heterozygous (tj>HopTum-l; hhts2/+; F) and hh mutant (tj>HopTum-l; hhts2; G) testes. Bam is green in E, Zfh1 is green in F,G; Vasa is red in E-G, Tj is blue in E and Eya is blue in F,G. (H,H′,J,J′) The stem cell marker Zfh1 (green, single channel in H′,J′) is expressed at lower levels in hhts2 (J,J′) than in hhts2/+ siblings (H,H′). Eya (blue) expression is largely absent in hhts2/+ siblings (H) but is readily detectable in hhts2 mutants (J), where it is co-expressed with Zfh1. Vasa is red in H,J. (I,K) The germ cells present in tumors induced in hhts2 mutant testes (K) are larger than in control siblings (I) and display large, branched fusomes (1B1, green) compared with the small, round fusomes in hhts2/+ siblings, which are indicative of a stem-like state in GSCs. (L) Model of genetic interactions occurring at the testis stem cell niche. The hub (green) produces Upd and Hh, which are required in the CySC (blue) for self-renewal. The CySC requires the Stat92E targets Zfh1 and Chinmo. Stat92E and its targets also regulate BMP production in the CySC, and these BMPs signal to the GSC (red) together with hub-derived BMPs for self-renewal, but Hh signaling does not contribute to this function.
However, it is apparent that the genetic network is more complex in the testis niche. hh expression by hub cells has been known for some time (Forbes et al., 1996). In the absence of Hh, its receptor Patched (Ptc), a 12-pass transmembrane protein, inhibits the activation of Smoothened (Smo), a seven-pass transmembrane protein required for Hh signal transduction (Fig. 1C, ‘OFF’) (Alcedo et al., 1996; Chen and Struhl, 1996; van den Heuvel and Ingham, 1996). With Smo inhibited, the full-length form of the transcription factor Cubitus interruptus (Ci) is cleaved by a complex that includes Protein kinase A (PKA) and the scaffolding protein Costal2 (Costa – FlyBase) (Kalderon, 2005). This results in a shortened form of Ci (called Cirep) that can translocate to the nucleus and bind regulatory elements of Hh target genes but represses their expression. When Hh binds to Ptc, Smo is no longer inhibited and it in turn can prevent the cleavage of Ci (Fig. 1C, ‘ON’). This results in a full-length Ci protein called Ciact that induces transcription of target genes (Domínguez et al., 1996; Aza-Blanc et al., 1997). One such gene is ptc, which, in addition to encoding the Hh receptor, is also a well-established Hh pathway target (Hidalgo and Ingham, 1990; Tabata and Kornberg, 1994; Chen and Struhl, 1996; Goodrich et al., 1996).
Here, we dissect the role of Hh signaling in the Drosophila testis and describe a requirement for Hh signal reception in CySC maintenance, similar to the role that Hh signaling fulfils in somatic stem cells in the ovary. We find that Hh and JAK/STAT signaling act independently and cannot substitute for one another in CySC maintenance. We also demonstrate that, in contrast to JAK/STAT signaling, Hh pathway activity does not impart ‘extended niche’ function to CySCs and that Hh only regulates CySC self-renewal.
MATERIALS AND METHODS
Clonal analysis and fly husbandry
Temperature-sensitive mutants were raised at 16°C. Adult males were collected every 3 days and shifted to 29°C for 10 days. eyaA3-gal4 crosses were kept at room temperature and adult males were shifted to 29°C for 10 days for maximum Gal4 activity. For clonal analysis, flies were raised at 25°C, collected for 2 days and heat shocked at 37°C for 1 hour and returned to 25°C until dissection, 2 or 7 days post-clone induction. The following stocks were used for clonal analyses:
yw,hsflp122; FRT40A ubi-GFP;
yw,hsflp122; FRT40A;
yw,hsflp122, UAS-nls-GFP, tub-gal4; tub-gal80, FRT40A;
yw,hsflp122, UAS-CD8-GFP;; tub-gal4, FRT82B tub-gal80.
Fly stocks
The following stocks were used and are described in FlyBase: Oregon-R, FRT40A smo2, FRT40A smo119B6, FRT40A smoD16, FRT40A smoD16 Pka-C1H2, FRT40A chinmo1, ptc-lacZ, hhP30 (hh-lacZ), hhts2 (Ma et al., 1993); Stat92EF, FRT82B Stat92E85c9 (Stat92EF/Stat92E85c9 corresponds to the Stat92Ets mutation), UAS-Ciact (UAS-Ci5Ncm5, UAS-Ci5m30; from D. Kalderon, Columbia University, NY, USA), UAS-ptc RNAi (Vienna Drosophila RNAi Center), UAS-Cirep (UAS-CiN-ZN; from R. Holmgren, Northwestern University, IL, USA), UAS-3HA-Stat92E, UAS-mys, UAS-DE-Cadherin, UAS-HopTum-l, eyaA3-gal4 and UAS-Hh [from J. Treisman (Azpiazu et al., 1996)].
Immunohistochemistry
The following antibodies were used: chicken anti-β-galactosidase (β-gal) (1:250; Immunology Consultants Lab), guinea-pig anti-Traffic jam (Tj) (1:3000; gift of D. Godt, University of Toronto, ON, Canada), goat anti-Vasa (1:400; Santa Cruz), guinea-pig anti-Zfh1 (1:1000; gift of J. Skeath, Washington University, MO, USA), rabbit anti-Zfh1 (1:5000; gift of R. Lehmann, New York University School of Medicine, NY, USA), rabbit anti-GFP (1:500; Invitrogen), mouse anti-GFP (1:500; Invitrogen), mouse anti-Eyes absent (Eya) [1:20; Developmental Studies Hybridoma Bank (DSHB)], mouse anti-Fas3 (1:50; DSHB), mouse anti-Adducin (Hts – FlyBase) (1B1, 1:20; DSHB), mouse anti-Ptc (1:200; DSHB), rat anti-Ci (1:20; DSHB), rabbit anti-Hh (1:50; gift of I. Guerrero, CBMSO, Madrid, Spain) and rabbit anti-Stat92E (1:500; Flaherty et al., 2010). Testes were dissected and fixed in 4% formaldehyde and processed as described (Flaherty et al., 2010).
RESULTS
Hh pathway components are expressed in the testis niche
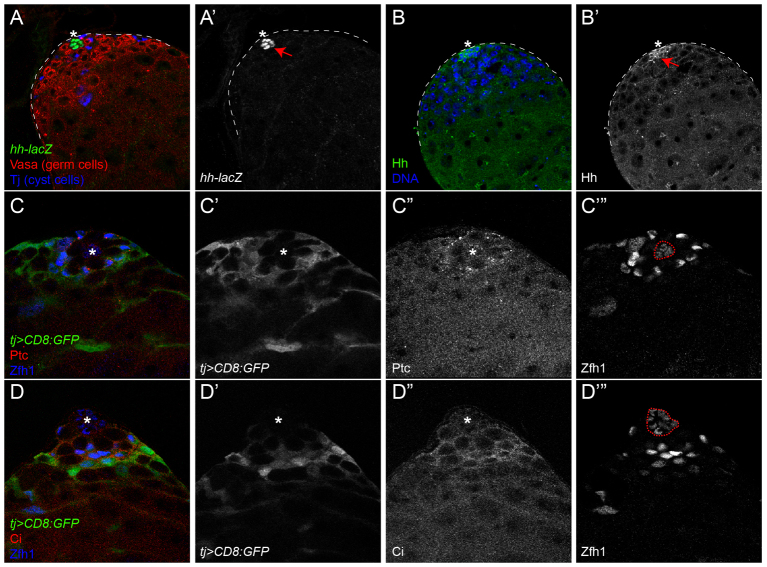
It has been reported that an enhancer trap in the hh locus called hhP30 is expressed in hub cells in the testis (Forbes et al., 1996; Dinardo et al., 2011). We confirm that hh is transcribed in hub cells (Fig. 2A,A′) and furthermore show that Hh protein is concentrated on and around the hub (Fig. 2B,B′). We also analyzed the expression of components required cell-autonomously for response to the Hh signal (see Fig. 1C). Colabeling somatic membranes with GFP reveals that Ptc, which is the Hh receptor and an Hh pathway target, is present in the somatic lineage, one cell diameter away from the hub, in presumptive CySCs (Fig. 2C-C″′). Ci, the transcription factor that mediates Hh signaling, is expressed in an overlapping, but larger, subset of somatic cells including the CySCs and their daughters (Fig. 2D-D″′). The Ci antibody we used recognizes only full-length Ci (Motzny and Holmgren, 1995; Aza-Blanc et al., 1997), i.e. the Ciact form that has not yet been cleaved into Cirep and is competent for activation. Taken together, the patterns of Ptc and Ci show that Hh is secreted from the hub and that CySCs are competent to respond to it.
Fig. 2.
Expression of Hh pathway components at the testis niche. (A,A′) Expression of hh-lacZ is seen in the hub (arrow in A′). (B,B′) Hh protein is also found at high levels in and around the hub (arrow in B′). (C-D″′) tj-gal4>UAS-CD8::GFP was used to label somatic cell membranes (single channel in C′,D′). CySCs co-express Ptc (red; single channel in C″) and Zfh1 (blue; single channel in C″′) adjacent to the hub. Ci (red; single channel in D″) is expressed in the cyst lineage, including Zfh1-positive CySCs. Asterisks indicate the hub, which is outlined (red dotted lines) in C″′,D″′.
Hh signaling is required for CySC self-renewal
In order to test whether Hh signaling plays a role in stem cell self-renewal at the testis niche, we made clones of cells mutant for smo, which is essential for transduction of the Hh signal. Mutant negatively marked clones were generated by heat shock-induced recombination in adults and were scored at 2 or 7 days post-clone induction (dpci) to assess whether clones could be generated and maintained, respectively. We compared smo clones to neutral negatively marked clones made with the same FRT site but on a wild-type chromosome (i.e. FRT40A). We used three different loss-of-function alleles of smo and obtained similar results. We scored GSCs as Vasa-positive cells that contacted the hub and scored CySCs as Zfh1-positive or Tj-positive cells with their nuclei one cell diameter away from the hub (i.e. on the other side of the GSCs). The hub was identified as a cluster of cells expressing low levels of Zfh1 or Tj surrounded by a rosette of Vasa-positive GSCs. In all cases, we were able to induce and recover smo mutant GSCs at comparable rates to control clones, indicating that Hh signaling is dispensable for GSC self-renewal (Fig. 3G; supplementary material Table S1). However, marked CySCs were recovered at low frequency at 2 dpci and very rarely at 7 dpci (Fig. 3G; supplementary material Table S1).
Fig. 3.
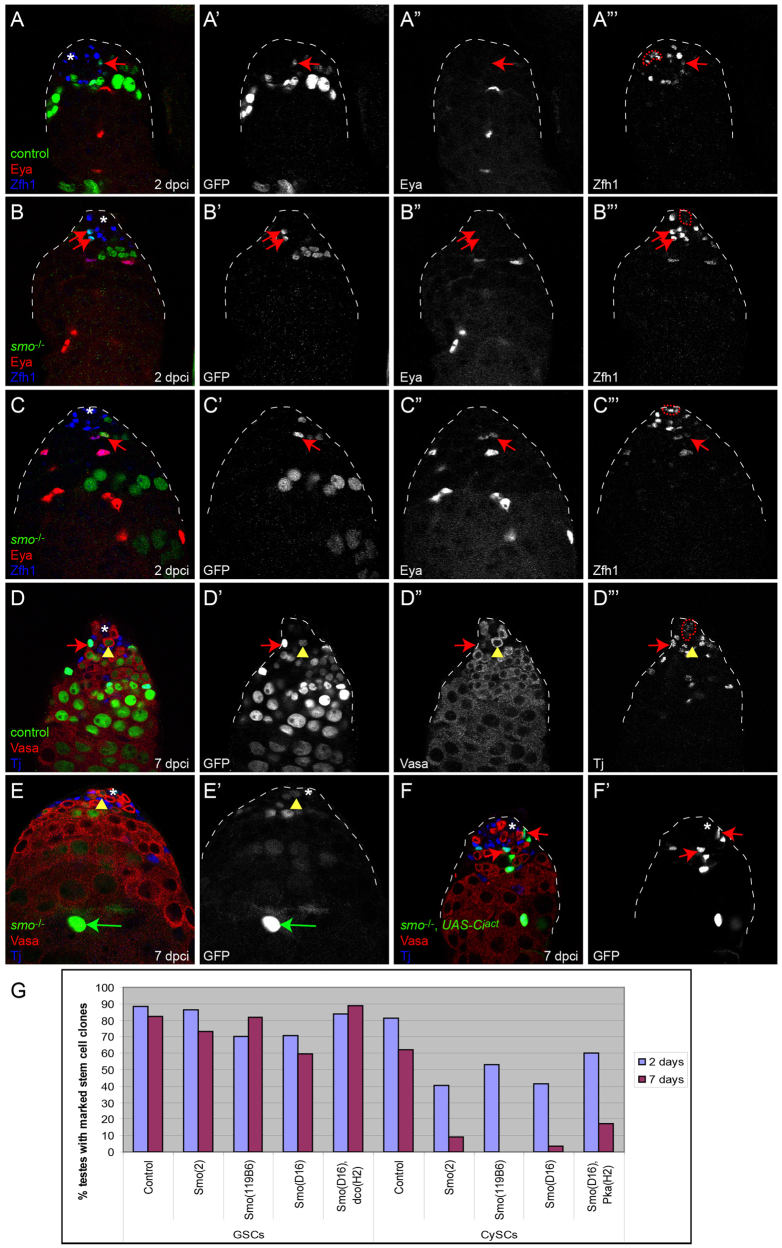
Hh signaling is required for CySC self-renewal. (A-F′) MARCM clonal analysis showing control (A,D) or smo mutant clones (B,C,E,F). (A-C″′) Control FRT40A CySC clones at 2 dpci can be recovered, as assessed by Zfh1 expression (blue) and lack of Eya (red) (A). smo mutant CySC clones can be recovered at 2 dpci (B) and express Zfh1, whereas other smo mutant clones initiate normal differentiation and begin to express Eya (C). Single channel for GFP is shown in A′-C′, for Eya in A″-C″ and for Zfh1 in A″′C″′. (D-D″′) Control FRT40A MARCM clone (green) at 7 dpci showing a marked CySC (red arrow) and GSC (yellow arrowhead) adjacent to the hub. (E-F′) smo mutant MARCM clone at 7 dpci showing a labeled GSC (yellow arrowhead) but no CySCs (E). The green arrow points to a marked differentiated cyst cell (E). smo mutant CySC clones in which Ciact is overexpressed can be recovered at 7 dpci (red arrows indicate two mutant CySCs) (F). See Table 1 for values. Vasa is red and Tj is blue in D-F. Single channel for GFP is shown in D′-F′, for Vasa in D″ and for Tj in D″′. In A-F′, the position of the hub is indicated with an asterisk in merged images and is outlined (dotted red line) in the Tj or Zfh1 single channel. (G) The recovery rates for labeled GSCs (left) and CySCs (right) at 2 and 7 dpci in negatively labeled clones. See supplementary material Table S1 for n values.
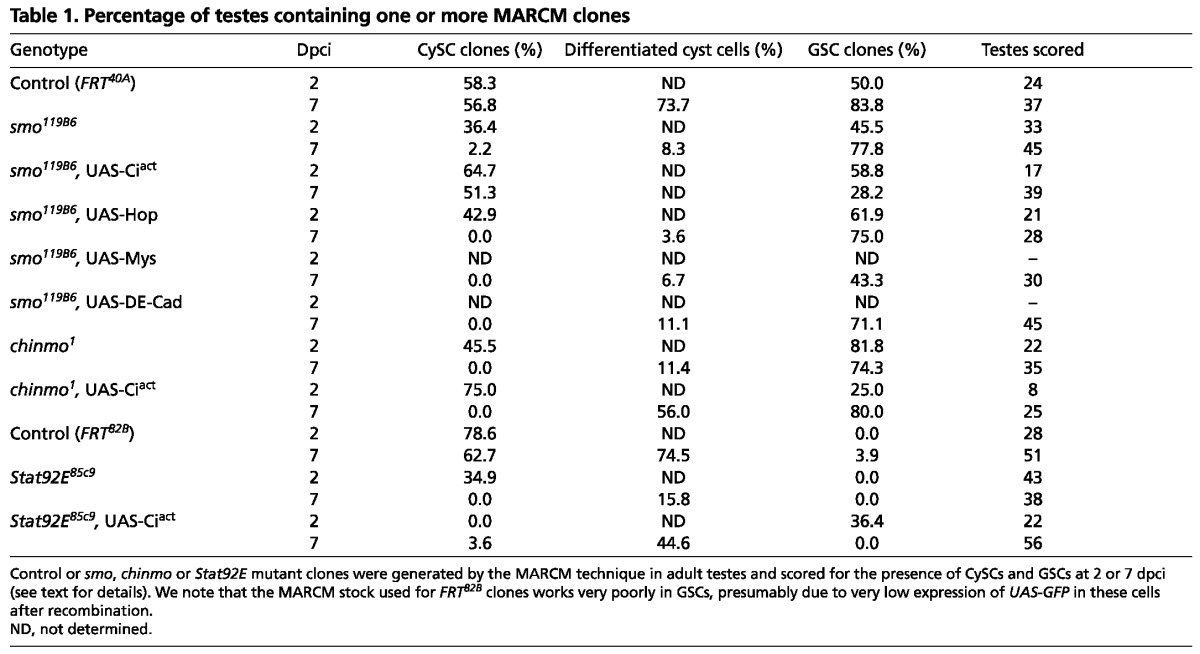
We also performed similar experiments using positively marked clones generated by the MARCM technique (Lee and Luo, 1999). Control FRT40A MARCM clones in CySCs, which were identified by high levels of Zfh1, could be recovered at 2 dpci (Fig. 3A-A″′). These control clones were maintained over time and could be readily identified at 7 dpci (Fig. 3D-D″′; Table 1). smo MARCM clones in CySCs were recovered less frequently than controls (Fig. 3B; Table 1), but when they were recovered they either (1) expressed Zfh1 and lacked the differentiation marker Eya (suggesting that they are still CySCs) (Fig. 3B-B″′, arrows) or (2) downregulated Zfh1 and began to express Eya (Fig. 3C-C″′, arrow). By 7 dpci, we were unable to recover smo-deficient CySCs (Fig. 3E,E′; Table 1). By contrast, we were able to identify smo mutant GSCs at 7 dpci at rates similar to control MARCM clones (Fig. 3D-E′, arrowheads; Table 1). Therefore, both negatively and positively marked clonal analyses confirm that GSCs are largely, if not entirely, unaffected by loss of Hh signaling, whereas CySCs require Hh for self-renewal.
Table 1.
Percentage of testes containing one or more MARCM clones
We carried out several experiments to confirm that the lack of CySC recovery in smo mutant clones was due to specific loss of the Hh signal. Hh signaling activates the transcription factor Ci, which is cleaved in the absence of Hh to form a repressor. This cleavage requires the enzyme PKA. We reasoned that smo clones, which are unable to transduce Hh signaling, might be at least partially rescued if the full-length Ci in these clones was unable to be cleaved to the repressor form. To test this hypothesis, we made clones that were doubly mutant for smo and Pka-C1 (the Drosophila gene that encodes PKA) and assessed CySC recovery rates in these clones at 2 and 7 dpci. Indeed, smo Pka-C1 double-mutant CySCs were present more often than single smo mutant CySCs at both 2 and 7 dpci (P<0.04; supplementary material Table S1). However, this is not a full rescue as PKA loss alone is insufficient to fully activate Ci (Price and Kalderon, 1999) and double-mutant clones are still lost with time. Finally, we used the MARCM technique to make smo mutant clones expressing an uncleavable form of Ci, termed Ciact, which behaves as a dominant-active protein. In this case, CySCs could be recovered at 90% of control rates, indicating robust rescue of smo mutants (Fig. 3F,F′; Table 1).
Together, these data indicate that transcription downstream of Hh reception and signal transduction is required in CySCs, but not GSCs, for self-renewal.
Hh levels control CySC number
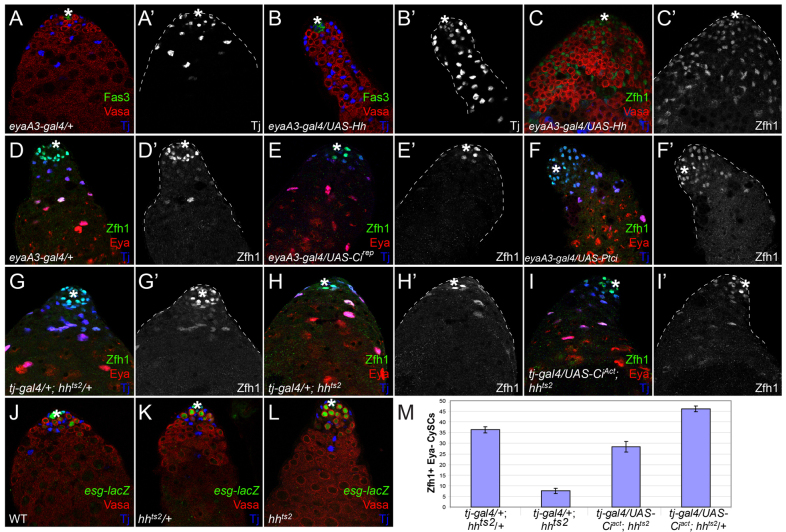
Next, we assayed the effect of Hh gain-of-function in the testis. Expressing Hh under the control of a somatic cell driver (eyaA3-gal4) causes an increase in the number of Traffic jam-positive somatic cells (Fig. 4B,B′, compare with 4A,A′). Further analysis showed that these excess somatic cells express Zfh1, which is most highly expressed in CySCs. These data suggest that the excess somatic cells in eyaA3>Hh are stem cells (Fig. 4C,C′). As Hh is secreted, this result could be due to cell-autonomous effects on cyst cells or to non-cell-autonomous signaling to the hub or germ cells. These possibilities were distinguished by expressing either the Ci repressor (Cirep) or an RNAi against ptc to block or enhance Hh signaling, respectively, within the somatic lineage. Loss of ptc results in ligand-independent Hh signaling (Ingham et al., 1991; Chen and Struhl, 1996). We counted 44.6±1.2 Zfh1-positive, Eya-negative cells in testes from eyaA3-gal4/+ control males (n=20), consistent with the previously reported 36-50 Zfh1-positive cells per testis (Leatherman and Dinardo, 2010; Inaba et al., 2011). Ptc knockdown causes a statistically significant increase in the number of Zfh1-positive, Eya-negative CySCs to 73.2±3.1 (n=15, P<9.7×10–8) (Fig. 4D,F). We observed a similar expansion of Zfh1-positive cells when we overexpressed Ciact using eyaA3-gal4 (not shown). Conversely, the number of CySCs was reduced again in a statistically significant manner when Hh signaling was impaired cell-autonomously (29.8±3.2 CySCs; n=10, P<1×10–3 compared with control; Fig. 4D,E).
Fig. 4.
Hh levels modulate CySC number. (A-B′) Control eyaA3-gal4-expressing testis tip showing the hub (Fas3, green), germ cells (Vasa, red) and somatic cells (Tj, blue single channel in A′,B′). Expression of Hh (eyaA3>Hh) leads to an increase in Tj-positive somatic cell number (B). (C,C′) The additional somatic cells in eyaA3>Hh testes are stem cells as evidenced by expression of Zfh1 (green; single channel in C′); Vasa is red and Tj is blue in C. (D-F′) Cell-autonomous modulation of Hh levels within somatic cells is sufficient to alter CySC number. Compared with eyaA3-gal4 controls (D,D′), expression of Cirep (E,E′) or knockdown of Ptc by RNAi (F,F′) leads to loss or gain, respectively, of CySCs, assessed as Zfh1-positive, Eya-negative cells. (G,G′) An hhts2/+ sibling exhibits a normal complement of CySCs. (H,H′) By contrast, an hhts2 mutant displays a loss of CySCs after 10 days at the non-permissive temperature. (I,I′) However, CySCs in the hhts2 mutant can be rescued by expression of Ciact in the somatic lineage. In D-I, Zfh1 is green (single channel in D′-I′), Eya is red and Tj is blue. (J-L) GSCs [Vasa-positive (red), esg-lacZ-positive (green)] are maintained in hhts2 mutants (L) as compared with wild-type (WT) (J) and hhts2/+ sibling controls (K). Tj is blue. The hub is indicated by an asterisk in all images. (M) Quantification of CySC numbers for the specified genotypes. CySCs were defined as Zfh1-positive, Eya-negative cells. Error bars indicate s.e.m.
In order to address further the in vivo role of Hh in the adult testis, a temperature-sensitive mutant allele of hh (hhts2) was analyzed. Adult males were allowed to develop normally by raising the embryos, larvae and pupae at the permissive temperature of 16°C and were then shifted after eclosion to the restrictive temperature of 29°C. CySC numbers were strongly reduced from 36±1.5 in heterozygous controls (n=19) to 7.6±1.3 in hhts2 mutant animals (n=15, P<1.3×10–15; Fig. 4G-H,M). The autonomy and the specificity of this phenotype were tested by restoring Hh signaling downstream of the ligand using a somatic cell driver to express Ciact. In this situation, CySC numbers were nearly restored to control levels (28±2.5; n=14, P<4.6×10–7 compared with hhts2; Fig. 4I,M). These data demonstrate that Hh signaling through Ci-activated transcription is necessary to specify the correct number of somatic stem cells at the testis niche.
We also investigated the presence of GSCs in hhts2 mutants at the restrictive temperature. We used the M5-4 enhancer trap line, which reports expression at the escargot (esg) locus and is expressed in GSCs, gonialblasts and hub cells in wild-type testes (Fig. 4J) (Gönczy and DiNardo, 1996). We compared GSCs (Vasa-positive, esg-lacZ-positive cells in contact with the hub) in hhts2/+ heterozygous siblings with hhts2 mutants raised under the same conditions. hhts2/+ heterozygous siblings had 8.6±0.3 GSCs (n=18; Fig. 4K). By contrast, hhts2 mutants had 6.3±0.4 GSCs (n=16), a statistically significant decrease (P<6.2×10–5; Fig. 4L). This change is due to the requirement for Hh, as the same animals raised at the permissive temperature did not display significantly different numbers of GSCs [10.4±0.5 in hhts2/+ (n=14) and 11.2±0.4 in hhts2 (n=5); differences not significant by Student’s t-test]. The reduction in GSCs in hhts2 mutants could be due to a reduction in hub size. We ruled out this possibility because the number of hub cells in mutant animals did not differ from that of controls [11.5±0.5 (n=17) for hhts2/+ animals and 11.1±0.5 hub cells in hhts2 (n=15); differences not significant by Student’s t-test]. We noted that, unlike Stat92E temperature-sensitive mutants (see Fig. 6B,B″) (Leatherman and Dinardo, 2010), GSCs were still present in hhts2 mutants. This is despite the reduction in CySCs, which constitute part of the niche for GSCs and are necessary to support their self-renewal (Leatherman and Dinardo, 2010).
STAT and Hh act independently on self-renewal
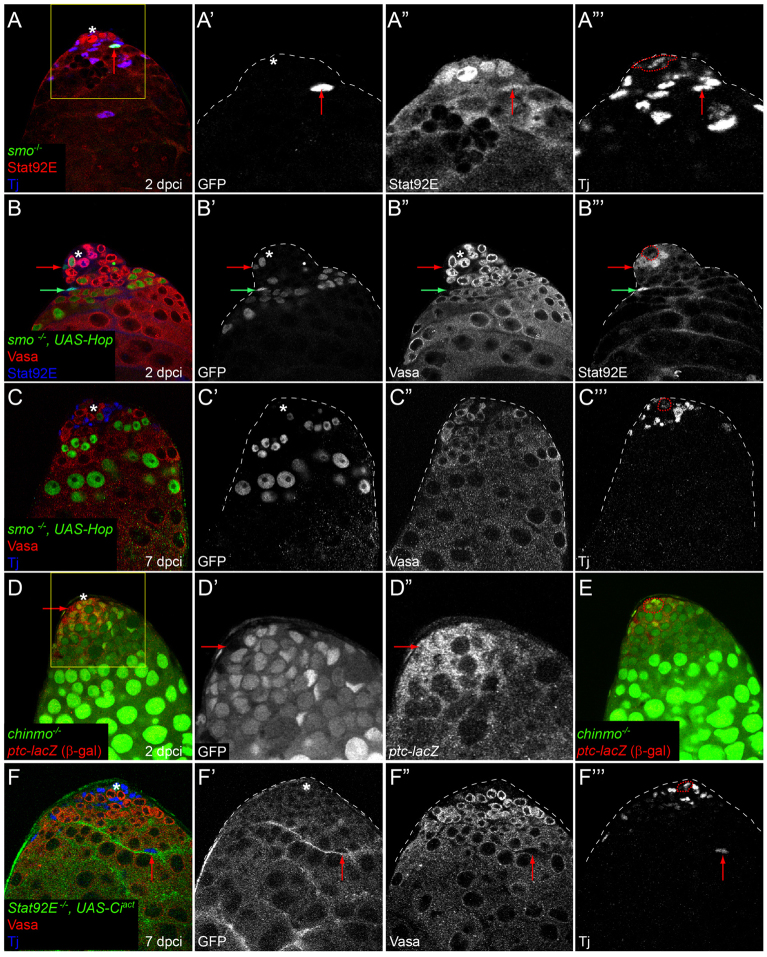
As JAK/STAT signaling is essential for CySC self-renewal (Leatherman and Dinardo, 2008), we examined whether Hh could be acting on stem cell maintenance by affecting the JAK/STAT pathway, or, conversely, whether JAK/STAT affects Hh signaling. Stabilization of the Stat92E protein is often used as a readout for JAK/STAT pathway activity (Chen et al., 2002; Flaherty et al., 2010). Mutant clones for smo accumulated Stat92E protein normally when found close to the hub, suggesting that JAK/STAT signaling is unaffected in these cells (Fig. 5A-A″′). We tried to rescue smo-deficient CySCs by expressing Hop in them, which is sufficient to activate Stat92E when overexpressed (Ekas et al., 2006). We confirmed that Stat92E is indeed activated in smo MARCM clones expressing Hop both in CySCs and in differentiating cyst cells (Fig. 5B-B″′, red and green arrows, respectively). This, combined with the fact that Ciact overexpression can rescue loss of CySCs in smo mutant clones (Fig. 3F,F′; Table 1), suggests that the MARCM technique is a valid tool for attempting to rescue CySC maintenance. We cannot rule out the possibility that perdurance of the Gal80 protein might prevent full CySC rescue. However, ectopic activation of the JAK/STAT pathway cannot rescue CySCs lacking smo (Fig. 5C-C″′; Table 1).
Fig. 5.
Relationship between Hh and JAK/STAT signaling in CySC self-renewal. (A-A″′) No change in Stat92E expression is detected in positively labeled smo mutant cells (A,A″′; arrow indicates smo mutant clone) at 2 dpci. Boxed area in A is enlarged in A′-A″′. GFP is green (single channel in A′), Stat92E is red (single channel in A″) and Tj is blue (single channel in A″′). (B-B″′) Stat92E is stabilized in smo MARCM clones that express Hop. Red arrow indicates a smo mutant CySC and the green arrow indicates a differentiating cyst cell. GFP is green (single channel in B′), Vasa is red (single channel in B″) and Stat92E is blue (single channel in B″′). (C-C″′) Activation of Stat92E by overexpression of Hop cannot rescue loss of smo MARCM mutants from the stem cell niche at 7 dpci, despite the stabilization of Stat92E protein in these clones (see B,B″′). GFP is green (single channel in C′), Vasa is red (single channel in C″) and Tj is blue (single channel in C″′). (D-D″) ptc-lacZ (red; single channel in D″) expression is unaltered in negatively marked chinmo mutants (arrow points to a ptc-lacZ-expressing mutant CySC in D-D″). Boxed area in D is enlarged in single channels in D′,D″. chinmo clones lack GFP (single channel in D′). (E) Adjacent optical z-section to D, showing where the hub (red dotted outline) is located. (F-F″′) Ciact expression cannot rescue Stat92E MARCM mutant CySCs at 7 dpci, but differentiated cyst cells can be recovered (red arrow indicates marked cyst cell; clones were labeled with membrane-targeted GFP). See also Table 1. The position of the hub is indicated with an asterisk or outlined in the Tj single channel.
We also addressed the possibility that smo-deficient CySCs are being outcompeted from the niche by wild-type CySCs. It has been reported that niche competition is due to differential Integrin-based adhesion downstream of Stat92E (Issigonis et al., 2009). We tested this hypothesis by overexpressing Myospheroid (Mys; the Drosophila βPS-integrin homolog) in smo mutant MARCM clones. CySC recovery was not observed in this experiment (Table 1). Additionally, we asked whether DE-Cadherin-based adhesion was responsible for the loss of smo mutant CySCs. We expressed DE-Cadherin (called Shotgun in Drosophila) in smo mutant clones by the MARCM technique, but CySCs were not recovered at 7 dpci (Table 1). These two experiments strongly suggest that smo-deficient CySCs are lost as a direct consequence of a failure to self-renew and not as a result of niche competition.
We next tested whether readouts of Hh signaling were affected when the JAK/STAT pathway was disrupted. Since ptc-lacZ – the best-established readout of Hh signaling – and Stat92E reside on the same chromosome, we were unable to assess ptc levels in Stat92E clones. As a proxy, we examined ptc-lacZ in chinmo clones, as we previously showed that chinmo is key a functional effector of Stat92E in numerous cell types including CySCs (Flaherty et al., 2010). We found that ptc-lacZ was unchanged in chinmo mutant CySCs at 2 dpci, suggesting that Hh signaling is unaffected in CySCs lacking a key Stat92E functional effector (Fig. 5D-D″). Consistent with this observation, we were unable to obtain significant rescue of either Stat92E or chinmo mutant CySCs by ectopic activation of Hh signaling through misexpression of Ciact (Fig. 5F-F″′; Table 1). It should be noted that Stat92E and chinmo mutant CySCs differentiate very quickly (Leatherman and Dinardo, 2008; Issigonis et al., 2009; Flaherty et al., 2010), meaning that few differentiated cyst cells can be seen at 7 dpci (Table 1). By contrast, differentiated Stat92E and chinmo mutant cyst cells overexpressing Ciact are readily recovered (Fig. 5F-F″′, arrow; Table 1). This suggests that Hh activation can delay the loss of Stat92E- or chinmo-deficient CySCs, even though this is ultimately insufficient for their maintenance.
Furthermore, we were unable to detect a genetic interaction between hh and Stat92E. Animals that were transheterozygous for both mutations (hhts2/Stat92E85C9) appeared to have a normal complement of stem cells at the testis niche (data not shown). In addition, CySC numbers in controls (hhts2/+) and transheterozygous animals were not statistically different (means of 36 and 34, respectively; P<0.6). Thus, we conclude that the Hh and JAK/STAT pathways provide non-redundant signals for the self-renewal of CySCs.
Hh signaling does not contribute to CySC niche function
In a series of elegant experiments, Leatherman and DiNardo established that, in addition to being the source of cyst cells, CySCs have a second function, providing part of the ‘extended niche’ for GSCs (Leatherman and Dinardo, 2008; Leatherman and Dinardo, 2010). We made use of their model to examine whether Hh signaling contributes to this extended niche function of CySCs. As shown by Leatherman and Dinardo, Stat92Ets animals lose both CySCs and GSCs (Fig. 6A-B″). Both stem cell populations can be restored by resupplying wild-type Stat92E to the somatic lineage only (Fig. 6C-C″). We used tj-gal4 to express Ciact and activate Hh signaling in the somatic lineage of Stat92Ets mutant testes (Fig. 6D-D″). Strikingly, we observed that CySCs are rescued in large numbers and fill up the testis tip, as assessed by Zfh1 expression (and the lack of Eya). These data suggest, in accordance with our previous results, that the Hh and JAK/STAT pathways independently regulate CySC fate. Surprisingly, no GSCs or early germ cell cysts were present in these Stat92Ets testes somatically supplied with ectopic Hh signaling, indicating that CySCs with activated Hh signaling alone are not able to support GSC maintenance.
Stem cell markers are lost in Hop-induced tumors in the absence of Hh
Next, we asked whether Hh signaling is required for the stem cell tumors observed when Upd or Stat92E is hyperactivated (Kiger et al., 2001; Tulina and Matunis, 2001; Leatherman and Dinardo, 2008). Expressing HopTum-l, a dominant-active form of Hop (Hanratty and Dearolf, 1993), in the somatic lineage is sufficient to cause overproliferation of stem-like cells, both cell-autonomously in CySCs and non-cell-autonomously in GSCs (Leatherman and Dinardo, 2008) (compare Fig. 6E with 6F). Markers of stemness of both populations are visible, including the presence of Zfh1 and absence of Eya for CySCs, and small, rounded germ cells with dot- or dumbbell-shaped fusomes for GSCs (Fig. 6H-I). Expressing HopTum-l in the somatic lineage in an hhts2 mutant animal also gave rise to expanded testes (compare Fig. 6G with 6E). However, the expanded population of somatic cells in these testes expressed lower levels of Zfh1 and higher levels of Eya than controls, suggesting that they have initiated differentiation (Fig. 6J). Similarly, germ cells in hhts2 tumors are larger than in control tumors as they appear to be part of transit-amplifying cysts, and their fusomes display a branched morphology indicative of a more differentiated germ cell type (compare Fig. 6K with 6I). Thus, Hh is not required for tumor formation but is required for those tumors to be composed of stem cells.
DISCUSSION
We have shown that Hh from the Drosophila testis niche is a self-renewal factor for CySCs and that Hh signaling does not contribute to the role of CySCs as a niche for GSCs. This supports the model that the Hh and JAK/STAT pathways act independently within CySCs (Fig. 6L). Our results therefore confirm those recently reported by another group (Michel et al., 2012), who showed that Hh regulates CySC self-renewal, and extend their results by demonstrating the genetic independence of Hh and the other pathway (i.e. JAK/STAT) that is crucial in CySC function.
Separating niche and stem cell roles
It is notable that two signals regulate CySC self-renewal but only JAK/STAT signaling contributes to the GSC niche. Moreover, despite the drastic reduction in CySCs in hhts2 testes (from ∼36 in controls to ∼8), GSCs do remain in hh mutant animals albeit at reduced numbers. The reduction in GSCs in hh mutants is not due to changes in the size of the hub. These data suggest that most CySCs are dispensable for their niche function and that only a few BMP-producing CySCs are needed to maintain GSC self-renewal. This raises the question as to whether, in a wild-type animal, there are distinct populations of CySCs, some with activated Stat92E that produce BMPs and act as a niche for GSCs, and others with activated Hh signaling that participate only in self-renewal and the production of cyst progeny. This is consistent with the fact that, despite the presence of ∼36 Zfh1-positive CySCs, elevated Stat92E is only seen in a few CySCs (Fig. 5A-B″′) (Leatherman and Dinardo, 2008; Flaherty et al., 2010). However, it is also conceivable that all Zfh1-positive CySCs are equivalent and that high Stat92E correlates, for instance, with a specific phase of the cell cycle, such as the repositioning of the spindle during anaphase that brings the nucleus of the CySC closer to the hub interface (Cheng et al., 2011) and might expose that CySC to more Upd ligand. This possibility implies a much more dynamic stem cell niche for the GSCs than has been previously appreciated.
Multiple signals for homeostasis
Our results indicate that the Hh and JAK/STAT pathways act mostly in parallel, although activating Hh may delay the differentiation of CySCs that are deficient for JAK/STAT pathway components. It is unclear why the CySC would require both signaling inputs to be maintained. However, it should be noted that these inputs contribute different information, as JAK/STAT signaling imparts niche potential (Leatherman and Dinardo, 2008; Flaherty et al., 2010; Leatherman and Dinardo, 2010), and Hh signaling additionally ensures that the right number of CySCs are present and provide cyst cells for normal spermatogonial development (this study) (Michel et al., 2012). Future work will establish whether self-renewal in CySCs depends on two sets of genes controlled separately by the Hh and JAK/STAT pathways or whether they converge on the same targets. The first possibility is supported by the fact that Hh does not contribute to the niche function of STAT in CySCs, indicating that different targets (presumably BMPs) are regulated differently.
Conservation of the role of Hh
One consequence of this work is to lead us to re-evaluate the differences between male and female gonad development in Drosophila (Losick et al., 2011). Indeed, Hh signaling is an essential regulator of the self-renewal and the number of follicle stem cells, the offspring of which carry out a comparable function to cyst cells by ensheathing germ line cysts (Forbes et al., 1996; Zhang and Kalderon, 2000; Zhang and Kalderon, 2001). In the ovary, as in the testis, JAK/STAT signaling in somatic cells is required for the maintenance of GSCs via BMP production (López-Onieva et al., 2008; Leatherman and Dinardo, 2010). However, in the ovary, the escort cells and cap cells are the JAK/STAT-responsive niche cells (López-Onieva et al., 2008; Wang et al., 2008), implying that CySCs in the male gonad fulfil the function of two cell types in the female gonad and require both the signals used in the female to do so. Finally, our data evoke the interesting possibility that Hh has a conserved ancestral role in male gonads. Mutation in one of the three mammalian hh homologs, desert hedgehog (Dhh), causes male sterility (Bitgood et al., 1996) and a loss of somatic support cells called Leydig cells (Clark et al., 2000; Yao et al., 2002). However, the cellular niche for spermatogenesis in mammals is less well understood than in Drosophila and it remains to be established whether the Hh pathway orchestrates similar cellular functions.
Supplementary Material
Acknowledgments
We thank G. Struhl, D. Godt, R. Lehmann, I. Guerrero, J. Treisman, D. Kalderon, R. Holmgren, F. Schöck, T. Harris, J. Skeath, the DSHB and the Bloomington Stock Center for flies and antibodies; Y. Mavromatakis for helpful comments on the manuscript; the NYU fly community for discussion; and we are very grateful to Christian Bökel for communicating results.
Footnotes
Funding
This work was supported by grants from the National Institutes of Health [R01-GM085075] and New York State Department of Health (NYSTEM) [C024284] to E.A.B. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.086413/-/DC1
References
- Alcedo J., Ayzenzon M., Von Ohlen T., Noll M., Hooper J. E. (1996). The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86, 221–232 [DOI] [PubMed] [Google Scholar]
- Arbouzova N. I., Zeidler M. P. (2006). JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133, 2605–2616 [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P., Ramírez-Weber F. A., Laget M. P., Schwartz C., Kornberg T. B. (1997). Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 89, 1043–1053 [DOI] [PubMed] [Google Scholar]
- Azpiazu N., Lawrence P. A., Vincent J. P., Frasch M. (1996). Segmentation and specification of the Drosophila mesoderm. Genes Dev. 10, 3183–3194 [DOI] [PubMed] [Google Scholar]
- Bitgood M. J., Shen L., McMahon A. P. (1996). Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 6, 298–304 [DOI] [PubMed] [Google Scholar]
- Chen D., McKearin D. (2003). Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr. Biol. 13, 1786–1791 [DOI] [PubMed] [Google Scholar]
- Chen Y., Struhl G. (1996). Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553–563 [DOI] [PubMed] [Google Scholar]
- Chen H. W., Chen X., Oh S. W., Marinissen M. J., Gutkind J. S., Hou S. X. (2002). mom identifies a receptor for the Drosophila JAK/STAT signal transduction pathway and encodes a protein distantly related to the mammalian cytokine receptor family. Genes Dev. 16, 388–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Tiyaboonchai A., Yamashita Y. M., Hunt A. J. (2011). Asymmetric division of cyst stem cells in Drosophila testis is ensured by anaphase spindle repositioning. Development 138, 831–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. M., Garland K. K., Russell L. D. (2000). Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol. Reprod. 63, 1825–1838 [DOI] [PubMed] [Google Scholar]
- de Cuevas M., Matunis E. L. (2011). The stem cell niche: lessons from the Drosophila testis. Development 138, 2861–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decotto E., Spradling A. C. (2005). The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev. Cell 9, 501–510 [DOI] [PubMed] [Google Scholar]
- DiNardo S., Okegbe T., Wingert L., Freilich S., Terry N. (2011). lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development 138, 1687–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez M., Brunner M., Hafen E., Basler K. (1996). Sending and receiving the hedgehog signal: control by the Drosophila Gli protein Cubitus interruptus. Science 272, 1621–1625 [DOI] [PubMed] [Google Scholar]
- Ekas L. A., Baeg G. H., Flaherty M. S., Ayala-Camargo A., Bach E. A. (2006). JAK/STAT signaling promotes regional specification by negatively regulating wingless expression in Drosophila. Development 133, 4721–4729 [DOI] [PubMed] [Google Scholar]
- Flaherty M. S., Salis P., Evans C. J., Ekas L. A., Marouf A., Zavadil J., Banerjee U., Bach E. A. (2010). chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev. Cell 18, 556–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A. J., Lin H., Ingham P. W., Spradling A. C. (1996). hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 122, 1125–1135 [DOI] [PubMed] [Google Scholar]
- Gönczy P., DiNardo S. (1996). The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122, 2437–2447 [DOI] [PubMed] [Google Scholar]
- Goodrich L. V., Johnson R. L., Milenkovic L., McMahon J. A., Scott M. P. (1996). Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 10, 301–312 [DOI] [PubMed] [Google Scholar]
- Hanratty W. P., Dearolf C. R. (1993). The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol. Gen. Genet. 238, 33–37 [DOI] [PubMed] [Google Scholar]
- Hidalgo A., Ingham P. (1990). Cell patterning in the Drosophila segment: spatial regulation of the segment polarity gene patched. Development 110, 291–301 [DOI] [PubMed] [Google Scholar]
- Inaba M., Yuan H., Yamashita Y. M. (2011). String (Cdc25) regulates stem cell maintenance, proliferation and aging in Drosophila testis. Development 138, 5079–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham P. W., Taylor A. M., Nakano Y. (1991). Role of the Drosophila patched gene in positional signalling. Nature 353, 184–187 [DOI] [PubMed] [Google Scholar]
- Issigonis M., Tulina N., de Cuevas M., Brawley C., Sandler L., Matunis E. (2009). JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science 326, 153–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D. (2005). The mechanism of hedgehog signal transduction. Biochem. Soc. Trans. 33, 1509–1512 [DOI] [PubMed] [Google Scholar]
- Kawase E., Wong M. D., Ding B. C., Xie T. (2004). Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 131, 1365–1375 [DOI] [PubMed] [Google Scholar]
- Kiger A. A., Jones D. L., Schulz C., Rogers M. B., Fuller M. T. (2001). Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542–2545 [DOI] [PubMed] [Google Scholar]
- Leatherman J. L., Dinardo S. (2008). Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 3, 44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman J. L., Dinardo S. (2010). Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat. Cell Biol. 12, 806–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 [DOI] [PubMed] [Google Scholar]
- López-Onieva L., Fernández-Miñán A., González-Reyes A. (2008). Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development 135, 533–540 [DOI] [PubMed] [Google Scholar]
- Losick V. P., Morris L. X., Fox D. T., Spradling A. (2011). Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev. Cell 21, 159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Zhou Y., Beachy P. A., Moses K. (1993). The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell 75, 927–938 [DOI] [PubMed] [Google Scholar]
- Michel M., Kupinski A. P., Raabe I., Bökel C. (2012). Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development 139, 2663–2669 [DOI] [PubMed] [Google Scholar]
- Morris L. X., Spradling A. C. (2011). Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 138, 2207–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzny C. K., Holmgren R. (1995). The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech. Dev. 52, 137–150 [DOI] [PubMed] [Google Scholar]
- Price M. A., Kalderon D. (1999). Proteolysis of cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development 126, 4331–4339 [DOI] [PubMed] [Google Scholar]
- Shivdasani A. A., Ingham P. W. (2003). Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr. Biol. 13, 2065–2072 [DOI] [PubMed] [Google Scholar]
- Spradling A., Fuller M. T., Braun R. E., Yoshida S. (2011). Germline stem cells. Cold Spring Harb. Perspect. Biol. 3, a002642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T., Kornberg T. B. (1994). Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 76, 89–102 [DOI] [PubMed] [Google Scholar]
- Tulina N., Matunis E. (2001). Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546–2549 [DOI] [PubMed] [Google Scholar]
- van den Heuvel M., Ingham P. W. (1996). smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature 382, 547–551 [DOI] [PubMed] [Google Scholar]
- Wang L., Li Z., Cai Y. (2008). The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J. Cell Biol. 180, 721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T., Spradling A. C. (1998). decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251–260 [DOI] [PubMed] [Google Scholar]
- Yao H. H., Whoriskey W., Capel B. (2002). Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 16, 1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Kalderon D. (2000). Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development 127, 2165–2176 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kalderon D. (2001). Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599–604 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.